K+ intercalated MnO2 with ultra-long cycling life for high-performance aqueous magnesium-ion hybrid supercapacitors†
Abstract
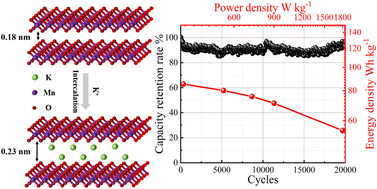
Aqueous magnesium-ion hybrid supercapacitors (MHSs) are very safe, low-cost, and green energy storage systems but confronted with several weaknesses such as low-capacity cathode materials and limited cycling life. In this work, K+ intercalated MnO2 is developed as a cathode material for MHSs to maintain reversible phase transition during cycling. The interplanar spacing is expanded by introducing K+ ions between the MnO2 layers, which shortens the ion diffusion path and exposes more ion reaction/storage sites. Therefore, the K–MnO2-2 electrode shows a high specific capacitance of 333.3 F g−1 and good rate capability. Furthermore, the aqueous MHS based on the K–MnO2-2 cathode and AC anode exhibits a superior energy density of 85.2 W h kg−1 at a power density of 360 W kg−1 and an amazing cycling life with a capacity retention of 96.7% after 20 000 cycles, surpassing most of the Mn-based electrode materials for MHSs reported in the literature. This strategy to design high-performance cathodes for aqueous MHSs will provide new opportunities to develop high-efficiency energy storage devices for practical applications.
- This article is part of the themed collection: Sustainable Energy & Fuels Recent HOT Articles


 Please wait while we load your content...
Please wait while we load your content...