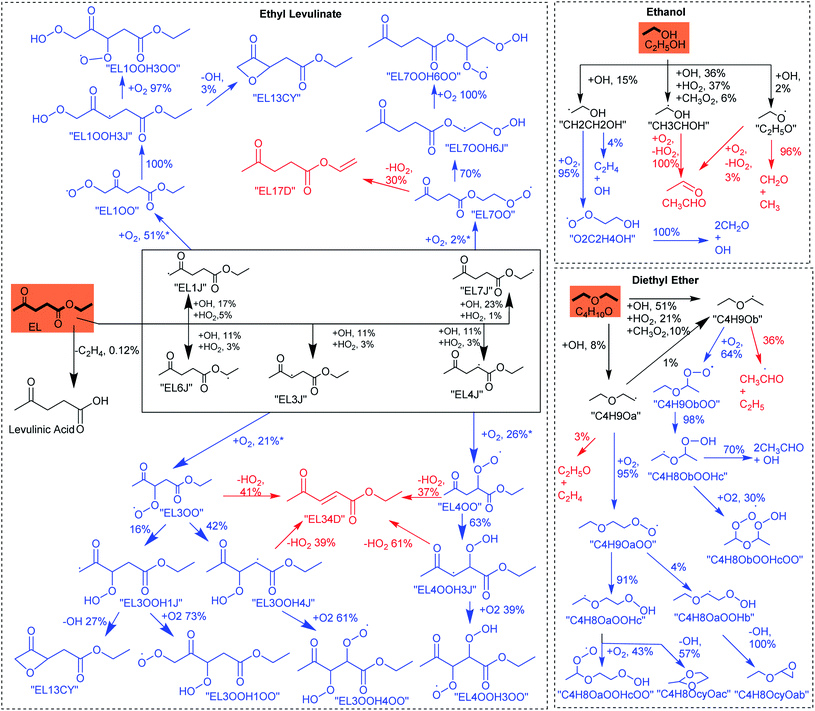
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Ethanolic gasoline, a lignocellulosic advanced biofuel†
Mícheál Séamus
Howard‡
ab,
Gani
Issayev
c,
Nimal
Naser
c,
S. Mani
Sarathy
c,
Aamir
Farooq
*c and
Stephen
Dooley‡
ab
aDepartment of Chemical Sciences, University of Limerick, Ireland
bSchool of Physics, Trinity College Dublin, Ireland
cKing Abdullah University of Science and Technology (KAUST), Clean Combustion Research Centre, Physical Sciences and Engineering Division, Thuwal, Saudi Arabia. E-mail: aamir.farooq@kaust.edu.sa
First published on 5th June 2022
Abstract
Correction for ‘Ethanolic gasoline, a lignocellulosic advanced biofuel’ by Mícheál Séamus Howard et al., Sustainable Energy Fuels, 2019, 3, 409–421, https://doi.org/10.1039/C8SE00378E.
The ignition delay time (IDT) data reported in this article1 were recently found to be erroneous. Two issues caused errors in the previous data, (i) a fraction of the volatile fuel, diethyl ether (DEE), was lost during mixture preparation, and (ii) a fraction of the high boiling point fuel, ethyl levulinate (EL), was lost due to condensation on the walls of the combustion chamber. These issues were realized after the publication of the original paper while working on another project. After fixing these problems, we obtained new IDT results which deviated significantly from the previous data at low temperatures. New IDT data of the EtOH/DEE/EL blend showed NTC behavior, in contrast to previous data which primarily exhibited Arrhenius-like behavior. The revised Fig. 2 below plots the updated data, and these data are provided in tabular form in the ESI.†
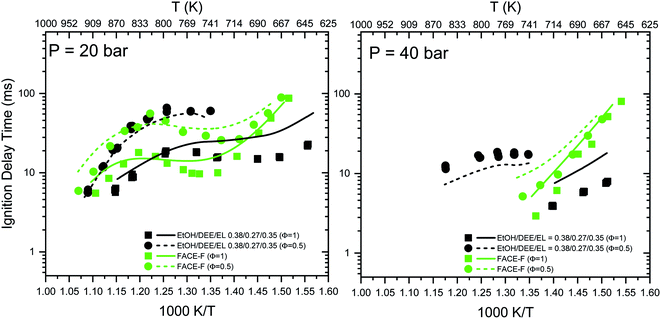 | ||
| Fig. 2 RCM ignition delay times for the EL/DEE/EtOH gasoline and FACE-F.2 Symbols correspond to measurements. Lines correspond to present model predictions. FACE-F experimental data are taken from Sarathy et al.2 | ||
The chemical kinetic model reported in the original paper was developed and tuned to match the erroneous experimental data originally reported. The new experimental data showed that our original model needed further improvements. Therefore, we have now revised the model based on the newly acquired IDT data. The predictions of the updated model are shown in the updated Fig. 2. The following modifications are made to the previous model:
(1) The gasoline related reactions (i.e., of large hydrocarbons) in the old model were removed in order to decrease its stiffness
(2) The DEE/EtOH sub-mechanism was replaced by the recently published and validated model from ref. 3
(3) The following EL low-temperature reaction rates were modified:
(a) EL6OO ↔ EL6OOH7J (activation energy reduced by 4 kcal mol−1)
(b) EL6OO ↔ EL67D + HO2 (A-factor decreased by a factor of 2)
(c) EL6OOH7OO ↔ EL6O7OOH + OH (A-factor decreased by a factor of 4)
The modified chemical kinetic model and ChemKin input files are provided in the ESI.† The modified model was used to update the kinetic analyses figures. The updated versions of Fig. 3–5 can be found below.
The corrected experimental ignition delay data and the updated modelling analyses alter some of the statements made in the original paper. Here, we summarize key inferences that may be drawn from the data and analyses presented in this correction:
• Ignition delay times of conventional hydrocarbon-based gasoline (FACE-F) and oxygenated gasoline (EL/DEE/EtOH) exhibit negative temperature coefficient (NTC) behavior at intermediate temperatures (see the updated Fig. 2). However, FACE-F shows steeper NTC compared to the oxygenated gasoline. Both fuels have similar high-temperature reactivity, but the oxygenated gasoline shows lower reactivity (longer ignition delays) than FACE-F at low temperatures.
• The nominally similar reactivity behavior of the two gasolines is in-line with their similar octane numbers. However, the flatter ignition delay profile of the oxygenated gasoline indicates that it should possess a larger octane sensitivity (S = RON − MON). This anomaly could result from the limitations of octane rating tests, as discussed in the original paper. Octane measurements of oxygenated fuels are quite complicated and may suffer from larger uncertainties compared to the well-established octane measurements of conventional gasoline.
• Reaction flux analysis (Fig. 3) shows that the OH radical leads the consumption of the three fuel components, consuming 73%, 59% and 53% of EL, DEE and EtOH, respectively. On the other hand, HO2 consumption of these fuels is 15%, 21%, and 37%, respectively. Therefore, modelling analysis (750 K, 25 bar) shows that EL is primarily consumed by OH radicals, while the HO2 radical plays a larger role in the consumption of EtOH. For this tri-blend, a large pool of reactive radicals is formed from DEE, which then promotes the oxidation of EL.
• The radical rate of production analysis (Fig. 4) indicates that HO2 formation is more significant for EL/DEE/EtOH compared to FACE-F at both low (750 K) and high (1200 K) temperatures. This augmented rate of production of HO2 for EL/DEE/EtOH stems from the dominance of RO2 and the alkylhydroperoxide radical elimination pathways.
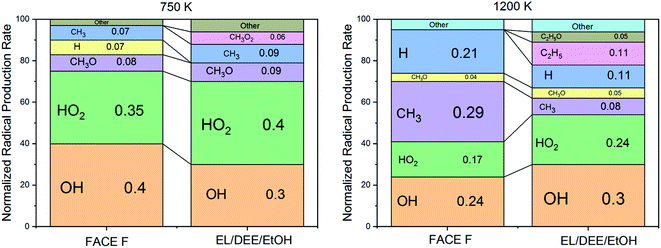 | ||
| Fig. 4 Normalized small radical rate of production for EL/DEE/EtOH gasoline and FACE-F2 after 65% of the computed ignition delay time at 750 K and 1200 K, 25 bar, and Φ = 1. | ||
• Sensitivity analyses (Fig. 5) show that OH consumption of the fuel components dictates the system reactivity at 750 K. In the case of FACE-F, for example, reactions of OH with iso-octane and n-heptane promote reactivity, while those with n-butane, cyclopentane, trimethylbenzene and the tertiary site of iso-octane inhibit reactivity. For EL/DEE/EtOH, the reaction of OH with DEE promotes reactivity, while those with EL and EtOH inhibit reactivity. At 1200 K, small radical chemistry dominates for both gasolines but an enhanced role of HO2 reactions and H2O2 decomposition is observed for EL/DEE/EtOH. Thus, the ignition modes of the two gasolines are somewhat different even though they have very similar reactivity at high temperatures.
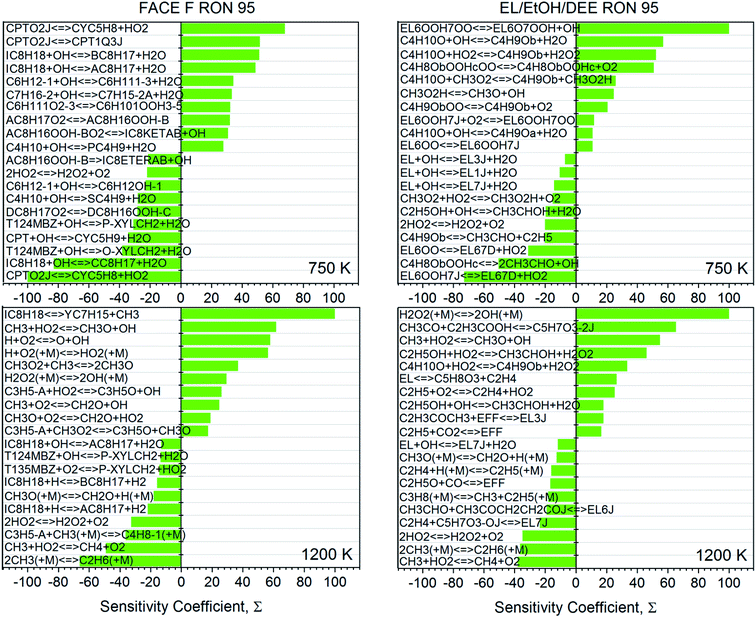 | ||
| Fig. 5 Sensitivity analysis to ΔT for EL/DEE/EtOH gasoline and FACE-F2 at 750 K and 1200 K, 25 bar, and Φ = 1. Top 20 sensitivity coefficients are shown, normalized to the absolute most sensitive reaction. Reactions are described in the direction of their net flux from left to right. | ||
• In conclusion, the petroleum gasoline (FACE-F) and ethanolic gasoline (EL/DEE/EtOH) with almost identical octane ratings have very similar high-temperature reactivity and both fuels show NTC behavior at intermediate temperatures. The ethanolic gasoline shows a flatter NTC profile and reduced reactivity at low temperatures, which are likely due to the increased role of HO2-related inhibition pathways.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- M. S. Howard, G. Issayev, N. Naser, S. M. Sarathy, A. Farooq and S. Dooley, Ethanolic gasoline, a lignocellulosic advanced biofuel, Sustainable Energy Fuels, 2019, 3, 409–421 RSC.
- S. M. Sarathy, G. Kukkadapu, M. Mehl, T. Javed, A. Ahmed and N. Naser, et al., Compositional effects on the ignition of FACE gasolines, Combust. Flame, 2016, 169, 171–193 CrossRef CAS.
- G. Issayev, S. Mani Sarathy and A. Farooq, Autoignition of diethyl ether and a diethyl ether/ethanol blend, Fuel, 2020, 279, 118553 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2se90024f |
| ‡ Mícheál Séamus Howard and Stephen Dooley could not be contacted prior to publication of this Correction. |
| This journal is © The Royal Society of Chemistry 2022 |