 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A steric hindrance alleviation strategy to enhance the photo-switching efficiency of azobenzene functionalized metal–organic frameworks toward tailorable carbon dioxide capture†
Qi
Huang
 a,
Junju
Mu
b,
Zhen
Zhan
d,
Feng
Wang
a,
Junju
Mu
b,
Zhen
Zhan
d,
Feng
Wang
 *b,
Shangbin
Jin
*c,
Bien
Tan
*b,
Shangbin
Jin
*c,
Bien
Tan
 *d and
Chunfei
Wu
*d and
Chunfei
Wu
 *a
*a
aSchool of Chemistry and Chemical Engineering, Queen's University Belfast, Belfast, BT7 1NN, UK. E-mail: c.wu@qub.ac.uk
bState Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023, China. E-mail: wangfeng@dicp.ac.cn
cSchool of Chemical Engineering and Technology, Xi'an Jiaotong University, China. E-mail: shangbin@xjtu.edu.cn
dSchool of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Luoyu Road No. 1037, Wuhan, 430074, China. E-mail: bien.tan@mail.hust.edu.cn
First published on 4th March 2022
Abstract
Photo-switching metal–organic frameworks are widely reported for low energy CO2 capture and release. However, owing to the steric hindrance caused by dense packing of MOF solids, the photo-switching efficiency was still severely restricted. Such an issue then further causes low CO2 switching capacity and poor regeneration of MOF adsorbents. Herein, we present a strategy to tailor the photo-switching efficiency of azobenzene functionalized MOFs via a steric hindrance alleviation approach. An azobenzene-containing Zn based MOF, U-mazo, was designed to decrease the steric hindrance of azo benzene pendants in U-pazo (PCN-123). For comparison, two MOFs without azobenzene, IRMOF-3 and CMOF-2, were also fabricated. Results suggested that compared to U-pazo, the cis isomer content in U-mazo increased by 50% upon UV light irradiation at 365 ± 10 nm, which contributed to about 34% enhancement of CO2 switching efficiency. Density functional theory calculations further explained that the optimized switching efficiency of U-mazo resulted from the lower energy cost for trans/cis isomerization of azo benzene pendants. Thereby, a promising strategy for optimizing the switching efficiency of the present photoresponsive MOF is explored and verified, and the structural steric hindrance of photoswitching units plays an important role in isomerization of azobenzene-containing MOFs.
Introduction
External stimuli such as light, temperature, pH or magnetic fields can trigger the release of stored substances from host materials, which has been widely researched.1–3 Among them, light responsive materials have attracted extensive interest owing to their high controllability with instantaneous responsiveness and high accuracy.4–6 Metal–organic frameworks (MOFs), as 3D crystalline porous organic–inorganic hybrid materials, have been utilized as ideal host materials for CO2 capture owing to their high porosity and surface area as well as structural tunability and chemical versatility.7–9 However, the high energy penalty related to MOF adsorbent regeneration through traditional gas liberation technologies strictly limited their large scale application.10Therefore, photo-responsive MOFs have been developed to overcome this challenge by triggering the release of CO2 under abundant and renewable light.11–13 Considering the high degree of structural tunability of MOFs, the integration of photochromic units such as azobenzene,14 diarylethene15 and spiropyran16 into MOFs as pendant groups,17 backbones18 or guest molecules19 has been reported to confer photo-switching features to MOFs. Particularly, azobenzene is among the most popular candidates for switching units, not only because of its versatile and simple integration approach,20 but also owing to its capability of providing alternative CO2 capture sites.21 Although many photo-responsive MOFs for low energy CO2 capture and release have been reported,22–24 the switching extent and efficiency were still sterically resisted by intermolecular interactions in dense packing of MOF solids.25 For Instance, Aida et al. reported an azobenzene-containing Zr-based AzoMOF. But only 21% of the AzoMOF was able to be converted to its cis state via UV exposure for enough time, which led to a 15% decrease of CO2 adsorption.26 Besides, many researchers reported that light triggered CO2 release was still restricted by the switching extent of photochromic units in frameworks.27–29 Some efforts have been made to enhance the CO2 switching efficiency, such as introducing CO2 target active sites, amine, into azo functionalized MOFs30 or constructing a dual stimuli responsive MOF system with both magnetic and light induction.31 Nevertheless, these methods cause more complexity of the light responsive system and higher difficulty of preparation, and then increase the cost and energy consumption, which is contrary to the original intention of designing photo-responsive MOFs. Therefore, a new strategy without increasing the cost and complexity of the light responsive system is in significant demand.
Herein, we employed a new strategy to enhance the photo-switching efficiency by tuning the steric hindrance of azo benzene pendants in U-pazo (PCN-123). A series of Zn-based MOFs with and without azobenzene functionalization were fabricated for controllable CO2 capture. The key to the strategy lies in the tuning of carboxyl acid groups sites on the azo-containing linkers-U-mazoL, which could alleviate the steric hindrance of azobenzene isomerization (Scheme 1). Such a structural tuning strategy was applied to enhance the photo-switching efficiency of U-pazo without increasing the cost and complexity of the light responsive system. The results suggested that integration of azobenzene pendant groups improved the CO2 capture capacity of U-mazo and U-pazo and endowed them with photo-switching features. Besides, the lower steric hindrance of U-mazoL contributed to the higher switching extent of U-mazo, and finally resulted in a remarkable improvement of CO2 switching efficiency up to 43% under static conditions. In addition, density functional theory calculation results suggested that ΔE (trans to cis conversion) of U-mazo was lower than that of U-pazo, which indicated a preferable trend for trans to cis conversion in U-mazo and further supported the stronger switching ability of U-mazo. The switching property was characterized by UV-visible spectroscopy before and after UV irradiation. The tailorable CO2 capture performance was demonstrated by adsorption isotherms of CO2.
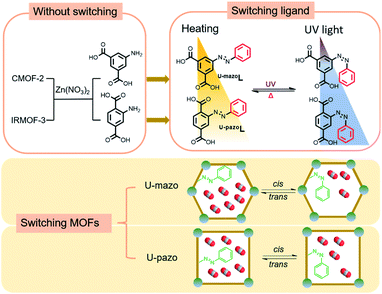 | ||
| Scheme 1 Trans-to-cis isomerization of the ligand of U-mazo and U-pazo trigged by UV irradiation and the cis-to-trans transition triggered by heat treatment. | ||
Experimental section
Materials and methods
Dimethyl 5-aminoisophthalate, nitrosobenzene, dimethyl aminoterephthalate, Zn(NO3)2·6H2O, sodium hydrogen carbonate, anhydrous magnesium sulfate, hydrochloric acid, glacial acetic acid and other common solvents were obtained from commercial suppliers such as Sigma-Aldrich without any further purification.Fourier-Transformed Infrared (FT-IR) spectra were measured using a Bruker Vertex 70 FTIR spectrometer. The sample was first collected on dried KBr disks, and the spectral range was 500–4000 cm−1. Ultraviolet-visible spectra were obtained by using a UV-VIS-NIR spectrophotometer (UV-3600, Shimadzu Japan) at room temperature. BaSO4 was used as the sample base, and the wavenumber range was set from 200 to 800 cm−1. 1H NMR experiments were performed to check the chemical structure and photo-responsive properties of linkages by using a 400 MHz Bruker with an automated tune and match multi nuclei probe. N2 and CO2 adsorption and desorption isotherms were obtained by using a Micromeritics ASAP 2020 M analyzer at 273 K. The temperature was maintained by a bath with refrigeration equipment. Before measurement, the samples were degassed under vacuum conditions (10−5 bar) at 80 °C for 8 hours. The CO2 adsorption heat of U-mazo was calculated from the adsorption isotherm at temperatures of 273 K and 298 K. Thermal stabilities of the samples were measured by Thermogravimetric analysis (TGA) with a PerkinElmer Pyris TGA. The test was performed under conditions of a N2 atmosphere with a heating rate of 10 °C min−1 from room temperature to 800 °C. Powder X-ray diffraction (XRD) was conducted on a Philips X′ Pert Pro. High resolution images of MOF morphologies were measured by using a FEI Sirion 200 field emission scanning electron microscope (FE-SEM).
Synthesis of U-mazo, U-pazo, IRMOF-3 and CMOF-2
A mixture of Zn(NO3)2·6H2O (1.8 mmol), U-mazoL/5-aminoisophthalic acid (0.6 mmol) and 50 mL DMA was sealed in a 100 mL Teflon-lined autoclave. At the same time, Zn(NO3)2·6H2O (1.8 mmol), U-pazoL/2-aminoterephthalic acid (0.6 mmol) and DMF (50 mL) were sealed in a 100 mL Teflon-lined autoclave. The two reaction systems were heated at 85 °C for 3 days at the same time. After cooling down the reaction system, the four product crystals were collected by filtration, and then washed with DMF and CH2Cl2 to get the final products U-mazo, CMOF-2, U-pazo and IRMOF-3, respectively.Characterization of photoisomerization in linkages and MOFs
Measurement of the cis isomer content in U-mazoL and U-pazoL: the samples were dissolved with DMSO-d6 in NMR tubes and then irradiated with UV LED strips (360 nm ± 10 nm) followed by heating with different time segments. The sampling tubes were shortly tested for the 1H NMR spectra to determine the cis isomer content. As shown in Fig. 2a and b, the signal at δ7.4 is assigned to the trans isomer of U-pazoL while those new blue peaks at δ6.8 correspond to the cis isomer. As for U-mazoL, the trans isomer signal was at δ8.6 while the cis isomer signal was at δ8.1. Therefore, the cis isomer content was defined as Icis/(Itrans + Icis).CO2 switching adsorption and desorption cycles: the original sample was first degassed under vacuum conditions (10−5 bar) at 80 °C for 8 hours and the CO2 adsorption and desorption isotherm test was carried out by using a Micromeritics ASAP 2020 M analyzer. After this test, the sample was sandwiched as a thin film between two quartz glass plates and then was uniformly irradiated with UV light from both directions. After 5 hour UV light irradiation, the sample was collected for the further ASAP CO2 adsorption and desorption test without the heating procedure. After finishing the test, the sample was degassed with heating for 8 hours again and then evaluated again by ASAP for the reversibility test. Such a process was repeated for three cycles for CO2 switching isomerization cycles of U-mazo.
Photoisomerization cycles of UV-Vis absorption spectra: BaSO4 was used as a reference sample. First, the sample was added on the surface of BaSO4 and tested for UV-Vis absorption spectra. The sample plate was then irradiated with UV light for 5 hours and then directly tested via UV-Vis spectroscopy again for characterization of trans to cis switching. After the UV-Vis spectroscopy test, the sample plate was then transferred to a vacuum oven for heating at 80 °C. After heating, the sample plate was tested via UV-Vis spectroscopy again for characterization of cis to trans back switching. Such a process was then repeated for five cycles for photoisomerization cycles of U-mazo.
Results and discussion
In this work, two azobenzene functionalized MOFs, U-mazo and U-pazo, were synthesized by a hydrothermal method, and CMOF-2 and IRMOF-3 were fabricated as the control samples without azobenzene. Compared to IRMOF-3 and CMOF-2, the obvious red-orange colour of U-mazo and U-pazo directly indicated the presence of azobenzene pendants in the frameworks (Fig. S2 in the ESI†). Besides, the incorporation of azobenzene bonding in U-mazo and U-pazo was further supported via the FTIR spectra (Fig. S3a in the ESI†) where the characteristic peaks at 1444 cm−1 corresponded to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonding of azo groups. For CMOF-2, the peaks centered at 1430, 1530 and 1610 cm−1 were attributed to the C
N bonding of azo groups. For CMOF-2, the peaks centered at 1430, 1530 and 1610 cm−1 were attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of azo phenyl groups. In addition, the two sharp bands around 1572 and 1362 cm−1 are attributed to the asymmetric (υs(C–O)) and symmetric (υs(C–O)) vibrations of carboxyl groups, respectively. Powder X-ray diffraction patterns (Fig. 1) were applied to estimate the crystal structure of the MOFs. Since the azobenzene group was relatively bulky, the integration of the groups could impact the crystallization process and then led to the different crystalline structures of U-mazo and CMOF-2. For the same reason, U-pazo and IRMOF-3 also suggested different crystalline structures. However, all peaks of U-mazo and U-pazo were completely consistent with reported PCN-123 (ref. 32) and JUC-128 (ref. 33) patterns that were synthesized using the same linkers and metals. It is demonstrated that the correct structure of U-mazo and U-pazo was developed.
C of azo phenyl groups. In addition, the two sharp bands around 1572 and 1362 cm−1 are attributed to the asymmetric (υs(C–O)) and symmetric (υs(C–O)) vibrations of carboxyl groups, respectively. Powder X-ray diffraction patterns (Fig. 1) were applied to estimate the crystal structure of the MOFs. Since the azobenzene group was relatively bulky, the integration of the groups could impact the crystallization process and then led to the different crystalline structures of U-mazo and CMOF-2. For the same reason, U-pazo and IRMOF-3 also suggested different crystalline structures. However, all peaks of U-mazo and U-pazo were completely consistent with reported PCN-123 (ref. 32) and JUC-128 (ref. 33) patterns that were synthesized using the same linkers and metals. It is demonstrated that the correct structure of U-mazo and U-pazo was developed.
Scanning electron microscopy (Fig. S4 in the ESI†) was employed to image the MOF samples. Both IRMOF-3 and U-pazo showed cubic crystals with particle sizes relatively larger than IRMOF-3. In addition, U-mazo and CMOF-2 indicated similar rod shapes. Therefore, the particle shape of either U-mazo or U-pazo was not influenced by the incorporation of azobenzene. To further investigate the elemental distribution of U-mazo and U-pazo, SEM mapping was also performed (Fig. S5 in the ESI†). Both U-mazo and U-pazo indicated uniform distribution of N, O, and Zn. Thermogravimetric (TGA) analysis was conducted to characterize the thermal stability of the MOF samples. As shown in Fig. S6,† each sample showed two steps of mass loss in TGA curves. The first and second stages of mass loss of these samples could be attributed to the dissolution of MOF frameworks and decomposition of linkages, respectively. The first mass loss of U-mazo and U-pazo starts at 200 and 170 °C, respectively, indicating lower thermal stability than CMOF-2 and IRMOF-3. Therefore, the incorporation of azobenzene pendants caused lower thermal stability of MOF frameworks of U-mazo and U-pazo. In addition, the second mass loss stage of U-mazo and U-pazo also occurred earlier than CMOF-2 and IRMOF-3, which was ascribed to the easier decomposition of azo groups of ligands under high temperature.
Photoswitching properties of linkages of U-mazo and U-pazo were characterized and compared using 1H NMR spectra in DMSO-d6 (Fig. 2a and b). Both original U-mazoL and U-pazoL (black coloured spectrum) were converted to the cis isomer (blue coloured spectrum) upon irradiation with UV light (360 ± 10 nm), and then underwent isomerization back to the trans form (red coloured spectrum) via simple heating. It is suggested that the isomerization process is reversible. However, as shown in Fig. S7 in the ESI,† the switching rate and extent of the two linkages were different. The trans to cis transition of U-mazoL upon UV exposure was established in 1 hour with a cis isomer content of 53%, while only 37% cis isomer was obtained for U-pazoL at the same time. Such results demonstrated the higher switching efficiency of U-mazoL, which was attributed to the lower steric hindrance of azobenzene in U-mazoL. Therefore, such a steric hindrance alleviation strategy by tuning carboxyl acid groups sites can successfully enhance the photoswitching efficiency of U-pazoL. However, after the coordination reaction, the switching condition in the solid state of frameworks could be totally different with free molecules dissolved in solution. The photo-switching efficiency of U-mazo and U-pazo as MOF solids also needs to be further investigated and compared.
The switching properties of U-mazo (Fig. 2c) and U-pazo (Fig. S8 in the ESI†) were also characterized and compared via UV/Vis spectroscopy. Under UV light irradiation, both U-mazo and U-pazo showed an enhancement of the n–π* transition band between 400 and 500 nm, corresponding to the occurrence of the trans to cis transition of azobenzene pendants in the frameworks. Through subsequent heating at 80 °C, the azobenzene pendants were converted back to trans isomers, indicating a reversible isomerization process of U-mazo and U-pazo, respectively. More importantly, a higher switching content of U-mazo was also observed in the UV/Vis spectra, which was consistent with the results of linkages. In detail, the photostationary state of U-mazo upon UV exposure was established at 14% cis isomer while U-pazo only achieved 8% cis isomer under the same conditions. Apart from that, such forward and backward photo-isomerization processes could be recycled three times with a slight decrease in second repeats and swiftly achieve stability for the subsequent cycles. These results further support that the lower steric hindrance of azobenzene side groups can greatly contribute to an optimized photo-switching efficiency of both U-mazoL and U-mazo.
The CO2 uptake ability of U-mazo and CMOF-2 was first investigated here at 273 K and 298 K. In addition, U-pazo and IRMOF-3 were also compared under the same conditions to explore the influence of the introduction of azobenzene groups on CO2 capture performance. As shown in Fig. 3a, the CO2 uptake capacity of U-mazo was far higher than that of its non-azo benzene functionalized CMOF-2, increasing around 7 times at 273 K and 1 bar. The same trend can also be observed for U-pazo and CMOF-2: azo benzene-containing U-pazo demonstrated around 4 times higher CO2 uptake capacity than IRMOF-3. These results suggested an enhanced CO2 adsorption performance of the MOF frameworks in the presence of azobenzene pendants, which was consistent with previous investigations.21
Notably, compared to previously reported U-pazo (PCN-123), U-mazo developed in this work exhibited an optimized dynamic photo-switching CO2 adsorption behavior upon trans/cis isomerization. After 5 hours of 360 nm UV light irradiation, the CO2 uptake capacity of U-mazo decreased from 0.83 to 0.49 mmol g−1 upon trans to cis isomerization, indicating an up to 43% decrease in CO2 uptake (Fig. 3c). In addition, such adsorption capacity could be recovered to its original state by simple heating at 80 °C, corresponding to a reversible CO2 switching process. However, U-pazo only showed a 32% decline of CO2 adsorption capacity upon UV exposure (Fig. 3d), reflecting an optimized CO2 switching efficiency of U-mazo. Such superior CO2 switching performance of U-mazo was comparable to those of many reported photo-responsive materials (Table S2 in the ESI†). Apart from that, the CO2 adsorption isotherm of CMOF-2 and IRMOF-3 under UV irradiation was also determined for controlled experiments (Fig. S9 in the ESI†). The results suggested that CO2 capture ability was still similar even under 5 hour UV irradiation, which demonstrated that the CO2 switching performance of U-mazo and U-pazo resulted from the incorporation of photochromic units. Therefore, azobenzene functionalization in U-mazo and U-pazo not only leads to a higher CO2 adsorption capacity but also endows them with tailorable CO2 capture ability. Moreover, the lower steric hindrance of azobenzene pendants in U-mazo led to faster and superior photoisomerization properties and then contributed to the enhanced CO2 switching efficiency of U-mazo.
ΔE values upon trans/cis isomerization of U-mazo and U-pazo were calculated by using the density functional theory method to support the experimental results. The ΔE(trans to cis) of U-mazo and U-pazo was calculated to be 47.2 and 54.2 kJ mol−1, respectively. The lower ΔE(trans to cis) of U-mazo confirmed the relatively easier trans to cis transition of azobenzene units in U-mazo, which further supported the greater switching extent and enhanced CO2 switching efficiency of U-mazo.
Regeneration of U-mazo was also studied as shown in Fig. 3e. The CO2 switching process upon trans/cis isomerization was performed three times, with a slight decrease of switching efficiency, indicating the reversibility and stability of the present adsorbents upon continuous UV exposure. In addition, the FTIR spectrum (Fig. S10 in the ESI†) showed minor changes even after 15 hours of UV irradiation, further confirming the structural stability of U-mazo. Since CO2 heat adsorption (Qads) is related to the interaction strength between CO2 molecules and MOF adsorbents, the Qads of U-mazo under its UV irradiated and non-irradiated state was also estimated to explore the mechanism for light induced CO2 switching behavior. As shown in Fig. 3f, the original Qads of U-mazo was around 31 kJ mol−1 in the trans isomer state, but the value decreased to 17 kJ mol−1 under UV light irradiation. Thus, the CO2 molecule showed weaker affinity toward the U-mazo framework in the UV irradiated cis state than its non-irradiated trans state. This explains the dramatic decrease of CO2 uptake capacity upon UV exposure, achieving a tailorable CO2 capture approach with low energy.34
N2 adsorption isotherms of all MOF samples were used for characterizing the CO2/N2 selectivity. Surprisingly, a clear decrease of N2 adsorption capacity of both U-mazo and U-pazo was observed upon the trans to cis isomerization of azobenzene units. These results further confirmed that steric hindrance played an important role in causing the CO2 adsorption capacity swings of U-mazo and U-pazo. In addition, the adsorption isotherms of U-mazo and U-pazo showed relatively low capacities for N2 adsorption (Fig. 4), indicating preferable binding with CO2 molecules and high selectivity for CO2 over N2. Compared to CMOF-2 and IRMOF-3 (Fig. S11 in the ESI†), the CO2/N2 selectivity of U-mazo and U-pazo was dramatically improved, indicating another advantage of the incorporation of azobenzene units. Apart from high CO2/N2 selectivity, a light induced selectivity swing was also observed. Henry's constants were used to estimate the CO2/N2 selectivity of U-mazo and U-pazo. It is suggested that U-mazo has a CO2/N2 selectivity of 334 in the trans state and 226 in the cis state at 273 K, indicating a light induced CO2/N2 selectivity swing. Interestingly, after 5 hour UV irradiation, U-pazo showed an increase of CO2/N2 selectivity from 100 to 148 at 273 K. Therefore, CO2/N2 selectivity could also be adjusted by remote light control during gas separation.
 | ||
| Fig. 4 CO2 and N2 adsorption isotherms of (a) U-mazo and (b) U-pazo at 273 K and the isotherms right after the first UV irradiation at 365 ± 10 nm (blue). | ||
As the performance of CO2 and N2 uptake is largely connected to pore properties, the BET surface area and pore size distributions of U-mazo and U-pazo in cis and trans isomer states were determined via N2 adsorption isotherms obtained at 77 K. As shown in Table S11 and Fig. S12,† both U-mazo and U-pazo indicated a relatively low BET surface area. The obtained CO2 uptakes could come from the restricted pores in which it is hard for N2 molecules to enter. However, the influence of isomerization on porosity changes can still be observed. Both U-pazo and U-mazo suggested a decrease of BET surface area and pore volume upon trans to cis isomerization, which then could lead to the decrease of CO2 and N2 uptake capacities. Apart from that, the pore size distribution of U-mazo and U-pazo also changed after the UV light irradiation. As shown in Fig. S13,† pristine U-mazo contained a small content of micropores. After the UV light irradiation, the micropore volume obviously decreased, which resulted from the light-induced isomerization of azo ligands. In addition, the UV light irradiation of U-pazo led to a decrease of micropore volume, which then could contribute to the decline of CO2 and N2 uptake capacities. These results demonstrate that photoisomerization of ligands in U-mazo and U-pazo could lead to pore size distribution changes, and then contribute to tailorable CO2 and N2 capture.
Conclusion
In summary, a series of Zn-based MOFs were fabricated, including U-mazo and U-pazo, which were functionalized with azobenzene groups to effectively tailor the CO2 capture properties. Experimental results indicated that the incorporation of azobenzene pendants not only enhanced their capacity and selectivity of CO2 adsorption but also endowed them with tailorable CO2 capture ability. More importantly, the lower steric hindrance of photochromic units in U-mazo contributed to enhanced higher CO2 switching efficiency, which was supported by the DFT calculation analysis. In addition, both U-mazo and U-pazo exhibited a high adsorption selectivity for CO2 over N2, and such selectivity could be switched upon trans/cis isomerization. Therefore, the enhanced tailorable CO2 capture combined with high selectivity via a structural strategy gives them more potential for gas separation in a realistic application. A promising strategy is then successfully developed to improve the CO2 switching efficiency of photoresponsive MOFs, promoting practical applications of using photo-switching MOFs for CO2 capture.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors sincerely appreciate the financial support from the China Scholarship Council. This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska–Curie grant agreement no. 823745.Notes and references
- J. Ge, E. Neofytou, T. J. Cahill, III, R. E. Beygui and R. N. Zare, ACS Nano, 2012, 6, 227–233 CrossRef CAS PubMed.
- R. P. Johnson, Y. Jeong, E. Choi, C. Chung, D. H. Kang, S. Oh, H. Suh and I. Kim, Adv. Funct. Mater., 2012, 22, 1058–1068 CrossRef CAS.
- M. Ishaq, M. A. Gilani, I. Arshad, M. R. Bilad, F. Ahmad and A. L. Khan, Carbon Capture Sci. Technol., 2021, 1, 100019 CrossRef.
- A. Modrow, D. Zargarani, R. Herges and N. Stock, Dalton Trans., 2011, 40, 4217–4222 RSC.
- R. Haldar, L. Heinke and C. Woll, Adv. Mater., 2019, e1905227 Search PubMed.
- S. Garg, H. Schwartz, M. Kozlowska, A. B. Kanj, K. Müller, W. Wenzel, U. Ruschewitz and L. Heinke, Angew. Chem., Int. Ed., 2019, 58, 1193–1197 CrossRef CAS PubMed.
- R. Lyndon, K. Konstas, B. P. Ladewig, P. D. Southon, P. C. Kepert and M. R. Hill, Angew. Chem., Int. Ed., 2013, 52, 3695–3698 CrossRef CAS PubMed.
- H. Demir, G. O. Aksu, H. C. Gulbalkan and S. Keskin, Carbon Capture Sci. Technol., 2022, 2, 100026 CrossRef.
- L. Gong Le, X. F. Feng and F. Luo, Inorg. Chem., 2015, 54, 11587–11589 CrossRef PubMed.
- A. B. Kanj, K. Müller and L. Heinke, Macromol. Rapid Commun., 2018, 39, 1700239 CrossRef PubMed.
- D. Hermann, H. A. Schwartz, M. Werker, D. Schaniel and U. Ruschewitz, Chemistry, 2019, 25, 3606–3616 CrossRef CAS PubMed.
- H. Agarkar and D. Das, J. Mol. Struct., 2019, 1184, 435–442 CrossRef CAS.
- C. T. Yang, A. R. Kshirsagar, A. C. Eddin, L. C. Lin and R. Poloni, Chemistry, 2018, 24, 15167–15172 CrossRef CAS PubMed.
- J. W. Brown, B. L. Henderson, M. D. Kiesz, A. C. Whalley, W. Morris, S. Grunder, H. Deng, H. Furukawa, J. I. Zink, J. F. Stoddart and O. M. Yaghi, Chem. Sci., 2013, 4, 2858–2864 RSC.
- I. M. Walton, J. M. Cox, J. A. Coppin, C. M. Linderman, D. G. Patel and J. B. Benedict, Chem. Commun., 2013, 49, 8012–8014 RSC.
- U. G. Randika Lakmali and C. V. Hettiarachchi, CrystEngComm, 2015, 17, 8607–8611 RSC.
- R. Huang, M. R. Hill, R. Babarao and N. V. Medhekar, J. Phys. Chem. C, 2016, 120, 16658–16667 CrossRef CAS.
- D. G. Patel, I. M. Walton, J. M. Cox, C. J. Gleason, D. R. Butzer and J. B. Benedict, Chem. Commun., 2014, 50, 2653–2656 RSC.
- D. Hermann, H. Emerich, R. Lepski, D. Schaniel and U. Ruschewitz, Inorg. Chem., 2013, 52, 2744–2749 CrossRef CAS PubMed.
- K. Müller, J. Wadhwa, J. Singh Malhi, L. Schottner, A. Welle, H. Schwartz, D. Hermann, U. Ruschewitz and L. Heinke, Chem. Commun., 2017, 53, 8070–8073 RSC.
- Y. Qiao, Z. Zhan, Y. Yang, M. Liu, Q. Huang, B. Tan, X. Ke and C. Wu, Mater. Today Commun., 2021, 27, 102338 CrossRef CAS.
- R. Lyndon, K. Konstas, A. W. Thornton, A. J. Seeber, B. P. Ladewig and M. R. Hill, Chem. Mater., 2015, 27, 7882–7888 CrossRef CAS.
- W. An, D. Aulakh, X. Zhang, W. Verdegaal, K. R. Dunbar and M. Wriedt, Chem. Mater., 2016, 28, 7825–7832 CrossRef CAS.
- S. Castellanos, A. Goulet-Hanssens, F. Zhao, A. Dikhtiarenko, A. Pustovarenko, S. Hecht, J. Gascon, F. Kapteijn and D. Bleger, Chem.–Eur. J., 2016, 22, 746–752 CrossRef CAS PubMed.
- F. Bigdeli, C. T. Lollar, A. Morsali and H. C. Zhou, Angew. Chem., Int. Ed., 2020, 59, 4652–4669 CrossRef CAS PubMed.
- H. Huang, H. Sato and T. Aida, J. Am. Chem. Soc., 2017, 139, 8784–8787 CrossRef CAS PubMed.
- H. Li, M. R. Martinez, Z. Perry, H. C. Zhou, P. Falcaro, C. Doblin, S. Lim, A. J. Hill, B. Halstead and M. R. Hill, Chem.–Eur. J., 2016, 22, 11176–11179 CrossRef CAS PubMed.
- W. C. Song, X. Z. Cui, Z. Y. Liu, E. C. Yang and X. J. Zhao, Sci. Rep., 2016, 6, 34870 CrossRef CAS PubMed.
- N. Prasetya and B. P. Ladewig, Sci. Rep., 2017, 7, 13355 CrossRef PubMed.
- Y. Jiang, P. Tan, S. C. Qi, X. Q. Liu, J. H. Yan, F. Fan and L. B. Sun, Angew. Chem., Int. Ed., 2019, 58, 6600–6604 CrossRef CAS PubMed.
- H. Li, M. M. Sadiq, K. Suzuki, C. Doblin, S. Lim, P. Falcaro, A. J. Hill and M. R. Hill, J. Mater. Chem. A, 2016, 4, 18757–18762 RSC.
- J. Park, D. Yuan, K. T. Pham, J. Li, A. Yakovenko and H. Zhou, J. Am. Chem. Soc., 2012, 134, 99–102 CrossRef CAS PubMed.
- H. He, J. Du, H. Su, Y. Yuan, Y. Song and F. Sun, CrystEngComm, 2015, 17, 1201–1209 RSC.
- N. Prasetya, B. C. Donose and B. P. Ladewig, J. Mater. Chem. A, 2018, 6, 16390–16402 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ta09270g |
| This journal is © The Royal Society of Chemistry 2022 |



