Two-dimensional MXenes for electrochemical energy storage applications
Abstract
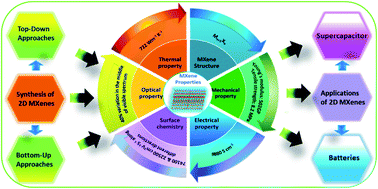
Since the discovery of Ti3C2Tx in early 2011, a newly emerging family of post-graphene two-dimensional transition metal carbides and nitrides (MXenes) has been rigorously investigated due to their high electrical conductivity and various stunning properties. MXenes have attracted significant research interest worldwide and have demonstrated promising potential in energy storage applications owing to their layered structure, superior hydrophilicity, metallic nature, high charge carrier mobility, tunable bandgap, and rich surface chemistry. To completely exploit their potential beyond the existing boundaries, unique functional nanostructures, monolayers, multilayer compounds, nanoparticles, and composites have been prepared through functionalization, hybridization, intercalation, etc. MXenes have shown novel and tunable properties, easy processing, and superior electrochemical performance, which make them potential candidates for application in electrochemical energy storage. Herein, we present a forward-looking review of MXene-based materials with their synthesis protocol, fundamental properties, and state-of-the-art electrochemical activity and performance in supercapacitors and rechargeable batteries. Finally, we discuss the challenges that must be addressed for future research, which will deepen the basic understanding of MXenes and their derivatives to promote further advancements in burgeoning energy storage technologies.
- This article is part of the themed collection: 2023 Journal of Materials Chemistry A Lunar New Year collection


 Please wait while we load your content...
Please wait while we load your content...