 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The nonlinear motion of cells subject to external forces†
Aondoyima
Ioratim-Uba
 *a,
Aurore
Loisy
*a,
Aurore
Loisy
 a,
Silke
Henkes
a,
Silke
Henkes
 ab and
Tanniemola B.
Liverpool
ab and
Tanniemola B.
Liverpool
 a
a
aSchool of Mathematics, University of Bristol, Bristol BS8 1UG, UK. E-mail: deems.ioratim-uba@bristol.ac.uk
bLorentz Institute for Theoretical Physics, Leiden University, Leiden 2333 CA, The Netherlands
First published on 10th November 2022
Abstract
To develop a minimal model for a cell moving in a crowded environment such as in tissue, we investigate the response of a liquid drop of active matter moving on a flat rigid substrate to forces applied at its boundaries. We consider two different self-propulsion mechanisms, active stresses and treadmilling polymerisation, and we investigate how the active drop motion is altered by these surface forces. We find a highly non-linear response to forces that we characterise using drop velocity, drop shape, and the traction between the drop and the substrate. Each self-propulsion mechanism gives rise to two main modes of motion: a long thin drop with zero traction in the bulk, mostly occurring under strong stretching forces, and a parabolic drop with finite traction in the bulk, mostly occurring under strong squeezing forces. In each case there is a sharp transition between parabolic, and long thin drops as a function of the applied forces and indications of drop break-up where large forces stretch the drop.
1 Introduction
Cells are highly adaptable and move themselves around in a variety of different conditions and environments.1–3 This is essential for biological functions such as wound repair,4 organ development,5 and in pathological processes such as cancer metastasis.6 Understanding individual cell motility and how it affects collective cell migration is key to understanding these processes. In particular, experiments show that cell–cell tugging plays an important role in collective migration,7–9 and that the force distribution within tissues may tell us something about pathological behaviour.10,11Cell motility is powered by the cytoskeleton, a dynamic network of interlinking protein filaments inside the cell.12–14 These filaments can collectively form anisotropic liquid crystalline (LC) phases.15 There are a number of mechanisms by which cell motility occurs.
The most studied is cell crawling,16–21 which combines the treadmilling (polymerisation/depolymerisation) of cytoskeletal actin filaments with strong adhesion to the substrate. In cells the likely source of this type of motion is actin polymerisation combined with acto-myosin contractility. Myosin II molecular motors cause actin filaments to slide relative to each other22,23 and generate an active stress that can be contractile (positive) or extensile (negative). A large class of hydrodynamic active liquid models of these processes have been built, using active gel theory,24–28 and also more detailed computational active nematic LC models.29 Such models can be augmented by including reaction-diffusion chemical feedback,30,31 and by adding confinement.32,33 It has been shown that even in the absence of actin treadmilling, spontaneous motion is still possible in LC active matter systems.34–38
A minimal description of cell motility is thus provided by the motion of a drop of anisotropic active LC matter. The possible modes of a such a drop freely moving on a flat rigid substrate have recently been classified by some of us in ref. 39. We identified three modes: motion due to active stresses, motion due to self-advection of active units along their direction of orientation, and motion due to contact angle mismatch. All modes are in principle present but one can consider regimes when one mode dominates. We also showed that a drop moving purely due to active stresses can do so without exerting traction on the substrate.40 This type of motion is relevant to fast migration in crowded environments,2,41 where cellular adhesions are unstable at high strain rates.42
Crowded environments also lead to significant forces on cells. However, many of the effects of external forces on cell-motility and migration remain a mystery. These forces, either coming from cell–ell tugging or from outside the cell, are important for the functionality of cells and tissues. Furthermore, experiments show that external forces can alter cell stiffness, induce migration, alter cell shape, induce remodelling, and alter cell phenotype.43–45 Hence a better handle on them promises to have a significant impact on our understanding of multicellular systems.
In this paper we study the dynamics of an active LC drop on a flat surface under external forces applied at its two ends. We model the anisotropy of the cytoskeleton in two ways, corresponding to dynamics dominated by the first two modes we identified in ref. 39. First, we probe an imposed LC director field, and the active stress that this generates (active contractile/extensile drop). The behaviour is controlled by the ratio  of the activity to the splay-bend winding number of the LC director, as measured from the surface to the top of the drop. Second, we study self-advection of LC units along their direction of orientation (active polymerising drop), whose behaviour is controlled by the ratio
of the activity to the splay-bend winding number of the LC director, as measured from the surface to the top of the drop. Second, we study self-advection of LC units along their direction of orientation (active polymerising drop), whose behaviour is controlled by the ratio  of the self-advection speed to the surface tension. We classify the motion of the drop according to (1) the difference between the forces on each end, i.e. whether it is being squeezed or stretched and (2) the sum of the forces applied to its two ends, i.e. if it is being pushed to the right (R) or to the left (L). For a passive drop (
of the self-advection speed to the surface tension. We classify the motion of the drop according to (1) the difference between the forces on each end, i.e. whether it is being squeezed or stretched and (2) the sum of the forces applied to its two ends, i.e. if it is being pushed to the right (R) or to the left (L). For a passive drop ( ), we find parabolic shapes with simple symmetric behaviour: it moves to the right (or left) if it is pushed in the right (or left) direction unless it is stretched above a critical stretching force where it tends to break up into smaller droplets. For both active drops, we find a much richer response to external forces. The active contractile/extensile drop (
), we find parabolic shapes with simple symmetric behaviour: it moves to the right (or left) if it is pushed in the right (or left) direction unless it is stretched above a critical stretching force where it tends to break up into smaller droplets. For both active drops, we find a much richer response to external forces. The active contractile/extensile drop ( ) drop moves to the R almost all the time except when the sum of forces is large in the L direction and it is being squeezed. We also find a wide variety of shapes. Parabolic shapes are observed only when the drop is being squeezed. When the drop is being stretched, we find (i) a double humped shape with a large hump at the front when the sum of forces is strongly to the R, (ii) a flat pancake shape which exerts almost no traction on the surface when the sum of forces is small and (iii) droplet break up when the sum of forces is strongly in the L direction. Our results hold for both contractile and extensile drops as our equations of motion are invariant: changing the sign of
) drop moves to the R almost all the time except when the sum of forces is large in the L direction and it is being squeezed. We also find a wide variety of shapes. Parabolic shapes are observed only when the drop is being squeezed. When the drop is being stretched, we find (i) a double humped shape with a large hump at the front when the sum of forces is strongly to the R, (ii) a flat pancake shape which exerts almost no traction on the surface when the sum of forces is small and (iii) droplet break up when the sum of forces is strongly in the L direction. Our results hold for both contractile and extensile drops as our equations of motion are invariant: changing the sign of  , which flips the directions L ↔ R, is equivalent to either moving from contractile to extensile stresses or to flipping the splay-bend winding number. The active polymerising drop (
, which flips the directions L ↔ R, is equivalent to either moving from contractile to extensile stresses or to flipping the splay-bend winding number. The active polymerising drop ( ) also moves to the R almost all the time, except when the sum of forces is large in the L direction and it is being squeezed. Again, parabolic drops are observed only when the drop is being squeezed. When the drop is being stretched, we find (i) a double humped shape with a large hump at the rear when the sum of forces is strongly to the L, (ii) a flat travelator shape which exerts almost no traction on the surface and (iii) droplet break up when the sum of forces is strongly in the R direction. Changing the sign of
) also moves to the R almost all the time, except when the sum of forces is large in the L direction and it is being squeezed. Again, parabolic drops are observed only when the drop is being squeezed. When the drop is being stretched, we find (i) a double humped shape with a large hump at the rear when the sum of forces is strongly to the L, (ii) a flat travelator shape which exerts almost no traction on the surface and (iii) droplet break up when the sum of forces is strongly in the R direction. Changing the sign of  flips the directions L ↔ R.
flips the directions L ↔ R.
2 Model
We model a single cell as a two dimensional incompressible active nematic drop moving on a flat rigid substrate subject to external forces at its boundaries (Fig. 1(a)). This can be thought of as a projection of the full 3d system to its average in 2d. We expect this minimal model to capture a large part of the phenomenology in 3d but not the full behaviour such as splay in the lamellipodial protrusion.29 We work in the (![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif) ,
,![[z with combining tilde]](https://www.rsc.org/images/entities/i_char_007a_0303.gif) ) plane, where the drop is characterised by the height
) plane, where the drop is characterised by the height ![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) (
(![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif) ,
,![[t with combining tilde]](https://www.rsc.org/images/entities/i_char_0074_0303.gif) ) of its free surface above the substrate, and moves with velocity
) of its free surface above the substrate, and moves with velocity  . Note that variables with tildes are dimension-full, while the un-tilded variables, which we introduce for the lubrication approximation,51 are dimension-less. We use the well established equations of active liquid crystal hydrodynamics46,47 where the motion of the coarse grained orientation of elongated units n = (cos
. Note that variables with tildes are dimension-full, while the un-tilded variables, which we introduce for the lubrication approximation,51 are dimension-less. We use the well established equations of active liquid crystal hydrodynamics46,47 where the motion of the coarse grained orientation of elongated units n = (cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ,sin
θ,sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), known as the director, is coupled to the fluid velocity ũ inside the drop. The velocity satisfies force balance equations at vanishing Reynolds number:
θ), known as the director, is coupled to the fluid velocity ũ inside the drop. The velocity satisfies force balance equations at vanishing Reynolds number:∂j![[small sigma, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e10d.gif) ij + ij + ![[f with combining tilde]](https://www.rsc.org/images/entities/i_char_0066_0303.gif) i = 0, i = 0, | (1a) |
![[small sigma, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e10d.gif) ij = − ij = −![[p with combining tilde]](https://www.rsc.org/images/entities/i_char_0070_0303.gif) δij + η(∂iũj+ ∂jũi) − δij + η(∂iũj+ ∂jũi) − ![[small alpha, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0dc.gif) ninj, ninj, | (1b) |
![[f with combining tilde]](https://www.rsc.org/images/entities/b_char_0066_0303.gif) =
= ![[f with combining tilde]](https://www.rsc.org/images/entities/b_char_0066_0303.gif) (
(![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif) ) is the external force per unit height,
) is the external force per unit height, ![[p with combining tilde]](https://www.rsc.org/images/entities/i_char_0070_0303.gif) is the pressure, ũ is the fluid velocity inside the drop, and
is the pressure, ũ is the fluid velocity inside the drop, and  is the active stress to leading order in a gradient expansion, which represents the coarse-grained stresses generated when cytoskeletal actin filaments slide relative to each other.48,49 Following,39,40 We perform calculations in the lubrication approximation51 for which changes in the height are much smaller than the width. In this approximation, higher order gradients in the stress tensor can be neglected because they scale with the ratio of the characteristic length to the characteristic height of the drop, which is small. We also work in the strong elastic limit, where the director relaxes instantaneously to follow changes in the drop height
is the active stress to leading order in a gradient expansion, which represents the coarse-grained stresses generated when cytoskeletal actin filaments slide relative to each other.48,49 Following,39,40 We perform calculations in the lubrication approximation51 for which changes in the height are much smaller than the width. In this approximation, higher order gradients in the stress tensor can be neglected because they scale with the ratio of the characteristic length to the characteristic height of the drop, which is small. We also work in the strong elastic limit, where the director relaxes instantaneously to follow changes in the drop height ![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) (
(![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif) ,
,![[t with combining tilde]](https://www.rsc.org/images/entities/i_char_0074_0303.gif) ). In this case, the dynamic equation for the director reduces to
). In this case, the dynamic equation for the director reduces to  .
.
For the active contractile/extensile drop, we anchor the director parallel to the substrate, i.e. θ(![[z with combining tilde]](https://www.rsc.org/images/entities/i_char_007a_0303.gif) = 0) = 0, and parallel to the free surface with an imposed winding, i.e. θ(
= 0) = 0, and parallel to the free surface with an imposed winding, i.e. θ(![[z with combining tilde]](https://www.rsc.org/images/entities/i_char_007a_0303.gif) =
= ![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) ) = ωπ+ arctan(h′). The winding number ω ∈
) = ωπ+ arctan(h′). The winding number ω ∈ ![[Doublestruck Z]](https://www.rsc.org/images/entities/i_char_e17d.gif) + counts the number of half turns of the director across the drop height (Fig. 2). At fixed activity, the winding of the director, through its chirality, breaks the left-right symmetry and sets the preferred direction of motion. With no external forces, at positive activity (extensile drop), the drop moves right for positive winding and left for negative winding. There is no active contribution to the drop velocity when ω = 0. For the active polymerising drop, we anchor the director parallel to the substrate i.e. θ(
+ counts the number of half turns of the director across the drop height (Fig. 2). At fixed activity, the winding of the director, through its chirality, breaks the left-right symmetry and sets the preferred direction of motion. With no external forces, at positive activity (extensile drop), the drop moves right for positive winding and left for negative winding. There is no active contribution to the drop velocity when ω = 0. For the active polymerising drop, we anchor the director parallel to the substrate i.e. θ(![[z with combining tilde]](https://www.rsc.org/images/entities/i_char_007a_0303.gif) = 0) = 0, and parallel to the free surface with no imposed winding, i.e. θ(
= 0) = 0, and parallel to the free surface with no imposed winding, i.e. θ(![[z with combining tilde]](https://www.rsc.org/images/entities/i_char_007a_0303.gif) =
= ![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) ) = arctan(h′).
) = arctan(h′).
 | ||
Fig. 3 Left: Droplet height profiles at  and and  . Here, π0 = 0.9226 and Ω = 1. Right: Drop length L as a function of . Here, π0 = 0.9226 and Ω = 1. Right: Drop length L as a function of  . . | ||
The external forces  ,
, ![[f with combining tilde]](https://www.rsc.org/images/entities/i_char_0066_0303.gif)
![[z with combining tilde]](https://www.rsc.org/images/entities/i_char_007a_0303.gif) = 0 are localised at the left and right drop borders, which leads to boundary conditions on the pressure
= 0 are localised at the left and right drop borders, which leads to boundary conditions on the pressure  , and
, and  is the Laplace pressure generated by the surface tension γ of an un-forced symmetric passive drop of length
is the Laplace pressure generated by the surface tension γ of an un-forced symmetric passive drop of length ![[L with combining tilde]](https://www.rsc.org/images/entities/i_char_004c_0303.gif) 0 and contact angle
0 and contact angle ![[small phi, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e2.gif) . For an active drop with no external forces,
. For an active drop with no external forces,  is a constant shift in the pressure. These boundary conditions can be derived by integrating the
is a constant shift in the pressure. These boundary conditions can be derived by integrating the ![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif) component of (1a) with respect to
component of (1a) with respect to ![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif) from
from ![[L with combining tilde]](https://www.rsc.org/images/entities/i_char_004c_0303.gif) /2 − Δ to
/2 − Δ to ![[L with combining tilde]](https://www.rsc.org/images/entities/i_char_004c_0303.gif) /2 + Δ and from −
/2 + Δ and from −![[L with combining tilde]](https://www.rsc.org/images/entities/i_char_004c_0303.gif) /2 − Δ to −
/2 − Δ to −![[L with combining tilde]](https://www.rsc.org/images/entities/i_char_004c_0303.gif) /2 + Δ, where
/2 + Δ, where ![[L with combining tilde]](https://www.rsc.org/images/entities/i_char_004c_0303.gif) is the length of an arbitrary drop with Δ > 0, and taking the limit Δ → 0+ (see Section 1.2 of ESI ref. 50). In addition to these boundary conditions, we use a free surface boundary condition for the stress at the free surface:
is the length of an arbitrary drop with Δ > 0, and taking the limit Δ → 0+ (see Section 1.2 of ESI ref. 50). In addition to these boundary conditions, we use a free surface boundary condition for the stress at the free surface: ![[small sigma, Greek, tilde]](https://www.rsc.org/images/entities/b_i_char_e10d.gif) ·m = γκm for the stress at the free surface, where γ is the surface tension, m is the unit normal vector (see Fig. 1(a)), and κ = ∇·m is the curvature of the free surface. The interaction of the drop with the rigid substrate is modelled by a partial slip boundary condition: ũ
·m = γκm for the stress at the free surface, where γ is the surface tension, m is the unit normal vector (see Fig. 1(a)), and κ = ∇·m is the curvature of the free surface. The interaction of the drop with the rigid substrate is modelled by a partial slip boundary condition: ũ![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif) =
= ![[l with combining tilde]](https://www.rsc.org/images/entities/i_char_006c_0303.gif) u
u![[small sigma, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e10d.gif)
![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif)
![[Z with combining tilde]](https://www.rsc.org/images/entities/i_char_005a_0303.gif) /η, where
/η, where ![[l with combining tilde]](https://www.rsc.org/images/entities/i_char_006c_0303.gif) u is a slip length.
u is a slip length.
We seek travelling wave solutions ![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) =
= ![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) (
(![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif) − Ṽt) and work in the reference frame of the drop. Mass conservation implies
− Ṽt) and work in the reference frame of the drop. Mass conservation implies
 | (2) |
![[w with combining tilde]](https://www.rsc.org/images/entities/i_char_0077_0303.gif) n describes the additional transport due to treadmilling self-advection at speed
n describes the additional transport due to treadmilling self-advection at speed ![[w with combining tilde]](https://www.rsc.org/images/entities/i_char_0077_0303.gif) of active units whose orientations are characterised by the director n – the active units essentially propel themselves along their own tangent. Since treadmilling self-advection only occurs near substrates, we choose the form
of active units whose orientations are characterised by the director n – the active units essentially propel themselves along their own tangent. Since treadmilling self-advection only occurs near substrates, we choose the form ![[w with combining tilde]](https://www.rsc.org/images/entities/i_char_0077_0303.gif) =
= ![[w with combining tilde]](https://www.rsc.org/images/entities/i_char_0077_0303.gif) 0e−
0e−![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) /
/![[l with combining tilde]](https://www.rsc.org/images/entities/i_char_006c_0303.gif) w, where
w, where ![[w with combining tilde]](https://www.rsc.org/images/entities/i_char_0077_0303.gif) 0 is a characteristic self-advection speed and
0 is a characteristic self-advection speed and ![[l with combining tilde]](https://www.rsc.org/images/entities/i_char_006c_0303.gif) w is the characteristic height over which the self-advection term decays in the direction normal to the substrate. This functional form was also used in previous work that simulates crawling cells.29 Then, combining mass conservation with
w is the characteristic height over which the self-advection term decays in the direction normal to the substrate. This functional form was also used in previous work that simulates crawling cells.29 Then, combining mass conservation with ![[p with combining tilde]](https://www.rsc.org/images/entities/i_char_0070_0303.gif) = −γ
= −γ![[p with combining tilde]](https://www.rsc.org/images/entities/i_char_0070_0303.gif) ′′, which can be derived by taking the leading order terms of the normal component of the stress boundary condition at the drop free surface, we obtain a non-linear ODE for
′′, which can be derived by taking the leading order terms of the normal component of the stress boundary condition at the drop free surface, we obtain a non-linear ODE for ![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) . After non-dimensionalisation, we retain the salient dimensionless parameters activity
. After non-dimensionalisation, we retain the salient dimensionless parameters activity  , advection speed
, advection speed  , and drop velocity
, and drop velocity  ,
, | (3) |
![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) /ε
/ε![[L with combining tilde]](https://www.rsc.org/images/entities/i_char_004c_0303.gif) x, coordinates x =
x, coordinates x = ![[x with combining tilde]](https://www.rsc.org/images/entities/i_char_0078_0303.gif) /
/![[L with combining tilde]](https://www.rsc.org/images/entities/i_char_004c_0303.gif) x, z =
x, z = ![[z with combining tilde]](https://www.rsc.org/images/entities/i_char_007a_0303.gif) /ε
/ε![[L with combining tilde]](https://www.rsc.org/images/entities/i_char_004c_0303.gif) x, slip length lu =
x, slip length lu = ![[l with combining tilde]](https://www.rsc.org/images/entities/i_char_006c_0303.gif) u/ε
u/ε![[L with combining tilde]](https://www.rsc.org/images/entities/i_char_004c_0303.gif) x, polymerisation height lw =
x, polymerisation height lw = ![[l with combining tilde]](https://www.rsc.org/images/entities/i_char_006c_0303.gif) w/ε
w/ε![[L with combining tilde]](https://www.rsc.org/images/entities/i_char_004c_0303.gif) x, pressure
x, pressure  , contact angle
, contact angle  , and forces f± =
, and forces f± = ![[f with combining tilde]](https://www.rsc.org/images/entities/i_char_0066_0303.gif) ±
±![[L with combining tilde]](https://www.rsc.org/images/entities/i_char_004c_0303.gif) x/γε, where
x/γε, where ![[L with combining tilde]](https://www.rsc.org/images/entities/i_char_004c_0303.gif) x is a characteristic length scale in the x direction. Then the drop shape satisfies the ODE
x is a characteristic length scale in the x direction. Then the drop shape satisfies the ODE | (4) |
 for the contractile/extensile drop and
for the contractile/extensile drop and  for the polymerising drop. The drop velocity
for the polymerising drop. The drop velocity  is a functional of h:
is a functional of h: | (5) |
 (positive means push right and negative means push left). We also use the quantity
(positive means push right and negative means push left). We also use the quantity  , which we term squeeze, in the analysis of our results (positive means squeeze, and negative means stretch). We set the height of the drop at its boundary to a finite h0 = h(±L/2), which represents a finite contact area with neighbouring cells or with obstacles. The drop length L is determined as part of the solution by requiring that the drop have constant volume Ω:
, which we term squeeze, in the analysis of our results (positive means squeeze, and negative means stretch). We set the height of the drop at its boundary to a finite h0 = h(±L/2), which represents a finite contact area with neighbouring cells or with obstacles. The drop length L is determined as part of the solution by requiring that the drop have constant volume Ω:  . The tangential traction exerted by the drop on the substrate is σxz|z= 0 = hh′′′. In terms of h alone, using (4), the traction can be written
. The tangential traction exerted by the drop on the substrate is σxz|z= 0 = hh′′′. In terms of h alone, using (4), the traction can be written  . The dimensionless parameters
. The dimensionless parameters  ,
,  ,
,  ,
,  , and
, and  all represent a ratio of stress to surface tension, and as we will show below, the interesting active phenomenology appears when they are all
all represent a ratio of stress to surface tension, and as we will show below, the interesting active phenomenology appears when they are all  . We translate these results back to biological parameters at the end, allowing us to make predictions for cell speeds and stresses in addition to cell shapes.
. We translate these results back to biological parameters at the end, allowing us to make predictions for cell speeds and stresses in addition to cell shapes.
2.1 Numerics
We obtained solutions to (4) by numerically solving the time dependent version of the force balance equation, ∂th+ ∂xI = 0, where , which represents overdamped dynamics of the height evolution. Solving the time dependent problem (starting from a known initial condition) allows us to iteratively evaluate the integrals in eqn (5) and gives us the potential to investigate the temporal dynamics of the drop. The steady state of the time evolution, ∂th = 0, is equivalent to eqn (4).
, which represents overdamped dynamics of the height evolution. Solving the time dependent problem (starting from a known initial condition) allows us to iteratively evaluate the integrals in eqn (5) and gives us the potential to investigate the temporal dynamics of the drop. The steady state of the time evolution, ∂th = 0, is equivalent to eqn (4).
We used an implicit method, a variant of the Crank–Nicholson algorithm, using finite difference coefficients to approximate the derivatives. The algorithm starts with an initial condition that has the correct values of h at the boundaries and advances in time until steady state. The algorithm advances in time according to hn+1i/Δt + Ii(hn+11,…,hn+1N)/2 = hni/Δt + Ii(hn1,…,hnN)/2, where hni is the discretised drop height evaluated at the ith spatial point at the nth time step, Ii(hn1,…,hnN) is the discretised version of I as defined above evaluated at the ith at the nth time step. The time stepping in the algorithm is adaptive, meaning that the time step is increased as steady state approaches.39 In the numerics, we apply both height and pressure boundary conditions (converted to boundary conditions on h′′) because the time evolution is a fourth order PDE:
| h(±L/2) = h0, h′′(±L/2) = ±f± − π0. | (6) |
The boundary conditions on h′′ are required for consistency with force balance, however we are in principle free to change the boundary conditions on h. In all simulations lu = 0.05, h0 = 0.1, Ω = 1, π0 = 0.9226, which corresponds to a contact angle of 1 for a free passive drop, ω = 1, and the spatial step size is 0.002. The value of the slip length was chosen to be consistent with ref. 39. Changing the slip length has different effects on the contractile/extensile mode and polymerisation mode: increasing the slip parameter at a fixed polymerisation speed decreases drop speed because motility via polymerisation requires strong adhesion.29,39 The opposite is true for the contractile/extensile drops – increasing the slip length increases drop speed.39
3 Results
The phase diagrams in Fig. 2(a)–(c) schematically show the regimes of drop shapes and motility that we find for the passive , the contractile/extensile case
, the contractile/extensile case  case, and the polymerising case
case, and the polymerising case  . Fig. 4 and 5 provide full results for drop velocity, drop shapes and substrate traction.
. Fig. 4 and 5 provide full results for drop velocity, drop shapes and substrate traction.
 | ||
Fig. 4 Contractile/extensile drops. (a) Velocity of a passive drop. (b) Velocity of a contractile/extensile drop. (c) Length of a contractile/extensile drop. (d) Traction between the drop and the substrate integrated over 70% of the drop length:  . Each square represents a single simulation and its colour corresponds to the legend. The solid line in each plot is an isoline corresponding to zero velocity, the dashed line is an isoline where the first moment of h, . Each square represents a single simulation and its colour corresponds to the legend. The solid line in each plot is an isoline corresponding to zero velocity, the dashed line is an isoline where the first moment of h,  , vanishes, and the dotted line is an isoline corresponding to the drop having equal contact angles. (e) 2nd moment and velocity , vanishes, and the dotted line is an isoline corresponding to the drop having equal contact angles. (e) 2nd moment and velocity  across the RP(R)/RH(R) boundary, indicating a first order transition. (f) Stable numerical solutions for h (top) and the corresponding traction σxz|z=0 (bottom) for across the RP(R)/RH(R) boundary, indicating a first order transition. (f) Stable numerical solutions for h (top) and the corresponding traction σxz|z=0 (bottom) for  , (g) , (g)  , (h) , (h)  . The colour of each curve matches the colour of its phase in Fig. 2(c). The legend labels are coordinates in the ( . The colour of each curve matches the colour of its phase in Fig. 2(c). The legend labels are coordinates in the ( , ,  ) phase plane. ) phase plane. | ||
 | ||
Fig. 5 Polymerising drops. (a) Length of a passive drop. (b) Length of a polymerising drop. (c) Velocity of a polymerising drop. (d) Traction between the drop and the substrate integrated over 70% of the drop length:  . Each square represents a single simulation and its colour corresponds to the legend. The solid line in each plot is an isoline corresponding to zero velocity, the dashed line is an isoline where the first moment of h, . Each square represents a single simulation and its colour corresponds to the legend. The solid line in each plot is an isoline corresponding to zero velocity, the dashed line is an isoline where the first moment of h, 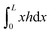 , vanishes, and the dotted line is an isoline corresponding to the drop having equal contact angles. (e) Jump in length and velocity going from parabolic to flat. (f) Stable numerical solutions for h (top) and the corresponding traction σxz|z=0 (bottom) for , vanishes, and the dotted line is an isoline corresponding to the drop having equal contact angles. (e) Jump in length and velocity going from parabolic to flat. (f) Stable numerical solutions for h (top) and the corresponding traction σxz|z=0 (bottom) for  , (g) Stable numerical solutions for h (top) and the corresponding traction σxz|z=0 (bottom) for , (g) Stable numerical solutions for h (top) and the corresponding traction σxz|z=0 (bottom) for  , (h) , (h)  . The colour of each curve matches the colour of its phase in Fig. 2(c). The legend labels are coordinates in the ( . The colour of each curve matches the colour of its phase in Fig. 2(c). The legend labels are coordinates in the ( , ,  ) phase plane. ) phase plane. | ||
3.1 Passive drop
We begin with a passive drop (Fig. 2(a)), both to provide a reference, but also to illuminate the limit when surface tension dominates over activity. Then all dimensionless parameters are small and eqn (4) can be approximated by h′′′(x) = 0, which is the equation for the passive drop with a parabolic solution. Moving to finite
(Fig. 2(a)), both to provide a reference, but also to illuminate the limit when surface tension dominates over activity. Then all dimensionless parameters are small and eqn (4) can be approximated by h′′′(x) = 0, which is the equation for the passive drop with a parabolic solution. Moving to finite  and
and  now, we observe two phases of motion, both with finite traction in the bulk: a left-moving right-parabola RP(L) for
now, we observe two phases of motion, both with finite traction in the bulk: a left-moving right-parabola RP(L) for  and a right-moving left-parabola LP(R) for
and a right-moving left-parabola LP(R) for  . These passive drops are qualitatively close in shape to an upside down parabola but are asymmetric with a first moment of h that is non-zero (characteristic shapes shown in Fig. 2(b)). Squeezing the drop
. These passive drops are qualitatively close in shape to an upside down parabola but are asymmetric with a first moment of h that is non-zero (characteristic shapes shown in Fig. 2(b)). Squeezing the drop  in general maintains the parabolic shape and increases the effective surface tension.
in general maintains the parabolic shape and increases the effective surface tension.
The passive solutions are antisymmetric along the push/pull  axis, and they move with opposite velocities that increase as the drop is squeezed (see Fig. 4(a)). All drops become longer and thinner as we move along the stretch/squeeze axis from squeeze to stretch, eventually reaching the region of drop breakup (DB), where the drop free surface reaches h < 0. For passive drops at
axis, and they move with opposite velocities that increase as the drop is squeezed (see Fig. 4(a)). All drops become longer and thinner as we move along the stretch/squeeze axis from squeeze to stretch, eventually reaching the region of drop breakup (DB), where the drop free surface reaches h < 0. For passive drops at  , there are no steady solutions in the DB region that satisfy volume conservation. Consider a passive drop with
, there are no steady solutions in the DB region that satisfy volume conservation. Consider a passive drop with  and
and  . The equation for drop height becomes
. The equation for drop height becomes
 | (7) |
 | (8) |
 . The drop length diverges at
. The drop length diverges at  (π0 is chosen so that the contact angle of a free passive drop is one), and goes negative for
(π0 is chosen so that the contact angle of a free passive drop is one), and goes negative for  . This means that volume conservation is not truly satisfied even though the condition
. This means that volume conservation is not truly satisfied even though the condition  is satisfied mathematically by a negative L. This is clear in the left plot in Fig. 3, where h < 0 for
is satisfied mathematically by a negative L. This is clear in the left plot in Fig. 3, where h < 0 for  and thus
and thus  , with |L| being the actual length of the drop.
, with |L| being the actual length of the drop.
3.2 Active contractile/extensile drop
With contractility, as shown in shown in Fig. 2(b), we find, in addition to RP(L), LP(R) and DB, a right-moving right-parabola RP(R) state with finite traction, and then two long and thin states (see length in Fig. 4(c)), the right-moving double hump DH(R) state, which has a small dip followed by a prominent frontal hump, and the right-moving pancake PC(R) drop, which is flat almost everywhere with the average height![[h with combining macron]](https://www.rsc.org/images/entities/i_char_0068_0304.gif) < h0.
< h0.
Both DH(R) and PC(R) drops have zero traction in the bulk (Fig. 4(d)). In,40 equal contact angles were imposed at the boundaries for all drops (rather than variable external force), and it was found that the tractionless DH(R) shapes emerged when the dimensionless active contractile stress  . Here, these tractionless drops can be found in the DH(R) region of the phase plane along the line of equal contact angle (dotted line, Fig. 4(b)–(d)). The drop partitions itself into a small region with large free surface curvature, and a larger region where the free surface is flat. There is large curvature at these humps, and the remaining 50–90% of the drop is flat. At lower activity
. Here, these tractionless drops can be found in the DH(R) region of the phase plane along the line of equal contact angle (dotted line, Fig. 4(b)–(d)). The drop partitions itself into a small region with large free surface curvature, and a larger region where the free surface is flat. There is large curvature at these humps, and the remaining 50–90% of the drop is flat. At lower activity  (see ESI ref. 50, Fig. S2), we find DH(R) and PC(R) shapes at large enough stretch (
(see ESI ref. 50, Fig. S2), we find DH(R) and PC(R) shapes at large enough stretch ( ), which shows that stretching can work together with activity to deform the drop. Therefore, in the DH(R) and PC(R) drops, we see the competition between active contractile/extensile stress plus stretch, and surface tension: activity plus stretch wants to deform the drop, and surface tension wants the free surface to be flat.
), which shows that stretching can work together with activity to deform the drop. Therefore, in the DH(R) and PC(R) drops, we see the competition between active contractile/extensile stress plus stretch, and surface tension: activity plus stretch wants to deform the drop, and surface tension wants the free surface to be flat.
The symmetry about the push/pull axis is broken because the drop is motile at zero force due to the imposed winding ω = 1 (Fig. 4(b)). Both the RP(L) and LP(R) regions become smaller with activity and are pushed towards the top-left (push-left/squeeze) and top-right (push-right/squeeze) of the phase plane respectively by the emergence of the RP(R) phase, which largely occupies the middle squeeze region that lies between RP(L) and LP(R). The stretch region of the phase plane is populated by DH(R) and PC(R), as well as DB. The region containing DH(R) and PC(R) shares a phase boundary with RP(R) determined by the drop length, and the distribution of substrate traction. The second moment of h (with drop length scaled to unity), which is a measure of the spread of the drop also determines the same phase boundary – see ESI.† The phase boundary is indicated in the schematic Fig. 2, as determined from the data in Fig. 4(c) and (d). Strikingly, the transition between RP(R) and DH(R)/PC(R) is sharp suggesting a first order transition.
The behaviour of the second moment and the drop velocity across the phase boundary is shown in Fig. 4(e). There is a jump in both quantities going from RP(R) to DH(R) and also from DH(R) to RP(R). The change in drop shape induced by moving from RP(R) to DH(R) and back to RP(R) at constant squeeze  is shown if Fig. 4(f). The drop shape changes continuously within the DH(R) region but changes sharply at the phase boundary. There are also indications of bi-stability in the region: we have obtained two stable solutions for two different initial conditions at the same point in the phase plane (see ESI at ref. 51, Fig. 3). In contrast, the transition between DH(R) and PC(R) is smooth and happens under increased stretching for constant push (Fig. 4(g)).
is shown if Fig. 4(f). The drop shape changes continuously within the DH(R) region but changes sharply at the phase boundary. There are also indications of bi-stability in the region: we have obtained two stable solutions for two different initial conditions at the same point in the phase plane (see ESI at ref. 51, Fig. 3). In contrast, the transition between DH(R) and PC(R) is smooth and happens under increased stretching for constant push (Fig. 4(g)).
3.3 Active polymerising drop
The polymerising drop, in addition to RP(L), LP(R), RP(R), and DB has two long and thin states (see length in Fig. 5(b)), the right-moving treadmilling state TM(R), which has a prominent hump at the rear followed by a small dip and a protrusion at the front, and the right-moving travelator, TL(R) which is flat almost everywhere, with the average height![[h with combining macron]](https://www.rsc.org/images/entities/i_char_0068_0304.gif) < h0. The TM(R) shape is a solution to the 2D equation for a free drop driven by actin polymerisation for large enough self-advection velocity.39 Similar shapes with flat frontal protrusions have been observed experimentally for motile keratocytes.16,19 Both TM(R) and TL(R) drops have zero traction in the bulk Fig. 5(d). In ref. 39, where equal contact angles (rather than variable force) were imposed, the TM(R) shapes emerged when the dimensionless polymerisation speed
< h0. The TM(R) shape is a solution to the 2D equation for a free drop driven by actin polymerisation for large enough self-advection velocity.39 Similar shapes with flat frontal protrusions have been observed experimentally for motile keratocytes.16,19 Both TM(R) and TL(R) drops have zero traction in the bulk Fig. 5(d). In ref. 39, where equal contact angles (rather than variable force) were imposed, the TM(R) shapes emerged when the dimensionless polymerisation speed  , showing that relatively small polymerisation speeds can generate stresses large enough to deform the drop. Again, there is large curvature at the humps, which take up 10–50% of the drop length, and the remaining drop is flat. At much lower polymerisation speed,
, showing that relatively small polymerisation speeds can generate stresses large enough to deform the drop. Again, there is large curvature at the humps, which take up 10–50% of the drop length, and the remaining drop is flat. At much lower polymerisation speed,  , (see ESI ref. 50), we find TM(R) and TL(R) shapes at large enough stretch (
, (see ESI ref. 50), we find TM(R) and TL(R) shapes at large enough stretch ( ), which again shows that that stretching can amplify the effect of activity to deform the drop. Therefore, in the TM(R) and TL(R) drops, we see the competition between stress generated by polymerisation plus stretch, and surface tension: activity plus stretch wants to deform the drop, and surface tension wants the free surface to be flat.
), which again shows that that stretching can amplify the effect of activity to deform the drop. Therefore, in the TM(R) and TL(R) drops, we see the competition between stress generated by polymerisation plus stretch, and surface tension: activity plus stretch wants to deform the drop, and surface tension wants the free surface to be flat.
Again, the symmetry about the push/pull axis is broken because the drop is motile at zero force, this time due to treadmilling self-advection,  . The RP(L) region becomes smaller with self-advection, and is pushed to the top-left (push-left, squeeze), while the LP(R) region becomes larger with self-advection and invades the left half of the squeeze region. The RP(R) phase emerges as a thin strip between RP(L) and LP(R). DB for the polymerising drop occurs in the bottom-right (push-right, stretch) rather than in the bottom-left (push-left, stretch) as is the case for the contractile/extensile drop. The stretch region of the phase plane is populated by TM(R) and TL(R), as well as DB. The region containing TM(R) and TL(R) shares a phase boundary with both RP(R) and LP(R) determined by the second moment of h as a measure of the spread of the drop, and the distribution of substrate traction. The phase boundary is indicated in the schematic Fig. 2(c), as determined from the data in Fig. 5(b) and (d). Strikingly, the transition between RP(R)/LP(R) and DH(R)/PC(R) is sharp suggesting a first order transition. The behaviour of the second moment and the drop velocity across the phase boundary is shown in Fig. 5(e). There is a jump in both quantities going from RP(R)/LP(R) to TM(R)/TL(R). The change in drop shape induced by moving from LP(R) to TL(R) is shown Fig. 5(f). The drop shape changes continuously within the TM(R)/TL(R) region but changes sharply at the phase boundary. In contrast, the transition between TM(R) and TL(R) is smooth.
. The RP(L) region becomes smaller with self-advection, and is pushed to the top-left (push-left, squeeze), while the LP(R) region becomes larger with self-advection and invades the left half of the squeeze region. The RP(R) phase emerges as a thin strip between RP(L) and LP(R). DB for the polymerising drop occurs in the bottom-right (push-right, stretch) rather than in the bottom-left (push-left, stretch) as is the case for the contractile/extensile drop. The stretch region of the phase plane is populated by TM(R) and TL(R), as well as DB. The region containing TM(R) and TL(R) shares a phase boundary with both RP(R) and LP(R) determined by the second moment of h as a measure of the spread of the drop, and the distribution of substrate traction. The phase boundary is indicated in the schematic Fig. 2(c), as determined from the data in Fig. 5(b) and (d). Strikingly, the transition between RP(R)/LP(R) and DH(R)/PC(R) is sharp suggesting a first order transition. The behaviour of the second moment and the drop velocity across the phase boundary is shown in Fig. 5(e). There is a jump in both quantities going from RP(R)/LP(R) to TM(R)/TL(R). The change in drop shape induced by moving from LP(R) to TL(R) is shown Fig. 5(f). The drop shape changes continuously within the TM(R)/TL(R) region but changes sharply at the phase boundary. In contrast, the transition between TM(R) and TL(R) is smooth.
4 Discussion and parameter estimates
The drastic changes in drop shape that we have observed are not associated with changes in direction of motion. Both the contractile/extensile drop and the polymerising drop remain as right-parabolas when their velocity is reversed at the RP(L)/RP(R) boundary. Stationary drop profiles are shown in Fig. 4(h). All drops we have considered here are fastest for strong push and strong squeeze, towards the top corners of the phase plane. The contractile/extensile drop slows down dramatically on entering the DH(R) region and continues to slow down as it enters the PC(R) region and approaches DB, however it does not stop before reaching DB. In contrast, the polymerising drop locally speeds up as it approaches the TM(R) region and continues to speed up as it enters the TL(R) region and approaches DB. We can also compute the active power generated by moving the drop against applied forces, showing that when the drop moves opposite the applied force, it acts as a motile engine (see Fig. 6). | ||
| Fig. 6 Left: Power (net force × velocity) for a passive drop. Middle: Power for an active contractile/extensile drop. Right: Power for an active polymerising drop. | ||
We now estimate dimensionful parameter values for our model to provide biological context and make predictions. In our active simulations we looked at  between 0.3 and 1. Assuming a surface tension of 0.01 mN m−1,52 a length scale of 25 μm, and a drop inverse aspect ratio ε = 0.1, we can use (3) to calculate
between 0.3 and 1. Assuming a surface tension of 0.01 mN m−1,52 a length scale of 25 μm, and a drop inverse aspect ratio ε = 0.1, we can use (3) to calculate ![[small alpha, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0dc.gif) to be between 0.02 pN (μm)−2 and 0.05 pN (μm)−2. This is comparable to a bottom-up estimate of activity parameter
to be between 0.02 pN (μm)−2 and 0.05 pN (μm)−2. This is comparable to a bottom-up estimate of activity parameter ![[small alpha, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0dc.gif) from the force produced by myosin motors cross-linking and walking across actin filaments, which we treated as rods in water. We estimated the force produced by each rod to be the Stokes drag on a rod, Frod ≈ 2πLηv, where L is the length of an actin filament, which we take to be 1 μm, η is the viscosity of water, which is 8.9 × 10−4 Pa s, and v is the myosin walking speed, which we take to be 200 nm s−1.53 Using these numbers we estimate the force produced by a single actomyosin bundle to be 5 nN. We take the density of actin monomers in a cell to be 100 mg mL−1,54 the mass of an actin monomer to be 42 kDa,55 the volume of a cell to be 10−15 m3,56 and the number of actin monomers to per filament to be 370,57 which gives 4 × 105 actin filaments per cell. Multiplying this number by the Stokes drag gives a total force of Fcell = 5 × 10−10 N. Since disordered actomyosin networks do not convert microscopic forces efficiently, we estimate the active force scale to be 0.01–0.1 Fcell/(Lcell2), where we have used Lcell ≈ 25 μm,56 so
from the force produced by myosin motors cross-linking and walking across actin filaments, which we treated as rods in water. We estimated the force produced by each rod to be the Stokes drag on a rod, Frod ≈ 2πLηv, where L is the length of an actin filament, which we take to be 1 μm, η is the viscosity of water, which is 8.9 × 10−4 Pa s, and v is the myosin walking speed, which we take to be 200 nm s−1.53 Using these numbers we estimate the force produced by a single actomyosin bundle to be 5 nN. We take the density of actin monomers in a cell to be 100 mg mL−1,54 the mass of an actin monomer to be 42 kDa,55 the volume of a cell to be 10−15 m3,56 and the number of actin monomers to per filament to be 370,57 which gives 4 × 105 actin filaments per cell. Multiplying this number by the Stokes drag gives a total force of Fcell = 5 × 10−10 N. Since disordered actomyosin networks do not convert microscopic forces efficiently, we estimate the active force scale to be 0.01–0.1 Fcell/(Lcell2), where we have used Lcell ≈ 25 μm,56 so ![[small alpha, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0dc.gif) ≈ 0.08–0.8 N m−2 = 0.08–0.8 pN (μm)−2.
≈ 0.08–0.8 N m−2 = 0.08–0.8 pN (μm)−2.
Similarly, we can calculate ![[w with combining tilde]](https://www.rsc.org/images/entities/i_char_0077_0303.gif) 0 and Ṽ viaeqn (3) using our assumed value of surface tension, and using the viscosity of a semi-flexible polymer gel, which we take to be 3 × 10−1 Pa s.58 We find
0 and Ṽ viaeqn (3) using our assumed value of surface tension, and using the viscosity of a semi-flexible polymer gel, which we take to be 3 × 10−1 Pa s.58 We find ![[w with combining tilde]](https://www.rsc.org/images/entities/i_char_0077_0303.gif) 0 between 10 nm s−1 and 30 nm s−1, comparable to the range of polymerisation speeds, 7 nm s−1 to 170 nm s−1, for motile cells.59 Our model predicts cell speeds around 10 nm s−1. We use values of push and squeeze ranging from 0.04 pN (μm)−2 to 0.4 pN (μm)−2, and our model predicts a stall force of around 60 pN (assuming a contact area of 2 × 25 (μm)2).
0 between 10 nm s−1 and 30 nm s−1, comparable to the range of polymerisation speeds, 7 nm s−1 to 170 nm s−1, for motile cells.59 Our model predicts cell speeds around 10 nm s−1. We use values of push and squeeze ranging from 0.04 pN (μm)−2 to 0.4 pN (μm)−2, and our model predicts a stall force of around 60 pN (assuming a contact area of 2 × 25 (μm)2).
5 Conclusion
In summary, we have studied the dynamics of a model cell (an active LC drop) on a flat surface under external forces applied at its two ends. Our phase diagrams in terms of the sum and differences of these forces and show how cell shape and motile behaviour is nonlinearly modulated by external forces. Our analysis has focused on two mechanisms of motility driven by contractile/extensile active stresses and actin polymerisation. Both mechanisms give rise to the same parabolic phases (RP(L), LP(R), RP(R)) under squeeze, and different phases under stretch: DH(R) and PC(R) for the contractile/extensile drop, and TM(R) and TL(R) for the polymerising drop.These unexpectedly strong shape and motility changes under applied forces should help us understand similar changes in experiment. Epithelia change from columnar (tall) to squamous (flat) shapes under tension, and there is a tradeoff between active traction with the substrate and at the apical (top) surface. Similarly, in the mesenchymal–epithelial transition from flat or humped, strongly motile mesenchymal cells slow down and become taller when squeezed in by other cells.1,5 The changes in shape that we have observed here can be exploited to control behaviour of different cell phenotypes and tissue remodelling. In particular, the relation that we have derived between cell-substrate traction and forces, and between cell velocity and traction could be investigated by measuring the cell-substrate force using e.g. traction force microscopy and pushing/pulling the cell with a micropipette.
Author contributions
TBL formulated the problem. AI, under the supervision of TBL and SH, derived the model equations, carried out the numerical calculations with the help of AL, and analysed the data. All authors contributed to writing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Part of this work was funded by a Leverhulme Trust Research Project Grant RPG-2016-147. TBL acknowledges support of BrisSynBio, a BBSRC/EPSRC Advanced Synthetic Biology Research Centre (grant number BB/L01386X/1). SH acknowledges support of BBSRC grant BB/N009150/2.Notes and references
- B. Alberts, Molecular Biology of the Cell, W.W. Norton, 2017 Search PubMed.
- E. K. Paluch, I. M. Aspalter and M. Sixt, Annu. Rev. Cell Dev. Biol., 2016, 32, 469–490 CrossRef CAS PubMed.
- T. Lämmermann and M. Sixt, Curr. Opin. Cell Biol., 2009, 21, 636–644 CrossRef PubMed.
- K. Chiou and E.-M. S. Collins, Dev. Biol., 2018, 433, 155–165 CrossRef CAS PubMed.
- T. Lecuit and P.-F. Lenne, Nat. Rev. Mol. Cell Biol., 2007, 8, 633–644 CrossRef CAS PubMed.
- C. L. Chaffer and R. A. Weinberg, Science, 2011, 331, 1559–1564 CrossRef CAS PubMed.
- X. Trepat, M. R. Wasserman, T. E. Angelini, E. Millet, D. A. Weitz, J. P. Butler and J. J. Fredberg, Nat. Phys., 2009, 5, 426–430 Search PubMed.
- S. Jain, V. M. Cachoux, G. H. Narayana, S. de Beco, J. D’alessandro, V. Cellerin, T. Chen, M. L. Heuzé, P. Marcq and R.-M. Mège, et al. , Nat. Phys., 2020, 16, 802–809 Search PubMed.
- D. T. Tambe, C. Corey Hardin, T. E. Angelini, K. Rajendran, C. Y. Park, X. Serra-Picamal, E. H. Zhou, M. H. Zaman, J. P. Butler and D. A. Weitz, et al. , Nat. Mater., 2011, 10, 469–475 CrossRef CAS PubMed.
- M. R. Ng, A. Besser, J. S. Brugge and G. Danuser, eLife, 2014, 3, e03282 CrossRef PubMed.
- A. Leal-Egaña, G. Letort, J.-L. Martiel, A. Christ, T. Vignaud, C. Roelants, O. Filhol and M. Théry, Mol. Biol. Cell, 2017, 28, 1612–1621 CrossRef PubMed.
- T. Mitchison and L. Cramer, Cell, 1996, 84, 371–379 CrossRef CAS PubMed.
- D. A. Fletcher and R. D. Mullins, Nature, 2010, 463, 485–492 CrossRef CAS PubMed.
- F. Juelicher, K. Kruse, J. Prost and J.-F. Joanny, Phys. Rep., 2007, 449, 3–28 CrossRef CAS.
- M. C. Marchetti, J.-F. Joanny, S. Ramaswamy, T. B. Liverpool, J. Prost, M. Rao and R. A. Simha, Rev. Mod. Phys., 2013, 85, 1143 CrossRef CAS.
- E. L. Barnhart, K.-C. Lee, K. Keren, A. Mogilner and J. A. Theriot, PLoS Biol., 2011, 9, e1001059 CrossRef CAS PubMed.
- A. B. Verkhovsky, T. M. Svitkina and G. G. Borisy, Curr. Biol., 1999, 9, 11–S1 CrossRef CAS PubMed.
- P. T. Yam, C. A. Wilson, L. Ji, B. Hebert, E. L. Barnhart, N. A. Dye, P. W. Wiseman, G. Danuser and J. A. Theriot, J. Cell Biol., 2007, 178, 1207–1221 CrossRef CAS PubMed.
- K. Keren, Z. Pincus, G. M. Allen, E. L. Barnhart, G. Marriott, A. Mogilner and J. A. Theriot, Nature, 2008, 453, 475–480 CrossRef CAS PubMed.
- E. Anon, X. Serra-Picamal, P. Hersen, N. C. Gauthier, M. P. Sheetz, X. Trepat and B. Ladoux, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 10891–10896 CrossRef CAS PubMed.
- C. W. Wolgemuth, J. Stajic and A. Mogilner, Biophys. J., 2011, 101, 545–553 CrossRef CAS PubMed.
- M. Murrell, P. W. Oakes, M. Lenz and M. L. Gardel, Nat. Rev. Mol. Cell Biol., 2015, 16, 486–498 CrossRef CAS PubMed.
- P. Pandya, J. L. Orgaz and V. Sanz-Moreno, Curr. Opin. Cell Biol., 2017, 48, 87–96 CrossRef CAS PubMed.
- A. Callan-Jones, J.-F. Joanny and J. Prost, Phys. Rev. Lett., 2008, 100, 258106 CrossRef CAS PubMed.
- P. Recho and L. Truskinovsky, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2013, 87, 022720 CrossRef PubMed.
- C. Blanch-Mercader and J. Casademunt, Phys. Rev. Lett., 2013, 110, 078102 CrossRef CAS PubMed.
- T. Putelat, P. Recho and L. Truskinovsky, Phys. Rev. E, 2018, 97, 012410 CrossRef CAS PubMed.
- P. Recho, T. Putelat and L. Truskinovsky, New J. Phys., 2019, 21, 033015 CrossRef CAS.
- E. Tjhung, A. Tiribocchi, D. Marenduzzo and M. Cates, Nat. Commun., 2015, 6, 1–9 Search PubMed.
- B. A. Camley, Y. Zhao, B. Li, H. Levine and W.-J. Rappel, Phys. Rev. E, 2017, 95, 012401 CrossRef PubMed.
- K. Doubrovinski and K. Kruse, Phys. Rev. Lett., 2011, 107, 258103 CrossRef CAS PubMed.
- I. Lavi, N. Meunier, R. Voituriez and J. Casademunt, Phys. Rev. E, 2020, 101, 022404 CrossRef CAS PubMed.
- R. J. Hawkins, M. Piel, G. Faure-Andre, A. Lennon-Dumenil, J. Joanny, J. Prost and R. Voituriez, Phys. Rev. Lett., 2009, 102, 058103 CrossRef CAS PubMed.
- E. Tjhung, D. Marenduzzo and M. E. Cates, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 12381–12386 CrossRef CAS PubMed.
- D. Khoromskaia and G. P. Alexander, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2015, 92, 062311 CrossRef PubMed.
- L. Giomi and A. DeSimone, Phys. Rev. Lett., 2014, 112, 147802 CrossRef PubMed.
- R. J. Hawkins, R. Poincloux, O. Bénichou, M. Piel, P. Chavrier and R. Voituriez, Biophys. J., 2011, 101, 1041–1045 CrossRef CAS PubMed.
- T. Sanchez, D. T. Chen, S. J. DeCamp, M. Heymann and Z. Dogic, Nature, 2012, 491, 431–434 CrossRef CAS PubMed.
- A. Loisy, J. Eggers and T. B. Liverpool, Soft Matter, 2020, 16, 3106–3124 RSC.
- A. Loisy, J. Eggers and T. B. Liverpool, Phys. Rev. Lett., 2019, 123, 248006 CrossRef CAS PubMed.
- Y.-J. Liu, M. Le Berre, F. Lautenschlaeger, P. Maiuri, A. Callan-Jones, M. Heuzé, T. Takaki, R. Voituriez and M. Piel, Cell, 2015, 160, 659–672 CrossRef CAS PubMed.
- U. S. Schwarz and S. A. Safran, Rev. Mod. Phys., 2013, 85, 1327 CrossRef CAS.
- Y. Li, M. Wu, Z. Zhang, J. Xia, Z. Wang, X. Chen, X. Xiao, F. Lu and Z. Dong, Tissue Eng., Part A, 2019, 25, 1614–1622 CrossRef CAS PubMed.
- A. Saez, M. Ghibaudo, A. Buguin, P. Silberzan and B. Ladoux, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8281–8286 CrossRef CAS PubMed.
- B. L. Ricca, G. Venugopalan, S. Furuta, K. Tanner, W. A. Orellana, C. D. Reber, D. G. Brownfield, M. J. Bissell and D. A. Fletcher, eLife, 2018, 7, e26161 CrossRef PubMed.
- M. C. Marchetti, J.-F. Joanny, S. Ramaswamy, T. B. Liverpool, J. Prost, M. Rao and R. A. Simha, Rev. Mod. Phys., 2013, 85, 1143 CrossRef CAS.
- F. Jülicher, S. W. Grill and G. Salbreux, Rep. Prog. Phys., 2018, 81, 076601 CrossRef PubMed.
- Y. Hatwalne, S. Ramaswamy, M. Rao and R. A. Simha, Phys. Rev. Lett., 2004, 92, 118101 CrossRef PubMed.
- T. B. Liverpool and M. C. Marchetti, Phys. Rev. Lett., 2006, 97, 268101 CrossRef CAS PubMed.
- see ESI† at [to be inserted].
- A. Oron, S. H. Davis and S. G. Bankoff, Rev. Mod. Phys., 1997, 69, 931 CrossRef CAS.
- N. C. Gauthier, T. A. Masters and M. P. Sheetz, Trends Cell Biol., 2012, 22, 527–535 CrossRef CAS PubMed.
- J. Howard and R. Clark, Appl. Mech. Rev., 2002, 55, B39–B39 CrossRef.
- P. A. Janmey, Curr. Opin. Cell Biol., 1991, 3, 4–11 CrossRef CAS PubMed.
- J. Mair, B. Puschendorf and G. Michel, Adv. Clin. Chem., 1994, 31, 63–98 CAS.
- E. Hannezo, J. Prost and J.-F. Joanny, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 27–32 CrossRef CAS PubMed.
- W. J. Lennarz and M. D. Lane, Encyclopedia of biological chemistry, Academic Press, 2013 Search PubMed.
- P. A. Valberg and H. A. Feldman, Biophys. J., 1987, 52, 551–561 CrossRef CAS PubMed.
- A. E. Carlsson, Annu. Rev. Biophys., 2010, 39, 91–110 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2sm00934j |
| This journal is © The Royal Society of Chemistry 2022 |


