 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enrichment of low concentration methane: an overview of ventilation air methane
Zhuxian
Yang
*a,
Mian Zahid
Hussain
a,
Pablo
Marín
 b,
Quanli
Jia
c,
Nannan
Wang
d,
Salvador
Ordóñez
b,
Quanli
Jia
c,
Nannan
Wang
d,
Salvador
Ordóñez
 b,
Yanqiu
Zhu
b,
Yanqiu
Zhu
 a and
Yongde
Xia
a and
Yongde
Xia
 *a
*a
aCollege of Engineering, Mathematics and Physical Sciences, University of Exeter, Exeter, EX4 4QF, UK. E-mail: z.yang@exeter.ac.uk; y.xia@exeter.ac.uk
bCatalysis, Reactors and Control Research Group (CRC), Department of Chemical and Environmental Engineering, University of Oviedo, Facultad de Química, Julián Clavería 8, 33006 Oviedo, Spain
cHenan Key Laboratory of High Temperature Functional Ceramics, Zhengzhou University, Zhengzhou, 450052, PR China
dGuangxi Institute for Fullerene Technology (GIFT), Key Laboratory of New Processing Technology for Nonferrous Metals and Materials, Ministry of Education, School of Resources, Environment and Materials, Guangxi University, Guangxi 530004, China
First published on 16th February 2022
Abstract
Methane (CH4) is the second most important greenhouse gas after carbon dioxide (CO2), but its global warming potential is 21–28 times that of CO2. Coal mining accounts for 9% of global CH4 emissions, among which 60–70% is contributed by ventilation air methane (VAM). Currently the simplest way to reduce CH4 emissions from ventilation air is to thermally oxidize it to CO2; however the low and changeable CH4 concentrations (0.1–1.5% CH4) and the large volume of ventilation air make it a challenge since conventional technologies used for CH4 separation/purification in natural gas (CH4 concentration 55–98%) are not suitable for VAM enrichment. It is therefore highly desirable to concentrate VAM up to levels for further harnessing, as the utilization of VAM can not only reduce CH4 emissions but also provide extra economic benefits to relevant industries. Herein, for the first time, we present a review on both unconventional technologies and materials for VAM enrichment. The feasibility of technologies including vortex tubes, mechanical towers, gas hydrates, membranes and adsorption-based processes has been discussed, with focus on adsorption-based processes. Given that the adsorbents used in adsorption-based processes are one of the key factors for gas enrichment performance, materials including zeolites, porous carbon materials and metal–organic frameworks for methane separation have been critically analyzed and overviewed, covering the summary of the textural properties, CH4 adsorption capacity, CH4/N2 equilibrium selectivity and CO2/CH4 equilibrium selectivity of these materials under ambient conditions and highlighting some new synthesis strategies to achieve high CH4 adsorption capacity and CH4/N2 equilibrium selectivity. This review not only provides state-of-the-art technologies and materials for VAM enrichment (also applicable to other low grade CH4), which will inspire further studies to better mitigate and utilize VAM and other low grade CH4, but also supports the upcoming low-carbon economy.
1. Introduction
It is more urgent than ever to cut down on greenhouse emissions given that we are confronting frequent extreme weather events. As the second most important greenhouse gas after carbon dioxide (CO2), methane (CH4) possesses global warming potential (GWP) 21–28 times that of CO2 for a 100 year time horizon.1 CH4 is released mainly from enteric fermentation (livestock), energy industry, landfills, wastewater treatment, rice cultivation, etc. The estimated global anthropogenic CH4 emissions by the source in 2020 is 9390 million metric tons of CO2 equivalent, of which 9% is contributed by coal mining. Fig. 1 shows the estimated global anthropogenic CH4 emissions by sources.2 The global CH4 emissions from coal mining continue growing even with the declining coal production.3 | ||
| Fig. 1 Estimated global anthropogenic CH4 emissions by sources. Adapted with permission.2 Copyright 2020, Global Methane Initiative website. | ||
Coal production releases CH4 trapped in coal seams and surrounding strata, which can be categorized into three types of CH4:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH4 drained from the seam before mining (60–95% CH4), CH4 drained from worked areas of the mine (30–95% CH4) and CH4 diluted through ventilation fans (0.1–1.5% CH4) while extracting coal.4,5 Drained gas with CH4 concentration more than 30% normally can be upgraded for pipeline quality gases or readily used by industries for heating or power generation.6 The diluted CH4 through ventilation fans is discharged to the atmosphere via mine exhausts and is called ventilation air methane (VAM). The VAM gas mixture is characterized by large volume (200–385 m3 s−1), the majority being air (nitrogen ∼79%, oxygen ∼20%, carbon dioxide 0.13–0.19% and small amounts of other gases), low and changeable VAM concentration (0.1–1.5%), high relative humidity (70–100%) and high dust loading (0.13–4.47 mg m−3).4,7 VAM contributes about 60–70% of total CH4 emissions from coal mining activities.6,8 Therefore, the utilization of VAM can not only reduce greenhouse gas emissions, but also provide extra economic benefits to the mining industry.
CH4 drained from the seam before mining (60–95% CH4), CH4 drained from worked areas of the mine (30–95% CH4) and CH4 diluted through ventilation fans (0.1–1.5% CH4) while extracting coal.4,5 Drained gas with CH4 concentration more than 30% normally can be upgraded for pipeline quality gases or readily used by industries for heating or power generation.6 The diluted CH4 through ventilation fans is discharged to the atmosphere via mine exhausts and is called ventilation air methane (VAM). The VAM gas mixture is characterized by large volume (200–385 m3 s−1), the majority being air (nitrogen ∼79%, oxygen ∼20%, carbon dioxide 0.13–0.19% and small amounts of other gases), low and changeable VAM concentration (0.1–1.5%), high relative humidity (70–100%) and high dust loading (0.13–4.47 mg m−3).4,7 VAM contributes about 60–70% of total CH4 emissions from coal mining activities.6,8 Therefore, the utilization of VAM can not only reduce greenhouse gas emissions, but also provide extra economic benefits to the mining industry.
The key challenges of VAM mitigation and utilization are the low and changeable CH4 concentrations (0.1–1.5% CH4), the large volume, the high relative humidity, and the high dust loading of ventilation air.4 Currently the simplest way to reduce CH4 emissions from ventilation air is to thermally oxidize it via the reaction CH4 + 2O2 → CO2 + 2H2O.9 In the oxidation processes, VAM is used as either an ancillary fuel or the principal fuel in lean-burn gas turbines, recuperative gas turbines, thermal flow reversal reactors (TFRRs) and catalytic flow reversal reactors (CFRRs).8,10–12
By converting CH4 into CO2, the effect of CH4 on climate change can be significantly reduced given that the GWP of CH4 is 21–28 times that of CO2. In addition, the thermal energy produced from the CH4 oxidation process can be readily utilized for domestic or industrial applications. Technologies for energy recovery are commercially available, but they are not very effective when the CH4 concentration is less than 0.5%.13 In addition, for lean-burn gas turbines, a CH4 concentration of 0.8–2% (a minimum CH4 concentration of 0.8%) is required for self-sustaining operation.8,10,14 Therefore, it is highly desirable to concentrate VAM up to levels for further harnessing. If CH4 can be concentrated to approximately 30% or higher, it can be used for conventional gas turbines without significant modifications to generate electricity.8
There have been excellent reviews on the emission sources and mitigation options for the major CH4 sources,6,8,15–17 in addition, the latest developments and opportunities in CH4 mitigation technologies have been presented by Pratt et al. recently.18 However, to the best of our knowledge, there has been no comprehensive analysis and overview on the enrichment and mitigation of VAM. Given that VAM contributes about 60–70% of total CH4 emissions from coal mining activities,6,8 it is of great importance to present a dedicated review on the enrichment of VAM, to discuss the current advance in technologies and materials for VAM enrichment and to inspire further studies in relevant areas, and consequently to better mitigate and utilize VAM, which will not only cut down on the VAM emission, but also provide extra economic benefits to the mining industry.
Herein, for the first time, we present a review to discuss and analyze the technologies and materials for VAM enrichment. Because the conventional technologies widely used for CH4 separation/purification from natural gas (CH4 concentration of 55–98%) are not suitable for VAM enrichment, we first discuss the unconventional technologies including vortex tubes,19–23 mechanical towers,24,25 gas hydrates,26–28 membranes29,30 and adsorption based processes,31–35 with focus on adsorption-based processes. Since the adsorbents used in adsorption-based processes are one of the key factors for gas separation/enrichment performance, we then discuss various materials including zeolites, porous carbon materials and metal–organic frameworks (MOFs) that are potentially useful as adsorbents for VAM and other low grade CH4 enrichment, with focus on porous carbon materials and MOFs. Apart from the summary on the textural properties, CH4 adsorption capacity, CH4/N2 equilibrium selectivity and CO2/CH4 equilibrium selectivity of these materials under ambient conditions, new synthesis strategies for materials with high CH4 adsorption capacity and high CH4/N2 equilibrium selectivity have been highlighted. Finally, we draw conclusions and provide prospective research directions in this field.
2. Technologies for CH4 separation from ventilation air
Generally, gas separation may involve one of the following mechanisms: phase creation by heat transfer to or from the mixture, absorption in a liquid solvent, adsorption on a solid adsorbent or permeation through a membrane. The differences in gas properties, such as the kinetic diameter, polarizability, quadrupole and dipole moments, could be harnessed for gas separation.36 The key challenge to separate CH4 from ventilation air is the similar chemical and physical properties of CH4, CO2 and N2, as listed in Table 1.37 Due to these similar properties, the adsorbents with high CH4 uptake capacities also exhibit high CO2 uptake capacities.29,36 The significant difference in the polarizability between CH4 and N2 could be exploited to separate CH4 from N2, which has been demonstrated by some MOFs.38–40| Gas | Molecular weight (g mol−1) | Kinetic diameter (nm) | Polarizability (× 10−25 cm3) | Dipole moment (× 1018 esu cm) | Quadrupole moment (×10−26 esu cm2) |
|---|---|---|---|---|---|
| CH4 | 16 | 0.38 | 26.0 | 0.00 | 0.00 |
| N2 | 28 | 0.364 | 17.6 | 0.00 | 1.52 |
| CO2 | 44 | 0.33 | 26.5 | 0.00 | 4.30 |
Conventional technologies for natural gas (containing 55–98% CH4) separation include cryogenic distillation to remove N2 and higher hydrocarbons, dehydration by adsorption to remove H2O, and gas sweetening by amine absorption to remove acidic gases (CO2 and H2S). Cryogenic distillation requires a gas–liquid phase change, which will add a significant energy cost. These processes become uneconomic or impractical when the CH4 content is below about 40%. In the case of VAM where the CH4 concentration varies in the range of 0.1–1.5%, it is highly desirable to separate or selectively adsorb CH4 itself from the gas mixture (the majority is air), so as to concentrate or enrich CH4 for further utilization.41 In this section, we will first discuss the feasibility of unconventional technologies for CH4 separation/enrichment from ventilation air.
Unconventional technologies that have been investigated for gas separation include vortex tubes,19–23 mechanical towers,24,25 gas hydrates,26–28 membranes29,30 and adsorption based separation.31–35 The first three (vortex tubes, gas hydrates and mechanical towers) are technologies/concepts without commercial applications currently, but the last two (membrane separation and adsorption-based separation) have been used on a commercial scale for natural gas and other gas separation. We will discuss the feasibility of these technologies in the application of VAM enrichment in the following part.
2.1. Vortex tubes
A vortex tube, also known as a Ranque–Hilsch vortex tube, is a simple mechanical device. It separates a compressed fluid that tangentially enters the vortex chamber through inlet nozzles into two streams, the cold one at one end of the tube and the hot one at the other end. It was hypothesized that a stream of a compressed gas mixture flew into the vortex tube could be separated into individual gas streams based on the differential centrifugal forces acting on them.19 Linderstrom-Lang experimentally studied the gas separation of three gas mixtures (O2/N2, O2/CO2, and O2/He) in a vortex tube in detail. They found that gas separation was created by centrifugation, supporting the centrifugation hypothesis.20 Gas separation by using a vortex tube is relatively less investigated.19–23Kulkarni et al. studied the separation of a gas mixture of CH4 and N2 with a vortex tube to verify if gas separation occurs. Fig. 2 shows the schematic experimental setup. The two gases were mixed before passing through the vortex tube. Gas samples at the exits of the vortex tube were collected in gas sampling bags and were injected into a gas chromatograph for analysis. The two parameters including the inlet pressure and cold flow fraction (the ratio of mass of gases exiting the cold exit to the mass of gases entering the vortex tube) were studied. The results showed that gas separation took place in the vortex tube as evidenced by the CH4 concentration at the cold and hot ends. Moreover, the experimental values and the calculated ones of gas separation were comparable. The inlet pressure was identified to be the most dominant factor affecting the separative power of a vortex tube of fixed geometry, and the higher the inlet pressure, the higher the separative power of the vortex tube.19
 | ||
| Fig. 2 Schematic experimental setup for the separation of a gas mixture of CH4 and N2 with a vortex tube. Adapted with permission.19 Copyright 2002, American Society of Civil Engineers. | ||
The advantage of a vortex tube is that it has no moving parts and does not break or wear, so it requires little maintenance. However, this technique does demand the inlet gas stream to be pressurized (165–248 kPa was used in the above study).19 Given that the volume of ventilation air is huge, pressurizing it is a big issue. Further feasibility studies on CH4 separation/enrichment from ventilation air via a vortex tube are very much needed.
2.2. Mechanical towers
Wang et al. designed a mechanical tower for the enrichment of VAM by using CH4 buoyancy under free diffusion conditions (namely, the gas mixture moves freely under the force of gravity) based on the fact that the density of CH4 is lower than that of air.24 The enrichment characteristics of segregated (CH4 and N2 enter the system separately) and non-segregated (CH4 and N2 are pre-mixed before entering the system) mixtures under free diffusion conditions were studied. For a CH4/N2 mixture with 0.5% CH4, a CH4 concentration of 0.54% was monitored for the non-segregated mixture, which is not promising.Recently Wang et al. carried out a follow up study using a similar mechanical tower for the VAM (0.5% CH4) enrichment, under free diffusion conditions and weak eddy conditions, respectively. For a non-segregated mixture, under weak eddy field conditions, the maximum CH4 concentration is 0.54% (0.55%) in the top (middle) tower.25 This enrichment from 0.5% to 0.55% is far from enough for lean-burn gas turbines, which require a minimum CH4 concentration of 0.8% for self-sustaining operation.8,10,14 Since this technique is based on the difference in the density of the gases to be separated, significant improvement in the CH4 enrichment performance with this technique seems difficult.
2.3. Gas hydrates
Gas hydrates (clathrate hydrates) are crystalline solid structures formed via the inclusion of gas molecules (such as CH4, N2, O2, CO2, H2, etc.) into cavities of the frame built from hydrogen-bound water molecules without forming a chemical bond between the gas molecules and the water molecules. Generally, low temperature and high pressure are required for the formation of gas hydrates.42 However, the use of thermodynamic promoters, such as tetra-n-butyl ammonium bromide (TBAB), tetra-n-butyl ammonium chloride (TBAC), tetrabutyl phosphonium bromide (TBPB) etc., has been reported to be able to reduce the hydrate formation pressure.43–45CH4 separation from coal mine methane (CMM) via hydrate formation has been extensively investigated,44,46,47 but only a few studies on CH4 separation from ventilation air via hydrate formation have been reported. In particular, Adamova et al. theoretically calculated the hydrate formation of VAM (0.5% CH4, 75% N2, and 24.5% O2) in the temperature range of 258 to 273 K and the pressure range of 0.1 to 35.5 MPa.26 At 273 K and 17.96 MPa, CH4 concentration in the gas clathrate is 1.9%, nearly 4 times that in the gas phase (0.5%). Based on this theoretical study and the formation of methane hydrate under milder conditions (282.7–291.5 K and 0.15–5.10 MPa) achieved with the addition of TBPB,45 Du et al. investigated phase equilibrium conditions for gas hydrates formed from simulated mine ventilation air in the presence of TBPB,27 tri-n-butyl phosphine oxide (TBPO) or TBAB.28 With 37.1 wt% TBPB, at 278 K and 4 MPa, CH4 was enriched from 0.5% to 1.75% in the hydrate phase,27 and with an addition of 5 wt% TBPO, approximately 3-fold CH4 enrichment could be achieved in the hydrate phase.28 They also proposed the concept of a hydrate-based CH4 concentrator shown in Fig. 3, in which with 5 wt% TBPO, at 281 K and pressure higher than 0.91 MPa, CH4 could be enriched to a level meeting the requirements of lean-burn CH4 utilization technologies.
 | ||
| Fig. 3 Conceptual diagram of CH4 enrichment via hydrate crystallization. The product gas released from hydrate decomposition can be fed to lean-burn turbines. Adapted with permission.28 Copyright 2015, Elsevier. | ||
Although the results of CH4 separation from coal mine methane via hydrate formation are promising, the conditions (281 K and >0.91 MPa) for the formation of VAM hydrate are still a concern, because it would be a great challenge to cool and pressurize a large volume of ventilation air. Further studies on the energy cost and feasibility of VAM enrichment via a gas hydrate are urgently needed. Breakthroughs in the improvement of VAM hydrate formation conditions could make this method viable for practical application.
2.4. Membrane separation
Gas separation using membranes can offer the advantages of lower cost and smaller equipment size, lower energy consumption and ease of operation compared to conventional processes.48 The usage of membranes has been widely employed for natural gas separation in industry for the removal of acidic gases, such as CO2 and H2S.29,30 However, currently, membranes are not suitable for separating low concentration CH4 from ventilation air because of the high costs of initial capital investment and membrane maintenance or replacement due to fouling.13 Advances to make membrane separation systems commercially viable for VAM are highly demanded.492.5. Adsorption based processes
Accordingly, there are two types of selectivity, equilibrium selectivity and kinetic selectivity. Equilibrium selectivity (or thermodynamic selectivity) depends on the difference in adsorption at equilibrium, and kinetic selectivity depends on the difference in adsorption rates.
The equilibrium selectivity for a binary gas mixture of components i and j is defined as:
 | (1) |
The equilibrium selectivity for a binary gas mixture of components i and j can be estimated by using eqn (2). The value in eqn (2) is a constant and only valid at a very low gas pressure and low adsorption loading on the adsorbent.58
 | (2) |
In a kinetically controlled separation process, both the kinetic and equilibrium effects determine the actual kinetic selectivity, which is given by the following equation in the linear range of the isotherms:59,60
 | (3) |
Fluid bed concentrators, widely used for volatile organic compounds, were proposed to concentrate CH4 of very dilute VAM flows up to levels that might be beneficial as a fuel in a gas turbine, reciprocating engine, or support oxidation in a TFRR or CFRR.61 The concentration principle is as follows: the bed concentrator consists of a series of perforated plates or trays supporting an adsorbent medium (e.g., activated carbon beads). The VAM stream enters from the bottom of the concentrator and passes upward through the trays where it fluidizes the adsorbent medium to enhance the capture of CH4. The CH4 saturated adsorbent drops to the bottom of the concentrator, where it can be discharged to the storage vessel and then the desorber. The adsorbent is regenerated by increasing the temperature to release the adsorbed CH4 into a stream of a lower flow rate and higher CH4 concentration.61 However, experimental results on a fluidized bed concentrator using simulated ventilation air with 0.5% CH4 from Environmental C & C, Inc. in 2003 were not promising, and the trials were stopped.8,61,62 It is worth mentioning that a lot of new and advanced adsorbents with high CH4 adsorption capacities (up to 2.90 mmol g−1) and high CH4/N2 selectivity (up to 12.5) have been prepared and studied since the trial.38,63–71 Studies on the performance of concentrators with these newly developed adsorbents are highly desirable, which could lead to high performance and cost-effective concentrators.
In swing mode adsorption processes, at least two columns packed with adsorbents are required to carry out the separation process. The feed gas mixture passes through the first column, and the adsorbent selectively adsorbs one of the components till it is saturated. Then, the feed gas mixture is directed to the second column. Meanwhile, the first column is regenerated by desorbing the adsorbed component. When the second column is saturated, the feed gas mixture is directed toward the first column and the second column is regenerated.29 In practice, multiple columns are employed in a swing-mode to make the process continuous and additional steps are added to maximize the productivity and reduce the energy consumption.72 The adsorbed component is desorbed from the adsorbent in the regeneration process, which results in the enrichment of the adsorbed component. When the adsorbent is regenerated by desorption at a lower pressure than that used for the adsorption phase of the cycle, the process is termed pressure swing adsorption (PSA).73 In the case of regeneration that takes place under vacuum, it is called vacuum pressure swing adsorption (VPSA) or vacuum swing adsorption (VSA).74,75 When the adsorbent is regenerated by desorption at a higher temperature than that used for the adsorption phase of the cycle, it is called temperature swing adsorption (TSA).76 Normally the feed gas is pressurized in the adsorption step, but in the case of VAM enrichment, given the large volume of the ventilation air flow, it would not be practical to pressurize the feed gas flow. Therefore, the operating pressure close to atmospheric pressure is preferred.77
Due to their low operational costs, high separation efficiency and flexibility compared to mature separation technologies, such as absorption and distillation, swing mode separation processes are widely used for natural gas separation and purification, the production of hydrogen, the separation of O2 and N2 from air, etc.33 Recently, these processes have been extensively studied for the enrichment of low-grade CH4 gas78–80 and VAM7,77,81–87 too. VAM enrichment has been investigated by several research groups, including University of Science and Technology Beijing,81–84 Dalian University of Technology,85 East China University of Science and Technology,88 and Commonwealth Scientific and Industrial Research Organization (CSIRO).7,77,86
2.5.2.1. VPSA process. Early studies on VAM enrichment were carried out via the VPSA process.81,82,85 For example, Liu et al. used coconut shell-based activated carbon as an adsorbent via the VPSA process at an adsorption pressure of 150 to 250 kPa, and the CH4 concentration was improved from 0.3% to 0.73%.87 Although the enriched VAM concentration was still below 1% in these early reports, it triggered further investigations in VAM enrichment via PSA processes. Later Quyang et al. studied VAM enrichment using a coconut shell-based activated carbon adsorbent (CH4/N2 selectivity of 6.18) via the VPSA process with a feed gas of 0.42% CH4 and 99.58% N2.85 The concentration of CH4 was enriched from 0.42% to 1.09%, which can satisfy the requirements of flow-reversal reactors or lean-burn combustion turbines to generate power.
Based on their early study,87 Yang et al. recently carried out the VAM enrichment via a three-bed VPSA process unit which was equipped with a new vacuum exhaust step for the VPSA process.84 The coconut shell-based active carbon with an equilibrium CH4/N2 selectivity of 5 was used as the adsorbent. On a lab scale, the VAM was enriched from 0.2% to 0.4% and 0.69% as the vacuum exhaust ratio increased from 0 to 3.1, respectively. Moreover, a pilot-scale test system has been built at Julong Mine, Jizhong Energy Handan Mig., Handan, China.84 It uses a two-stage three-bed separation unit which can handle a flow rate of 500 m3 h−1. As shown in Fig. 4, the feed gas is compressed with a Roots blower (P1) and transferred to the adsorption bed of the first stage–stage separation unit. The CH4-rich gas is vacuumed with a vacuum pump (VP1) and transferred to the second separation unit with a Roots blower (P2). The product is collected from the vacuum pump (VP2). The VAM was enriched from 0.2% to over 1.2% in this system, which is well above the minimum CH4 concentration of 0.8% required for self-sustaining operation of lean-burn gas turbines,8,10,14 making it promising for VAM enrichment. Further studies are expected to evaluate the cost, efficiency, etc. for commercial possibility.
 | ||
| Fig. 4 Process diagram for the pilot plant. BED-adsorber; PT-pressure transmitter; TT-temperature transmitter; V-gas buffer; KV-pneumatic valve; FT-flowmeter; MV-gas-manual control valve; P1/P2-pump; VP1/VP2-vacuum pump; A-methane sensor.84 | ||
2.5.2.2. Vacuum swing (VS) followed by a temperature and vacuum swing adsorption (TVSA) process. Bae et al. developed a prototype VAM capture unit with a honeycomb monolithic carbon fiber composite (HMCFC) adsorbent and employed it in a large-scale test for VAM capture under various conditions in 2014.5,77 In the regeneration stage, a new vacuum and temperature swing process is employed to desorb CH4 from the adsorbent, involving initial VS followed by TVSA. The initial VS was found to be vital for the final CH4 concentration. A VAM of less than 1% can be enriched by 5 or 11 times by one-step adsorption, and a CH4 concentration of more than 25% can be achieved by two-step adsorption, as shown in Fig. 5. A VAM of less than 5% obtained from the one-step adsorption can be utilized as a principal fuel for lean burn turbines.
 | ||
| Fig. 5 Enrichment of simulated VAM with monolithic carbon fiber composites (MCFCs) by vacuum swing (VS) followed by combined temperature and vacuum swing adsorption (TVSA). Adapted with permission.77 Copyright 2014, American Chemical Society. | ||
Based on the above study,77 Bae et al. built up a two-stage prototype VAM enrichment unit with VTVSA in accordance with relevant Australian Standards and local mine site regulations. The enrichment of simulated ventilation air from 0.30%, 0.60%, and 0.98% CH4 up to 19.28%, 24.24%, and 36.92% CH4, respectively was demonstrated, avoiding the explosion range of 5–15% CH4.86 Very recently they have successfully carried out site trials of a two-stage VTVSA process using carbon fiber composites for VAM enrichment at an Australian coal mine.7
The schematic diagram of the prototype VAM enrichment unit is shown in Fig. 6. About 22–60 L min−1 VAM from the outlet of an oxidation unit was fed to the prototype unit by using a blower with a variable speed drive, until CH4 was detected in the exhaust stream of the first column. Then the first column was vacuumed until a predetermined pressure was reached. The HMCFC in the first column was then heated up to 383 K by circulating a heated thermal fluid (about 20 L min−1), followed by vacuuming to desorb gas out of the column. The desorbed gas stream was fed to the second column. The hot thermal fluid in the first column was drained and cooled down to ambient temperature for the next test. These processes were repeated until the second column was saturated, which has undergone the same regeneration processes to produce the final product. The inlet VAM was enriched from 0.54–0.73% to 4.34–4.94% after the first stage and 27.62–35.89% after the second stage.7 As mentioned by the authors, these VTVSA processes were scalable and portable to cope with changes in mine site operation and specifications, but the involvement of the energy intensive step of the temperature swing adsorption (TSA) process is an issue for practical applications. In this regard, the VPSA with CO2 displacement process (VPSA-CO2DIS) (discussed below) could be a solution to tackle this energy consultation issue.
 | ||
| Fig. 6 Schematic diagram of the prototype VAM enrichment unit equipped with two-stage adsorption processes and installed at an Australian coal mine site. Adapted with permission.7 Copyright 2020, American Chemical Society. | ||
2.5.2.3. VPSA with CO2 displacement process (VPSA-CO2DIS). Compared to CH4, CO2 is more strongly adsorbed by activated carbon, so in the VPSA-CO2DIS process, after the column has adsorbed CH4, the feed gas is switched to CO2 and the adsorbed CH4 is displaced and pushed out of the column by CO2.89 In 2011, Liu et al. studied the VPSA-CO2DIS process with activated carbon as the adsorbent for coal bed CH4 enrichment (CH4/N2 mixture of 17% CH4). The results demonstrated a CH4 enrichment from 17% up to 90% with CH4 recovery higher than 98%.89 Based on this study, Yang et al. investigated the VPSA-CO2DIS process with activated carbon beads as the adsorbent for the enrichment of VAM in 2016.88 CH4 enrichment from 1% to 53.5% was achieved, demonstrating that the VPSA-CO2DIS process is promising for VAM enrichment. More studies on the cost, efficiency, scalability, etc. of the VPSA-CO2DIS process for real VAM enrichment are highly demanded.
2.6. Summary on technologies for VAM enrichment
Among various technologies investigated for VAM enrichment, pressure swing adsorption (PSA)-based process has been the most studied and the most promising technology for VAM enrichment. Specifically, a pilot-scale study with the VPSA process and a site trial with the VTVSA process for VAM enrichment have been carried out by two research groups, respectively. Nevertheless, further studies on the cost, efficiency, scalability, etc., are needed. Meanwhile, as discussed in the following part, many adsorbents with high CH4 adsorption capacities (up to 2.90 mmol g−1) and high CH4/N2 selectivity (up to 12.5) have been reported recently.38,63–71 The use of some of these highly efficient adsorbents in PSA and/or TSA processes is expected to lead to improved VAM enrichment performance.3. Materials for selective adsorption of CH4 from CH4–N2 mixtures
The performance of PSA and TSA units for the enrichment of VAM is mainly determined by the adsorbents employed for the separation process, though the engineering of PSA and TSA units is also very important.90 Adsorbents with simultaneously high CH4/N2 selectivity and high CH4 adsorption capacity are preferred, as the former can reduce the number of cycles for the treatment of an input stream to reach a desired CH4 concentration and the latter may lower the overall cost.70 Therefore, information on the adsorption equilibrium and kinetic data of adsorbents is essential for the design of adsorption columns.59,91Due to their porous nature, zeolites, carbon materials and metal–organic frameworks (MOFs) have been extensively studied as adsorbents for gas storage and separation.29,36,92–96 For natural gas purification, studies have been devoted to the separation of N2 and CO2 from CH4, in which case CH4 is the major component and N2 and CO2 are impurities, so adsorbents with high N2 or CO2 adsorption capacities and high N2/CH4 selectivity or CO2/CH4 selectivity are preferred. Excellent reviews on natural gas separation/purification and related adsorbents can be found in the literature.29,36,72
In the case of VAM (and other low concentration CH4) enrichment, the above adsorbents are naturally good candidates. However, adsorbents with high CH4 adsorption capacity and high CH4/N2 selectivity are prerequisites given the low concentration of CH4 (0.1–1.5%) in ventilation air and the large volume of ventilation air. It has been demonstrated that the CH4 uptake amount is almost linearly related to the surface area of the adsorbent;90,97 meanwhile, it has also been revealed that the optimum pore diameter for CH4 adsorption is within the range of 0.7–1.0 nm.98–100 Therefore, an adsorbent with a high surface area associated with an optimal pore size is the key prerequisite for high CH4 adsorption capacity. Given that N2 and CO2 are present in ventilation air, low CO2/CH4 selectivity of the adsorbent is also important. In addition, due to the high relative humidity (70–100%) of ventilation air, the effect of water on the performance of the adsorbents also needs to be taken into account. Therefore, in the following part, we discuss the textural properties, CH4 adsorption capacity, CH4/N2 equilibrium selectivity and CO2/CH4 equilibrium selectivity of these materials under ambient conditions unless otherwise specified, the effect of water on the adsorbents, and the advantages and disadvantages of the widely studied three types of adsorbents (zeolites, carbon materials and MOFs). In addition, we highlight new synthesis strategies to achieve high CH4 adsorption capacity and high CH4/N2 equilibrium selectivity, including the combination of the advantages of zeolites and MOFs, or the advantages of carbon and MOFs, heteroatom (N or S) doping, and the surface property modification for porous carbons, the creation of coordinatively unsaturated metal centers for MOFs and the pore wall environment control of MOFs. It is worth noting that materials with relatively lower CH4 adsorption capacity or lower CH4/N2 selectivity (compared to those listed in the tables) are excluded from the tables; if several samples were studied in the same paper, only some of them are listed in the tables.
3.1. Zeolites
Zeolites are 3-dimensional aluminosilicate frameworks constituted by Si and Al tetrahedra linked through bridged oxygen atoms giving rise to a regular distribution of pores and cavities of molecular dimensions. In the case of VAM separation, due to the close kinetic diameters of CH4 (0.38 nm) and N2 (0.364 nm), it is extremely difficult to separate them by using the molecular sieving effect; therefore the separation of CH4 from N2 by using the adsorption equilibrium difference between CH4 and N2 would be a better choice. Consequently, zeolites with high CH4 adsorption capacity and high CH4/N2 selectivity are required.The polarizability, dipole and quadrupole moments of a gas determine the strength of the interaction between the gas and the zeolite surface. Due to their large dipole and quadrupole moments, H2O and CO2 are usually the strongest adsorbed species on hydrophilic zeolites.101 Given that ventilation air is usually saturated with H2O and contains CO2, zeolites with high CH4/CO2 selectivity are preferred for the separation of CH4 from ventilation air. Kim et al. studied the CH4 capture effectiveness of two classes of materials by computer modelling, including liquid solvents and zeolites, for a low-quality natural gas and VAM, respectively. Among the 190 experimentally realized International Zeolite Association (IZA) structures and over 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 predicted crystallography open database (PCOD) structures, they found that zeolites ZON and FER with the largest adsorbed CH4/CO2 ratio and KH(CH4) (Henry's constant) of 1.29 and 1.12 mol kg−1 kPa−1, respectively, are excellent candidates for concentrating a dilute CH4 stream into moderate concentration.41
000 predicted crystallography open database (PCOD) structures, they found that zeolites ZON and FER with the largest adsorbed CH4/CO2 ratio and KH(CH4) (Henry's constant) of 1.29 and 1.12 mol kg−1 kPa−1, respectively, are excellent candidates for concentrating a dilute CH4 stream into moderate concentration.41
Wu et al. introduced the subunits of ZIFs into zeolite Y and zeolite ZSM-5 for CH4/N2 separation, based on a theoretical study that the preferential adsorption sites for CH4 on ZIF-8 are in specific regions close to the organic imidazolate. The results showed that the CH4/N2 selectivity of the zeolites was greatly improved to above 8 at 100 kPa and 298 K, which was higher than that of zeolites and even better than that of ZIFs.102 However, the CH4 adsorption capacity is low, only 0.11–0.27 mmol g−1. Nevertheless, this strategy provides a way to combine the advantages of the low cost and mature synthesis technology of zeolites and the high CH4/N2 selectivity of MOFs, avoiding the complicated and high costs of synthesis processes of MOFs. Other combinations of zeolites and MOFs could result in both high CH4/N2 selectivity and high CH4 adsorption capacity.
Table 2 summarizes the textural properties, CH4 adsorption capacities, CH4/N2 selectivity and CO2/CH4 selectivity of zeolites under ambient conditions. As shown in Table 2, the CH4/N2 selectivity of commercial zeolites 5A and 13X is less than 3.2 and the CH4 adsorption capacities are less than 0.82 mmol g−1.50,58,103,104 The majority of zeolites in Table 2 show CH4 adsorption capacities below 1 mmol g−1, but ion-exchanged CHA (Li-CHA) shows a high CH4 adsorption capacity of 1.47 mmol g−1 with a CH4/N2 selectivity of 4.7 and a CO2/CH4 selectivity of 32.105 All the zeolites with available CO2/CH4 selectivity listed in Table 2 show that their CO2/CH4 selectivity is higher than their CH4/N2 selectivity, except for silicalite-I reported by Yang et al. with a CH4/N2 selectivity of 3.60 and a CO2/CH4 selectivity of 2.55. However, its CH4 adsorption capacity is only 0.65 mmol g−1.106 A theoretical study using silicalite-I as the adsorbent for the concentration of CH4 from low concentration CH4/N2 mixtures by a concentration thermal swing adsorption (CTSA) process has been reported by Delgado et al.107 It would be interesting to see similar simulation work for VAM concentration with silicalite-I as the adsorbent for PSA processes.
| Zeolites | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | CH4 uptake (mmol g−1) | Equilibrium selectivity CH4/N2 | Equilibrium selectivity CO2/CH4 | Ref. |
|---|---|---|---|---|---|---|
| a Calculated from the IAST model and values in brackets are the gas mixture ratio used for the calculation. b Measured by CO2 adsorption at 273 K. c Calculated from Henry's selectivity. d Obtained at 303 K. e Calculated from Langmuir equation parameters. f Obtained at 305 K. g Obtained at 302 K. h Obtained at 313 K. i Obtained at 295 K. j Calculated from the ratio of adsorption amounts at 273 K. | ||||||
| Silicalite-I | 439 | 0.19 | 0.65 | 3.60a | 2.55a | 106 |
| Silicalite-I-H | 323 | 0.137 | 0.67 | 3.9a | 5.63a | 108 |
| Silicalite-I-M | 342 | 0.130 | 0.67 | 3.9a | 5.83a | 108 |
| K-KFI | 430.4b | 0.10b | 0.78 | 8.5 | 35 | 105 |
| Na-KFI | 556.2b | 0.15b | 0.79 | 4.1 | 92 | 105 |
| Li-KFI | 550.6b | 0.14b | 0.71 | 3.0 | 80 | 105 |
| Ca-KFI | 333.5b | 0.07b | 0.72 | 3.0 | 19 | 105 |
| K-CHA | 278.5b | 0.07b | 0.85 | 14.5 | 24 | 105 |
| Na-CHA | 594.8b | 0.16b | 1.35 | 4.3 | 42 | 105 |
| Li-CHA | 638.0b | 0.17b | 1.47 | 4.7 | 32 | 105 |
| 5A | — | — | 0.81 | 0.94c | 256.47c | 58 |
| 5A | — | — | 0.69f | 1.96f | — | 103 |
| 13X | — | — | 0.59 | 2.36e | 14.9e | 50 |
| Linde 4A | — | 0.108 | 0.95g | 3.4g | 6.5g | 109 |
| H-ZSM-5 | — | — | 0.71h | 3.28d,h | 6.11d,h | 110 |
| H-clinoptilolite | — | — | 0.93 | 3.91e,i | — | 111 |
K/Na (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50)-clinoptilolite 50)-clinoptilolite |
— | — | 1.05 | 3.27e,i | — | 111 |
| Zeolite X/AC composite | 802 | 0.64 | 0.77 | 3.4j | — | 112 |
The disadvantages of zeolites as sorbents for methane adsorption include low surface area, low CH4 adsorption capacities and hydrophilic surface. As mentioned above, an adsorbent with high surface area associated with optimal pore size is the key prerequisite for methane adsorption, but the surface area of zeolites is generally low compared to porous carbon materials, which results in a low CH4 adsorption capacity. In addition, the hydrophilic surface of zeolites will reduce the adsorption capacity in the presence of moisture (given the high relative humidity in ventilation air methane). Furthermore, zeolites with polar surfaces that possess high electric-field gradients have stronger interaction with CO2 than with the non-polar CH4 and N2 molecules.113 As can be observed from Table 2, most zeolites have a tendency to adsorb CO2 over CH4. The advantages of zeolites as the adsorbents are the low cost and mature synthesis technology of zeolites compared to MOFs. A new synthesis strategy that combines the advantages of zeolites (low cost and mature synthesis technology) and MOFs (high CH4/N2 selectivity), avoiding the complexity and high costs of synthesis processes of MOFs, is highly preferred to generate zeolites with both high CH4/N2 selectivity and high CH4 adsorption capacity.
3.2. Porous carbon materials
Porous carbon materials, including high surface area activated carbons (ACs),114,115 activated carbon fibers (ACFs),92,116 and carbon molecular sieves (CMSs),117,118 have been extensively studied as the adsorbents in adsorption based processes for low concentration CH4 recovery from unconventional natural gases115,119,120 and VAM enrichment.7,77,84–86Table 3 summarizes the textural properties, CH4 adsorption capacity, CH4/N2 selectivity and CO2/CH4 selectivity of porous carbon materials under ambient conditions unless otherwise specified.| Carbon materials | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | CH4 uptake (mmol g−1) | Equilibrium selectivity CH4/N2 | Equilibrium selectivity CO2/CH4 | Ref. |
|---|---|---|---|---|---|---|
| a Carbon has been studied for VAM enrichment. b N-doped carbon. c Calculated from the IAST model and values in brackets are the gas mixture ratio used for the calculation. d Micropore volume. e Calculated from Henry's law constants. f Calculated from the initial slopes of adsorption isotherms at very low pressures. g Calculated at 303 K. h Calculated from Langmuir equation parameters. i Calculated from the initial slopes of adsorption isotherms at relevant pressures (CH4 0.69 kPa and N2 79.99 kPa). | ||||||
| ACa (from coconut shell) | 329 | 0.145 | — | 6.18c (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
— | 84 and 85 |
| ACF compositesa | 577 | 0.135d | — | 5.9 /4.2f | 3.29f | 77 |
| AC (GCTAC) | 700 | — | 1.21 | 9.8c (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97.5) 97.5) |
— | 67 |
| BPL AC | — | — | — | 4.5 | 2.5e | 31 |
| AC (CGUC-1-6) | 624 | 0.267 | 1.01 | 5.9c (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
16.9c (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) 60) |
134 |
| AC (CGUC-1-8) | 1071 | 0.439 | 1.34 | 5.0c (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
9.4c (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) 60) |
134 |
| AC (PRCs) | 776 | — | 1.12 | 5.7c (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85) 85) |
— | 128 |
| AC beads | 1457 | 0.57 | 1.06 | 5.5c,g (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
3.6c,g (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
135 |
| Granular AC | 1227 | 0.505 | 1.59 | 3.2g | — | 136 |
| AC (SCs) | 914 | 0.35 | 1.86 | 5.7c (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
— | 137 |
| ACb (N-WAPC) | 862 | 0.5 | 1.01 |
7.62
(50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 11.76c (15 50) 11.76c (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85) 13.71c (5 85) 13.71c (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95) 95) |
3.19c (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 5.98c (15 50) 5.98c (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85) 8.66c (5 85) 8.66c (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95) 95) |
63 |
| ACb (ClCTF-1-650) | 974 | 0.37 | 1.47 |
8.6
(50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
8.1c (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) 99) |
64 |
| ACb (ClCTF-1-550) | 986 | 0.37 | 1.27 |
8.1
(50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
7.8c (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) 99) |
64 |
| ACb (NAPC-2-6) | 1247 | 0.69 | 1.3 | 4.1c (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
10.1c (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
124 |
| ACb (OTSS-1-550) | 778 | 0.31 | 1.49 | 5.22c (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
8.15c (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) 90) |
138 |
| ACb (SNMC-1-600) | 1021 | 0.43 | 1.45 | 5.1c (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
6.9c (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) 60) |
139 |
| ACb (SNMC-2-600) | 1884 | 0.78 | 1.57 | 4.2c (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
4.3c (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) 60) |
139 |
| ACb (SNMC-3-600) | 3049 | 1.4 | 1.17 |
3.6
(50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
3.2c (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) 60) |
139 |
| ACb (ACSs-N) | 697 | 0.46 | 1.30 | 3.76c | 7.49c | 125 |
| S-doped AC (CSK-7) | 1324 | 0.81 | 1.23 | 2.92c | 2.3c | 126 |
| CMS (1023) | 389 | 0.182d | 1.33 | 4.74h | — | 130 |
| Mesoporous carbon | 599 | 0.66 | 1.05 | 5.8e | — | 133 |
| Mesoporous carbon (sOMC) | 2255 | 2.17 | 0.90 |
3.7
(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) 90) |
2.8c (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) 90) |
132 |
| Carbon composite (MNS-derived) | — | — | 1.48 | 6.3e/10.4i | — | 65 |
| Carbon nanoplates (PCNPs) | 690 | — | 1.17 | 10c (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) 70) |
— | 66 |
In addition, heteroatom-doped (N or S) porous carbons have also been extensively studied, and some N-doped carbons show very high CH4/N2 selectivity up to 8.6 for a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixture.64,124–126 Most of the reported N-doped porous carbons showed CH4/N2 selectivity lower than CO2/CH4 selectivity. However, N-doped AC with a high N content (>10 wt%) reported by Yao et al. showed a high CH4 adsorption capacity of up to 1.47 mmol g−1 at 298 K and 100 kPa and high CH4/N2 selectivity up to 8.6, which was attributed to the unusually high N content as well as the suitably narrow ultramicropore size distribution.64 In addition, it is worth pointing out that Li et al. reported that a N-doped AC (14.48 wt% N content) derived from waste wool showed a high CH4/N2 selectivity of 7.6 for a 50
50 mixture.64,124–126 Most of the reported N-doped porous carbons showed CH4/N2 selectivity lower than CO2/CH4 selectivity. However, N-doped AC with a high N content (>10 wt%) reported by Yao et al. showed a high CH4 adsorption capacity of up to 1.47 mmol g−1 at 298 K and 100 kPa and high CH4/N2 selectivity up to 8.6, which was attributed to the unusually high N content as well as the suitably narrow ultramicropore size distribution.64 In addition, it is worth pointing out that Li et al. reported that a N-doped AC (14.48 wt% N content) derived from waste wool showed a high CH4/N2 selectivity of 7.6 for a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixture and 13.7 for a 5
50 mixture and 13.7 for a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 mixture with a decent CH4 adsorption of 1.01 mmol g−1.63
95 mixture with a decent CH4 adsorption of 1.01 mmol g−1.63
Apart from the textural properties and N-doping, the surface chemistry properties of the porous carbons have also been found to be important for the gas separation performance.127,128 For example, Tang et al. prepared binderless particle rice-based carbon materials (PRCs) with narrow micropore distribution by carbonization of rice followed by CO2 activation, which showed a high CH4 uptake of 1.12 mmol g−1 and a CH4/N2 selectivity of 5.7 at 298 K and 100 kPa.128 Simulation calculations showed that the surface carboxyl groups played an important role in the improvement of the CH4/N2 selectivity. Fixed-bed experiments demonstrated that this carbon material can greatly separate CH4/N2 mixtures under ambient conditions.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70N2 mixture.66 Further studies on the CO2/CH4 selectivity of these carbon nanoplates and their performance of VAM enrichment with SPA processes are highly needed.
70N2 mixture.66 Further studies on the CO2/CH4 selectivity of these carbon nanoplates and their performance of VAM enrichment with SPA processes are highly needed.
As shown in Table 3, a lot of carbon materials with high CH4 adsorption capacity and high CH4/N2 selectivity have been reported recently. So far the highest CH4 adsorption capacity of 1.86 mmol g−1 with a CH4/N2 selectivity of 5.7 (for a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 gas mixture) has been reported by Du et al.137 and the highest CH4/N2 selectivity of 13.7 (for a 5
50 gas mixture) has been reported by Du et al.137 and the highest CH4/N2 selectivity of 13.7 (for a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 mixture) with a CH4 adsorption capacity of 0.5 mmol g−1 has been reported by Li et al.63 It is noteworthy that some of these ACs show CH4/N2 selectivity higher than the CO2/CH4 selectivity.31,63,64,132,139 Given that ventilation air usually contains CO2, it is highly desirable to study the VAM enrichment with SPA processes using these adsorbents. In addition, those carbon materials without available data on CO2/CH4 selectivity but with high CH4 adsorption capacity and high CH4/N2 selectivity are potentially attractive too for VAM enrichment studies.65–67,128,130
95 mixture) with a CH4 adsorption capacity of 0.5 mmol g−1 has been reported by Li et al.63 It is noteworthy that some of these ACs show CH4/N2 selectivity higher than the CO2/CH4 selectivity.31,63,64,132,139 Given that ventilation air usually contains CO2, it is highly desirable to study the VAM enrichment with SPA processes using these adsorbents. In addition, those carbon materials without available data on CO2/CH4 selectivity but with high CH4 adsorption capacity and high CH4/N2 selectivity are potentially attractive too for VAM enrichment studies.65–67,128,130
Although porous carbon materials are generally hydrophobic, both experimental and theoretical studies show that microporous carbons can adsorb a large amount of water vapor.140–142 Different from non-polar fluids, the adsorption mechanism of water in the micropores of carbon is determined by the balance between fluid–solid and fluid–fluid interactions, and it has been observed that the adsorption of water occurs via cluster formation.142 It has also been demonstrated that the presence of water vapor adversely affects the adsorption capacity of microporous carbon and limits its effectiveness as an adsorbent material in humid environments.140 Considering the high relative humidity of ventilation air, it is important to study the effect of humidity on the adsorption capacities and selectivity of adsorbents in humid environments. In this respect, the adsorbent (carbon fiber composites) used in the site study for VAM enrichment by Bae et al. shows that the presence of moisture in ventilation air has no effect on CH4 capture.7 It is important to investigate the effect of water vapor on the adsorption performance of other porous carbon materials (with high CH4 adsorption capacity and high CH4/N2 selectivity) that are potentially useful for VAM enrichment.
Compared to zeolites and MOFs, the downside of porous carbon materials would be the less control over the pore size distribution. However, porous carbon materials possess the advantages including high CH4 adsorption capacity, high CH4/N2 selectivity, hydrophobicity, excellent chemical stability, low cost and ease of production. So far porous carbon materials have been the only adsorbents that have been used for site studies, demonstrating their potential in practical VAM enrichment.7,84
3.3. MOFs
MOFs, formed by coordination bonds between metal clusters and organic linkers, have great potential for gas adsorption and separation because of their high surface areas, tunable pore sizes, and versatile chemistry. Some MOFs have been investigated by molecular simulation studies, while plenty of MOFs have been experimentally studied for CH4 separation from a CH4/N2 gas mixture. Table 4 summarizes the textural properties, CH4 adsorption capacity, CH4/N2 selectivity and CO2/CH4 selectivity of MOFs at 100 kPa and 298 K unless otherwise specified.| Carbon materials | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | CH4 uptake (mmol g−1) | Selectivity CH4/N2 | Selectivity CO2/CH4 | Ref. |
|---|---|---|---|---|---|---|
| a Calculated from the IAST model and values in brackets are the gas mixture ratio used for the calculation. b Calculated from Langmuir equation parameters. c Micropore volume. d Calculated from Henry's law constants. e Calculated with the dual-site Langmuir model and values in brackets are the gas mixture ratio used for the calculation. | ||||||
| [Co3(C4O4)2(OH)2] | 76 | — | 0.40 |
12.5
(50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
— | 69 |
| Cu-MOF [Cu(hfipbb)(H2hfipbb)0.5] | 105 | — | 0.46 | 6.9b | 3.1b | 146 |
| Cu-MOF | 110 | — | 0.63 | 11.18 | — | 70 |
| [Ni3(HCOO)6] | 293 | 0.097c | 0.82 | 6.18d | — | 40 |
| Ni(DOBDC) | 1018 | 0.49 | 1.90 | 3.80a (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
30a (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
151 |
| 3D Ni-MOF | 1168 | 0.807 | 1.76 | 7.0 | — | 152 |
| [Cu(INA)2] | 252 | 0.12 | 0.80 | 8.34d | — | 147 |
| Cu(Me-4py-trz-ia) | 1473 | 0.586 | 1.12 | 4.2d | — | 156 |
| ATC-Cu | 600 | 0.23 | 2.90 |
9.7
(50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
— | 68 |
| CAU-21-BPDC (Al based MOF) | 523 | 0.38 | 0.99 |
11.9
(50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
— | 38 |
| Co(DOBDC) | 1089 | 0.50 | 1.95 | 3.2a (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
40a (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
151 |
| ZIF-94 | 597 | — | 1.5 | 7a (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) (50 70) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
— | 148 |
| ROD-8 (CdII-MOF) | 369 | — | 0.77 |
9.1
(50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
3.3e (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
71 |
| ACF@[Ni3(HCOO)6] | 1053 | 0.453 | 1.10 | 6.22 | — | 149 |
| Ni-MA-BPY | 464 | 0.160c | 1.01 | 7.4 | — | 150 |
Liu et al. carried out molecular simulation studies on the separation of CH4/N2 mixtures by using MOFs including Cu-BTC, MIL-47 (V), IRMOF-1, IRMOF-12, IRMOF-14, IRMOF-11, and IRMOF-13,143 and ZIFs including ZIF-68 and ZIF-69.144 The highest CH4/N2 selectivity of 4.7 was achieved for MIL-47 (V).143 Peng et al. studied the adsorption and separation of CH4/N2 and CO2/CH4 of UMCM-1 and UMCM-2 using a hybrid method of computer simulation and adsorption theory.145 A CH4/N2 selectivity of 2 was achieved for both MOF materials, which was insensitive to the pressure.
As listed in Table 4, for those frequently studied MOFs, so far the highest CH4/N2 selectivity of 12.5 has been reported by Li et al.69 for an ultramicroporous squarate-based MOF, [Co3(C4O4)2(OH)2] (C4O42− = squarate); however, the CH4 adsorption capacity is low (0.40 mmol g−1). In addition, a number of MOFs with very high CH4/N2 selectivity (6–12.5) have been reported in recent years,38,40,68–71,146–152 with some of them also showing decent or extremely high CH4 adsorption capacity (up to 2.9 mmol g−1), making them very promising candidates for VAM or low grade CH4 enrichment. Further studies on VAM enrichment using these MOFs with high CH4/N2 selectivity and decent or high CH4 adsorption capacity are very much demanded.
The high CH4/N2 selectivity of those MOFs has been ascribed to the properties of the MOFs under study, including the uniform ultra-micropores and optimal polarizability,39,40 or the suitable SOD cage size (0.84 nm) acting as a strong adsorption potential,148 or the optimal coupling of the polarizability and structure of the MOF framework,153 or strong interactions between CH4 molecules and their coordinatively unsaturated metal sites,68,151 or an increased number of polar sites,38etc. For example, it is known that coordinatively unsaturated metal centers can bind CH4 significantly.151 Niu et al. reported a CH4 nano-trap that features oppositely adjacent open metal sites and dense alkyl groups in a MOF, ATC-Cu.68 As illustrated in Fig. 7, when two opposite coordinatively unsaturated metal centers are adjacent to each other, a nano-trap for CH4 can be generated based on the cooperative coulombic interaction between the CH4 molecules and the two metal centers. This MOF showed a record-high CH4 adsorption capacity of 2.90 mmol g−1 and a high CH4/N2 selectivity of 9.7 at 298 K and 100 kPa. Breakthrough experiments showed a complete separation of mixtures of 50CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50N2 and 30CH4
50N2 and 30CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70N2 and good results for a 15CH4
70N2 and good results for a 15CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85N2 gas mixture.
85N2 gas mixture.
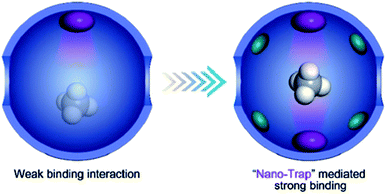 | ||
| Fig. 7 The schematic comparison of a traditional CH4 adsorbent and nano-trap. The purple and green ellipsoids represent coordinatively unsaturated metal centers and alkyl groups, respectively. Adapted with permission.68 Copyright 2019, Wiley-VCH. | ||
Given that CH4 has a greater polarizability than N2, Lv et al. demonstrated a strategy to improve CH4/N2 selectivity by controlling the pore wall environment of two isomeric Al-based metal–organic frameworks (MOFs) with four highly symmetric polar sites for strengthened adsorption affinity toward CH4 over N2. At 298 K and 100 kPa, CAU-21-BPDC with four highly symmetric polar sites in the pore walls showed a CH4 uptake capacity of 0.99 mmol g−1 and a CH4/N2 selectivity of 11.9, which was 2.4 times higher than that of the MOF without four highly symmetric polar sites.38
Apart from high CH4/N2 selectivity and high CH4 adsorption capacity, considering that ventilation air is saturated with water, good water stability is also important for MOFs to be used as adsorbents for VAM separation/enrichment. It has been reported that one of the decisive factors in determining the water stability of MOFs is their metal clusters, with trinuclear chromium clusters more stable than copper paddlewheel clusters, which are more stable than basic zinc acetate clusters.154 Other factors that affect the water stability including the strength of the bond between the metal oxide cluster and the bridging linker, the functionality of the ligand (hydrophilic or hydrophobic), the intrinsic textual properties, etc., have been proposed.155 Water stable MOFs with high CH4/N2 selectivity, such as [Ni3(HCOO)6], three-dimensional Cu-MOF, squarate-based MOFs, Co-MA-BPY and Ni-MA-BPY, have been reported.40,69,70,150 Moreover, due to the high relative humidity of ventilation air, apart from the water stability of MOFs, it is highly desirable to investigate the effect of water vapor on the adsorption performance of VAM for those promising MOF candidates with high CH4 adsorption capacity and high CH4/N2 selectivity.
In addition, it is worth mentioning that Liu et al. prepared a formate metal–organic framework and activated carbon fiber composite, ACF@[Ni3(HCOO)6], with a CH4/N2 selectivity of 6.22 and a CH4 adsorption capacity of 1.10 mmol g−1.149 This strategy could be adopted for the preparation of other carbon/MOF composites with desired CH4/N2 selectivity and CH4 adsorption capacity for gas separation/enrichment.
For most of the MOFs with CO2/CH4 selectivity available listed in Table 4, their CH4/N2 selectivities are lower than their CO2/CH4 selectivities, except in a few cases. In particular, Cu-MOF [Cu(hfipbb)(H2hfipbb)0.5] showed a CH4/N2 selectivity of 6.9, CO2/CH4 selectivity of 3.1, and adsorption capacity of 0.46 mmol g−1,146 and ROD-8 (CdII-MOF) showed a CH4/N2 selectivity of 9.1, CO2/CH4 selectivity of 3.3 and adsorption capacity of 0.77 mmol g−1.71 Considering the presence of CO2 in ventilation air, these MOFs with CH4/N2 selectivity higher than their CO2/CH4 selectivity are very promising candidates for VAM enrichment.
The disadvantages of MOFs lie in the complexity and high cost of synthesis processes. On the other hand, MOFs have more flexibility in tuning the pore size and surface properties to achieve adsorbents with desired properties (e.g., high CH4 adsorption capacity, high CH4/N2 selectivity, and water stability). Some MOFs (in Table 4) show the highest CH4 adsorption capacity among the widely studied three types of adsorbents (zeolites, porous carbon materials and MOFs), making them promising candidates for VAM enrichment.
3.4. Combination of adsorbents
Given that zeolites have stronger adsorption for CO2 than for CH4 and N2, while carbon molecular sieves (CMSs) can kinetically separate CH4 from N2, a combination of zeolites and CMSs in a PSA system has been employed to separate the ternary mixtures of CH4/CO2/N2. For example, ACs, CMS, and zeolite 13X in a specially designed PSA has shown that CO2, CH4 and N2 could apparently be enriched in the top, side and bottom product streams, respectively.157 In addition, a layered pressure swing adsorption (LPSA) system based on the assumption that CO2 would be almost completely adsorbed in 13X followed by kinetic separation of CH4/N2 in the CMS layer has been demonstrated to be able to enrich CH4 from 60% to 86%.35 The combination of different types of adsorbents in PSA processes for VAM enrichment could be a choice to simultaneously collect CH4, CO2 and N2 separately, which could add value to the processes.3.5. Summary of adsorption materials
In general, the limitation of zeolites as the adsorbent for CH4 separation lies in the low CH4/N2 selectivity and low CH4 adsorption capacity compared to porous carbons and MOFs, and the hydrophilic surface. A number of porous carbons and MOFs exhibiting both high CH4/N2 selectivity and high CH4 adsorption capacity are very promising candidates for VAM enrichment, especially those with CH4/N2 selectivity higher than CO2/CH4 selectivity, and some MOFs show the highest CH4 adsorption capacity among the widely studied three types of adsorbents. So far porous carbon materials have been the only sorbents that have been used in site studies for VAM ventilation. However, further studies on the VAM enrichment performance, regenerability, kinetics, compatibility and cost are highly desirable for their practical applications. Porous carbons have the advantages of excellent chemical stability, low cost and ease of production, while MOFs have more flexibilities on the pore size, surface property, etc. New strategies to produce novel porous materials that combine the advantages of zeolites and MOFs or the advantages of carbon and MOFs could lead to promising adsorbents with desired properties for gas enrichment, such as high CH4/N2 selectivity, high CH4 adsorption capacity, easy regenerability, good chemical stability, etc.4. Conclusions and prospects
We have presented an overview on the technologies and materials for VAM enrichment with focus on adsorption-based processes (PSA and TSA). PSA processes have been the most studied and the most promising technology for VAM enrichment so far. A pilot-scale study with VPSA and a site trial with VTSA for VAM enrichment have been carried out, respectively. Further studies on the cost, efficiency, scalability etc. of these technologies are needed.The adsorbents used in the adsorption processes (PSA and TSA) are one of the key factors for gas separation/enrichment performance. We have summarized materials that are potentially useful for VAM enrichment, including zeolites, porous carbon materials and MOFs with focus on those with high CH4/N2 selectivity and high CH4 adsorption capacity and highlighted new synthesis strategies to achieve high CH4 adsorption capacity and CH4/N2 equilibrium selectivity. In general, zeolites exhibit low CH4/N2 selectivity and low CH4 adsorption capacity compared to porous carbons and MOFs. Several porous carbons and MOFs exhibiting both high CH4/N2 selectivity (up to 12.5) and high CH4 adsorption capacity (up to 2.90 mmol g−1) are very promising candidates for VAM enrichment,38,63–71 especially those with CH4/N2 selectivity higher than CO2/CH4 selectivity. Making use of these adsorbents in PSA and/or TSA processes is likely to result in desirable VAM enrichment performance.
New synthesis strategies including the combination of the advantages of zeolites and MOFs, or the advantages of carbon and MOFs, heteroatom (N or S) doping, and surface property modification for porous carbons, the creation of coordinatively unsaturated metal centers for MOFs and the pore wall environment control of MOFs could lead to novel adsorbents with desirable properties such as high CH4/N2 selectivity, high CH4 adsorption capacity, easy regenerability, good chemical stability, etc. for VAM enrichment.
This review will inspire further studies and advances for VAM enrichment to better mitigate and utilize VAM or other low grade CH4, which will not only cut down on CH4 emissions, but also provide extra economic benefits to the mining industry and ultimately contribute to the future low carbon economy.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the EU RFCS [RFCS-2016-754077] and Leverhulme Trust [RPG-2018-320].References
- IPCC, Global Warming Potential Values. Intergovernmental Panel on Climate Change, http://ghgprotocol.org/sites/default/files/ghgp/Global-Warming-Potential-Values%20(Feb%2016%202016).pdf, 2016 Search PubMed.
- Global Methane Emissions and Mitigation Opportunities, Global Methane Initiative, https://www.globalmethane.org/documents/gmi-mitigation-factsheet.pdf, 2020 Search PubMed.
- N. Kholod, M. Evans, R. C. Pilcher, V. Roshchanka, F. Ruiz, M. Cote and R. Collings, J. Cleaner Prod., 2020, 256, 120489 CrossRef CAS PubMed.
- S. Su, H. W. Chen, P. Teakle and S. Xue, J. Environ. Manage., 2008, 86, 44–62 CrossRef CAS PubMed.
- R. Thiruvenkatachari, S. Su and X. X. Yu, J. Hazard. Mater., 2009, 172, 1505–1511 CrossRef CAS PubMed.
- I. Karakurt, G. Aydin and K. Aydiner, Renewable Sustainable Energy Rev., 2011, 15, 1042–1049 CrossRef CAS.
- J. S. Bae, S. Su, X. X. Yu, J. J. Yin, A. Villella, M. Jara and M. Loney, Ind. Eng. Chem. Res., 2020, 59, 15732–15741 CrossRef CAS.
- S. Su, A. Beath, H. Guo and C. Mallett, Prog. Energy Combust. Sci., 2005, 31, 123–170 CrossRef CAS.
- P. Hinde, I. Mitchell and M. Riddell, Johnson Matthey Technol. Rev., 2016, 60, 211–221 CrossRef CAS.
- J. J. Yin, S. Su, X. X. Yu, J. S. Bae, Y. G. Jin, A. Villella, M. Jara, M. Ashby, M. Cunnington and M. Loney, Energy Fuels, 2020, 34, 9885–9893 CrossRef CAS.
- D. Ursueguía, P. Marín, E. Díaz and S. Ordóñez, J. Nat. Gas Sci. Eng., 2021, 88, 103808 CrossRef.
- P. Marín, F. V. Díez and S. Ordóñez, Chem. Eng. Process., 2019, 135, 175–189 CrossRef.
- X. Jiang, D. Mira and D. L. Cluff, Prog. Energy Combust. Sci., 2018, 66, 176–199 CrossRef.
- R. I. Holmes, Journal of Applied Engineering Sciences, 2016, 6, 41–50 CrossRef.
- L. Hoglund-Isaksson, Atmos. Chem. Phys., 2012, 12, 9079–9096 CrossRef.
- I. Karakurt, G. Aydin and K. Aydiner, Renewable Energy, 2012, 39, 40–48 CrossRef CAS.
- R. O. Yusuf, Z. Z. Noor, A. H. Abba, M. A. Abu Hassan and M. F. M. Din, Renewable Sustainable Energy Rev., 2012, 16, 5059–5070 CrossRef CAS.
- C. Pratt and K. Tate, Environ. Sci. Technol., 2018, 52, 6084–6097 CrossRef CAS PubMed.
- M. R. Kulkarni and C. R. Sardesai, J. Energy Eng., 2002, 128, 1–12 CrossRef.
- C. U. Linderstrøm-Lang, Int. J. Heat Mass Transfer, 1964, 7, 1195–1206 CrossRef.
- R. Manimaran, Aust. J. Mech. Eng., 2020, 29, 1816735 Search PubMed.
- S. Mohammadi and F. Farhadi, Sep. Purif. Technol., 2014, 138, 177–185 CrossRef CAS.
- J. Yun, Y. Kim and S. Yu, Int. J. Heat Mass Transfer, 2018, 126, 353–361 CrossRef CAS.
- W. Wang, H. Wang, H. M. Li, D. Y. Li, H. B. Li and Z. H. Li, Energies, 2018, 11, 428 CrossRef.
- W. Wang, J. D. Ren, X. J. Li, H. B. Li, D. Y. Li, H. M. Li and Y. Song, Sci. Rep., 2020, 10, 7276 CrossRef CAS PubMed.
- T. P. Adamova, O. S. Subbotin, L. J. Chen and V. R. Belosludov, J. Eng. Thermophys., 2013, 22, 62–68 CrossRef CAS.
- J. W. Du, H. J. Li and L. G. Wang, Ind. Eng. Chem. Res., 2014, 53, 8182–8187 CrossRef CAS.
- J. W. Du, H. J. Li and L. G. Wang, Chem. Eng. J., 2015, 273, 75–81 CrossRef CAS.
- D. Saha, H. A. Grappe, A. Chakraborty and G. Orkoulas, Chem. Rev., 2016, 116, 11436–11499 CrossRef CAS PubMed.
- S. Sridhar, B. Smitha and T. M. Aminabhavi, Sep. Purif. Rev., 2007, 36, 113–174 CrossRef CAS.
- S. Sircar and T. C. Golden, Sep. Sci. Technol., 2000, 35, 667–687 CrossRef CAS.
- J. G. Jee, J. S. Lee and C. H. Lee, Ind. Eng. Chem. Res., 2001, 40, 3647–3658 CrossRef CAS.
- S. U. Nandanwar, D. R. Corbin and M. B. Shiflett, Ind. Eng. Chem. Res., 2020, 59, 13355–13369 CrossRef CAS.
- A. Olajossy, A. Gawdzik, Z. Budner and J. Dula, Chem. Eng. Res. Des., 2003, 81, 474–482 CrossRef CAS.
- S. Cavenati, C. A. Grande and A. E. Rodrigues, Chem. Eng. Sci., 2006, 61, 3893–3906 CrossRef CAS.
- T. E. Rufford, S. Smart, G. C. Y. Watson, B. F. Graham, J. Boxall, J. C. D. da Costa and E. F. May, J. Pet. Sci. Eng., 2012, 94–95, 123–154 CrossRef CAS.
- T. C. Golden and S. Sircar, J. Colloid Interface Sci., 1994, 162, 182–188 CrossRef CAS.
- D. F. Lv, Y. Wu, J. Y. Chen, Y. H. Tu, Y. N. Yuan, H. X. Wu, Y. W. Chen, B. Y. Liu, H. X. Xi, Z. Li and Q. B. Xia, AIChE J., 2020, 66, e16287 CrossRef CAS.
- X. Y. Ren, T. J. Sun, J. L. Hu and S. D. Wang, Microporous Mesoporous Mater., 2014, 186, 137–145 CrossRef CAS.
- Y. Guo, J. L. Hu, X. W. Liu, T. J. Sun, S. S. Zhao and S. D. Wang, Chem. Eng. J., 2017, 327, 564–572 CrossRef CAS.
- J. Kim, A. Maiti, L. C. Lin, J. K. Stolaroff, B. Smit and R. D. Aines, Nat. Commun., 2013, 4, 1694 CrossRef PubMed.
- A. Eslamimanesh, A. H. Mohammadi, D. Richon, P. Naidoo and D. Ramjugernath, J. Chem. Thermodyn., 2012, 46, 62–71 CrossRef CAS.
- M. Arjmandi, A. Chapoy and B. Tohidi, J. Chem. Eng. Data, 2007, 52, 2153–2158 CrossRef CAS.
- J. Z. Zhao, Y. S. Zhao and W. G. Liang, Energy Technol., 2016, 4, 864–869 CrossRef CAS.
- T. Suginaka, H. Sakamoto, K. Iino, Y. Sakakibara and R. Ohmura, Fluid Phase Equilib., 2013, 344, 108–111 CrossRef CAS.
- D. L. Zhong, Y. Ye, C. Yang, Y. Bian and K. Ding, Ind. Eng. Chem. Res., 2012, 51, 14806–14813 CrossRef CAS.
- J. Cai, C. G. Xu, Z. M. Xia, Z. Y. Chen and X. S. Li, Appl. Energy, 2017, 204, 1526–1534 CrossRef CAS.
- T. Rodenas, M. van Dalen, E. Garcia-Perez, P. Serra-Crespo, B. Zornoza, F. Kapteijn and J. Gascon, Adv. Funct. Mater., 2014, 24, 249–256 CrossRef CAS.
- P. Marín, Z. Yang, Y. Xia and S. Ordóñez, J. Nat. Gas Sci. Eng., 2020, 81, 103420 CrossRef.
- S. Cavenati, C. A. Grande and A. E. Rodrigues, J. Chem. Eng. Data, 2004, 49, 1095–1101 CrossRef CAS.
- S. Cavenati, C. A. Grande and A. E. Rodrigues, Sep. Sci. Technol., 2005, 40, 2721–2743 CrossRef CAS.
- Q. Wu, L. Zhou, J. Q. Wu and Y. P. Zhou, J. Chem. Eng. Data, 2005, 50, 635–642 CrossRef CAS.
- G. Watson, E. F. May, B. F. Graham, M. A. Trebble, R. D. Trengove and K. I. Chan, J. Chem. Eng. Data, 2009, 54, 2701–2707 CrossRef CAS.
- Z. Huang, L. Xu, J. H. Li, G. M. Guo and Y. Wang, J. Chem. Eng. Data, 2010, 55, 2123–2127 CrossRef CAS.
- C. A. Grande, Advances in Pressure Swing Adsorption for Gas Separation, ISRN Chem. Eng., 2012, 982934 Search PubMed.
- M. B. Kim, Y. S. Bae, D. K. Choi and C. H. Lee, Ind. Eng. Chem. Res., 2006, 45, 5050–5058 CrossRef CAS.
- J. F. Yang, R. Krishna, J. M. Li and J. P. Li, Microporous Mesoporous Mater., 2014, 184, 21–27 CrossRef CAS.
- D. Saha, Z. B. Bao, F. Jia and S. G. Deng, Environ. Sci. Technol., 2010, 44, 1820–1826 CrossRef CAS PubMed.
- Y. Yang, A. M. Ribeiro, P. Li, J. G. Yu and A. E. Rodrigues, Ind. Eng. Chem. Res., 2014, 53, 16840–16850 CrossRef CAS.
- T. E. S. Rufford, G. C. Y. Watson, B. F. Graham, J. Boxall, J. C. Diniz da Costa and E. F. Maya, J. Pet. Sci. Eng., 2012, 94–95, 32 Search PubMed.
- US EPA, Assessment of the worldwide market potential for oxidizing coal mine ventilation air methane, United States Environmental Protection Agency, EPA 430-R-03-002, July 2003 Search PubMed.
- P. Franklin, US Environmental Protection Agency VAM Vendor's Workshop Summary, Washington DC, 2nd and 3rd June 2004 Search PubMed.
- Y. Li, R. Xu, B. B. Wang, J. P. Wei, L. Y. Wang, M. Q. Shen and J. Yang, Nanomaterials, 2019, 9, 266 CrossRef CAS PubMed.
- K. X. Yao, Y. L. Chen, Y. Lu, Y. F. Zhao and Y. Ding, Carbon, 2017, 122, 258–265 CrossRef CAS.
- J. S. Bae, Y. G. Jin, C. Huynh and S. Su, J. Environ. Manage., 2018, 217, 373–380 CrossRef CAS PubMed.
- S. Xu, W.-C. Li, C.-T. Wang, L. Tang, G.-P. Hao and A.-H. Lu, Angew. Chem., Int. Ed., 2020, 60, 6339–6343 CrossRef PubMed.
- G. P. Hu, Q. H. Zhao, L. F. Tao, P. Xiao, P. A. Webley and K. G. Li, Chem. Eng. Sci., 2021, 229, 116152 CrossRef CAS.
- Z. Niu, X. L. Cui, T. Pham, P. C. Lan, H. B. Xing, K. A. Forrest, L. Wojtas, B. Space and S. Q. Ma, Angew. Chem., Int. Ed., 2019, 58, 10138–10141 CrossRef CAS PubMed.
- L. Y. Li, L. F. Yang, J. W. Wang, Z. G. Zhang, Q. W. Yang, Y. W. Yang, Q. L. Ren and Z. B. Bao, AIChE J., 2018, 64, 3681–3689 CrossRef CAS.
- M. Chang, Y. J. Zhao, Q. Y. Yang and D. H. Liu, ACS Omega, 2019, 4, 14511–14516 CrossRef CAS PubMed.
- R. J. Li, M. Li, X. P. Zhou, S. W. Ng, M. O'Keeffe and D. Li, CrystEngComm, 2014, 16, 6291–6295 RSC.
- M. Tagliabue, D. Farrusseng, S. Valencia, S. Aguado, U. Ravon, C. Rizzo, A. Corma and C. Mirodatos, Chem. Eng. J., 2009, 155, 553–566 CrossRef CAS.
- D. Tondeur and P. C. Wankat, Sep. Purif. Methods, 1985, 14, 157–212 CrossRef CAS.
- S. Cavenati, C. A. Grande and A. E. Rodrigues, Energy Fuels, 2006, 20, 2648–2659 CrossRef CAS.
- C. A. Grande and A. E. Rodrigues, Ind. Eng. Chem. Res., 2007, 46, 4595–4605 CrossRef CAS.
- G. S. Duarte, B. Schurer, C. Voss and D. Bathen, ChemBioEng Rev., 2017, 4, 277–288 CrossRef.
- J. S. Bae, S. Su and X. X. Yu, Environ. Sci. Technol., 2014, 48, 6043–6049 CrossRef CAS PubMed.
- Y. Zhou, Q. Fu, Y. H. Shen, W. N. Sun, D. H. Zhang, D. D. Li and H. Y. Yan, Energy Fuels, 2016, 30, 1496–1509 CAS.
- Y. L. Li, Y. S. Liu and X. Yang, Sep. Sci. Technol., 2013, 48, 1201–1210 CrossRef CAS.
- D. L. Qu, Y. Yang, Z. L. Qian, P. Li, J. G. Yu, A. M. Ribeiro and A. E. Rodrigues, Chem. Eng. J., 2020, 380, 122509 CrossRef CAS.
- Y. L. Li, Y. Meng, Y. S. Liu, X. Yang and C. Z. Zhang, Adv. Mechan. Design, Parts 1–3, ed. W. Z. Chen, P. Dai, Y. L. Chen, Q. T. Wang and Z. Jiang, 2012, 479–481, pp. 648–653 Search PubMed.
- Y. L. Li, C. Z. Zhang, Y. S. Liu, X. Yang and Y. Meng, Adv. Mechan. Design, Parts 1–3, ed. W. Z. Chen, P. Dai, Y. L. Chen, Q. T. Wang and Z. Jiang, 2012, 479–481, pp. 260–265 Search PubMed.
- X. Yang, Y. S. Liu and Y. L. Li, Chem. Eng. Mater. Properties, Parts 1 and 2, ed. H. M. Zhang and B. Wu, 2012, 391–392, pp. 1253–1258 Search PubMed.
- X. Yang, Y. S. Liu, Z. Y. Li, C. Z. Zhang and Y. Xing, Energies, 2018, 11, 1030 CrossRef.
- S. B. Ouyang, S. P. Xu, N. Song and S. L. Jiao, Fuel, 2013, 113, 420–425 CrossRef CAS.
- J. S. Bae, S. Su and X. X. Yu, Ind. Eng. Chem. Res., 2019, 58, 21700–21707 CrossRef CAS.
- Y. S. Liu, X. Yang, Y. L. Li, H. J. Yang, C. Z. Zhang and Y. Meng, Energy Educ. Sci. Technol., Part A, 2012, 29, 217–226 CAS.
- Y. Yang, Y. J. Wu, H. Q. Liu, A. M. Ribeiro, P. Li, J. G. Yu and A. E. Rodrigues, Chem. Eng. Sci., 2016, 149, 215–228 CrossRef CAS.
- C. M. Liu, Y. P. Zhou, Y. Sun, W. Su and L. Zhou, AIChE J., 2011, 57, 645–654 CrossRef CAS.
- V. C. Menon and S. Komarneni, J. Porous Mater., 1998, 5, 43–58 CrossRef CAS.
- K. F. Loughlin, M. M. Hassan, A. I. Fatehi and M. Zahur, Gas Sep. Purif., 1993, 7(4), 264–273 CrossRef CAS.
- G. Q. Lu and M. RostamAbadi, Gas Sep. Purif., 1996, 10, 89–90 CrossRef CAS.
- J. W. Yoon, H. Chang, S. J. Lee, Y. K. Hwang, D. Y. Hong, S. K. Lee, J. S. Lee, S. Jang, T. U. Yoon, K. Kwac, Y. Jung, R. S. Pillai, F. Faucher, A. Vimont, M. Daturi, G. Ferey, C. Serre, G. Maurin, Y. S. Bae and J. S. Chang, Nat. Mater., 2017, 16, 526–531 CrossRef CAS PubMed.
- D. E. Jaramillo, D. A. Reed, H. Z. H. Jiang, J. Oktawiec, M. W. Mara, A. C. Forse, D. J. Lussier, R. A. Murphy, M. Cunningham, V. Colombo, D. K. Shuh, J. A. Reimer and J. R. Long, Nat. Mater., 2020, 19, 517–521 CrossRef CAS PubMed.
- J. H. Zhao, S. H. Mousavi, G. K. Xiao, A. H. Mokarizadeh, T. Moore, K. F. Chen, Q. F. Gu, R. Singh, A. Zavabeti, J. Z. Liu, P. A. Webley and G. K. Li, J. Am. Chem. Soc., 2021, 143, 15195–15204 CrossRef CAS PubMed.
- F. F. Zhang, K. J. Li, J. Chen, X. R. Zhang, K. B. Li, H. Shang, L. Ma, W. J. Guo, X. L. Wu, J. F. Yang and J. P. Li, Sep. Purif. Technol., 2022, 281, 119951 CrossRef CAS.
- A. Policicchio, R. Filosa, S. Abate, G. Desiderio and E. Colavita, J. Porous Mater., 2017, 24, 905–922 CrossRef CAS.
- J. Alcaniz-Monge, D. Lozano-Castello, D. Cazorla-Amoros and A. Linares-Solano, Microporous Mesoporous Mater., 2009, 124, 110–116 CrossRef CAS.
- C. Guan, F. B. Su, X. S. Zhao and K. Wang, Sep. Purif. Technol., 2008, 64, 124–126 CrossRef CAS.
- D. Lozano-Castello, D. Cazorla-Amoros, A. Linares-Solano and D. F. Quinn, Carbon, 2002, 40, 989–1002 CrossRef CAS.
- N. Kosinov, J. Gascon, F. Kapteijn and E. J. M. Hensen, J. Membr. Sci., 2016, 499, 65–79 CrossRef CAS.
- Y. Q. Wu, D. H. Yuan, D. W. He, J. C. Xing, S. Zeng, S. T. Xu, Y. P. Xu and Z. M. Liu, Angew. Chem., Int. Ed., 2019, 58, 10241–10244 CrossRef CAS PubMed.
- J. A. C. Silva, A. Ferreira, P. A. P. Mendes, A. F. Cunha, K. Gleichmann and A. E. Rodrigues, Ind. Eng. Chem. Res., 2015, 54, 6390–6399 CrossRef CAS.
- G. M. Nam, B. M. Jeong, S. H. Kang, B. K. Lee and D. K. Choi, J. Chem. Eng. Data, 2005, 50, 72–76 CrossRef CAS.
- J. F. Yang, Q. Zhao, H. Xu, L. B. Li, J. X. Dong and J. P. Li, J. Chem. Eng. Data, 2012, 57, 3701–3709 CrossRef CAS.
- J. F. Yang, J. M. Li, W. Wang, L. B. Li and J. P. Li, Ind. Eng. Chem. Res., 2013, 52, 17856–17864 CrossRef CAS.
- J. A. Delgado, V. I. Agueda, J. Garcia and S. Alvarez-Torrellas, Sep. Purif. Technol., 2019, 209, 550–559 CrossRef CAS.
- J. F. Yang, N. Yuan, M. Xu, J. Q. Liu, J. P. Li and S. G. Deng, Microporous Mesoporous Mater., 2018, 266, 56–63 CrossRef CAS.
- N. K. Jensen, T. E. Rufford, G. Watson, D. K. Zhang, K. I. Chan and E. F. May, J. Chem. Eng. Data, 2012, 57, 106–113 CrossRef CAS.
- P. J. E. Harlick and F. H. Tezel, Sep. Sci. Technol., 2002, 37, 33–60 CrossRef CAS.
- A. Jayaraman, A. J. Hernandez-Maldonado, R. T. Yang, D. Chinn, C. L. Munson and D. H. Mohr, Chem. Eng. Sci., 2004, 59, 2407–2417 CrossRef CAS.
- C. L. Xue, W. P. Cheng, W. M. Hao, J. H. Ma and R. F. Li, J. Chem., 2019, 2019, 2078360 Search PubMed.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- I. Esteves, M. S. S. Lopes, P. M. C. Nunes and J. P. B. Mota, Sep. Purif. Technol., 2008, 62, 281–296 CrossRef CAS.
- T. L. Saleman, G. Li, T. E. Rufford, P. L. Stanwix, K. I. Chan, S. H. Huang and E. F. May, Chem. Eng. J., 2015, 281, 739–748 CrossRef CAS.
- G. M. Kimber, M. Jagtoyen, Y. Q. Fei and F. J. Derbyshire, Gas Sep. Purif., 1996, 10, 131–136 CrossRef CAS.
- S. Cavenati, C. A. Grande and A. E. Rodrigues, Sep. Sci. Technol., 2005, 40, 2721–2743 CrossRef CAS.
- A. Jayaraman, A. S. Chiao, J. Padin, R. T. Yang and C. L. Munson, Sep. Sci. Technol., 2002, 37, 2505–2528 CrossRef CAS.
- G. F. Zhao, P. Bai, H. M. Zhu, R. X. Yan, X. M. Liu and Z. F. Yan, Asia-Pac. J. Chem. Eng., 2008, 3, 284–291 CrossRef CAS.
- M. Gu, B. Zhang, Z. D. Qi, Z. J. Liu, S. Duan, X. D. Du and X. F. Xian, Sep. Purif. Technol., 2015, 146, 213–218 CrossRef CAS.
- P. Kluson, S. Scaife and N. Quirke, Sep. Purif. Technol., 2000, 20, 15–24 CrossRef CAS.
- C. M. Liu, Y. Y. Dang, Y. P. Zhou, J. Liu, Y. Sun, W. Su and L. Zhou, Adsorption, 2012, 18, 321–325 CrossRef CAS.
- D. S. Yuan, Y. N. Zheng, Q. Z. Li, B. Q. Lin, G. Y. Zhang and J. F. Liu, Powder Technol., 2018, 333, 377–384 CrossRef CAS.
- J. Wang, P. X. Zhang, L. Liu, Y. Zhang, J. F. Yang, Z. L. Zeng and S. G. Deng, Chem. Eng. J., 2018, 348, 57–66 CrossRef CAS.
- J. Wang, R. Krishna, J. F. Yang, K. P. R. Dandamudi and S. G. Deng, Mater. Today Commun., 2015, 4, 156–165 CrossRef CAS.
- W. Su, L. Yao, M. Ran, Y. Sun, J. Liu and X. J. Wang, J. Chem. Eng. Data, 2018, 63, 2914–2920 CrossRef CAS.
- H. Y. Pan, Y. Yi, Q. Lin, G. Y. Xiang, Y. Zhang and F. Liu, J. Chem. Eng. Data, 2016, 61, 2120–2127 CrossRef CAS.
- R. L. Tang, Q. B. Dai, W. W. Liang, Y. Wu, X. Zhou, H. Y. Pan and Z. Li, Chem. Eng. J., 2020, 384, 123388 CrossRef CAS.
- S. Cavenati, C. A. Grande and A. E. Rodrigues, Sep. Sci. Technol., 2005, 40, 2721–2743 CrossRef CAS.
- J. H. Zhang, S. J. Qu, L. T. Li, P. Wang, X. F. Li, Y. F. Che and X. L. Li, J. Chem. Eng. Data, 2018, 63, 1737–1744 CrossRef CAS.
- M. Suzuki, Carbon, 1994, 32, 577–586 CrossRef CAS.
- B. Yuan, X. F. Wu, Y. X. Chen, J. H. Huang, H. M. Luo and S. G. Deng, Environ. Sci. Technol., 2013, 47, 5474–5480 CrossRef CAS PubMed.
- B. Yuan, X. F. Wu, Y. X. Chen, J. H. Huang, H. M. Luo and S. G. Deng, J. Colloid Interface Sci., 2013, 394, 445–450 CrossRef CAS PubMed.
- P. X. Zhang, J. Wang, W. Fan, Y. Zhong, Y. Zhang, Q. Deng, Z. L. Zeng and S. G. Deng, Chem. Eng. J., 2019, 375, 121931 CrossRef CAS.
- Y. J. Wu, Y. Yang, X. M. Kong, P. Li, J. G. Yu, A. M. Ribeiro and A. E. Rodrigues, J. Chem. Eng. Data, 2015, 60, 2684–2693 CrossRef CAS.
- D. L. Qu, Y. Yang, K. Lu, L. Yang, P. Li, J. G. Yu, A. M. Ribeiro and A. E. Rodrigues, Adsorption, 2018, 24, 357–369 CrossRef CAS.
- S. J. Du, Y. Wu, X. J. Wang, Q. B. Xia, J. Xiao, X. Zhou and Z. Li, AIChE J., 2020, 66, e16231 CrossRef CAS.
- Y. Zhang, L. Liu, P. X. Zhang, J. Wang, M. Xu, Q. Deng, Z. L. Zeng and S. G. Deng, Chem. Eng. J., 2019, 355, 309–319 CrossRef CAS.
- P. X. Zhang, Y. Zhong, J. Ding, J. Wang, M. Xu, Q. Deng, Z. L. Zeng and S. G. Deng, Chem. Eng. J., 2019, 355, 963–973 CrossRef CAS.
- B. P. Russell and M. D. LeVan, Ind. Eng. Chem. Res., 1997, 36, 2380–2389 CrossRef CAS.
- T. Ohba and K. Kaneko, Langmuir, 2011, 27, 7609–7613 CrossRef CAS PubMed.
- J. Bahadur, C. I. Contescu, D. K. Rai, N. C. Gallego and Y. B. Melnichenko, Carbon, 2017, 111, 681–688 CrossRef CAS.
- B. Liu and B. Smit, Langmuir, 2009, 25, 5918–5926 CrossRef CAS PubMed.
- B. Liu and B. Smit, J. Phys. Chem. C, 2010, 114, 8515–8522 CrossRef CAS.
- X. Peng, X. Cheng and D. P. Cao, J. Mater. Chem., 2011, 21, 11259–11270 RSC.
- X. F. Wu, B. Yuan, Z. B. Bao and S. G. Deng, J. Colloid Interface Sci., 2014, 430, 78–84 CrossRef CAS PubMed.
- J. L. Hu, T. J. Sun, X. W. Liu, Y. Guo and S. D. Wang, RSC Adv., 2016, 6, 64039–64046 RSC.
- Q. Shi, J. Wang, H. Shang, H. H. Bai, Y. Zhao, J. F. Yang, J. X. Dong and J. P. Li, Sep. Purif. Technol., 2020, 230, 115850 CrossRef CAS.
- X. W. Liu, J. L. Hu, T. J. Sun, Y. Guo, T. D. Bennett, X. Y. Ren and S. D. Wang, Chem.–Asian J., 2016, 11, 3014–3017 CrossRef CAS PubMed.
- X. W. Liu, Y. M. Gu, T. J. Sun, Y. Guo, X. L. Wei, S. S. Zhao and S. D. Wang, Ind. Eng. Chem. Res., 2019, 58, 20392–20400 CrossRef CAS.
- L. B. Li, J. F. Yang, J. M. Li, Y. Chen and J. P. Li, Microporous Mesoporous Mater., 2014, 198, 236–246 CrossRef CAS.
- C. E. Kivi, B. S. Gelfand, H. Dureckova, H. T. K. Ho, C. Ma, G. K. H. Shimizu, T. K. Woo and D. T. Song, Chem. Commun., 2018, 54, 14104–14107 RSC.
- J. L. Hu, T. J. Sun, X. Y. Ren and S. D. Wang, Microporous Mesoporous Mater., 2015, 204, 73–80 CrossRef CAS.
- K. A. Cychosz and A. J. Matzger, Langmuir, 2010, 26, 17198–17202 CrossRef CAS PubMed.
- W. Zhang, Y. L. Hu, J. Ge, H. L. Jiang and S. H. Yu, J. Am. Chem. Soc., 2014, 136, 16978–16981 CrossRef CAS PubMed.
- J. Mollmer, M. Lange, A. Moller, C. Patzschke, K. Stein, D. Lassig, J. Lincke, R. Glaser, H. Krautscheid and R. Staudt, J. Mater. Chem., 2012, 22, 10274–10286 RSC.
- F. Dong, H. M. Lou, A. Kodama, M. Goto and T. Hirose, Sep. Purif. Technol., 1999, 16, 159–166 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |



