Stabilization of ruthenium nanoparticles over NiV-LDH surface for enhanced electrochemical water splitting: an oxygen vacancy approach†
Abstract
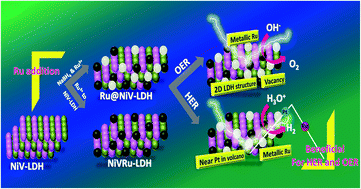
In a search of alternates for oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), out of various transition metal-based electrocatalysts, layered double hydroxide (LDH) based materials have attracted more attention. Engineering them via tri- and tetra metallic LDHs have been crucially studied for expanding them as suitable electrocatalysts for OER. However, their poor electronic conductance towards HER still lacks in LDHs. Here, for the first time, we developed a new way by the addition of trimetallic Ru3+ and metallic Ru(0) to the NiV-LDH system that exhibits outstanding alkaline OER and acidic HER activities. To reach 10 mA cm−2 current density for OER and HER, Ru@NiV-LDH demands only 272 and 135 mV overpotential, respectively, with an ultra-low mass loading of just 0.1 mg cm−2. In comparison to bare NiV-LDH with the same loading, Ru@NiV-LDH required a less overpotential value of 118 and 289 mV to achieve 10 mA cm−2 current density for OER and HER, respectively. The successful formation of oxygen vacancy led to the confinement of Ru NPs, which leads to a low coagulation of the same and hence provides a greater number of active sites towards HER. We also claim our findings with the first principles density functional theory (DFT) approach for the successful addition of metallic Ru in vacancy sites. Moreover, it was observed that during Ru(0) addition to the LDH surface, an oxygen-rich vacancy surface is generated that enhances the OER and HER activities. It also revealed that the formation of oxygen vacancy stabilized the LDH surface when different Ru clusters are added by adsorption energy value of 3.8 ± 0.5 eV. Further, the total density of state (TDOS) ensured a greater number of electronic states near the Fermi level for Ru@NiV-LDH that enhanced the electrochemical activity. Further, it also makes the catalyst a better candidate for the formation of active intermediate for OER, as can be seen from the calculated ΔG values for intermediate formation.
- This article is part of the themed collection: 2022 Journal of Materials Chemistry A Most Popular Articles


 Please wait while we load your content...
Please wait while we load your content...