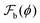Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermodynamics predicts a stable microdroplet phase in polymer–gel mixtures undergoing elastic phase separation†
Subhadip
Biswas
 ,
Biswaroop
Mukherjee
,
Biswaroop
Mukherjee
 and
Buddhapriya
Chakrabarti
and
Buddhapriya
Chakrabarti
 *
*
Department of Physics, University of Sheffield, Hicks Building, Hounsfield Road, Sheffield, S3 7RH, UK. E-mail: b.chakrabarti@sheffield.ac.uk
First published on 23rd September 2022
Abstract
We study the thermodynamics of binary mixtures with the volume fraction of the minority component less than the amount required to form a flat interface and show that the surface tension dominated equilibrium phase of the mixture forms a single macroscopic droplet. Elastic interactions in gel–polymer mixtures stabilize a phase with multiple droplets. Using a mean-field free energy we compute the droplet size as a function of the interfacial tension, Flory parameter, and elastic moduli of the gel. Our results illustrate the role of elastic interactions in dictating the phase behavior of biopolymers undergoing liquid-liquid phase separation.
1 Introduction
Membraneless compartmentalisation in cells that are driven by phase-separation processes due to changes in temperature or pH, and maintained by a non-vanishing interfacial tension, is one of the most exciting recent biological discoveries.1–4 These membraneless compartments composed bio-molecular condensates have been implicated in important biological processes such as transcriptional regulation,5 chromosome organisation6 and in several human pathologies e.g. Huntington's, ALS etc.7 Self-assembly processes that lead to organelle formation however need to be tightly regulated such that the phase separated droplets do not grow without bound and remain small compared to the cell size. Understanding the regulatory processes that control droplet size in cellular environments is therefore a crucial interdisciplinary question. The two candidate mechanisms proposed for arresting droplet growth are (i) incorporation of active forces that break detailed balance,8,9 and (ii) non-equilibrium reaction mechanisms which couple to the local density field.4 Although biological systems are inherently out of equilibrium, an estimation of diffusion constant of bio-molecules indicates that non-equilibrium effects are negligible on length-scales beyond microns and timescales beyond microseconds. Hence, the framework of equilibrium thermodynamics can be readily applied to analyse biological phase separation in cells.10For synthetic polymer mixtures, in the absence of active processes, droplet growth is limited by the elastic interactions of the background matrix that alters the thermodynamics of phase separation.11–13 Recent experiments on mixtures of liquid PDMS and fluorinated oil in a matrix of cross-linked PDMS show the dependence of the droplet size on the nucleation temperature and the network stiffness.14,15 Despite theoretical attempts16,17 a complete understanding of elasticity mediated arrested droplet growth is still lacking.
The connection between coarsening phenomena and network elasticity is an important, and exciting area of research across several disciplines, biological regulation of cellular function,1–4 tailoring mechanical properties of materials,18–20 controlling morphology,21–23 size of precipitates in food products,24,25 and even growth of methane bubbles in aquatic sediments.26–28
In this paper, we develop a consistent thermodynamic formalism to compute the equilibrium radius of the droplet of the minority phase in (a) binary polymer, and (b) a polymer–gel mixture, using mean-field theories utilising the Flory–Huggins29 and the Flory–Rehner30 free energies, respectively. A parallel tangent construction for droplets, used to obtain the densities of coexisting phases is presented. This procedure is a generalisation of the common tangent construction for flat interfaces and in the thermodynamic limit allows us to compute the equilibrium radius of a single droplet. For phase separation processes in mixtures with a gel component, elastic interactions limit droplet growth stabilising a phase with multiple droplets, in the correct parameter regime.31
2 Model
Consider a binary mixture of a gel and a solvent, close to but below the gelation temperature, where the gel-formation and the phase-separation are competing processes. The physical system considered is different from the recent experimental14,15 and theoretical studies.16,17 The experiments have been performed on mixtures of liquid PDMS (uncrosslinked PDMS polymers) and fluorinated oil in a matrix of cross-linked PDMS, thus it is a ternary system. The two previous theoretical attempts16,17 however approach this by describing the thermodynamics of a binary mixture (oil and uncrosslinked PDMS) in the background of the elastic matrix (crosslinked PDMS), where the volume-fraction of the matrix does not enter the calculation. The matrix only provides an elastic background in which the phase separation of the binary mixture occurs. On the other hand, the elastic matrix is considered in reference,14,15 but the translational entropy of the gel has been explicitly put to zero. However, this is a contentious issue, as we discuss later in the manuscript, and it leads to unstable solutions for a binary mixture of a gel and a solvent. References14,15 does not encounter this issue as they do not perform the parallel tangent construction, which is a condition that arises from the minimisation of the free-energy, and they bypass this by assuming that the dispersed microdroplets of the solvents can be described as an ideal gas.The thermodynamic formalism to understand phase separation is as follows: an unstable mixture of composition ϕ0 splits into two coexisting phases in a slab-like geometry respecting volume and mass conservation, with the equilibrium configuration being a minimum of the free energy (Fig. 1(a)). The volume fraction of the two coexisting phases are ϕin, and ϕout respectively, with Vd denoting the volume occupied by phase with density ϕin, and  is the Helmholtz free-energy per unit volume (in units of
is the Helmholtz free-energy per unit volume (in units of 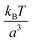 ). The solvent fraction is
). The solvent fraction is 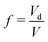 , and the free-energy density
, and the free-energy density  of the planar configuration (Fig. 1(a)) is given by,
of the planar configuration (Fig. 1(a)) is given by,
 | (1) |
 = 2γV−1/3, corresponds to the surface energy with γ being the surface tension, V the volume of the system considered, and λ a Lagrange multiplier that enforces the mass conservation constraint.
= 2γV−1/3, corresponds to the surface energy with γ being the surface tension, V the volume of the system considered, and λ a Lagrange multiplier that enforces the mass conservation constraint.
A calculation of the equilibrium thermodynamics proceeds via minimising the free energy in eqn (1) w.r.t to the independent quantities ϕin, ϕout, f, and λ. The constrained minimization of the free-energy function in eqn (1) w.r.t. ϕin, ϕout and f leads to the common tangent construction
| μ(ϕin) = μ(ϕout), and Π(ϕin) = Π(ϕout), | (2) |
 (ϕin, ϕout, f, λ)/∂λ = 0, which yields,
(ϕin, ϕout, f, λ)/∂λ = 0, which yields, 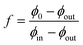 . For a planar interface, the surface energy term does not explicitly depend on the solvent volume fraction f. Consequently, the minimisation conditions lead to four uncoupled equations (ESI†) and a knowledge of the coexistence volume fractions ϕin and ϕout is enough to determine f. As evident from eqn (1), the effect of the surface energy term vanishes in the thermodynamic limit, i.e., as volume V → ∞. In contrast, a spherical droplet geometry introduces a non-trivial coupling among the minimisation conditions and a knowledge of the volume, V, of the system is required to obtain the equilibrium configuration.
. For a planar interface, the surface energy term does not explicitly depend on the solvent volume fraction f. Consequently, the minimisation conditions lead to four uncoupled equations (ESI†) and a knowledge of the coexistence volume fractions ϕin and ϕout is enough to determine f. As evident from eqn (1), the effect of the surface energy term vanishes in the thermodynamic limit, i.e., as volume V → ∞. In contrast, a spherical droplet geometry introduces a non-trivial coupling among the minimisation conditions and a knowledge of the volume, V, of the system is required to obtain the equilibrium configuration.
A spherical droplet
Spherical droplets of the minority phase arise in finite systems when the volume fraction is less than a critical value.32,33 The thermodynamics in such situations differ from the common tangent construction and leads to the classical Gibbs-Thomson relations.4Fig. 1(b) shows an unstable system of volume fraction ϕ0, that phase separates into a background matrix of volume fraction ϕout and a single droplet of radius R of volume fraction ϕin in a finite box of volume V. Assuming an ansatz of a phase separated mixture comprising of N spherical droplets of identical radius R, (referred to as the micro-droplet phase henceforth), the solvent fraction is given by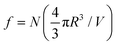 . The free energy of the micro-droplet phase is therefore
. The free energy of the micro-droplet phase is therefore ![[scr F, script letter F]](https://www.rsc.org/images/entities/char_e141.gif) = f
= f ![[scr F, script letter F]](https://www.rsc.org/images/entities/char_e141.gif) b (ϕin) + (1 − f)
b (ϕin) + (1 − f) ![[scr F, script letter F]](https://www.rsc.org/images/entities/char_e141.gif) b (ϕout) +
b (ϕout) + ![[scr F, script letter F]](https://www.rsc.org/images/entities/char_e141.gif) s(f), where
s(f), where  , accounts for the interfacial energy between the droplet and the background phase. By imposing the mass conservation constraint and expressing the surface energy in terms of the solvent fraction f, the free energy per unit volume is given by
, accounts for the interfacial energy between the droplet and the background phase. By imposing the mass conservation constraint and expressing the surface energy in terms of the solvent fraction f, the free energy per unit volume is given by | (3) |
The surface energy of the droplet depends on the solvent fraction f on account of the its spherical shape. The equilibrium conditions therefore lead to four coupled equations, involving the yet unknown system volume V. The chemical and mechanical equilibrium conditions for the micro-droplet phase involving the coexisting densities translates to, μ(ϕin) = μ(ϕout) and  , where the extra term in the pressure equation accounts for the Laplace pressure acting across the interface. We carry out a minimisation procedure akin to the planar interface to obtain the solvent volume fraction f, and the coexistence volume fractions inside and outside the droplet, ϕin and ϕout respectively for a given box volume V. In the absence of elastic interactions the equilibrium phase corresponds to a single droplet of the minority phase, i.e. N = 1 in eqn (3). The radius of the drop is determined in terms of the coexistence densities and is given by
, where the extra term in the pressure equation accounts for the Laplace pressure acting across the interface. We carry out a minimisation procedure akin to the planar interface to obtain the solvent volume fraction f, and the coexistence volume fractions inside and outside the droplet, ϕin and ϕout respectively for a given box volume V. In the absence of elastic interactions the equilibrium phase corresponds to a single droplet of the minority phase, i.e. N = 1 in eqn (3). The radius of the drop is determined in terms of the coexistence densities and is given by
| R = νL. | (4) |
 , and L = V1/3 is the length of the cubic box. We apply the framework to compute the radius of the minority phase droplet of a binary polymer mixture described by a Flory–Huggins free energy in the thermodynamic limit i.e. V → ∞, performing our calculation for different box volumes V. The surface tension γ for the micro-droplet phase is taken to be the same as that of a planar interface.
, and L = V1/3 is the length of the cubic box. We apply the framework to compute the radius of the minority phase droplet of a binary polymer mixture described by a Flory–Huggins free energy in the thermodynamic limit i.e. V → ∞, performing our calculation for different box volumes V. The surface tension γ for the micro-droplet phase is taken to be the same as that of a planar interface.
The thermodynamics of binary polymer mixtures is well described by the Flory–Huggins free-energy 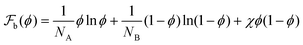 , where NA, and NB are the lengths of A and B polymers respectively, and χ is the mixing parameter. For χ > χc, where χc is the value of the mixing parameter at criticality, the mixture is unstable and spontaneously phase separates into low and high volume fraction phases determined by the minimisation conditions. We consider an unstable polymer mixture with NA = 100, NB = 200, and an initial composition ϕ0 = 0.35, and χ = 1.2χc. Fig. 1(a) shows the common tangent construction which yields the coexistence volume fractions ϕα = 0.235 and ϕβ = 0.885 for a flat interface. If the amount of material is not enough, the minority phase forms a droplet whose coexistence volume fractions outside ϕout and inside ϕin are determined by the parallel tangent construction (Fig. 1(b)) as a function of the box volume V. A combination of the parameters ϕ0, ϕα and ϕβ determines that the fraction of the solvent-rich phase f ≈ 0.17. The equilibrium phase is a single drop. To obtain the coexistence volume fractions and the droplet radius in the thermodynamic limit, we perform parallel tangent constructions for cubic boxes of lengths L = 160, … 104 using eqn (4).
, where NA, and NB are the lengths of A and B polymers respectively, and χ is the mixing parameter. For χ > χc, where χc is the value of the mixing parameter at criticality, the mixture is unstable and spontaneously phase separates into low and high volume fraction phases determined by the minimisation conditions. We consider an unstable polymer mixture with NA = 100, NB = 200, and an initial composition ϕ0 = 0.35, and χ = 1.2χc. Fig. 1(a) shows the common tangent construction which yields the coexistence volume fractions ϕα = 0.235 and ϕβ = 0.885 for a flat interface. If the amount of material is not enough, the minority phase forms a droplet whose coexistence volume fractions outside ϕout and inside ϕin are determined by the parallel tangent construction (Fig. 1(b)) as a function of the box volume V. A combination of the parameters ϕ0, ϕα and ϕβ determines that the fraction of the solvent-rich phase f ≈ 0.17. The equilibrium phase is a single drop. To obtain the coexistence volume fractions and the droplet radius in the thermodynamic limit, we perform parallel tangent constructions for cubic boxes of lengths L = 160, … 104 using eqn (4).
Fig. 2 shows a finite size scaling analysis of the droplet radius R in units of the box-size L, (R/L) as a function of 1/L. The thermodynamic limit 1/L→0 corresponds to the y-intercept R/L ≈ 0.34 for the Flory parameters listed above. The numerical derivative of R/L w.r.t. L approaches zero in this limit (Fig. 2 inset). The solvent fraction f, is also a function of the systems size (f ∼ (R/L)3). The coexistence densities calculated from eqn (4) are functions of L and can be quantified in terms of their deviation from the coexistence volume fractions for a planar interface, i.e., ![[small phi, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e2.gif) in = (ϕin−ϕβ)/ϕβ and
in = (ϕin−ϕβ)/ϕβ and ![[small phi, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e2.gif) out = (ϕout−ϕα)/ϕα. As shown in Fig. 2ϕout → ϕα, and ϕin → ϕβ in the thermodynamic limit.
out = (ϕout−ϕα)/ϕα. As shown in Fig. 2ϕout → ϕα, and ϕin → ϕβ in the thermodynamic limit.
 | ||
| Fig. 2 Finite size scaling of equilibrium drop radius R(L)/L, of a phase separated binary polymer mixture using a Flory–Huggins free energy functional with parameters described in Fig. 1. Coexistence volume fractions inside and outside the droplet ϕin and ϕout approaches the coexistence values obtained from a common tangent construction as L → ∞. Inset shows the rate of change of the radius approaches zero as L → ∞. | ||
A microdroplet phase
The Helmholtz free-energy per unit volume of the micro-droplet configuration of a gel–solvent mixture, with N droplets (see Fig. 4(d) inset), is given by | (5) |
 is the Flory–Huggins free energy given by
is the Flory–Huggins free energy given by 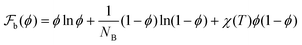 . We consider a situation where the strand length of the gel, NB, is considered to be finite in these calculations (NB = 25 and NA = 1). The reason for this, and not letting NB → ∞, is based on stability arguments and is discussed in the ESI† and we set χ(T) = 1.38χc in our calculations. The surface-energy per unit volume in eqn (5),
. We consider a situation where the strand length of the gel, NB, is considered to be finite in these calculations (NB = 25 and NA = 1). The reason for this, and not letting NB → ∞, is based on stability arguments and is discussed in the ESI† and we set χ(T) = 1.38χc in our calculations. The surface-energy per unit volume in eqn (5),  is expressed in terms of the solvent fraction f using the relation between the drop radius and the number density, i.e.,
is expressed in terms of the solvent fraction f using the relation between the drop radius and the number density, i.e.,  . The elastic part of the free-energy density in eqn (5) can be expressed as a function of the solvent fraction, f, (see ESI†) and is given by
. The elastic part of the free-energy density in eqn (5) can be expressed as a function of the solvent fraction, f, (see ESI†) and is given by | (6) |
To incorporate the effects of the finite stretch-ability of the gel, we adopt the Gent model.34,35 The elastic free energy density has the form, 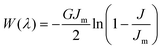 , where J = λr2 + λθ2 + λϕ2− 3, with λ's corresponding to the strains in the radial, azimuthal, and polar directions, Jm ∼ 106 is the stretching limit of the network, and G is the shear modulus. The shear modulus is related to the microscopic parameters via the relation,
, where J = λr2 + λθ2 + λϕ2− 3, with λ's corresponding to the strains in the radial, azimuthal, and polar directions, Jm ∼ 106 is the stretching limit of the network, and G is the shear modulus. The shear modulus is related to the microscopic parameters via the relation,  , where ndry and R0 are the average cross-link density and the mesh size of the dry gel respectively36 (see ESI†). Due to the volume-preserving nature of the deformation, λr = 1/λ2 and λϕ = λθ = λ and its magnitude is bounded, i.e., 0 < J/Jm < 1.34 The energy minimisation conditions w.r.t the independent variables as outlined earlier, leads to a modified equilibrium conditions: μ(ϕin) = μ(ϕout) and
, where ndry and R0 are the average cross-link density and the mesh size of the dry gel respectively36 (see ESI†). Due to the volume-preserving nature of the deformation, λr = 1/λ2 and λϕ = λθ = λ and its magnitude is bounded, i.e., 0 < J/Jm < 1.34 The energy minimisation conditions w.r.t the independent variables as outlined earlier, leads to a modified equilibrium conditions: μ(ϕin) = μ(ϕout) and 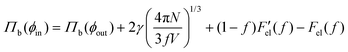 . These conditions lead to a set of coupled equations that we solve numerically to yield the four unknown variables, ϕin, ϕout, f, and λ, associated with each droplet number, N. A geometrical interpretation of these equations lead to the construction of parallel tangents.
. These conditions lead to a set of coupled equations that we solve numerically to yield the four unknown variables, ϕin, ϕout, f, and λ, associated with each droplet number, N. A geometrical interpretation of these equations lead to the construction of parallel tangents.
We substitute the equilibrium values of the coexistence volume fractions and solvent fraction into the original free-energy expression in eqn (5), to obtain a free energy ![[F with combining tilde]](https://www.rsc.org/images/entities/i_char_0046_0303.gif) (N), as a function of the number of droplets N. The minimisation of
(N), as a function of the number of droplets N. The minimisation of ![[F with combining tilde]](https://www.rsc.org/images/entities/i_char_0046_0303.gif) (N) w.r.t N yields Nm, the optimal number of droplets of the micro-droplet phase.
(N) w.r.t N yields Nm, the optimal number of droplets of the micro-droplet phase.
Fig. 3(a) shows the free-energy ![[F with combining tilde]](https://www.rsc.org/images/entities/i_char_0046_0303.gif) (N) =
(N) =  (N) −
(N) − (1) (eqn (5)) as a function of the number of droplets, once the coexistence volume fractions have been obtained for a cubic box of side L = 200 and the surface tension γ = 1.67 × 10−3 (in units of kBT/a2). It is evident that this is a convex function, with a well defined minimum occurs around Nm ≈ 23. The inset shows the contrasting behaviour of
(1) (eqn (5)) as a function of the number of droplets, once the coexistence volume fractions have been obtained for a cubic box of side L = 200 and the surface tension γ = 1.67 × 10−3 (in units of kBT/a2). It is evident that this is a convex function, with a well defined minimum occurs around Nm ≈ 23. The inset shows the contrasting behaviour of ![[F with combining tilde]](https://www.rsc.org/images/entities/i_char_0046_0303.gif) (N) for a binary polymer mixture. In the absence of elastic interactions, surface tension dominates the thermodynamics and a phase with a single droplet is the equilibrium state corresponding to the free energy minimum. The convex nature of the free energy
(N) for a binary polymer mixture. In the absence of elastic interactions, surface tension dominates the thermodynamics and a phase with a single droplet is the equilibrium state corresponding to the free energy minimum. The convex nature of the free energy ![[F with combining tilde]](https://www.rsc.org/images/entities/i_char_0046_0303.gif) (N) arises from a balance between the surface, elastic, and bulk free energies of the micro-droplet phase. As the number of droplets N increases, the surface energy monotonically increases on account of the increase of the total interfacial area. In contrast, the elastic energy monotonically decreases as a function of N, since an increase in the number of droplets translates to smaller sized drops and less deformation of the gel matrix. The elastic free energy has a lower bound corresponding to a minimum droplet of size R/L ∼ a, length of a monomer. The combined effect of these two contributions to the free energy therefore stabilizes the micro-droplet phase. The bulk free energy is nearly independent of N. Fig. 3(b) shows the variation of the different components of the total free energy as a function of the number of droplets N, while Fig. 3(c) shows the variation of number density n = Nm/V, and droplet radius R as a function of the shear modulus G. The shear modulus G is tuned by varying the mesh size, R0, of the gel. We compute the number density by minimizing
(N) arises from a balance between the surface, elastic, and bulk free energies of the micro-droplet phase. As the number of droplets N increases, the surface energy monotonically increases on account of the increase of the total interfacial area. In contrast, the elastic energy monotonically decreases as a function of N, since an increase in the number of droplets translates to smaller sized drops and less deformation of the gel matrix. The elastic free energy has a lower bound corresponding to a minimum droplet of size R/L ∼ a, length of a monomer. The combined effect of these two contributions to the free energy therefore stabilizes the micro-droplet phase. The bulk free energy is nearly independent of N. Fig. 3(b) shows the variation of the different components of the total free energy as a function of the number of droplets N, while Fig. 3(c) shows the variation of number density n = Nm/V, and droplet radius R as a function of the shear modulus G. The shear modulus G is tuned by varying the mesh size, R0, of the gel. We compute the number density by minimizing ![[F with combining tilde]](https://www.rsc.org/images/entities/i_char_0046_0303.gif) (N) w.r.t. N and determine the drop radius using R(Nm) = (ν/Nm1/3)L for a given shear modulus G. As shown, the radii of the droplets decrease (and hence the number density n increases commensurately) as the gel becomes stiffer.
(N) w.r.t. N and determine the drop radius using R(Nm) = (ν/Nm1/3)L for a given shear modulus G. As shown, the radii of the droplets decrease (and hence the number density n increases commensurately) as the gel becomes stiffer.
The convex nature of ![[F with combining tilde]](https://www.rsc.org/images/entities/i_char_0046_0303.gif) (N) as a function of N is independent of the system size L as shown in Fig. 4(a). Fig. 4(b) shows the dependence of
(N) as a function of N is independent of the system size L as shown in Fig. 4(a). Fig. 4(b) shows the dependence of ![[F with combining tilde]](https://www.rsc.org/images/entities/i_char_0046_0303.gif) (N) as a function of the surface tension, γ = 0.0025, … 0.004, while keeping the shear modulus of the gel–solvent mixture fixed at G = 1.9 × 10−4 (in units of kBT/a3). The free energy minimum shifts to smaller values of Nm with increasing surface tension as shown in Fig. 4(b). The inset of Fig. 4(b) shows that for γ < γc ≈ 4.0 × 10−3, a micro-droplet phase is the equilibrium configuration, with Nm, monotonically increasing with decreasing γ. Fig. 4(c) shows that the equilibrium number density of droplets n = Nm/V, and the droplet radius R(Nm) have reached a thermodynamic limit and are independent of the system size L. Fig. 4(d) shows the phase boundary demarcating regions of a stable macro-droplet and dispersed micro-droplet phases in the γ−G plane. The mean field phase-boundary (symbols) qualitatively agrees with the scaling results31 (red dashed line) for softer gels while significant deviations are observed for stiffer ones. The mean-field phase boundary (symbols) is now a function of the gel-strand length NB, a variable that is associated with the network heterogeneity of the system. Such quenched disorder dramatically modifies the equilibrium thermodynamics of gel networks.
(N) as a function of the surface tension, γ = 0.0025, … 0.004, while keeping the shear modulus of the gel–solvent mixture fixed at G = 1.9 × 10−4 (in units of kBT/a3). The free energy minimum shifts to smaller values of Nm with increasing surface tension as shown in Fig. 4(b). The inset of Fig. 4(b) shows that for γ < γc ≈ 4.0 × 10−3, a micro-droplet phase is the equilibrium configuration, with Nm, monotonically increasing with decreasing γ. Fig. 4(c) shows that the equilibrium number density of droplets n = Nm/V, and the droplet radius R(Nm) have reached a thermodynamic limit and are independent of the system size L. Fig. 4(d) shows the phase boundary demarcating regions of a stable macro-droplet and dispersed micro-droplet phases in the γ−G plane. The mean field phase-boundary (symbols) qualitatively agrees with the scaling results31 (red dashed line) for softer gels while significant deviations are observed for stiffer ones. The mean-field phase boundary (symbols) is now a function of the gel-strand length NB, a variable that is associated with the network heterogeneity of the system. Such quenched disorder dramatically modifies the equilibrium thermodynamics of gel networks.
Fig. 5 (a), which is similar to Fig. 4(d), shows the contour-plot of the dimensionless ratio between the surface energy and the elastic energy, h/α, has been shown in the γ–G plane, where α is equal to 2.5 (see ESI† for a discussion on this). Also shown is the phase boundary from the mean field theory calculations (inverted triangles, the inverted triangle and the dashed line are similar to that presented in Fig. 4(d)). Simple scaling arguments would suggest that the phase boundary would occur at h/α equal to unity (see the dashed line in Fig. 5(a)) and we observe that for small values of the shear modulus, G, this is indeed the case. However, as the value of G increases deviations between the mean-field phase boundary (inverted triangles) and the h/α equal to unity increase. In order to facilitate comparison with present and future experiments, we have studied how the equilibrium number of droplets evolve as a function of a tuning parameters (shear modulus or surface tension in this case) as one crosses the phase boundary along the principal directions in the phase plane. Panel (b) shows the transition from a dispersed micro-droplet to a single macro-droplet as one crosses the phase boundary while keeping G fixed and increasing γ. For γ < γc, the dependence of the number of droplets on the surface tension follows the linear relationship, 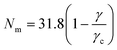 . Similarly, panel (c) shows the transition from a single macro-droplet to a dispersed micro-droplet state when one keeps γ constant and increases G and here the dependence of the number of droplets on the elastic modulus again follows a linear dependence
. Similarly, panel (c) shows the transition from a single macro-droplet to a dispersed micro-droplet state when one keeps γ constant and increases G and here the dependence of the number of droplets on the elastic modulus again follows a linear dependence  . The linear dependence of the number of droplets on the elastic modulus of the matrix is a result of the mean-field theory calculations (and not an assumptions as in16) and has been observed in the experiments.14
. The linear dependence of the number of droplets on the elastic modulus of the matrix is a result of the mean-field theory calculations (and not an assumptions as in16) and has been observed in the experiments.14
In summary, we consider phase separation in an elastic medium, where the background matrix influences the equilibrium thermodynamics of the. Previous studies consider the background matrix as an inert phase.16,17 For composition regimes where the solvent is a minority phase and there is a dearth of material to form a flat interface, solvent-rich droplets coexist with the majority phase. We demonstrate, via a mean-field theory that the dispersed micro-droplet phase is indeed a thermodynamic minimum for a binary gel–solvent mixture. A competition between surface tension and network elasticity stabilizes this phase. When the surface-tension exceeds a critical value, a single macroscopic droplet is the stable thermodynamic phase. Though the Flory–Huggins functional has been used to describe polymer mixtures, our results are generic and valid for any bistable potential.37
3 Discussion
A macroscopic gel would possess intrinsic heterogeneities in the mesh size resulting in different values of NB in different that leads to a micro-droplet phase with different sized droplets.38 Thus our mean-field theory needs to be extended to incorporate a distribution of mesh sizes, i.e. P(NB). Assuming that the disorder correlation length is ψ, our mean-field theory is applicable for length scales![[small script l]](https://www.rsc.org/images/entities/i_char_e146.gif) ≤ ψ from which the coexistence densities ϕin and ϕout can be obtained. The coexistence densities are functions of NB, a parameter in the FH free-energy. Thus a distribution of mesh sizes, P(NB), leads to a distribution of coexistence volume fractions (akin to “mosaic state” in spin-glass models39) within the sample, which can be computed using the formalism presented here. The differing coexistence volume fractions in different parts of the sample corresponding to different local values of NB would result in additional surface energy cost between domains that has not been considered in the present calculation. However, this would have effect on the thermodynamics of the mixture gel–solvent mixture.13 Upon investigating the slope of the common-tangent for the bulk free-energy,
≤ ψ from which the coexistence densities ϕin and ϕout can be obtained. The coexistence densities are functions of NB, a parameter in the FH free-energy. Thus a distribution of mesh sizes, P(NB), leads to a distribution of coexistence volume fractions (akin to “mosaic state” in spin-glass models39) within the sample, which can be computed using the formalism presented here. The differing coexistence volume fractions in different parts of the sample corresponding to different local values of NB would result in additional surface energy cost between domains that has not been considered in the present calculation. However, this would have effect on the thermodynamics of the mixture gel–solvent mixture.13 Upon investigating the slope of the common-tangent for the bulk free-energy,  , for different values of NB, we infer that at a constant temperature (and hence constant χ(T)) the effect of increasing NB leads to the lowering of the slope of the common tangent. Thus, a heterogeneous mesh-size would result in random slopes of the common tangent to
, for different values of NB, we infer that at a constant temperature (and hence constant χ(T)) the effect of increasing NB leads to the lowering of the slope of the common tangent. Thus, a heterogeneous mesh-size would result in random slopes of the common tangent to  . The situation is analogous to the behaviour of random-field Ising models, where the relative depth of the bistable free-energy is set by the value of the field h(x).40
. The situation is analogous to the behaviour of random-field Ising models, where the relative depth of the bistable free-energy is set by the value of the field h(x).40
The effect of network disorder and its relation to the thermodynamics of random field Ising models would be studied in a future work. Elastically mediated phase transitions admit a third thermodynamic phase, where the gel network partially wets and intrudes the solvent rich droplets.31 A variational calculation allowing for polydisperse droplets and their associated wetting behaviour is currently underway and will be reported elsewhere.
We place our work in context of previous work in this exciting area. The importance of elastic interactions in modifying the equilibrium state of phase separating system was first discussed in context of a ternary system with the elastic network and a polymer interacting with a solvent.14,15 The stability of a droplet phase is argued along the lines of classical nucleation theory, using the Gibbs free energy (eqn (1) of15) to relate the work done by an expanding drop against the pressure exerted by the bounding polymer network. The droplet is identified as a dilute solvent and the ideal gas equation is used to determine the chemical potential difference 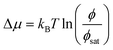 . Based on this formalism (and the eqn (1)–(9) of the ESI†) the authors argue that when ϕ < ϕsat, the mixture is stable, independent of elasticity. When ϕ > ϕcond, the mixture is unstable. While in the interim region ϕsat < ϕ < ϕcond a microdroplet phase is stabilised. This experimental situation is closely modelled by Kothari et al.17 who focus on the kinetics of a three-component system written in terms of the volume fractions of liquids A (uncrosslinked part of the background gel) ϕA, part of liquid B ϕB that resides within the gel, and ϕD, the part of liquid B which exists in droplet form. Our model bears resemblance with the model free energy proposed by Wei et al.,16 though differing significantly in detail. Perhaps the work that is most relevant to the present study is the beautiful scaling theory backed by simulation data by Ronceray et al.31 We believe that our work is the first calculation against which these results can be compared. In fact, the schematic phase diagram (Fig. 2 of31) can be derived from the thermodynamic treatment presented in the present manuscript. In addition, deviations from the scaling theory can also be captured within our model. We hope that our work will prompt careful experimental and theoretical studies in this area. Lastly, we note that our thermodynamic formalism does not capture the exciting non-equilibrium effects.38 A time-dependent Ginzburg-Landau formalism based on the free energy form explored in this article that incorporates network inhomogeneity, and adhesion of droplets to gel matrices will be explored in a future study. We hope that our theoretical work will instigate experimental work on binary gel–polymer mixtures towards a complete understanding of this fascinating problem.
. Based on this formalism (and the eqn (1)–(9) of the ESI†) the authors argue that when ϕ < ϕsat, the mixture is stable, independent of elasticity. When ϕ > ϕcond, the mixture is unstable. While in the interim region ϕsat < ϕ < ϕcond a microdroplet phase is stabilised. This experimental situation is closely modelled by Kothari et al.17 who focus on the kinetics of a three-component system written in terms of the volume fractions of liquids A (uncrosslinked part of the background gel) ϕA, part of liquid B ϕB that resides within the gel, and ϕD, the part of liquid B which exists in droplet form. Our model bears resemblance with the model free energy proposed by Wei et al.,16 though differing significantly in detail. Perhaps the work that is most relevant to the present study is the beautiful scaling theory backed by simulation data by Ronceray et al.31 We believe that our work is the first calculation against which these results can be compared. In fact, the schematic phase diagram (Fig. 2 of31) can be derived from the thermodynamic treatment presented in the present manuscript. In addition, deviations from the scaling theory can also be captured within our model. We hope that our work will prompt careful experimental and theoretical studies in this area. Lastly, we note that our thermodynamic formalism does not capture the exciting non-equilibrium effects.38 A time-dependent Ginzburg-Landau formalism based on the free energy form explored in this article that incorporates network inhomogeneity, and adhesion of droplets to gel matrices will be explored in a future study. We hope that our theoretical work will instigate experimental work on binary gel–polymer mixtures towards a complete understanding of this fascinating problem.
Author contributions
B. M., and B. C. designed the research. B. M. and S. B. contributed equally to this work. B. C. obtained funding for the research. All authors contributed to the paper.Conflicts of interest
The authors declare that no competing interests exist.Acknowledgements
S. B., and B. C. thank University of Sheffield, IMAGINE: Imaging Life grant for financial support. S. B., B. M., and B. C. acknowledge funding support from EPSRC via grant EP/P07864/1, and P & G, Akzo-Nobel, and Mondelez Intl. Plc. The authors thank Dr S. Kundu for a critical reading of the manuscript.References
- C. P. Brangwynne, C. R. Eckmann, D. S. Courson, A. Rybarska, C. Hoege, J. Gharakhani, F. Jülicher and A. A. Hyman, Science, 2009, 324, 1729–1732 CrossRef CAS.
- A. A. Hyman, C. A. Weber and F. Jülicher, Annu. Rev. Cell Dev. Biol., 2014, 30, 39–58 CrossRef CAS PubMed.
- J. Berry, C. P. Brangwynne and M. Haataja, Rep. Prog. Phys., 2018, 81, 046601 CrossRef PubMed.
- C. A. Weber, D. Zwicker, F. Jülicher and C. F. Lee, Rep. Prog. Phys., 2019, 82, 064601 CrossRef CAS.
- D. Hnisz, K. Shrinivas, R. A. Young, A. K. Chakraborty and P. A. Sharp, Cell, 2017, 169, 13–23 CrossRef CAS.
- S. Sanulli, M. Trnka, V. Dharmarajan, R. Tibble, B. Pascal, A. Burlingame, P. Griffin, J. Gross and G. Narlikar, Nature, 2019, 575, 390–394 CrossRef CAS PubMed.
- Y. Shin and C. P. Brangwynne, Science, 2017, 357, eaaf4382 CrossRef PubMed.
- E. Tjhung, C. Nardini and M. E. Cates, Phys. Rev. X, 2018, 8, 031080 CAS.
- R. Singh and M. Cates, Phys. Rev. Lett., 2019, 123, 148005 CrossRef CAS PubMed.
- A. W. Fritsch, A. F. Diaz-Delgadillo, O. Adame-Arana, C. Hoege, M. Mittasch, M. Kreysing, M. Leaver, A. A. Hyman, F. Jülicher and C. A. Weber, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2102772118 CrossRef CAS PubMed.
- J. Krawczyk, S. Croce, T. McLeish and B. Chakrabarti, Phys. Rev. Lett., 2016, 116, 208301 CrossRef PubMed.
- B. Mukherjee and B. Chakrabarti, Polymers, 2020, 12, 1576 CrossRef CAS PubMed.
- M. S. Dimitriyev, Y.-W. Chang, P. M. Goldbart and A. Fernández-Nieves, Nano Futures, 2019, 3, 042001 CrossRef CAS.
- R. W. Style, T. Sai, N. Fanelli, M. Ijavi, K. Smith-Mannschott, Q. Xu, L. A. Wilen and E. R. Dufresne, Phys. Rev. X, 2018, 8, 011028 CAS.
- K. A. Rosowski, T. Sai, E. Vidal-Henriquez, D. Zwicker, R. W. Style and E. R. Dufresne, Nat. Phys., 2020, 16, 422–425 Search PubMed.
- X. Wei, J. Zhou, Y. Wang and F. Meng, Phys. Rev. Lett., 2020, 125, 268001 CrossRef CAS PubMed.
- M. Kothari and T. Cohen, J. Mech. Phys. Solids, 2020, 145, 104153 CrossRef.
- F. Tancret, J. Laigo, F. Christien, R. Le Gall and J. Furtado, Mater. Sci. Technol., 2018, 34, 1333–1343 CrossRef CAS.
- T. Smith, B. Esser, N. Antolin, A. Carlsson, R. Williams, A. Wessman, T. Hanlon, H. Fraser, W. Windl and D. McComb, et al. , Nat. Commun., 2016, 7, 1–7 Search PubMed.
- F. R. N. Nabarro, Proc. R. Soc. London, Ser. A, 1940, 175, 519–538 CAS.
- M. Doi, T. Miyazaki and T. Wakatsuki, Mater. Sci. Eng., 1985, 74, 139–145 CrossRef CAS.
- P. Fratzl, O. Penrose and J. L. Lebowitz, J. Stat. Phys., 1999, 95, 1429–1503 CrossRef.
- S. Y. Karpov, Research, 1998, 3, 16 Search PubMed.
- P. Lonchampt and R. W. Hartel, Eur. J. Lipid Sci. Technol., 2004, 106, 241–274 CrossRef CAS.
- Y. H. Roos, in Handbook of food engineering, CRC Press, 2006, pp. 299–364 Search PubMed.
- B. D. Johnson, B. P. Boudreau, B. S. Gardiner and R. Maass, Mar. Geol., 2002, 187, 347–363 CrossRef.
- C. K. Algar and B. P. Boudreau, J. Geophys. Res.: Earth Surf., 2010, 115(F3), 1–12 Search PubMed.
- L. Liu, Environ. Sci. Technol., 2018, 52, 2007–2015 CrossRef CAS PubMed.
- M. Rubinstein and R. H. Colbyet al., Polymer physics, Oxford university press; New York, 2003, vol. 23 Search PubMed.
- L. G. Treloar, The physics of rubber elasticity, OUP Oxford, 1975 Search PubMed.
- P. Ronceray, S. Mao, A. Košmrlj and M. P. Haataja, Europhys. Lett., 2022, 137, 67001 CrossRef.
- M. Schrader, P. Virnau and K. Binder, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2009, 79, 061104 CrossRef PubMed.
- K. Binder, B. J. Block, P. Virnau and A. Tröster, Am. J. Phys., 2012, 80, 1099–1109 CrossRef CAS.
- S. Raayai-Ardakani, Z. Chen, D. R. Earl and T. Cohen, Soft Matter, 2019, 15, 381–392 RSC.
- J. Zhu, T. Li, S. Cai and Z. Suo, J. Adhes., 2011, 87, 466–481 CrossRef CAS.
- T. Tanaka, Phys. Rev. Lett., 1978, 40, 820 CrossRef CAS.
- P. M. Chaikin, T. C. Lubensky and T. A. Witten, Principles of condensed matter physics, Cambridge university press; Cambridge, 1995, vol. 10 Search PubMed.
- E. Vidal-Henriquez and D. Zwicker, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2102014118 CrossRef CAS PubMed.
- G. Parisi, Phys. A, 2007, 386, 611–624 CrossRef.
- T. Nattermann, Spin Glasses and Random Fields, World Scientific, 1997 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2sm01101h |
| This journal is © The Royal Society of Chemistry 2022 |