Dealloying layered PdBi2 nanoflakes to palladium hydride leads to enhanced electrocatalytic N2 reduction†
Abstract
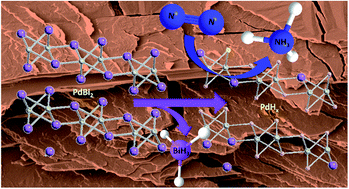
Electrochemical catalysis of nitrogen to ammonia is an environmentally friendly alternative strategy to the traditional and damaging Haber–Bosch process. Nevertheless, it is limited by a low ammonia yield and faradaic efficiency due to (i) the complexity associated with the breaking of the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and (ii) the competing hydrogen evolution reaction. Herein, we report that the electrochemical dealloying of exfoliated PdBi2 nanoflakes into palladium hydride greatly enhances the electrocatalytic nitrogen reduction reaction. The electrochemical decomposition of bismuth atoms creates vacancies in the PdBi2 crystal lattice enabling hydrogen atoms to be absorbed and instigating the initial protonation of N2 molecules necessary for the eventual NH3 formation. The average ammonia yield and faradaic efficiency were 30.06 ± 1.16 μg cm−2 h−1 and 15.25 ± 1.29% when the potential was held at −1.5 V vs. sat. Ag/AgCl after 6 h at room temperature. Our conclusions are supported by the first-principles simulations and this work emphasizes the potential implementation of palladium compounds for future N2 reduction reaction electrocatalysts.
N bond and (ii) the competing hydrogen evolution reaction. Herein, we report that the electrochemical dealloying of exfoliated PdBi2 nanoflakes into palladium hydride greatly enhances the electrocatalytic nitrogen reduction reaction. The electrochemical decomposition of bismuth atoms creates vacancies in the PdBi2 crystal lattice enabling hydrogen atoms to be absorbed and instigating the initial protonation of N2 molecules necessary for the eventual NH3 formation. The average ammonia yield and faradaic efficiency were 30.06 ± 1.16 μg cm−2 h−1 and 15.25 ± 1.29% when the potential was held at −1.5 V vs. sat. Ag/AgCl after 6 h at room temperature. Our conclusions are supported by the first-principles simulations and this work emphasizes the potential implementation of palladium compounds for future N2 reduction reaction electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...