Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrodynamic and geometric effects in the sedimentation of model run-and-tumble microswimmers
Andrea
Scagliarini
 *ab and
Ignacio
Pagonabarraga
*ab and
Ignacio
Pagonabarraga
 cde
cde
aIAC-CNR, Istituto per le Applicazioni del Calcolo “Mauro Picone”, Via dei Taurini 19, 00185 Rome, Italy. E-mail: andrea.scagliarini@cnr.it
bINFN, Sezione Roma “Tor Vergata”, Via della Ricerca Scientifica 1, 00133 Rome, Italy
cCECAM, Centre Européen de Calcul Atomique et Moléculaire, Ecole Polytechnique Fédérale de Lausanne, Batochimie, Avenue Forel 2, 1015 Lausanne, Switzerland
dDepartament de Física de la Matèria Condensada, Universitat de Barcelona, Carrer de Martí i Franquès 1, 08028 Barcelona, Spain
eUniversitat de Barcelona, Institute of Complex Systems (UBICS), Universitat de Barcelona, 08028 Barcelona, Spain
First published on 16th February 2022
Abstract
The sedimentation process in an active suspension is the result of the competition between gravity and the autonomous motion of particles. We carry out simulations of run-and-tumble squirmers that move in a fluid medium, focusing on the dependence of the non-equilibrium steady state on the swimming properties. We find that for large enough activity, the density profiles are no longer simple exponentials; we recover the numerical results through the introduction of a local effective temperature, suggesting that the breakdown of the Perrin-like exponential form is a collective effect due to fluid-mediated dynamic correlations among particles. We show that analogous concepts can also fit the case of active non-motile particles, for which we report the first study of this kind. Moreover, we provide evidence of scenarios where the solvent hydrodynamics induces non-local effects which require the full three-dimensional dynamics to be taken into account in order to understand sedimentation in active suspensions. Finally, analyzing the statistics of the orientations of microswimmers, the emergence of a height-dependent polar order in the system is discussed.
1 Introduction
A number of microorganisms (bacteria, algae, etc.…) have the ability to swim in a liquid environment through the generation of autonomous motion at the expense of their metabolism, thus being intrinsically out-of-equilibrium. As such, these systems lead to new challenges such as the understanding of how collective phenomena and self-organization emerge from the relevant features of the propulsion mechanism.1–4 In this perspective a suspension of active particles is qualitatively different from a suspension of passive ones. Maybe the simplest, yet not trivial, example of this is the case of a constant external forcing on the suspension, such as gravity in the sedimentation process. In fact, when thermal fluctuations are negligible (as in the case of particles above the micron size), while passive particles would inevitably precipitate, active suspensions maintain a finite sedimentation length that grows with the self-propulsion speed. This result was predicted theoretically for dry suspensions (i.e. where the solvent hydrodynamics is neglected) of non-interacting run-and-tumble particles5,6 and, then, confirmed in numerical simulations with point-like dipoles7 and experimentally in suspensions of active colloids.8 Suspensions of self-propelled particles under gravity have been also reported to display a complex orientational dynamics, with the development of an associated polar order9,10 or even, in the case of bottom-heavy particles, to the inversion of the sedimentation profiles.11 In this paper we present a computational study of sedimentation in active suspensions, where hydrodynamics is fully resolved near and far from the particle surface. We provide evidence that hydrodynamic correlations induce important deviations from the phenomenology for dry suspensions in the steady state of both self-propelled swimmers and “shakers”, namely active particles that stir the fluid around them without achieving a directed motion, for which, to the best of our knowledge, this study represents the first of this kind. The sedimentation profiles observed when activity is intense are captured through a simple extension of a drift-diffusion model with height dependent effective temperature. We show that pullers develop a distal region of constant density (a supernatant) whose emergence depends on both the activity/gravity ratio and the confining geometry (i.e. the cell aspect-ratio). We also address the statistics of the microswimmer orientation, finding that, in the regime of small tumbling frequency, the suspension develops a polar order whose characteristics are strongly dependent on the type of swimmer.2 Numerical method and simulation details
The velocity field of the solvent (of dynamic viscosity η) is evolved by means of a lattice Boltzmann (LB) method12 with nineteen lattice speeds in three dimensions (D3Q19).13 Swimmers are modelled as solid spherical objects of radius R. The correct momentum exchange and mass conservation through the set of boundary links (between grid points in and out the sphere) representing the particles is implemented according to the bounce-back-on-links scheme.14–16 In order to mimic the surface deformations inducing the microswimmer motion, we adopt a simplified version of the squirmer model,17,18 whereby only the tangential polar component of the axisymmetric velocity prescribed at the particle surface is non-zero, us = us(θ)![[small straight theta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e12d.gif) . Furthermore, just the first two terms in the series expansion of us(θ) are retained:19,20
. Furthermore, just the first two terms in the series expansion of us(θ) are retained:19,20us(θ) = (B1 + B2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(θ))sin(θ), cos(θ))sin(θ), | (1) |
![[r with combining circumflex]](https://www.rsc.org/images/entities/b_char_0072_0302.gif) s) is the angle formed by the squirmer orientation unit vector, ê, and the position on the surface,
s) is the angle formed by the squirmer orientation unit vector, ê, and the position on the surface, ![[r with combining circumflex]](https://www.rsc.org/images/entities/b_char_0072_0302.gif) s = xs/R, relative to the particle centre of mass position (see Fig. 1 for a schematic representation of the model microswimmer just described). It should be pointed out that the prescription (1) cannot cope with unsteady flows, like those occurring when flagellar beating is involved, in short times, but it is effective on scales much longer than a typical flagellar or ciliary cycle.21 The parameter B1 > 0 in eqn (1) is related to the propulsion speed, which is
s = xs/R, relative to the particle centre of mass position (see Fig. 1 for a schematic representation of the model microswimmer just described). It should be pointed out that the prescription (1) cannot cope with unsteady flows, like those occurring when flagellar beating is involved, in short times, but it is effective on scales much longer than a typical flagellar or ciliary cycle.21 The parameter B1 > 0 in eqn (1) is related to the propulsion speed, which is  , whereas the second parameter, B2, determines the strength of the stresslet,
, whereas the second parameter, B2, determines the strength of the stresslet, ![[scr S, script letter S]](https://www.rsc.org/images/entities/char_e532.gif) ∝ηR2B2, generated by the swimmer in the surrounding fluid (and, hence, it is related to the amplitude of the injected vorticity).19 The ratio
∝ηR2B2, generated by the swimmer in the surrounding fluid (and, hence, it is related to the amplitude of the injected vorticity).19 The ratio  , such that β ∈ (−∞, + ∞), quantifies the relative intensity of apolar stresses and polar self-propulsion and classifies swimmers in “pushers”, β < 0 (including bacteria like, e.g., E. coli), “pullers”, β > 0 (such as the algae Chlamydomonas), and “potential” swimmers, β = 0 (i.e. swimmers that simply self-propel without generating vorticity, like the alga V. carteri or certain artificial swimmers).17,19,22–24 Every τ time step the particles randomise their orientation ê with uniform probability over the interval [0, π], thus accounting for the characteristic run-and-tumble mechanism, which can be seen as a source of diffusion for particles that, we recall here, are insensitive to thermal fluctuations.25,26 Different probability distributions of tumbling angles can, in principle, characterise actual microswimmers. For E. coli, for instance, the distribution is peaked around ∼65° and is rather skewed towards smaller values.25 It is known, though, that, when looked at over time scales t ≫ τ, the run-and-tumble motion leads to a diffusive dynamics, irrespective of the specific statistical properties of the tumbling events, but for a dependence of the diffusion coefficient on the mean angle.25,27–29
, such that β ∈ (−∞, + ∞), quantifies the relative intensity of apolar stresses and polar self-propulsion and classifies swimmers in “pushers”, β < 0 (including bacteria like, e.g., E. coli), “pullers”, β > 0 (such as the algae Chlamydomonas), and “potential” swimmers, β = 0 (i.e. swimmers that simply self-propel without generating vorticity, like the alga V. carteri or certain artificial swimmers).17,19,22–24 Every τ time step the particles randomise their orientation ê with uniform probability over the interval [0, π], thus accounting for the characteristic run-and-tumble mechanism, which can be seen as a source of diffusion for particles that, we recall here, are insensitive to thermal fluctuations.25,26 Different probability distributions of tumbling angles can, in principle, characterise actual microswimmers. For E. coli, for instance, the distribution is peaked around ∼65° and is rather skewed towards smaller values.25 It is known, though, that, when looked at over time scales t ≫ τ, the run-and-tumble motion leads to a diffusive dynamics, irrespective of the specific statistical properties of the tumbling events, but for a dependence of the diffusion coefficient on the mean angle.25,27–29
Our model, featuring finite size resolved particles, equipped with the squirming motion, is then able to capture hydrodynamic effects in the sedimentation of active suspensions, both in their far and near field manifestations, although when particles are close to contact or swimming takes place near the walls, the dynamics on short time scales might be not accurately described for flagellated microorganisms.
We simulate suspensions, of volume fraction ϕ = 0.07, in three-dimensional boxes of size L × L × H, with height H ≈ 80R and variable aspect-ratio Γ = L/H (see Fig. 1 for a graphical sketch). The height value is chosen to be large enough to exceed the maximum theoretically expected sedimentation length (over the explored range of parameters and for cases where such theoretical control is available), so as to guarantee that the upper bound will not affect the results. Two solid walls (with no-slip boundary conditions for the fluid velocity) confine the system in the z-direction, while periodic boundary conditions along the x, y directions hold. The number of particles, with radius R = 2.3 (in lattice-spacing units), range between ∼500 and ∼3 × 104). We introduce a reference velocity, vg = μFg (where Fg is the gravity force magnitude and μ = 1/(6πηR) is the particle mobility), i.e. the sedimentation velocity of a passive particle, and a reference time, tc = R/vp (where vp = |vp|), that is basically the time an isolated particle takes to displace its own radius. In terms of vg and tc, the following dimensionless parameters can be defined, namely
 | (2) |
![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) ≈ 4.3, corresponding to a run time much longer than the typical time the flow field takes to relax and adapt to the new orientation; the latter, given the low Reynolds number dynamics, can be taken as tν ∼ R2/ν, the viscous time of diffusion around the particle (ν being the solvent kinematic viscosity), so that τ/tν ≈ 30.
≈ 4.3, corresponding to a run time much longer than the typical time the flow field takes to relax and adapt to the new orientation; the latter, given the low Reynolds number dynamics, can be taken as tν ∼ R2/ν, the viscous time of diffusion around the particle (ν being the solvent kinematic viscosity), so that τ/tν ≈ 30.
3 Sedimentation profiles
We start each run with the active particles homogeneously distributed in space, with random orientations. To check that a (non-equilibrium) statistically steady state is reached, we follow the time evolution of the average height , where ρ(z, t) is the (unsteady) normalized particle density (i.e. ρ(z, t)dz is the probability of finding a particle centred between z and z + dz at the time t). We consider as the steady state the time interval during which h(t) fluctuates by less than ∼5%. All data shown hereafter are meant to be averaged over such time interval. Our aim is to study the impact that activity, in terms of χ1 and β, has on the squirmer sedimentation, and to characterize the emerging dynamical regimes, checking whether and how hydrodynamic effects come into play. According to the theory,5,6 as χ1 → 1, all particles concentrate at the bottom wall. Instead, when χ1 ≫ 1 (i.e., in the self-propulsion dominated regime) the steady state sedimentation profile should display an exponential form ρ(z) ∼ e−z/λ, with a sedimentation length depending on the single particle velocity (and, hence, on χ1) as
, where ρ(z, t) is the (unsteady) normalized particle density (i.e. ρ(z, t)dz is the probability of finding a particle centred between z and z + dz at the time t). We consider as the steady state the time interval during which h(t) fluctuates by less than ∼5%. All data shown hereafter are meant to be averaged over such time interval. Our aim is to study the impact that activity, in terms of χ1 and β, has on the squirmer sedimentation, and to characterize the emerging dynamical regimes, checking whether and how hydrodynamic effects come into play. According to the theory,5,6 as χ1 → 1, all particles concentrate at the bottom wall. Instead, when χ1 ≫ 1 (i.e., in the self-propulsion dominated regime) the steady state sedimentation profile should display an exponential form ρ(z) ∼ e−z/λ, with a sedimentation length depending on the single particle velocity (and, hence, on χ1) as | (3) |
![[small script l]](https://www.rsc.org/images/entities/i_char_e146.gif) = vpτ ≈ 4.3R ≈ 0.06H is the microswimmer's run length. This result has been found to be in agreement with experimental observations8 and numerical simulations.7 The exponential profile also characterizes equilibrium systems, as in the classical Perrin's experiment for (thermal) colloids;30 the sedimentation length is determined by the particle diffusivity, D, and the gravity force as λ(eq) = D/(μFg) and depends, therefore, through the Stokes–Einstein relation D = μkBT, on the system temperature T, namely λ(eq) = kBT/Fg. The formal analogy with the passive (equilibrium) case suggests, then, to introduce an effective temperature as follows:
= vpτ ≈ 4.3R ≈ 0.06H is the microswimmer's run length. This result has been found to be in agreement with experimental observations8 and numerical simulations.7 The exponential profile also characterizes equilibrium systems, as in the classical Perrin's experiment for (thermal) colloids;30 the sedimentation length is determined by the particle diffusivity, D, and the gravity force as λ(eq) = D/(μFg) and depends, therefore, through the Stokes–Einstein relation D = μkBT, on the system temperature T, namely λ(eq) = kBT/Fg. The formal analogy with the passive (equilibrium) case suggests, then, to introduce an effective temperature as follows: | (4) |
In Fig. 2 we plot the time-averaged steady state density profiles of potential swimmers (β = 0) for χ1 ∈ [1, 20]. This range of values is compatible with those expected for typical bacteria, such as E. coli or B. subtilis whose swimming speeds are vp ∼ 15–30 μm s−1,25,31,32 in terrestrial gravity (vg ∼ 1–2.5 μm s−1), for which it would be χ1 ∼ 6–30.6
 | ||
Fig. 2 Main panel: Density profiles in microswimmer suspensions for various values of the propulsion/gravity ratio χ1, at β = 0 and Γ = 0.35. For χ1 close to one, the particles accumulate at the bottom wall, showing a crystal order (as the regularly spaced peaks in ρ suggest). For large χ1 the expected exponential profile is recovered. Inset: Dependence of the sedimentation length λ (computed out of exponential fits of the density profiles) (□) on the propulsion/gravity ratio χ1. The dashed line depicts the theoretical expectation λ/χ1 = ![[small script l]](https://www.rsc.org/images/entities/i_char_e146.gif) /3 ≈ 1.45R, eqn (3), valid for χ1 ≫ 1. /3 ≈ 1.45R, eqn (3), valid for χ1 ≫ 1. | ||
For values close to one, as expected, microswimmers uniformly fall down under the action of gravity; however, due to the finite size of particles, the sedimentation length remains finite. The particles in the sediment tend to organize themselves in layers with a crystal-like order, noticeable from the peaks in the density profile, close to the bottom wall, displaced from each other by about one diameter (2R), as found also in a previous computational study.10 At increasing χ1, swimmers occupy an increasingly larger volume of liquid and, correspondingly, ρ(z) shows, over the whole box length, the predicted exponential profile6 with a sedimentation length growing linearly with χ1 (see inset of Fig. 2).
If we increase |β| (thus intensifying the activity) to large enough values, for a fixed χ1, the deviation from the exponential profile can be important, as one can see from Fig. 3, where we plot the particle density ρ(z) for three cases with same χ1 = 10 and β = 0, ±10. For the sake of comparison of the chosen values of β with those expected for actual microswimmers, consider that, e.g., E. coli swims at a speed vp ∼ 20 μm s−1,25 exerting a force dipole of amplitude f ∼ 0.4 pN and length δ ∼ 2 μm;31 therefore,  and |B2| ∼
and |B2| ∼ ![[scr S, script letter S]](https://www.rsc.org/images/entities/char_e532.gif) /(ηδ2) ∼ f/(ηδ) ≈ 200 μm s−1, where
/(ηδ2) ∼ f/(ηδ) ≈ 200 μm s−1, where ![[scr S, script letter S]](https://www.rsc.org/images/entities/char_e532.gif) ∼ fδ is the stresslet, give |β| = |B2|/B1≈7.
∼ fδ is the stresslet, give |β| = |B2|/B1≈7.
 | ||
Fig. 3 Main panel: Density profiles in microswimmer suspensions with χ1 = 10, β = 0, ±10 and Γ = 0.35 (data are vertically shifted for clarity). The lines represent the predictions coming from the numerical integration of eqn (7) with λ = 15 and α1 = 1 (see the text for the discussion of the model parameters) for β = 0 (dashed line), β = −10 (solid line) and β= + 10 (dotted line). Inset: Fluid velocity fluctuations  as a function of the system height for the case β = − 10. as a function of the system height for the case β = − 10. | ||
In the pushers/pullers case (β ≠ 0), dynamic correlations are so intense that recovering a Perrin-like form just with the introduction of a global effective diffusion coefficient as coming from single particle dynamics is no longer possible.8 The larger the |β|, the stronger is the departure of the sedimentation profile from being exponential; indeed we found that deviations start to be relevant from |β| ≈ 5.7
4 Extended diffusive model
4.1 Local effective temperature
Due to hydrodynamic correlations the dynamics of an active particle in the suspension is affected by the presence of the others through the generation of motion within the liquid, which acts as a bath at an effective temperature (measuring the fluid agitation). We can understand these effects extending a diffusive model proposed to describe sedimentation in active colloidal suspensions,8 based on the Smoluchowski equation ∂tρ = −∇·J, determined by the flux J = −![[D with combining tilde]](https://www.rsc.org/images/entities/i_char_0044_0303.gif) ∇ρ +
∇ρ + ![[small mu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e0.gif) Fgρ. The ratio of the local diffusion coefficient,
Fgρ. The ratio of the local diffusion coefficient, ![[D with combining tilde]](https://www.rsc.org/images/entities/i_char_0044_0303.gif) , and particle mobility,
, and particle mobility, ![[small mu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e0.gif) , by virtue of a generalized Stokes–Einstein relation, represents the effective temperature field. Assuming that in the steady state the density will depend only on z, the zero flux boundary conditions at the walls gives
, by virtue of a generalized Stokes–Einstein relation, represents the effective temperature field. Assuming that in the steady state the density will depend only on z, the zero flux boundary conditions at the walls gives | (5) |
We propose an effective temperature of the form Teff =T(1p)eff + T(coll)eff, consisting of two terms: the single-particle effective temperature, eqn (4), accounting for the self-propulsion, plus a contribution proportional to the fluid velocity fluctuations, T(coll)eff, capturing the collective effects due to hydrodynamic interactions. However, since in the steady state microswimmers are distributed inhomogeneously over the volume (with a density increasing from top to bottom), the fluid velocity fluctuations  (where
(where  ) are also expected to vary with z (as indeed it can be seen in the inset of Fig. 3). This entails a height dependent effective temperature Teff(z) = T(1p)eff + T(coll)eff(z), leading, upon insertion in (5), to an equation for the sedimentation density which can be recast in the following form:
) are also expected to vary with z (as indeed it can be seen in the inset of Fig. 3). This entails a height dependent effective temperature Teff(z) = T(1p)eff + T(coll)eff(z), leading, upon insertion in (5), to an equation for the sedimentation density which can be recast in the following form:
 | (6) |
 | (7) |
4.2 The case of shakers
Another striking instance of how crucial the role played by hydrodynamics can be is provided by the regime where |β| → ∞, i.e. B1 goes to zero while B2 stays finite. This regime corresponds to active suspensions where particles do not self-propel but generate motion in the fluid and are relevant for microswimmers known as shakers,1,33 like, e.g., melanocytes.34 Since both their propelling velocity and the effect of thermal fluctuations are negligible, such a suspension would undergo a gravitational collapse, if one could completely neglect the presence of the solvent. However, as shown in Fig. 4, the steady state density profiles develop a sedimentation layer, whose width increases with χ2 (defined in (2)). The observed width cannot be interpreted simply as a result of the close packing of the particles, which would imply, in fact, a value of around 8R, much smaller than the measured one. We try to recover the sedimentation profiles of shakers following the same ideas of the previous section. We must integrate numerically eqn (5), with a vanishing one-particle contribution to the effective temperature, T(1p)eff = 0, since for shakers B1 = 0 ⇔ vp = 0, such that Teff(z) = T(coll)eff(z) = α2(σδu2(z)/vB22) (here we indicate the phenomenological parameter as α2 in order to distinguish it from that of self-propellers). The reference speed vB2 = |B2|/3 is the magnitude of the velocity field generated by a shaker, averaged over its surface, i.e. , where us is given by eqn (1). The stationary Smoluchowski equation, then, reads
, where us is given by eqn (1). The stationary Smoluchowski equation, then, reads | (8) |
 | ||
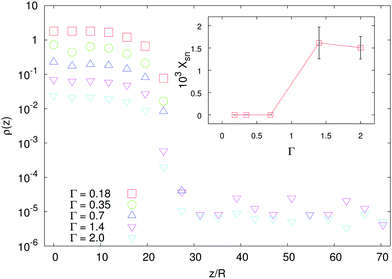
| Fig. 4 Main panel: Density profiles of shakers with two different χ2 < 0 and Γ = 0.35 (here and in the inset data are vertically shifted for clarity). The larger the |χ2| the longer the density tail (i.e. the wider is the region occupied by particles). The lines are the theoretical predictions coming from the numerical integration of eqn (8), where the function σδu2(z) is taken from the simulations, with α2 = 4.4. Inset: Density profiles of shakers with |χ2| = 5 (green triangles), |χ2| = 10 (red squares) and |χ2| = 20 (blue circles). Data for both pullers (χ2 > 0, full symbols) and pushers (χ2 < 0, empty symbols) are reported; notice the formation of the supernatant in the puller case for large enough χ2. | ||
Analogously to the case of pullers, shakers with χ2 > 0 develop (for χ2 large enough) a distal region of constant density in the sedimentation profile (see the inset of Fig. 4). The emergence of such supernatant is due to the sediment which acts as a pump and generates motion in higher layers of fluid. It is, then, a genuinely three-dimensional and non-local effect, two features which make also our formalism based on a height dependent effective temperature fail. To support this picture, we show that, for a fixed value of χ2, the supernatant disappears when decreasing the aspect-ratio Γ of the cell below unity (see Fig. 5).
This is, indeed, a manifestation of three-dimensionality: using an analogy with a Rayleigh–Bénard system,35 we argue that the geometry favors (or does not) the development of a large scale flow which can (or cannot) sustain the supernatant. In fact, the difference in the fluid flow pattern generated by a single particle, either a pusher or a puller, is not strong enough to sustain the different macroscopic patterns observed if the swimmers are randomly oriented (as a matter of fact, no supernatant is observed for pushers, or shakers with χ2 < 0). Hence, a collective organization of the swimmers is required to produce the observed macroscopic flows. We will next address the emergence of orientational order in the sedimentation profiles of microswimmers.
5 Orientational statistics
The emergence and the dynamical relevance of anisotropic ordering in active fluid systems has been widely recognized in the literature.1,9,36 We study the orientational statistics measuring the joint probability distribution function (PDF) of the particle elevation and vertical component of the squirmer characteristic vector, P(z, êz). For squirmers with χ1 = 10, β = 0 and run time![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) ≈ 4.3 we find a bimodal distribution symmetrically peaked at êz = ±1 (with a slight imbalance towards êz = −1), for any z, as expected under the assumption of a factorized joint PDF, P(z, êz) ∼ ρ(z)Φ(êz).26 However, it was shown theoretically, in the context of active Brownian particles, that such factorization could only be possible for the vanishing Péclet number;9 otherwise, when Pe ∼ O(1), the suspension develops a polar order which is non-trivially correlated with the height. We recall, here, that for athermal, run-and-tumble particles an effective diffusivity can be defined, proportional to the tumbling rate,
≈ 4.3 we find a bimodal distribution symmetrically peaked at êz = ±1 (with a slight imbalance towards êz = −1), for any z, as expected under the assumption of a factorized joint PDF, P(z, êz) ∼ ρ(z)Φ(êz).26 However, it was shown theoretically, in the context of active Brownian particles, that such factorization could only be possible for the vanishing Péclet number;9 otherwise, when Pe ∼ O(1), the suspension develops a polar order which is non-trivially correlated with the height. We recall, here, that for athermal, run-and-tumble particles an effective diffusivity can be defined, proportional to the tumbling rate, ![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) −1;26 hence the effective Péclet number grows at Pe ∼
−1;26 hence the effective Péclet number grows at Pe ∼ ![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) . We increase, therefore,
. We increase, therefore, ![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) to probe this regime. We remark, incidentally, that it is also possible to modulate the effective diffusivity by changing the mean tumbling angle; thus in real systems one must expect that the statistics of reorientations also affects the polar order. Indeed, for
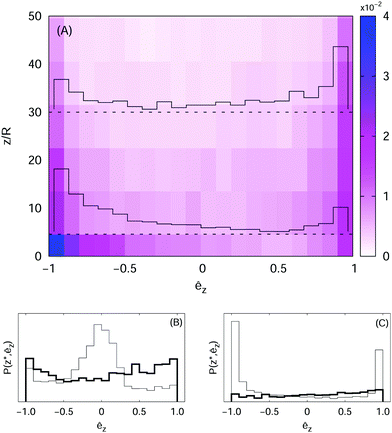
to probe this regime. We remark, incidentally, that it is also possible to modulate the effective diffusivity by changing the mean tumbling angle; thus in real systems one must expect that the statistics of reorientations also affects the polar order. Indeed, for ![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) ≈ 30, we observe from the joint PDF, shown in Fig. 6(A) as a colour map, a larger probability of finding downward oriented particles close to the wall, whereas the opposite trend appears at higher elevations, which means that in the bulk the active particles preferentially swim upwards (i.e. against gravity), in line with the theoretical results.9 To highlight this behaviour we also report the orientation PDFs, P(z*, êz)/ρ(z*), as histograms at two heights, z* = 2R and z* = 30R.
≈ 30, we observe from the joint PDF, shown in Fig. 6(A) as a colour map, a larger probability of finding downward oriented particles close to the wall, whereas the opposite trend appears at higher elevations, which means that in the bulk the active particles preferentially swim upwards (i.e. against gravity), in line with the theoretical results.9 To highlight this behaviour we also report the orientation PDFs, P(z*, êz)/ρ(z*), as histograms at two heights, z* = 2R and z* = 30R.
It is worth noting that the chosen value of ![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) is comparable, for instance, with that of E. coli, for which, having vp ≈ 25 μm s−1 and a typical run time of ∼1 s
is comparable, for instance, with that of E. coli, for which, having vp ≈ 25 μm s−1 and a typical run time of ∼1 s![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25,37 and size R ∼ 1 μm, one gets
25,37 and size R ∼ 1 μm, one gets ![[small tau, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d4.gif) = vpτ/R ≈ 25.
= vpτ/R ≈ 25.
For β ≠ 0 we expect this scenario to break down, because the generation of fluid motion acts as an effective source of noise; in fact, we observe that, close to the wall, the PDF is peaked around êz ≈ 0 for β < 0, and it is bimodal (with a higher peak at êz ≈ −1) for β > 0, while in the bulk it is rather uniform in both cases (panels (B) and (C) of Fig. 6). As anticipated in the previous section, such different orientational ordering between pushers and pullers turns out to have an impact also on the swimmers’ distribution in space, as indicated by the sedimentation profiles.
6 Conclusions
We have presented a computational study of suspensions of run-and-tumble squirmers under gravity. Thanks to the built-in properties of the mesoscopic approach adopted we could take into account both the finite size of particles and the hydrodynamics of the solvent. In the case of potential swimmers, agreement has been found with theoretical predictions regarding (i) the dependence of the density profiles on the activity/gravity ratio and (ii) the emergence of a polar order from the inspection of distributions of particle orientations. We have provided evidence, not reported so far, that, for pushers and pullers with large enough β, the hydrodynamic flows induced by their collective motion determine sedimentation profiles that cannot be understood in terms of a single swimmer response to the gravitational field. This feature appeared particularly distinctive in the emblematic case of shakers, whose sedimentation problem was never studied before. We have, then, proposed a generalised diffusive model, based on the concept of a height dependent collective effective temperature that proved to be able to recover the observed sedimentation profiles. Moreover, we showed that the profiles of pullers and shakers with positive β may develop a tail in the bulk of roughly constant density, signalling the presence of a supernatant, depending on the activity/gravity ratio (the parameter χ2) but also on the system geometry (the aspect-ratio Γ). The emergence of such a supernatant, no previous observation of which we are aware of, is probably connected to the complex correlation between spatial organization of the microswimmers in the sediment and generated flows in the solvent, which deserves further investigation.Overall, our findings, along the lines of recent studies,10,38 emphasize the importance of exploring, theoretically, numerically and experimentally, the full three-dimensional dynamics for the sake of a better understanding of the sedimentation phenomenology in active suspensions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge MICINN and DURSI for financial support under Project No. PGC2018-098373-B-I00, and 2017SGR- 884, respectively. IP acknowledges SNSF for financial support under Project No. 200021-175719. This work was possible thanks to the access to the MareNostrum Supercomputer at Barcelona Supercomputing Center (BSC) and also through the Partnership for Advanced Computing in Europe (PRACE).Notes and references
- M. Marchetti, J.-F. Joanny, S. Ramaswamy, T. Liverpool, J. Prost, M. Rao and R. Simha, Rev. Mod. Phys., 2013, 85, 1143–1189 CrossRef CAS.
- J. Elgeti, R. Winkler and G. Gompper, Rev. Prog. Phys., 2015, 78, 056601 CrossRef CAS PubMed.
- R. Simha and S. Ramaswamy, Phys. Rev. Lett., 2002, 89, 058101 CrossRef PubMed.
- I. Llopis and I. Pagonabarraga, Europhys. Lett., 2006, 75, 999–1005 CrossRef CAS.
- J. Tailleur and M. Cates, Phys. Rev. Lett., 2008, 100, 21803 CrossRef PubMed.
- J. Tailleur and M. Cates, Europhys. Lett., 2009, 86, 60002 CrossRef.
- R. Nash, R. Adhikari, J. Tailleur and M. Cates, Phys. Rev. Lett., 2010, 104, 258101 CrossRef CAS PubMed.
- J. Palacci, C. Cottin-Bizonne, C. Ybert and L. Bocquet, Phys. Rev. Lett., 2010, 105, 088304 CrossRef PubMed.
- M. Enculescu and H. Stark, Phys. Rev. Lett., 2011, 107, 058301 CrossRef PubMed.
- J.-T. Kuhr, J. Blaschke, F. Rühle and H. Stark, Soft Matter, 2017, 13, 7548–7555 RSC.
- K. Wolff, A. Hahn and H. Stark, Eur. Phys. J. E: Soft Matter Biol. Phys., 2013, 36, 43 CrossRef CAS PubMed.
- S. Succi, The lattice Boltzmann equation for complex states of flowing matter, Oxford University Press, 2018 Search PubMed.
- J.-C. Desplat, I. Pagonabarraga and P. Bladon, Comput. Phys. Commun., 2001, 134, 273–290 CrossRef CAS.
- A. Ladd, J. Fluid Mech., 1994, 271, 285–309 CrossRef CAS.
- A. Ladd, J. Fluid Mech., 1994, 271, 311–339 CrossRef CAS.
- N.-Q. Nguyen and A. Ladd, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2002, 66, 046708 CrossRef PubMed.
- M. Lighthill, Comm. Pure Appl. Math., 1952, 5, 109–118 CrossRef.
- J. Blake, J. Fluid Mech., 1971, 46, 199–208 CrossRef.
- T. Ishikawa, M. Simmonds and T. Pedley, J. Fluid Mech., 2006, 568, 119–160 CrossRef.
- R. Matas-Navarro and I. Pagonabarraga, Eur. Phys. J. E: Soft Matter Biol. Phys., 2010, 33, 27–39 CrossRef CAS PubMed.
- L. Zhu, E. Lauga and L. Brandt, J. Fluid Mech., 2013, 726, 285–311 CrossRef.
- K. Drescher, R. Goldstein, N. Michel, M. Polin and I. Tuval, Phys. Rev. Lett., 2010, 105, 168101 CrossRef PubMed.
- S. Thutupalli, R. Seemann and S. Herminghaus, New J. Phys., 2011, 13, 073021 CrossRef.
- A. Evans, T. Ishikawa, T. Yamaguchi and E. Lauga, Phys. Fluids, 2011, 23, 111702 CrossRef.
- H. Berg and D. Brown, Nature, 1972, 239, 500–504 CrossRef CAS PubMed.
- A. Solon, M. Cates and J. Tailleur, Eur. Phys. J. E: Soft Matter Biol. Phys., 2015, 224, 1231–1262 CAS.
- P. Lovely and F. Dahlquist, J. Theor. Biol., 1975, 50, 477–496 CrossRef CAS.
- J. Saragosti, P. Silberzan and A. Baguin, PLoS One, 2012, 7, e35412 CrossRef CAS PubMed.
- A. Villa-Torrealba, C. Chávez-Raby, P. de Castro and R. Soto, Phys. Rev. E, 2020, 101, 062607 CrossRef CAS PubMed.
- J. Perrin, Ann. Chim. Phys., 1909, 8, 5–114 Search PubMed.
- K. Drescher, J. Dunkel, L. Cisneros, S. Ganguly and R. Goldstein, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 10940–10945 CrossRef CAS PubMed.
- A. Hamby, D. Vig, S. Safonova and C. Wolgemuth, Sci. Adv., 2018, 4, eaau0125 CrossRef CAS PubMed.
- S. Ramachandran, P. S. Kumar and I. Pagonabarraga, Eur. Phys. J. E: Soft Matter Biol. Phys., 2006, 20, 151–158 CrossRef CAS PubMed.
- H. Gruler, U. Dewald and M. Eberhardt, Eur. Phys. J. B, 1999, 11, 187–192 CrossRef CAS.
- S. Chandrasekhar, Hydrodynamic and hydromagnetic stability, Oxford University Press, 1961 Search PubMed.
- S. Ramaswamy, Annu. Rev. Condens. Matter Phys., 2010, 1, 323–345 CrossRef.
- T. Ishiwaka, G. Sekiya, Y. Imai and T. Yamaguchi, Biophys. J., 2007, 93, 2217–2225 CrossRef PubMed.
- F. Rühle and H. Stark, Eur. Phys. J. E: Soft Matter Biol. Phys., 2020, 43, 26 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |