Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Perspectives and prospects of underground hydrogen storage and natural hydrogen
Emmanuel I.
Epelle
a,
Winifred
Obande
b,
Godwin A.
Udourioh
c,
Inioluwa Christianah
Afolabi
d,
Kwaghtaver S.
Desongu
e,
Uzezi
Orivri
f,
Burcu
Gunes
 *g and
Jude A.
Okolie
h
*g and
Jude A.
Okolie
h
aSchool of Computing, Engineering & Physical Sciences, University of the West of Scotland, Paisley PA1 2BE, UK
bInstitute for Materials and Processes (IMP), School of Engineering, The University of Edinburgh, The King's Buildings, Edinburgh, EH9 3FB, UK
cAnalytical/Material Chemistry Laboratory, Department of Pure and Applied Chemistry, Faculty of Natural and Applied Sciences, Veritas University, P. O. Box 6523, Garki, Abuja, Nigeria
dDepartment of Pure and Applied Chemistry, Ladoke Akintola University of Technology, P. M. B. 4000, Ogbomoso, Oyo State, Nigeria
eDepartment of Chemical Engineering, Federal University of Technology, Minna, Nigeria
fSchool of Engineering, University of Aberdeen, Scotland, UK
gSchool of Biotechnology and DCU Water Institute, Dublin City University, Glasnevin, Dublin, Ireland
hSt. Peter's College Muenster, Saskatchewan, Canada. E-mail: jude.okolie@usask.ca
First published on 17th June 2022
Abstract
Hydrogen is considered the fuel of the future due to its cleaner nature compared to methane and gasoline. Therefore, renewable hydrogen production technologies and long-term, affordable, and safe storage have recently attracted significant research interest. However, natural underground hydrogen production and storage have received scant attention in the literature despite its great potential. As such, the associated formation mechanisms, geological locations and future applications remain relatively under-explored, thereby requiring further investigation. In this review, the global natural hydrogen formation along with reaction mechanisms (i.e., metamorphic processes, pyritization and serpentinization reactions) as well as the suitable geological locations (i.e., ophiolites, organic-rich sediments, fault zones, igneous rocks, crystalline basements, salt bearing strata, and hydrocarbon-bearing basins) are discussed. Moreover, the underground hydrogen storage mechanisms are detailed and compared with underground natural gas and CO2 storage. Techno-economic analyses of large-scale underground hydrogen storage are presented along with the current challenges and future directions.
1. Introduction
The demand for hydrogen has increased tremendously recently due to its application in several industries. Hydrogen can mainly be used in heavy oil and conventional petroleum upgrading, ammonia production, hydrogenation, metallurgical industries and hydrodesulfurization.1 Additionally, hydrogen is preferred as an energy carrier and a promising alternative as sustainable fuel due to its renewability and environmentally friendly properties. Moreover, the combustion of hydrogen releases water vapour, and its mass calorific value (141.9 kJ g−1) is three times higher than that of gasoline (47 kJ g−1) and 2.6 times greater than that of natural gas (54 kJ g−1).2 Compared with other fuels such as ethanol and natural gas, hydrogen is lighter.3 On the other hand, the volume calorific values for hydrogen, gasoline and natural gas are 12.7, 34.2 and 40.6 MJ m−3, respectively. Thus, more space is required to store the same amount of energy as gasoline or natural gas. Although the energy required to produce hydrogen can be higher than its energy yield, it is still considered an efficient energy carrier.4–6Despite being promising, hydrogen faces several challenges in production and storage.3 Significant quantities of hydrogen produced today are from steam methane reforming of natural gas.7 A process that is not environmentally benign and generates tons of greenhouse gases. Moreover, hydrogen can also be produced from renewable sources such as biomass gasification,8 water splitting,9 dark fermentation,10 and the water–shift reactions in syngas fermentation processes.11
As noted earlier, storing hydrogen cheaply and safely is very difficult. Additionally, the onboard hydrogen storage in vehicles is another bottleneck because of the stringent requirements in its storage. Currently, hydrogen is primarily stored in the gaseous or liquid form in pressurized or cryogenic tanks.12 However, these technologies are insufficient to meet the requirements of large-scale storage. Therefore, there is a need to develop cost-effective, reliable, and safe storage systems to foster the development of a hydrogen economy. For broader context, the pros and cons of surface and subsurface hydrogen storage methods in practice are elucidated in Table 1. However, surface storage technologies are beyond the scope of this present work.
| Underground hydrogen storage5,13–18 | Compressed gas storage19 | Liquid hydrogen storage19 | Metal hydride-based storage19 | Carbon-based storage19 | Shallow-depth buried pipe storage20,21 | |
|---|---|---|---|---|---|---|
| Pros | Lower fire risk factors | Matured, established, and low-cost technology | Matured, established, and low-cost technology | Solid-state storage is characterized by good design flexibility | Lightweight, low-cost, and affords high storage density | Minimal contaminants/impurities will accumulate in the stored hydrogen during standard operations |
| High energy density: 250 W h L−1 | It can last up to 20 years | They are typically considered safe | Intermediate energy density: 125 W h L−1 | It can last up to 50 years | ||
| It can last up to 50 years | ||||||
| Cons | Risk of hydrogen-consuming reactions and damage to the storage infrastructure under certain conditions | Limited capacity at typical operational pressures | It can be expensive | Relatively high-cost technology | Still in technological infancy | The maximum pressure is ∼100 bar. Shallow depths limit them |
| Still in technological infancy | Low energy density: 85 W h L−1 | High risk of hydrogen loss | Requires cooling circuit during filling | Must be developed to improve reversibility and discharge rates | Highly susceptible to thermal expansion effects during injection and withdrawal, increasing the risk of structural damage20 | |
| Further development is still required to advance high-pressure (>700 bar) storage. Existing technology is relatively expensive | Liquid hydrogen production is energy intensive | Not lightweight and can degrade over time |
Underground hydrogen storage in geological formations could be a cheap and environmentally friendly medium- and long-term storage route. Hydrogen can be stored underground in different layers such as aquifers, porous rocks, and salt caverns.22 It should be mentioned that salt caverns do not exist naturally. Instead, they are artificial cavities in underground salt formations, that are created by the controlled dissolution of rock salt through water injection during the solution mining process.23 Although underground hydrogen storage is similar to natural gas storage and has been demonstrated in salt caverns in the USA and the UK, challenges such as the selection of geological structures, process hazards and economics, and legal and social implications could hinder its commercial application. These challenges have been well documented in a previous study by Tarkowski and Uliasz-Misiak.24 In another study, the same authors reviewed the barriers hindering the large-scale utilization of underground hydrogen storage.25 Factors such as increasing cost of CO2 emission allowances and declining “green hydrogen” costs are critical considerations for large-scale implementation of underground hydrogen storage.
Natural H2 has been discovered in many locations worldwide, including Oman, New Zealand, Russia, Philippines, Japan, China, and the Italian and French Western Alps10,26–28 (with a cumulative yearly emission rate of 23 Tg)29. Its occurrence has been investigated and surprisingly discovered in some continental wells in Mali,30 during hydrocarbon exploration in Kansas,31 and in subsurface rocks and mines.32 H2 seepages have also been realized in sedimentary basins in Russia,33 Brazil34 and near the San Andreas Fault in California.35,36 If discoveries of natural H2 accumulations are exploited, it holds considerable potential as a key element in the energy mix either as a chemical raw material or as an energy carrier/fuel for transport. Nikolaidis and Poullikkas37 demonstrated that all current methods of H2 production are still too expensive to compete with fossil fuels favourably. Thus, natural H2 discovery will likely yield a cost-effective improvement to the hydrogen economy. Moreover, combining natural H2 discovery with underground storage could help mitigate the challenges of H2 production and storage.
Several studies have reported the prospects and possibilities of storing hydrogen in underground formations.5,38,39 Panfilov et al.38 outlined the technical challenges of underground hydrogen storage. Tarkowski39 presented the possibility of storing hydrogen in underground deposits in Poland. In another study, the mechanisms of underground hydrogen storage and the process feasibility were comprehensively documented.4 Despite the prevalence of underground H2 storage in literature, a comprehensive review that comparatively discusses the prospects of artificially stored and naturally-occurring hydrogen is scarce. The present study provides a complementary overview of the necessary deployment and formation mechanisms involved in both scenarios, with insights into exploring and extracting this vital resource. It is hoped that the provided recommendations provided herein will not only inform researchers on the knowledge gaps to focus on in the coming years but also guide future large-scale investments in these technologies.
2. Underground hydrogen storage in geological structures
Underground hydrogen storage (UHS) is a promising route to addressing the demand-supply gap caused by the characteristic fluctuations of renewable energies. By exploiting the high specific energy (i.e., stored energy by mass) of hydrogen, the surplus generated energy can be readily converted to hydrogen and stored underground as a buffer for subsequent surges in demand.4 Additionally, the UHS concept can offer additional safety advantages with respect to conventional supra-surface storage alternatives because it limits contact of the stored hydrogen with atmospheric oxygen (for example, in aquifers).4,5Robust global technology road-mapping efforts highlight the potentials of UHS for addressing global energy- and emissions-related challenges.14,40 Further demonstrating the timeliness and relevance of UHS technology is the fact that several projects over the last decade (e.g., H2STORE,41–43 HYSTOREPORT,16,17,44 HyUnder,45 InSpEE46–49 and HyINTEGER50) have focused on investigating different aspects of geological hydrogen gas storage and utilization. Moreover, it has been suggested that the reliability and cost-effectiveness of global energy systems should have improved sufficiently by 2050 to facilitate 17–22 TW h of annual subsurface hydrogen storage.51
While the properties of hydrogen as a gas in its pure state are more or less understood, hydrogen within multiphase systems such as in porous media is highly complex and is still in research infancy.17 Moreover, the effects of robustness under cyclic loading and overall reversibility have been shown by Pfeiffer and Bauer52 by using simulations of porous media hydrogen storage that under optimal conditions, extraction rates do not attenuate over four consecutive cycles. The authors remarked that further improvements in storage performance could be realized by adopting an optimized injection scheme to decrease the pressure levels, improving well injectivity. The importance of geological models cannot be understated.
In recent years, depleted gas/oil reservoirs, aquifers, and artificial underground cavities (such as salt and rock caverns) have been the subject of research attention for UHS.4–6,17,39 These geologic formations have attractive attributes, which include but are not limited to: (i) good gas tightness; (ii) high wall (sealing) thicknesses compared to tanks for conventional storage; and (iii) extensive subsurface depths, which can minimize the risks posed to safety.
It is worth noting that UHS can benefit from the technological maturity of the geologic storage of natural gas and CO2, which are associated with decades of established knowledge. However, H2 is invariably more chemically, biologically, and microbially reactive, which presents unique challenges that are yet to be fully understood.4,14
Compared with natural gas storage, hydrogen storage in porous media (either aquifers or depleted reservoirs) similarly requires suitable geological structures such as well confined porous and permeable formations bounded by impermeable cap rock or seal to accommodate (accumulate) hydrogen safely at minimal losses.4,53 Apart from the differences in the extraction (withdrawal) frequency of the gas, which is frequent in the case of hydrogen storage, most of the existing knowledge of hydrogen storage, especially in underground systems, has been learned from the natural gas storage experiences despite the different physiochemical properties of hydrogen in comparison with natural gases.4 Leakage issues and loss of hydrogen are more common and severe in UHS than in natural gas storage (NGS) due to the lower density, viscosity, and molecule size of hydrogen.54 In comparison to natural gas, the flow rate of hydrogen should be higher to avoid diffusion in porous media because of its lower viscosity and increased mobility. Wellbore of larger size may mitigate this issue.4,54
Similarly, carbon-geo storage (CGS)) requires the injection of CO2 into porous geological formations located at least 800 m under the Earth's surface to realize pressures and temperatures to attain a liquid or supercritical phase.55,56 At the CO2 storage site, CO2 is injected under pressure into the geological formation and once injected, it moves up through the storage site until it reaches an impermeable layer of rock called cap rock, overlaying the storage site, which traps the carbon dioxide in the storage formation. This storage mechanism is called “structural storage”. It is the primary storage mechanism equivalent to the same process that has kept oil and natural gas securely trapped under the ground for millions of years.55,57,58
However, from the literature, a higher amount of CO2 can be stored in the same structural volume compared to hydrogen.57,59 CO2 storage is intended to be a long-term and permanent storage known as CO2 sequestration. The CO2 storage operation is not cyclic, due to the characteristic absence of the withdrawal stage to meet the main goals of CO2 storage – its removal from the atmosphere.56,59 The co-production of CO2 with other existing fluids in the porous media is not a concern in the case of CO2 storage; besides, the problems such as withdrawal rates, number of cycles, and idle time between injection/withdrawal, are not encountered in CGS operation. Unlike UHS, different aspects of leakage risks from the caprock and operation facilities, and other failures resulting from corrosion need to be considered, in the case of CGS. More so, CO2 is prone to reactions with rock and in situ fluids that promote the dissolution of the caprock minerals, further increasing the risk of leakage.60,61
According to Pan et al.,59 the benchmark data from CGS and NGS projects are more often used to estimate or predict the behaviours of H2 or the occurrences in underground hydrogen storage (UHS) reservoirs. However, relying entirely on such a benchmark may be misleading since CO2, H2, CH4 and other hydrocarbon fluids exhibit different physical and chemical properties.59 Although it is an economic requirement to compare the existing data to assess any means of meaningful extrapolations and avoid serious pitfalls, it should be noted that only solid properties such as absolute permeability (Ka) and effective porosity (ϕeff) show a consistent trend among NGS, CGS and UHS. Fluid properties such as density (ρ), viscosity (μ), and fluid–fluid interfacial tension (γFF), and solid–fluid interactions such as capillary pressure (Pc), relative permeability (Kr), mobility ratio (M), adsorption–desorption and chemical reactions are significantly different. These differences imply that conclusions drawn from CGS and NGS reservoirs cannot be used directly in UHS.62–64 Knowing that there are some subtle differences, as stated, calls for more detailed studies on the possible impacts and economic viability before embarking on UHS projects. Fig. 1 is a schematic representation showing NGS, CGS and UHS.
 | ||
| Fig. 1 Illustrations of viable geologic formations and schematic representations for (a) general underground storage of various substances (including H2) in depleted hydrocarbon deposits, salt formations and aquifers;65 (b) natural gas, liquid hydrocarbons, compressed air, hydrogen, and brine storage in salt caverns (showing cross-section through a salt dome);66 (c) carbon geo-storage (CGS);58,67 (d) integrated CO2 and CH4 storage.68 | ||
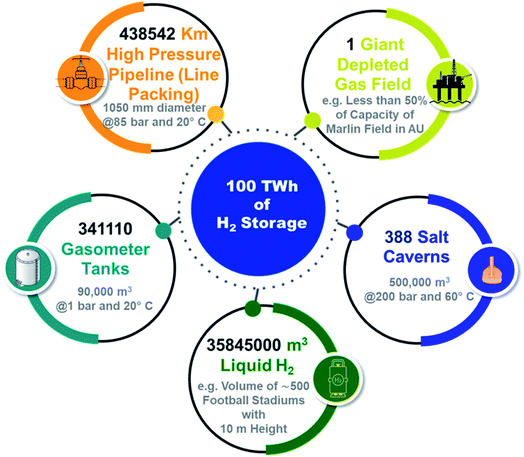 | ||
| Fig. 2 Volumes of different media require to store 100 TW h of H2 energy. The equivalent H2 storage volume of 1 depleted reservoir is 388 salt caverns.72 | ||
Typically, the process requires the pre-injection of cushion gas (e.g., nitrogen or methane) before H2 injection. Cushion gas can readily expand and be compressed during injection and extraction cycles to maintain the desired hydrogen pressures and flow rates without being consumed. As such, it constitutes a critical part of subsurface H2 storage.4 Already, its importance has been demonstrated for natural gas storage, where methane as the working gas can be injected and utilized without the extraction of cushion gas, which is maintained to provide pressure support.55
Since natural gas is denser than hydrogen; the storage of hydrogen gas of the same mass requires more pressure, which in turn influences the storage capacity.69 However, a higher amount of CO2 can be stored in the same underground storage site compared to hydrogen because of its density, compressibility, and solubility.13,56 A lower amount of H2 can be stored in caverns, aquifers and depleted reservoirs than CO2. As hydrogen is less dense than natural gas, the storage of hydrogen gas of the same mass often requires more pressure. This point raises the importance of storage capacity during a hydrogen storage operation.13,69 Given the storage efficiencies of the NGS, CGS and UHS processes, a comparative overview based on location suitability, leakages, in situ reactions, displacement dynamics, water production during extraction, contaminants or purity issues, environmental issues, and costs is presented in Table 2. The interested reader may also consult the studies of Tarkowski et al.39,56,57,70 for a more elaborate comparison.
| Factors | Natural gas storage (NGS) | Carbon geo-storage (CGS) | Underground hydrogen storage (UHS) |
|---|---|---|---|
| Types of underground storage options | Depleted gas/oil reservoirs, aquifers, and salt caverns56 | Depleted gas/oil reservoirs, aquifers, and salt caverns56 | Depleted gas/oil reservoirs, aquifers, and salt caverns14,56 |
| Storage adequacy | Caverns: favourable71,72 | Caverns: favourable73,74 | Caverns: very favourable75,76 |
| Aquifers: least favourable71,72 | Aquifers: very favourable73,74 | Aquifers: least favourable75,76 | |
| Depleted reservoirs: very favourable (Fig. 2)71,72 | Depleted reservoirs: favourable73,74 | Depleted reservoirs: favourable75,76 | |
| Location suitability | Some restrictions in geographical locations for storage options6,69,77 | There are fewer restrictions on geographical locations for all storage options6 | Restrictions in geographical locations and capacity exist for cavern storage69 |
| Leakages | Problems of leakage are minimized due to its high molecular weight, and high density making it less diffusive through overburden layers as compared to hydrogen78,79 | CO2 is more viscous and denser than H2; hence may experience less leakage. However, the risk of corrosion of the downhole facilities when they are in contact with CO2 (corrosive in nature) can increase the possibility of subsequent failures and consequent leakage78,79 | Leakage of hydrogen to the surface is likely to happen due to its low molecular weight and low density, making it highly diffusive through overburden layers as compared to CO2, or CH4. Additionally, H2 embrittlement may ensue in wellbore casings, causing corrosion and leading to further leakages78,79 |
| In situ reactions | Fewer reactions with in situ fluids15 | CO2 often reacts with rock and in situ fluids that promote the dissolution of the caprock minerals, which further increases the risk of leakage38 | Biochemical reactions can be considerably challenging for porous rock UHS, particularly where sulfate-reducing bacteria, methanogens and homoactogens are prevalent15,38,44,80 |
| Displacement dynamics | Experiences less hydrodynamic displacement due to high density and low mobility compared to H2 (ref. 53) | More viscous and low mobility relative to H2 (ref. 53) | Low viscosity and high mobility, which lead to unusual hydrodynamic (unfavourable displacement) behavior such as fingering, gas rising, and overriding53 |
| Water production during extraction | There is a relatively low occurrence of the water-coning phenomenon. This occurs when water overrides gas and breaks through the production well due to the dominance of viscous forces over gravity force in the reservoir – happening above a critical extraction rate70 | There is a relatively low occurrence of water-coning phenomenon due to the dominance of viscous force over gravity force in the reservoir happening above a critical extraction rate70 | There is significant water production during hydrogen extraction from porous rocks. The amount of hydrogen extraction and associated water production increase with flow rate70 |
| Contaminants or purity issues | There are many pure methane storage projects both in porous formations and in salt caverns; however, practical experience with pure hydrogen storage is still limited81 | There are already well-established pure CGS facilities given that CGS has attained a relatively advanced stage of development70 | Most UHS projects are operated with mixtures of other gases. There are limited pure UHS facilities. The presence of impurities may hamper the feasibility and economy of the withdrawal stages and should be removed from the withdrawn gas stream52,70 |
| Cost and economic viability | Minimal cost compared to CGS56,58 | In addition to the capital, operational, etc. costs, corrosion inhibitory and leakage control costs usually add up to increase the cost of CGS compared to UHS58 | Moderate storage costs compared to CGS and NGS.58 Although further studies are required to assess the economic viability of the process |
| Environmental issues | Less environmental issues58 | Environmental corrosion of the downhole facilities and other materials in contact with CO2 (ref. 58) | No insurmountable or environmental problems with the storage of hydrogen in naturally formed underground structures58 |
2.1. Mechanisms
The primary mechanisms by which UHS can be realized are believed to be associated with diverse phenomena, including hydrodynamics, geochemical, physiochemical, biochemical and microbial reactions.4,6,38 Thus, further research and development in the area of UHS require a detailed understanding of the mechanism involved over time and with operational cycles.4Presently, some of the main challenges limiting the advancement of the UHS concept are linked with hydrogen flow behaviour in reservoirs, understanding geochemical reactions occurring during and after the injection process, microbial hydrogen-consuming interactions, and of course, the implications of storage on the geomechanical characteristics of the formation under consideration. Other major bottlenecks stem from restricted locations, limited capacity, and assuring tightness in geological formations.4,40 Ultimately, the applicability and development potentials of the UHS concept are dependent on the properties of the geologic structure.
Owing to this reactivity, toxic and corrosive gases can form. These can have deleterious effects on the wellbore, soil and atmosphere14 and are thus, particularly important when considering the sustainability of UHS systems and the longevity of well materials.14,51,82 For rock caverns, auxiliary components such as lining, pipework and compressors are often steel-based and can be compromised due to the inherent vulnerability of steel to hydrogen-induced embrittlement.80 Moreover, identifying the condition-specific susceptibilities of specific sites to hydrogen consumption via sulfate reduction, methanogenesis and homoacetogenesis cannot be undermined.44 Some of the most commonly encountered classes of microorganisms can consume up to 4533 nM hour−1 of hydrogen.17 Thus, site selection is a precarious exercise to account for these factors, often necessitating retrofitting exercises in the case of existing rock cavern storage sites,80 and avoiding sulfate-, carbonate-, and sulfide-rich geological formations, which are typically undesirable for UHS.14,15 Conversely, iron-rich formations should ideally be favoured.15
In terms of microbial interactions, many factors are still relatively unexplored and not fully understood; as such, more studies are needed, focusing on the development of robust predictive methods for microbial proliferation and hydrogen consumption across a broad range of geological hydrogen storage systems. Notably, more work on determining critical conditions (salinity, temperatures, pressures, etc.) can be highly insightful from a microbial interaction perspective.44 Nonetheless, some early works in this regard have shown that methanogens, sulfate reducers, homoacetogens and iron(III)-reducing bacteria all have optimum pH of 6–7.5. For the same microorganisms, optimum salinities are <60, <100, <40 and <40 g L−1, respectively. Finally, the optimum temperatures for sulfate reducers and homoacetogens are reported to be between 20–30 °C, while the ranges for methanogens and iron(III)-reducing bacteria are 30–40 °C and 0–30 °C, respectively.17
Owing to the low density and viscosity of hydrogen compared to other fluids, two unfavourable phenomena relate to the displacement efficiency during mixing upon injection. These are gravity override and viscous fingering (Fig. 3). Despite facilitating passive separation of the hydrogen gas from denser fluids such as cushion gas, the former can cause an accumulation of hydrogen gas above denser fluids (e.g., in aquifers), which renders it vulnerable to losses.16 The latter, which mainly occurs in the presence of native and cushion gases, can cause the hydrogen gas to extend beyond the desired displacement envelope of the well (typically anticlinal) and amplify other loss mechanisms. It is worth noting that in porous storage media, the low viscosity of hydrogen can favourably facilitate mobility and mixing.16,83 Ultimately, strategic injection methodologies can effectively yield stable displacement for UHS to address these physical issues.84,85
 | ||
| Fig. 3 Graphical illustrations of viscous fingering and gravity override phenomena. Note: MZ* indicates the mixing zone. Reproduced from ref. 16. | ||
Hassanpouryouzband et al.16 remarked on the effects of changing temperatures and pressures within a reservoir on the mineral composition of formation fluids over time. A minuscule amount of hydrogen dissolution (into the fluids) can occur upon injection; with chemical disequilibrium, the hydrogen may become contaminated with water vapour. Residual trapping of a small fraction of hydrogen can take place, driven by capillary forces, and clay mineral surface adsorption can also occur. These events can trigger mineral dissolution and progressively compromise the reservoir and caprock tightness. At elevated temperatures and in the presence of hydrogen, adverse geochemical reactions can occur, releasing highly toxic gases and altering the pH of the water within the reservoir. This can exacerbate any ongoing mineralogic dissolution. This is closely supported by Heinemann et al.,17 who suggests that hydrogen solubility in water can be significantly reduced under high-temperature and -salinity conditions, arguing that dissolved hydrogen does not directly affect the pH of the pore water.
Agreeing with previous studies, Hassanpouryouzband et al.,16 conclude that any mineral dissolution-driven reactions within the reservoir are, at best, indirectly affected by hydrogen with native chemical constituents of the formation fluids. These works highlight the complex interplay of the operational conditions and the formation geochemistry, warranting extensive exploration of hydrogeochemical interactions and effects for UHS. More importantly, both studies identify effused gases (e.g., H2S) from the aforementioned adverse reactions as key contaminants, which affect the quality of the stored hydrogen and induce unfavourable fluid–rock reactions; these constitute high risks from corrosion flammability and toxicity perspectives. This is also well supported by Hemme and Berk15 who, by modelling the losses of hydrogen resulting from bacterial and hydrogeochemical interactions, identified that bacterial-driven losses were governed by the availability of co-injected CO2 and native sulfate within the reservoir. Unsurprisingly, the authors also found a correlation between storage duration and loss, reporting a greater risk for hydrogen losses over more extended storage periods. However, owing to higher co-injected CO2 with successive injections, cumulative losses can be more significant due to a higher propensity for methanogenesis. The authors conclude that safer storage conditions can result after years of storage due to mineralogic attenuation over time with the consumption of anhydrite and calcite; bacterial sulfate reduction and methanogenesis are slowed due to the limited diffusion of sulfate and CO2.
A qualitative overview of the comparative attributes of geological formations for UHS is presented in Table 3. Note that where values are reported for storage options that are yet to be proven or under investigation, the interested reader may refer to the cited literature for further details.
 : poor;
: poor;  : fair;
: fair;  : good;
: good;  : very good;
: very good;  : site-specific;
: site-specific;  : low;
: low;  : moderate;
: moderate;  : high
: high
| Salt caverns | Rock caverns | Depleted reservoirs | Aquifers | |
|---|---|---|---|---|
| Safety |
 20,40
20,40
|
 20,40
20,40
|
 20,40
20,40
|
 20,40
20,40
|
| Gas tightness |
 20,56
20,56
|
 20
20
|
 20,56
20,56
|
 20,56
20,56
|
| Relative investment cost |
 20
20
|
 20
20
|
 20
20
|
 20
20
|
| Relative operational cost |
 20
20
|
 20
20
|
 20
20
|
 20
20
|
| Injection rate (kg h−1) |

|

|

|

|
 20
20
|
 20
20
|
 20
20
|
 20
20
|
|
| Withdrawal rates (kg h−1) |

|

|

|

|
 20
20
|
 20
20
|
 20
20
|
 20
20
|
|
| Working gas capacity/total gas capacity (%) |

|

|

|

|
 80
80
|
 80
80
|
 80
80
|
 80
80
|
|
| Feasible cycles per annum |
 80
80
|
 80
80
|
 80
80
|
 80
80
|
| Depth (m) |

|

|

|

|
300–1800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20,80 20,80 |
114–1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20,80 20,80 |
300–2700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20,80 20,80 |
400–2300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20,80 20,80 |
|
| Operating pressure (bar) |

|

|

|

|
35–270![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20,80,86 20,80,86 |
10–230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20,80 20,80 |
15–285![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20,80 20,80 |
30–315![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20,80 20,80 |
|
| Suitability for hydrogen | Proven,80,87,88 TRL: 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
First UHS trial in development to be commissioned 2022,80 TRL: 5–6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
Still under investigation. Proven for town gas with up to 50% hydrogen,80 TRL: 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
Proven for H2–CH4 blends with up to 10% H2. Pure H2 under investigation,80 TRL: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80,86 80,86 |
| Key factors & considerations | Salt domes are preferred over bedded salt formations | Metamorphic/igneous rock | Operational considerations; formation fluid and rock composite and microbial activity | Operational considerations; formation fluid and rock composite and microbial activity; for new developments: gas tightness |
| Current locations (operator, start date) | UK: Teesside (Sabic Petrochemicals, 1972) | None for hydrogen; established use with natural gas and air | None for hydrogen; established use with town gas and natural gas | None for hydrogen; established use with natural gas |
| USA: Clemens dome (Conoco Philips, 1983) | ||||
| Spindletop (air Liquide, 2016) | ||||
| Moss Bluff (Praxair, 2007) |
2.2. Important considerations
With the evolution of ongoing studies towards demonstrating the feasibility of the four main geologic hydrogen storage options, a myriad of critical factors must be considered, which may have wide-reaching implications on the future of UHS exploration and utilization. Thus, some of these vital considerations are summarised herein in addition to the review of research contributions in this area.• Caglayan et al.88 have argued that more attention should be paid to technical storage potential analysis and that land eligibility plays a massive role in the availability of viable sites for exploration. Stringent site exclusion criteria must be applied based on criteria such as proximity to urban and rural areas; major fault zones; land and transport infrastructure such as railway, major roads and gas pipelines; and natural protected areas and water bodies. Moreover, the authors demonstrate the importance of careful design and site specification, which must also account for geotechnical safety factors such as lithostatic pressure.
• Despite the marked favourability of salt caverns over other geological formations, the risk of hydrogen-consuming reactions can increase in the presence of thermophilic, salt-loving microbes.75
• However, the success of UHS in salt caverns will also chiefly depend on the availability of cavern-leaching water supply and appropriate brine disposal schemes.5
• Residual carbon-based species within depleted oil/gas reservoirs can have unfavourable interactions with stored H2.
• Besides the criticality of the leak tightness of geological formations to their successful application, site-specific safety factors such as inherent susceptibility to seismic activities must be carefully assessed.75 Additionally, site evaluations may require robust and accurate hazard predictions.5
• Limited availability of short-, mid-and long-term data may significantly inhibit site selection and performance assessment.
• Iron-rich formations can be considerably more beneficial, whereas sulfate-, carbonate-, and sulfide-rich formations should typically be avoided.
• As indicated in Table 3, while porous structures have specific dimensions, in the case of salt caverns, their volume depends primarily on the needs specified by the investor and the geological and mining conditions in the rock salt deposit.
Finally, despite the many research advancements in the field of underground H2 storage, it is evident from the presented discussions that many influencing factors on the viability of UHS are still unresolved. In fact, a review by Tarkowski5 argued that this technology is unlikely to be a feasible one to be practically adopted in the near future. Geological, technological, legal, economic and social factors were cited as the obstacles to its full-scale implementation. Furthermore, it was pointed out that the future potential for lowering hydrogen's production cost via electrolysis will be a significant influencing factor on the applicability of UHS on an industrial scale. However, the success of salt cavern UHS in the USA and UK would suggest long-term viability. Notwithstanding, in light of these points, we present the feasibility of natural H2, as a complementary alternative with a strong potential to ameliorate the effects of increasing energy demand in Section 3.
3. Natural occurrence of hydrogen
In this study, natural hydrogen refers to the occurrence of H2 within the earth, independent of human activity. In contrast, we use the acronym UHS to illustrate deliberate efforts made by mankind to store H2 in underground/subsurface formations.3.1 Mechanisms
Hydrogen may be formed by the contact of water with rock surfaces containing radicals and radioactive elements like uranium and thorium (water–rock interactions, e.g., cataclasis, radiolysis). Furthermore, the decay of dissolved or solid organic matter by thermal maturation is another formation mechanism; however, this requires significant burial depth to initiate the processes. The relatively low thicknesses of some sedimentary basins over which H2 has been discovered make this mechanism unlikely.35 Many bacteria (e.g. Escherichia coli and Clostridium pasteurianum) are also capable of generating energy via H2 oxidation.89,90 Nevertheless, H2 produced in this way can also be rapidly consumed by soil enzymes and methanogenic bacteria,91 thus yielding low overall concentrations. Desorption from subsurface rocks is also a possible mechanism governing H2 generation (particularly in seepages).34 It is worth mentioning that these processes may occur in ophiolites, organic-rich sediments, fault zones, igneous rocks, crystalline basements, salt bearing strata, and hydrocarbon-bearing basins.92,93 The many occurrences of this resource worldwide are a strong indication of a deep-seated origin. The geological location and suggested formation mechanism of the global natural underground hydrogen occurrence with a minimum 40% concentration is presented in Table 4. Additionally, Table 5 summarizes some of the hypotheses proposed in recent contributions.| Reference | Measured H2 (%) | Place & country | Geological location & formation mechanisms |
|---|---|---|---|
| Smith et al.,93 Lollar et al.94 | 57.8 | Sudbury, Canada | Water bodies (radiolysis and hydration reactions) |
| Morrill et al.95 | 50.9 | Camp Spring, USA | Ophiolites (serpentinization of cedar via shallow and deep water sources) |
| Vacqand96 | 97 | Bahla, Oman | Ophiolites (gas seeps from the surface – serpentinization) |
| Etiope et al.97 | 48.3 | Vaiceva Voda, Bosnia and Herzegovina | Ophiolites (serpentinization in water-free or unsaturated rocks hosting metal catalyst) |
| Coveney et al.98 | 96.3 | Hoffman, USA | Rift zone (abiogenic origin with reactions involving Fe2+) |
| Zgonnik29 | 80.4 | Iriklinskoe, Russian Federation | Igneous rocks (−) |
| Angino et al.99 | 80.5 | Nizhny Tagil, Russian Federation | Igneous rocks (−) |
| Sakai et al.100 | 57.3 | Namafjall, Iceland | Rift zone (reaction between reduced carbon and water in the magma) |
| Huntingdon and Sato101 | 57.8 | Etna, Italy | Volcanic gases (fumaroles) |
| Ward102 | 68.6 | Penneshaw, Australia | Precambrian rocks (−) |
| Symonds et al.103 | 51.5 | Augustine, USA | Volcanic gases (shallow crustal sedimentary rock) |
| Guelard et al.31 | 91.8 | Kansas, USA | Precambrian rocks (deep-seated H2: Water reduction associated with Fe oxidation; reactions occurring in the tubing attributed to high content of reduced iron) |
| Prinzhofer et al.30 | 98 | Bourakebougou, Mali | Sedimentary rocks (abiotic origins, associated with neo-Proterozoic sediments) |
| Nakamura and Maéda104 | 51.4 | Arima, Japan | Geysers and hot springs (−) |
| McElduff105 | 100 | Cyprus | Orebodies (chromites as podiform bodies in the mantle) |
| Dubessy et al.106 | 100 | Oklo, Gabon | Orebodies (−) |
| Angino et al.99 | 61.5 | Muhlhausen, Germany | Salt deposits (mixed origins) |
| Wood107 | 75.8 | Poison Bay, New Zealand | Sedimentary rocks (serpentinization reactions) |
| Молчанов108 | 81 | Pechora, Russian Federation | Coal basins (−) |
| Молчанов108 and Zgonnik29 | >50 | Wittelwheim, France | Salt deposits (−) |
| Reference | Hypothesis |
|---|---|
| Larin et al.109 | Primordial origin, with subsequent global degassing of H2 from deep down the earth into several sedimentary structures |
| Shcherbakov and Kozlova;110 Toulhoat et al.111 | Originally H2-enriched earth's interior |
| Sugisaki et al.112 | Degassing of the earth's mantle |
| Isaev et al.113 | Significant H2 concentrations in the earth's core |
| Freund et al.;114 Larin et al.33 | Bacterial activity within deep aquifers in sedimentary formations, in the presence of organic matter substrates |
| Smith115 Charlou et al.116 | Water hydrolysis (including water radiolysis, electrolysis, cataclasis, and ferrous metal oxidation) |
| Zgonnik et al.35 | Decay of organic matter via thermal maturation |
| Decomposition of methane and ammonia at temperatures above 600 °C during metamorphism | |
| Takai et al.117 | Serpentinization (a process by which ultrabasic rocks are oxidized by water into serpentine, with H2 produced) – water contact with reducing agents in the earth's mantle |
One of the earliest studies documenting the origin of natural underground hydrogen in the US demonstrated the abiogenic origin of H2 in 10 Kansas wells near the Mid-continent rift system.98,99 The average amounts of H2 ranged from 29–37 mole% H2, with the rest being mainly N2. The low concentrations of CO2 and CH4 (products of biogenic activity) led to the conclusion that Fe2+ oxidation (during serpentinization of ultramafites) is a more feasible explanation for molecular hydrogen's occurrence. Redox reactions of mafic minerals in Precambrian rocks were also suggested as a likely mechanism. However, direct outgassing from the earth's mantle was deemed a less likely mechanism because of the extremely high temperatures therein (unsuitable for H2 formation via serpentinization). This observation was also confirmed in the work of Zgonnik et al.35 Larin et al.33 reported the existence of subcircular structures (morphological depressions) harbouring hydrogen in the Russian part of the European craton (the Borisoglebsk–Novokhopersk area, Fig. 4f). In one of these structures, they estimated the daily hydrogen seepage at the surface of these structures to be between 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 27
000 and 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3. The highest H2 concentrations in these structures were obtained inside and along the border of the depressions; adjacent regions outside the structure's boundary did not yield a detectable amount of H2 gas. The observed depressions have been interpreted as the consequence of rock alteration along the migration pathways of subsurface H2. These are also prevalent in Azerbaijan and Latvia as shown in Fig. 4c and e, respectively.
000 m3. The highest H2 concentrations in these structures were obtained inside and along the border of the depressions; adjacent regions outside the structure's boundary did not yield a detectable amount of H2 gas. The observed depressions have been interpreted as the consequence of rock alteration along the migration pathways of subsurface H2. These are also prevalent in Azerbaijan and Latvia as shown in Fig. 4c and e, respectively.
 | ||
| Fig. 4 (a) Sensor positions for H2 concentration measurements in the circular depression of the San Francisco Basin (Brazil);34 (b) H2 concentration measurements in the Smith Bay area of North Carolina (USA), with solid circles illustrating the H2 concentration in ppm;35 (c) drilling for H2 in Latvia (with blue rectangle showing the drilling well location and the green rectangle showing a H2 degassing region);118 (d) circular depressions emitting high purity H2 in Mali, showing the concentration profile;30 (e) satellite images of local H2 sites in Azerbaijan formed above basaltic;118 (f) size distribution of H2 seepage depressions (rounded orange lines) in the Borisoglebsk–Novokhopersk area of the East European craton in Russia.33 | ||
The presence of H2 (98% purity) was confirmed in the Bourabougou field of Mali (Fig. 4d) after analyzing data from 12 exploratory wells in the region (8 km diameter).30 The produced H2 was utilized for electricity supply (via an internal combustion engine) to the nearby local village. This represents one of the 1st deployments of natural H2 for energy production. It was further concluded that the exploitation of 1 kg of natural H2 is within 2–10 times lower than that of manufactured H2. Significant concentrations of H2 have been detected in morphological depressions in North Carolina, USA (Carolina bays).35 The measurements from this study suggested that observed H2 concentrations are reflective of the complex fluid flow pathways for H2 gas from deep down the earth to the surface. The study was facilitated by a review of satellite images (in Fig. 4b) showing a high density of bays with varying dimensions and accessibilities. An estimation of the daily H2 flow from the considered bays was as high as 2700 m3 (Fig. 4b). The authors suggest a possible origin of the observed H2 – geochemical processes occurring under the sedimentary pile followed by migration of the produced gas to the surface. This pathway for gas migration causes gas–rock interactions that result in the formation of shallow pathways; this is similar to the conclusions derived from Larin et al.33
Bay-like H2 emitting features have been documented to sometimes occur along structural faults.33,119 These faults tend to act as fluid conduits (preferential migration pathways, due to their high permeability relative to the surrounding rock); they have been suggested as facilitators of H2 gas seepages observed in morphological depressions. The investigation of continuous H2 seepage in a circular depression in Brazil (Sao Francisco Basin, Fig. 4a) was studied as a function of space and time.34 The H2 emission profile obtained in this study follows temperature and irradiation curves. This indicated that H2 emission is likely correlated with an evaporation mechanism during soil evaporation. A daily recharge of H2 in soils was observed, indicating a source of H2 below the observable surface seepage. A similar observation was made in the San Andreas Fault area.35,36 Their results also demonstrate that the soil structure cannot only be considered a H2 sink, as shown in the work of Khdhiri et al.,120 but also a H2 emitter. Hundreds of soil gas measurements in Kansas, USA, also suggest that natural fractures are possible preferential channels for the vertical migration of H2.121
Arrouvel and Prinzhofer122 presented the main reactions responsible for the formation of H2via metamorphic processes. The authors conclude that pyritization and serpentinization are complementary reactions, which enhance H2 formation through an iron cycle. Pyritization refers to the replacement of a material by iron pyrites whereas, serpentinization is a process by which ultrabasic rocks are oxidized by water into serpentine, with H2 produced. Arrouvel and Prinzhofer122 also outlined the influence of this process for H2 production according to the equation shown below.
| Fe2O3(hematite) + 4H2S(g) → 2FeS2(pyrite) + 3H2O + H2 |
The mechanism of H2 generation is more likely a complex combination of several redox reactions, which involve water, sulphur and iron. Through simple thermodynamic calculations (evaluating Gibbs free energy as a function of depth), the authors provided evidence on the geochemical cycles of iron responsible for H2 production. Considerable H2 concentrations have also been observed in groundwater obtained from fractured rock samples in drilled wells located in South Africa. The highest concentrations were found in deeper and highly saline fractured aquifers.123
The commonly adopted hypotheses for H2 generation are that of water reduction through iron oxidation and the radiolysis of crustal rocks.122,124,125 Nonetheless, a combination of the highlighted processes in Table 3 may be responsible in some locations, and more research is required to prove and further quantify the respective contributions of the highlighted hypotheses. In all hypotheses, however, the formation and liberation of H2 are thought to be a continuous process. As pointed out by Prinzhofer et al.,34 the observed recharge was hardly buffered by the presence of water or bacterial activity. Furthermore, in continental Mali, the production of H2 has been active for 4 years, with wellhead pressures still suggesting continuous migration to the reservoir. Additionally, as previously shown in Table 4, ophiolites have received significant research attention because H2 from these locations has often been linked to the well-researched serpentinization process given in Fig. 5.
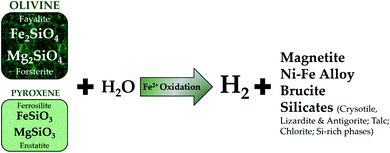 | ||
| Fig. 5 Serpentinization reaction of olivine and pyroxene for the production of H2, and various silicates, Ni–Fe alloys and oxides (adapted from ref. 126). | ||
3.2 Important considerations
The comprehensive review by Zgonnik et al.29 demonstrated that H2 exists in many more locations than currently identified. Commercial exploitation of this carbon-free resource would require the adaption of some lessons learned from the exploration and production of conventional hydrocarbon resources. We provide some key attributes of natural H2 systems, which are worth considering, as far as their exploitation is concerned.• H2 seepage from circular/elliptical depressions is a likely preliminary indicator for larger H2 deposits and thus further H2 exploration within these areas are imperative.
• The high porosity and permeability of most sedimentary or metamorphic rocks and the correspondingly high diffusivity of H2 may result in small H2 accumulations in these formations. However, carbonate rocks can absorb (up to 57 times more H2 than their original content) and retain it for days.29,127 Hence, these are also potential locations to look out for. Compared to a typical well-defined hydrocarbon system (composed of source rock, permeable carrier rock, and a structural trap/seal), this high H2 diffusivity makes defining a natural H2 system challenging.
• The highly diffusive nature of H2 also implies that it is unlikely to be retained in subsurface traps for extended periods; its reactive combination with O2 to give water facilitates its complete disappearance.64 Novel enhanced recovery methods might be required to localize its accumulation in a prospective region.
• The largest accumulations are thought to be found in the Precambrian basement,33,128 which is hardly the choicest location during hydrocarbon exploration and drilling campaigns. Furthermore, the gas's colourless, odourless, and non-toxic properties are possible explanations for its exploratory obscurity in previous drilling programs. Successful exploration campaigns would involve looking beyond sedimentary basins.
• Measured H2 concentrations in gas reservoirs have been observed to be 25 times less than H2 concentrations outside the reservoir's boundaries.129 It has also been reported that H2 concentrations are inversely related to hydrocarbon concentrations in an oil field.29 These occurrences may be an indication of H2 reaction with carbon-based fluids or materials. Thus, future H2 exploration may involve re-evaluating previously abandoned hydrocarbon wells or drilling new wells within their vicinity.
• The large spatial variation in natural hydrogen concentration may be problematic for exploration activities; however, advancements in deviated well drilling technology (via sidetracks as usually done in hydrocarbon drilling) will be particularly useful. Periodical changes (abrupt increases and falls34,112) would require continuous monitoring. Robust control schemes as already implemented in managed pressure drilling activities in the oil & gas industry will be very beneficial for safe drilling operations, as far as natural H2 is concerned.130
• H2 exploration & production will significantly depend on the accurate description of potential sealing horizons and high-permeability H2-conducting fracture zones. As such, the application of robust completion techniques will be vital.121
• It has been demonstrated that the upward migration of H2 through porous media saturated with water is a factor of 10 lower than hydrogen's flux through water. Thus, H2 migration could be retarded by aquifers.96 Therefore, water-saturated subsurface formations (if found in regions suspected to have H2) may act as good H2 traps.
• Since subsurface H2 is likely to be consumed by microorganisms, an accurate estimation of the H2 migration rate can be obtained only if the analysis is conducted below any zone/regions of biological activity.91 Thus, they should be accounted for during field development projects targeted at H2 discovery.
4. Complementary overview of underground hydrogen storage and natural hydrogen
Despite the prominent differences in the overall concept/philosophy of UHS and natural H2, there are considerable similarities in terms of their exploration, utilization and analytical methods (Fig. 6). For example, the significant presence of methanogens44,131 and homoactogens, which consume free H2 as an energy source, is essential in identifying potential storage formations and sites, which naturally harbor H2. On the other hand, the production techniques utilized for artificially stored H2 will probably be the same as natural H2. However, these will be affected by site-specific parameters, such as the reservoir pressure, permeability and porosity, which in turn determine the number type, geometry and length of wells. In addition, the difficult-to-store nature of H2 (ref. 56) implies that techniques which convert the gas to ammonia or formic acid would be beneficial to natural H2 and UHS projects. These chemicals can be easily transported in their liquid form and can be readily converted back to H2. Furthermore, identifying a proper sealing mechanism/subsurface rock capable of trapping the gas is crucial for both naturally occurring and artificially stored H2. Analytical methods capable of characterizing these effects are also likely to be the same (geological and engineering analyses of flow in porous media, as far as appraisal efforts and simulations of gas recovery are concerned).It is also worth emphasizing the similarities in the types of geological formations considered for artificial UHS and those in which significant natural H2 deposits have been realized, as evident in Fig. 7. Of all four candidates of artificial UHS concepts, salt caverns are ahead in terms of research and development, with four proven sites for pure hydrogen gas storage in the UK (Teesside) and the USA (Clemens Dome, Spindletop, Moss Bluff) demonstrating long-term storage (40–50 years) potentials. Perhaps, the gas–rock interaction in other subsurface formations where H2 is naturally occurring can provide further insights into prospective UHS geological locations. Furthermore, the fast diffusivity of H2 is an issue for subsurface geological storage and affects the extraction from natural or artificial sources through steel alloy pipes; also, the tightness level between connected drill pipes, applicable to conventional oil and natural gas production, may not be directly adaptable to H2 extraction systems.19–21,29,82,86,87
 | ||
| Fig. 7 Overview of proven salt caverns for UHS (USA: Moss Bluff, Clemens Dome, Spindletop, & UK: Teesside) denoted “A”; some potential salt caverns, denoted “◆”; salt deposits where natural H2 has been detected as free gases (>10% concentration); and some salt deposits across various countries. Figure produced based on the data obtained from the following ref. 19–21, 29, 51, 82, 86 and 87. | ||
Besides the technical considerations and challenges governing natural H2 and artificial UHS, the legal, social, environmental and economic aspects cannot be overlooked. As far as the legal requirements are concerned, land development activities of the storage site are expected to conform to national policies. In the UK, for example, it is likely that an open hearing/consultation is held for the public to view the development plan and express their concerns.132 Lessons can be drawn from the opposition posed by action groups in Yorkshire (UK), regarding the development of a natural gas storage cavern, following leakages observed in some underground storage areas.133 The potential for these leakages constitutes a significant environmental concern. It may also be argued that the exploitation of underground reservoirs may change the hydrological cycle from its natural condition.134 This in turn, may trigger adverse environmental effects, including nitrate accumulation in stored water.
A general overview of the economics of artificial UHS suggests that the required costs may be attributed to exploration (the cost of searching for viable storage sites), storage (the costs associated with transporting the gas to the desired subsurface formation and sealing it there), production/utilization (the costs incurred when the gas is extracted from the formation to the surface for energy generation), and transportation (the costs required to distribute the gas to locations, where it is needed). Conversely, only the exploration, production/utilization and transportation costs are applicable to natural H2 systems since no cost involvements are directly required for storage (if it is naturally occurring). Thus, when simultaneously considering long-term natural H2 and UHS projects, it is immediately apparent that additional operational costs will be incurred, with artificial UHS as a result of the double travel path (surface → subsurface & subsurface → surface) by the gas relative to natural H2 (which would only require H2 extraction – subsurface → surface). However, direct comparative analyses of these cost components for both systems are scarce in the literature. Also, the explorative costs for suitable storage sites have not been adequately quantified or reported in published literature. The explorative costs for UHS and natural H2 may be significantly different, despite the similarity in the factors (e.g. presence of seepages, permeability, porosity) considered during the search for viable sites. A more extensive seismic data collection and interpretation is likely to be the case for natural H2 exploration compared to UHS. The depth and configuration (vertical or deviated) of the wells required is also expected to contribute to this difference in explorative cost of both endeavors. Furthermore, a key difference between the production/extraction phases of UHS and natural H2 lies in the number of wells that will be required. It is expected that that the wells drilled during UHS will be readily applicable for its extraction. Although more extraction wells may be required with UHS, the exploratory wells drilled to prove the viability of a natural H2 deposit, will be insufficient for the full development of the discovered field. This difference is likely to make drilling cost of natural H2 exploitation twice as expensive as conventional UHS drilling or even greater, depending on the size of the hydrogen field. As far as H2 gas transportation is concerned, it has been reported that a 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg truck is required to transport only 300 kg of H2 gas – a very low transport efficiency.135 This paves the way for H2 liquefaction, which is very costly. The use of H2 pipelines appears unlikely until it has gained significant penetration into the energy mix as determined by the distribution economics.
000 kg truck is required to transport only 300 kg of H2 gas – a very low transport efficiency.135 This paves the way for H2 liquefaction, which is very costly. The use of H2 pipelines appears unlikely until it has gained significant penetration into the energy mix as determined by the distribution economics.
As with natural gas, the exploitation of natural H2, can be considered to consist of the exploration phase (searching for natural H2 deposits); the appraisal phase (investigating the volume of natural H2 reserves); the development phase (installing drilling & processing equipment); the production phase (extraction of natural H2 from identified deposits) and the abandonment phase (uninstalling facilities when the field is deemed non-viable). Despite the differences between natural gas and natural H2 exploitation considerations, and the absence of economic data on natural H2 exploitation, the economics of both endeavours have considerable similarities in several aspects; thus, we briefly present information on the economics of shale gas development in the UK and try to draw insights which may be beneficial to natural H2.
According the methodology proposed by Ahmed and Rezaei-Gomari136 for subsurface shale gas extraction in the UK, an analysis of the economic feasibility of natural H2 production, may begin by establishing 3 candidate development plans, after which a probabilistic financial model can be utilised to generate a distribution of potential gas prices. Based on their study, the average gas well drilling cost, (a significant component of the capital expenditure), in the UK (Bowland shale development) has been estimated to be $17 MM. However, it is important to mention that this cost includes fracking related technical costs (up to 20% of the original well development and completion costs), which may not be incurred, when drilling for natural H2 (depending on the properties of the formation containing natural H2). Another component of the capital expenditure – the land acquisition costs, has been given a range of $6 M–$16 M acre−1 for Bowland shale development in the work of Acquah-Andoh.137
Similarly, in the work of Ahmed and Rezaei-Gomari,136 a fixed annual operating expenditure (OPEX) of $25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was applied, together with a 15% overhead. Whereas, the variable operating expenditure, was assigned a mean value of $1.5 per Mcf. These values were derived from the comprehensive report of shale gas exploitation in the UK, where a variable OPEX was obtained as £0.5 MM per Bcf, or approximately $0.7 per Mcf in addition to 2.5% of the CAPEX each year138 Again, as with the CAPEX, these values for the OPEX, account for fracking costs, which may not be incurred during natural H2 production.
000 was applied, together with a 15% overhead. Whereas, the variable operating expenditure, was assigned a mean value of $1.5 per Mcf. These values were derived from the comprehensive report of shale gas exploitation in the UK, where a variable OPEX was obtained as £0.5 MM per Bcf, or approximately $0.7 per Mcf in addition to 2.5% of the CAPEX each year138 Again, as with the CAPEX, these values for the OPEX, account for fracking costs, which may not be incurred during natural H2 production.
In addition to the above costs, gas processing costs may be incurred to raise the purity of produced natural H2 to the desired levels. Most natural H2 discoveries have been accompanied with gases such as CH4, CO2, N2 and He. Membrane separation,139 pressure swing adsorption and cryogenic distillation technologies may be employed to achieve efficient separation. However, it is worth pointing out that the necessity of such separation and the degree to which it is applied depends on the geological location of the discovered H2 resource. For example, the discovery in Mali (98% H2 concentration) may be directly utilised for energy generation via internal combustion without this extra processing step. As far as the exploitation of natural H2 is concerned, currently identified seepages worldwide, may constitute locations for initial exploration, as conventionally done with oil and gas exploration. Nonetheless a deliberate exploration attempt has to made, if this resource is to be tapped, particularly because a majority of the currently highlighted discoveries were accidental. In some countries, H2 is not classified as a minable resource; thus, a reclassification of natural H2, is necessary in order to obtain exploration and production permits in different geographical locations. Besides the search for large natural H2 deposits, the degassing of water from wells drilled into fractured serpentines,140 may also prove a viable H2 source, to be further investigated, during which hydraulic fracturing technologies may be applicable.
In the absence of a detailed economic analysis of natural H2 exploration, extraction and transportation in literature (a subject beyond the scope of this review), we present a brief discussion on the economics of artificial UHS in Section 4.1. It is also important to highlight that the production/utilization-related costs are not captured – also beyond the scope of this review, but worthy of consideration in future research endeavors.
4.1. Economic analysis of artificial UHS
Underground hydrogen storage cost is dependent on the transportation, monitoring, storage, and injection cost.5 The cost of storage is also dependent on the location and properties of the geological storage site. A potential leak point in the storage site could increase the cost of storage, especially for porous rocks. In a scenario involving salt caverns, tightness tests are performed before each cavern is licensed, confirming whether the cavern is suitable for hydrogen storage. Earlier conceptual studies showed that large-scale hydrogen storage in underground deposits is inexpensive compared to other storage technologies.4 Moreover, the main identified capital costs incurred during UHS include the costs of gas compression, transformer installation, piping, transformer installation, new well drilling and wellhead equipment installation. Tarkowski et al.13 stated that the cost of constructing and operating hydrogen storage in aquifers is greater than that in depleted hydrocarbon reservoirs and caverns.5 Moreover, depleted natural gas reservoirs have a lower construction cost when compared with depleted oil reservoirs. In terms of storage cost, the cavern system has the lowest cost among the three media.5Abandoned reservoirs are the cheapest among all possible storage systems, followed by the solution and hard rock caverns.5 Techno-economic analysis of several caverns with different depths and volumes showed that the overall cost of the projects is identical. Although, each cost component differs. For instance, deep storage sites are associated with high surface installation primarily for gas compression. On the contrary, shallow sites have a lower surface installation cost as well as a very high cavern construction cost.141,142 In another study, the capital expenditures of hydrogen storage in different geological sites are assessed and presented in Fig. 8.143 It should be noted that the data and economic model used to estimate the capital cost are peculiar to the publication year 2014.
 | ||
| Fig. 8 (a) Comparative capital expenditures of hydrogen storage in different geological sites;143 (b–e) hydrogen storage costs versus storage density per acre for different storage systems.144 | ||
Based on the cost analysis (Fig. 8a), the depleted oil and gas reservoirs are the most economically viable storage medium with a levelized cost of 1.29 $ kg−1. The hard rock caverns system is the most expensive, with a levelized cost of 2.77 $ kg−1. In a more recent study, the techno-economic feasibility of large scale UHS in France was evaluated.145 The overall cost of hydrogen including storage cost ranges from €4.5 kg−1 to €6.6 kg−1 H2. The authors noted that the cost of UHS in salt caverns constitute about 5% of the total hydrogen cost.
Michalski et al.146 assessed the business potential of underground hydrogen storage in salt caverns in Germany. Macro- and microeconomic analysis was used to determine the dimensions and optimal location of promising hydrogen storage sites. They noted that the integration of UHS technology with electrolytic hydrogen production could foster power production and distribution systems in Germany.146 Recently, Singh144 performed a comparative techno-economic analysis of hydrogen storage in inactive horizontal shale gas wells and underground storage facilities (Fig. 8b–e). The analysis was performed by using a numerical model representing an hydraulically fractured depleted shale gas with the properties of Haynesville shale and its horizontal wells. Singh results shows that the capital expenditure of storing hydrogen in depleted shale wells ($0.73 kg−1) is lower than salt cavern ($1.51 kg−1). Additionally, the operating expenses is also lower ($0.11 kg−1) when compared to salt cavern ($0.14 kg−1). The author stated that the capital expenditure of hydrogen storage in shale lateral wells is dependent on the existing surface facilities such as the gas compressor station at the well – pad.144
Wu et al.147 presented a techno – economic assessment framework for hydrogen storage by considering four different case studies including the storage of hydrogen in an underground salt cavern for longer periods and fuel cell for regeneration. The other scenarios considered by the authors includes the direct injection of hydrogen into gas networks and the bulk sale as transportation fuel and industrial gas. Their result shows that the UHS in salt cavern is a promising technology with a present value of $47 million and benefit cost ratio of 1.28. In contrast, hydrogen injection into the gas network has a present value of $18.3 million and benefit cost ratio around one. However, detailed techno-economic and life cycle assessments are still required to compare the economic and environmental feasibility of various underground hydrogen storage sites.
5. Recommendations for future research
Based on the critical assessment of UHS and natural H2 systems presented, the following points constitute future research areas, which are worth investigating:• Geomechanical studies should be conducted to assess the widely reported high-cycling capabilities of rock salt, taking into account its rheological properties. These analyses, combined with thermodynamic conditions, should form the basis for simulations to predict accurate injection and withdrawal timings and ultimately exploit the high cycling potential.
• The potential of H2 escape and unwanted migration outside the subsurface boundary should be a key decision factor, as far as the acceptance or rejection of a candidate location is concerned. Thus, the accurate determination of site-specific timeframes over which H2 can be artificially stored (with minimal loss) is vital for the assessment of prospective underground locations. Furthermore, the cyclability of these sites is a crucial factor to be determined for long-term usage.
• To improve the reliability and durability of rock caverns, an intensive campaign of compatibility experiments must be conducted to build a database of materials, taking into account site variability and uncertainties associated with UHS.
• The areas of subsurface hydrogen (both natural and artificial) may well benefit from twinned explorations, mainly to build an understanding of the similarities and differences between factors like microbial interactions and formation stability. Lessons learnt can be mutually beneficial to communities within both areas and could progress research and development considerably.
• The sealing effect of natural H2 in the subsurface rock systems is still not well understood in relation to other gases present. Robust chemical modelling of gas–water interactions which govern gas migration is necessary. Advection and diffusion parameters as well as relative permeability and water solubility data, would be required for model development.
• Standard analytical methods for gas chromatography often utilize H2 as the carrier gas; this causes problems for large-scale detection of H2. Thus, there may be several occasions where this valuable resource has not been identified in H2-rich samples because of the lack of robust detection techniques for accurate measurements.
• Considering the uncertainty surrounding natural H2 exploration and production, further research is required on the techno-economic assessment of natural H2 exploratory projects. In addition, a comprehensive economic and lifecycle analysis of different types of UHS systems should be assessed. However, full-scale field development studies will be required to first, quantify the volume of natural H2, within an identified formation, before extensive economic analyses.
• Natural hydrogen exploration and production, will also benefit from conventional production optimisation methodologies applicable to oil and gas fields. Thus, similar studies to those reported in ref. 148 and 149 will be worth pursuing.
• Recently Proton Energies Ltd has devised a method of cheaply producing H2 from underground oil, gas and coal-bed fires. The procedure involves igniting subsurface hydrocarbon deposits by pumping air or oxygen. At temperatures above 500 °C, injected steam reacts with hydrocarbons to produce syngas, CO2, and more H2. The application of a novel Pd-alloy catalyst induces a selective diffusion of hydrogen to the surface, whereas other gases remain underground. While real-world testing of this technology is ongoing, there is a great potential of this technology to facilitate low-cost production of clean H2 if successful.
6. Conclusions
This review discussed the natural hydrogen production mechanisms and, most importantly, hydrogen storage technologies in detail. Underground hydrogen storage is suggested as a safe method considering the limited hydrogen contact with atmospheric oxygen. It is also effective in long-term (∼40–50 years) high energy storage density (up to 250 W h L−1). UHS in salt caverns was identified to be the most researched technology with four established sites in the USA (Clemens Dome, Spindletop, Moss Bluff) and the UK (Teesside). Leakage issues constitute a significant problem with UHS especially in porous rocks due to its molecular size, low density, and viscosity. According to the capital cost analysis, the depleted oil and gas reservoirs are determined to be the cheapest storage option with a cost approximation of 1.29 $ kg−1. In comparison, the hard rock caverns system is identified as the most expensive, with a Levelized cost reaching 2.77 $ kg−1. Additionally, the capital expenditure of storing hydrogen in depleted shale wells ($0.73 kg−1) is lower than salt cavern ($1.51 kg−1). With regard to natural H2, the successful deployment of this resource for energy production in Mali is proof that this technology has the potential to compete favourably with fossil fuel energy sources. Despite the scarcity of economic data on natural H2 projects, it is expected that the overall economics of its extraction will not be too different from natural gas. Nonetheless, this requires further substantiation via robust techno-economic analyses. An improved understanding of natural Hydrogen's formation mechanisms is also likely to facilitate future exploratory campaigns of this readily available resource. The exploitation of natural H2 will involve the 5 phases of conventional oil and gas exploitation, including: exploration, appraisal, development, production and abandonment phases. Further field-development studies, capturing these phases, will be required to prove its economic viability.Conflicts of interest
There are no conflicts to declare.References
- D. B. Levin and R. Chahine, Int. J. Hydrogen Energy, 2010, 35, 4962–4969 CrossRef CAS.
- S. Nanda, K. Li, N. Abatzoglou, A. K. Dalai and J. A. Kozinski, Bioenergy Systems for the Future: Prospects for Biofuels and Biohydrogen, 2017, pp. 373–418 Search PubMed.
- J. A. Okolie, B. R. Patra, A. Mukherjee, S. Nanda, A. K. Dalai and J. A. Kozinski, Int. J. Hydrogen Energy, 2021, 46, 8885–8905 CrossRef CAS.
- D. Zivar, S. Kumar and J. Foroozesh, Int. J. Hydrogen Energy, 2021, 46, 23436–23462 CrossRef CAS.
- R. Tarkowski, Renewable Sustainable Energy Rev., 2019, 105, 86–94 CrossRef CAS.
- N. Heinemann, M. G. Booth, R. S. Haszeldine, M. Wilkinson, J. Scafidi and K. Edlmann, Int. J. Hydrogen Energy, 2018, 43(45), 20861–20874, DOI:10.1016/j.ijhydene.2018.09.149.
- C. Acar and I. Dincer, Int. J. Hydrogen Energy, 2014, 39, 1–12 CrossRef CAS.
- J. Han, Y. Liang, J. Hu, L. Qin, J. Street, Y. Lu and F. Yu, Energy Convers. Manage., 2017, 153, 641–648 CrossRef CAS.
- S. E. Hosseini and M. A. Wahid, Renewable Sustainable Energy Rev., 2016, 57, 850–866 CrossRef CAS.
- M. D. Redwood, R. L. Orozco, A. J. Majewski and L. E. Macaskie, Bioresour. Technol., 2012, 119, 384–392 CrossRef CAS PubMed.
- B. Gunes, Renewable Sustainable Energy Rev., 2021, 143, 110950 CrossRef CAS.
- W. Liu, L. Sun, Z. Li, M. Fujii, Y. Geng, L. Dong and T. Fujita, Environ. Sci. Pollut. Res., 2020, 27, 31092–31104 CrossRef CAS PubMed.
- R. Tarkowski and G. Czapowski, Int. J. Hydrogen Energy, 2018, 21414–21427 CrossRef CAS.
- M. Bai, K. Song, Y. Sun, M. He, Y. Li and J. Sun, J. Pet. Sci. Eng., 2014, 124, 132–136 CrossRef CAS.
- C. Hemme and W. van Berk, Appl. Sci., 2018, 8(11), 2282 CrossRef CAS.
- A. Hassanpouryouzband, E. Joonaki, K. Edlmann and R. S. Haszeldine, ACS Energy Lett., 2021, 6, 2181–2186 CrossRef CAS.
- N. Heinemann, J. Alcalde, J. M. Miocic, S. J. T. Hangx, J. Kallmeyer, C. Ostertag-Henning, A. Hassanpouryouzband, E. M. Thaysen, G. J. Strobel, C. Schmidt-Hattenberger, K. Edlmann, M. Wilkinson, M. Bentham, R. Stuart Haszeldine, R. Carbonell and A. Rudloff, Energy Environ. Sci., 2021, 14 Search PubMed.
- A. Körner, C. Tam, S. Bennett and J. Gagné, Technology Roadmap: Hydrogen and Fuel Cells. International Energy Agency (IEA), 2015 Search PubMed.
- E. Tzimas, C. Filiou, S. D. Peteves and J.-B. Veyret, Hydrogen Storage: State-Of-The-Art and Future Perspective, 2003 Search PubMed.
- O. Kruck, F. Crotogino, R. Prelicz and T. Rudolph, Assessment of the Potential, the Actors and Relevant Business Cases for Large Scale and Seasonal Storage of Renewable Electricity by Hydrogen Underground Storage in Europe, 2013 Search PubMed.
- J. Andersson and S. Grönkvist, Int. J. Hydrogen Energy, 2019, 44, 11901–11919 CrossRef CAS.
- A. Keçebaş and M. Kayfeci, in Solar Hydrogen Production: Processes, Systems and Technologies, Elsevier, 2019, pp. 3–29 Search PubMed.
- S. Donadei and G.-S. Schneider, in Storing Energy, Elsevier, 2016, pp. 113–133 Search PubMed.
- R. Tarkowski and B. Uliasz-Misiak, Gospod. Surowcami Miner., 2021, 37(1), 141–160 Search PubMed.
- R. Tarkowski and B. Uliasz-Misiak, Renewable Sustainable Energy Rev., 2022, 162, 112451 CrossRef CAS.
- C. Vacquand, E. Deville, V. Beaumont, F. Guyot, O. Sissmann, D. Pillot, C. Arcilla and A. Prinzhofer, Geochim. Cosmochim. Acta, 2018, 223, 437–461 CrossRef CAS.
- Y. Hao, Z. Pang, J. Tian, Y. Wang, Z. Li, L. Li and L. Xing, Chem. Geol., 2020, 538, 119477 CrossRef CAS.
- E. Deville and A. Prinzhofer, Chem. Geol., 2016, 440, 139–147 CrossRef CAS.
- V. Zgonnik, Earth-Sci. Rev., 2020, 203, 103140 CrossRef CAS.
- A. Prinzhofer, C. S. Tahara Cissé and A. B. Diallo, Int. J. Hydrogen Energy, 2018, 43, 19315–19326 CrossRef CAS.
- J. Guelard, F. G. V. Beaumont, V. Rouchon and E. D. K. D. Newell, Geochem., Geophys., Geosyst., 2017, 1–26 Search PubMed.
- V. A. Nivin, V. V. Pukha, A. V. Lovchikov and R. G. Rakhimov, Dokl. Earth Sci., 2016, 471, 1261–1264 CrossRef CAS.
- N. Larin, V. Zgonnik, S. Rodina, E. Deville, A. Prinzhofer and V. N. Larin, Nat. Resour. Res., 2015, 24, 369–383 CrossRef.
- A. Prinzhofer, I. Moretti, J. Françolin, C. Pacheco, A. D'Agostino, J. Werly and F. Rupin, Int. J. Hydrogen Energy, 2019, 44, 5676–5685 CrossRef CAS.
- V. Zgonnik, V. Beaumont, E. Deville, N. Larin, D. Pillot and K. M. Farrell, Prog. Earth Planet. Sci., 2015, 2(31), 1–15 Search PubMed.
- M. Sato, A. J. Sutton, K. A. McGee and S. Russell-Robinson, J. Geophys. Res.: Solid Earth, 1986, 91(B12), 12315–12326 CrossRef.
- P. Nikolaidis and A. Poullikkas, Renewable Sustainable Energy Rev., 2017, 67, 597–611 CrossRef CAS.
- M. Panfilov, Compend. Hydrog. Energy, 2016, 91–115 CAS.
- R. Tarkowski, Int. J. Hydrogen Energy, 2017, 42, 347–355 CrossRef CAS.
- A. Körner, C. Tam, S. Bennett and J. Gagné, Technology Roadmap: Hydrogen and Fuel Cells, 2015 Search PubMed.
- S. Henkel, D. Pudlo, L. Werner, F. Enzmann, V. Reitenbach, D. Albrecht, H. Würdemann, K. Heister, L. Ganzer and R. Gaupp, Energy Procedia, 2014, 63, 8026–8035 CrossRef CAS.
- D. Pudlo, L. Ganzer, S. Henkel, M. Kühn, A. Liebscher, M. De Lucia, M. Panfilov, P. Pilz, V. Reitenbach, D. Albrecht, H. Würdemann and R. Gaupp, in Springer Series in Geomechanics and Geoengineering, 2013 Search PubMed.
- D. Pudlo, L. Ganzer, S. Henkel, A. Liebscher, M. Kühn, M. De Lucia, M. Panfilov, P. Pilz, V. Reitenbach, D. Albrecht, H. Würdemann and R. Gaupp, EGU Gen. Assem., 2013, 395–412 Search PubMed.
- E. M. Thaysen, S. McMahon, G. J. Strobel, I. B. Butler, B. T. Ngwenya, N. Heinemann, M. Wilkinson, A. Hassanpouryouzband, C. I. McDermott and K. Edlmann, Renewable Sustainable Energy Rev., 2021, 151, 111481 CrossRef CAS.
- J. Simon, A. M. Ferriz and L. C. Correas, Energy Procedia, 2015, 73, 136–144 CrossRef CAS.
- D. Zapf, B. Leuger, S. Donadei, P. L. Horváth, D. Zander-Schiebenhoefer, S. Fleig, M. Henneberg, J. Onneken, S. Gast, S. Roehling and A. Ruales, in 54th U.S. Rock Mechanics/Geomechanics Symposium, 2020 Search PubMed.
- D. Zapf, K. Staudtmeister, R. B. Rokahr, S. Donadei, D. Zander-Schiebenhöfer, P. L. Horvath, S. Fleig, L. Pollok, M. Hölzner and J. Hammer, in 49th US Rock Mechanics/Geomechanics Symposium, 2015, vol. 4 Search PubMed.
- S. Donadei, D. Zander-Schiebenhöfer, P. L. Horvath, D. Zapf, K. Staudtmeister, R. B. Rokahr, S. Fleig, L. Pollok, M. Hölzner, J. Hammer, S. Gast, C. Riesenberg and G. Von Goerne, in 3rd Sustainable Earth Sciences Conference and Exhibition: Use of the Sub-surface to Serve the Energy Transition, 2015 Search PubMed.
- L. Pollok, S. Gast, M. Hölzner, S. Fleig, C. Riesenberg, J. Hammer, G. Von Goerne, S. Donadei, P. L. Horvath, D. Zander-Schiebenhöfer, D. Zapf, K. Staudtmeister and R. B. Rokahr, in 3rd Sustainable Earth Sciences Conference and Exhibition: Use of the Sub-surface to Serve the Energy Transition, 2015 Search PubMed.
- T. Rudolph, in Underground Hydrogen Storage – Current Developments and Opportunities, 2019 Search PubMed.
- R. Groenenberg, Large-scale Energy Storage, TNO, 2020 Search PubMed.
- W. T. Pfeiffer and S. Bauer, Energy Procedia, 2015, 76, 565–572 CrossRef CAS.
- Z. Shi, K. Jessen and T. T. Tsotsis, Int. J. Hydrogen Energy, 2020, 45, 8757–8773 CrossRef CAS.
- B. Sorensen, Hydrog. Power Theor. Eng. Int. Symp., 2007, 1–9 Search PubMed.
- A. Di Gianfrancesco, in Materials for Ultra-supercritical and Advanced Ultra-supercritical Power Plants, Woodhead Publishing, 2017, pp. 643–687 Search PubMed.
- R. Tarkowski, B. Uliasz-Misiak and P. Tarkowski, Int. J. Hydrogen Energy, 2021, 46, 20010–20022 CrossRef CAS.
- L. Lankof and R. Tarkowski, Int. J. Hydrogen Energy, 2020, 45, 19479–19492 CrossRef CAS.
- S. Benson, P. Cook, J. Anderson, S. Bachu, H. B. Nimir, B. Basu, J. Bradshaw and G. Deguchi, in IPCC Special Report on Carbon Dioxide Capture and Storage, 2005 Search PubMed.
- B. Pan, X. Yin and S. Iglauer, Int. J. Hydrogen Energy, 2021, 46, 25578–25585 CrossRef CAS.
- T. Ajayi, J. S. Gomes and A. Bera, Pet. Sci., 2019, 16, 1028–1063 CrossRef CAS.
- H. Belhaj and A. Bera, Int. J. Pet. Eng., 2017, 3(1), 49–66 Search PubMed.
- Z. Hu, J. Klaver, J. Schmatz, J. Dewanckele, R. Littke, B. M. Krooss and A. Amann-Hildenbrand, Eng. Geol., 2020, 273, 105632 CrossRef.
- T. Chen, X. T. Feng, G. Cui, Y. Tan and Z. Pan, J. Nat. Gas Sci. Eng., 2019, 64, 1–14 CrossRef.
- X. Zhong, Y. Zhu, L. Liu, H. Yang, Y. Li, Y. Xie and L. Liu, J. Pet. Sci. Eng., 2020, 191, 107221 CrossRef CAS.
- A. Aberoumand, Underground Gas Storage, Dana Energy, 2022, accessed: 10th June 2022, https://www.danaenergy.com/en/media-menu/dana-magazine/conversations/underground-gas-storage Search PubMed.
- H. Karakilcik and M. Karakilcik, in Accelerating the Transition to 100% Renewable Energy Era, Springer, 2020, vol. 74, pp. 375–392 Search PubMed.
- P. J. Cook, Environ. Geosci., 1999, 6, 185–190 CrossRef.
- M. Kuhn, M. Streibel, N. Nakaten and T. Kempka, Energy Procedia, 2014, 59, 9–15 CrossRef.
- A. Lemieux, K. Sharp and A. Shkarupin, Int. J. Hydrogen Energy, 2019, 44, 15193–15204 CrossRef CAS.
- L. Katarzyna and T. Radosław, Int. J. Hydrogen Energy, 2020, 45, 2068–2083 CrossRef CAS.
- Energy Infrastructure, Underground Natural Gas Storage, 2022, accessed 10 June 2022, https://www.energyinfrastructure.org/energy-101/natural-gas-storage/ Search PubMed.
- A. Aftab, A. Hassanpouryouzband, Q. Xie, L. L. Machuca and M. Sarmadivaleh, Ind. Eng. Chem. Res., 2022, 61 Search PubMed.
- G. RPS, Undergr. Gas Storage, 2022, accessed 10 June 2022, https://www.rpsgroup.com/sectors/energy/storage/energy-and-low-carbon-storage-solutions/underground-gas-storage/#:%7E:text=The%20most%20common%20types%20of,long-term%20capture%20and%20storage Search PubMed.
- S. Kalam, T. Olayiwola, M. M. Al-Rubaii, B. I. Amaechi, M. S. Jamal and A. A. Awotunde, J. Pet. Explor. Prod. Technol., 2020, 11, 303–325 CrossRef.
- N. S. Muhammed, B. Haq, D. Al Shehri, A. Al-Ahmed, M. M. Rahman and E. Zaman, Energy Rep., 2022, 8, 461–499 CrossRef.
- C. Sambo, A. Dudun, S. A. Samuel, P. Esenenjor, N. S. Muhammed and B. Haq, Int. J. Hydrogen Energy, 2022, 47(54), 22840–22880 CrossRef.
- U. HSE, Salt Cavity Natural Gas Storage - Consent and Operational Issues, 2022, accessed 10 June 2022, https://www.hse.gov.uk/gas/supply/saltcavity.htm Search PubMed.
- D. R. Simbeck, Energy, 2004, 29(9–10), 1633–1641 CrossRef CAS.
- A. Amid, D. Mignard and M. Wilkinson, Int. J. Hydrogen Energy, 2016, 41, 5549–5558 CrossRef CAS.
- J. Cihlar, D. Mavins and K. van der Leun, Picturing the Value of Underground Gas Storage to the European Hydrogen System, 2021 Search PubMed.
- S. Cornot-Gandolphe, CEDIGAZ Insights: Underground Gas Storage in the World - 2018 Status, 2018 Search PubMed.
- R. Groenenberg, J. Juez-Larré, C. Goncalvez, L. Wasch, H. Dijkstra, B. Wassing, B. Orlic, L. Brunner, K. van der Valk, T. H. van der Meulen and K. Kranenburgh-Bruinsma, Techno-economic Modelling of Large-Scale Energy Storage Systems, 2020 Search PubMed.
- L. Paterson, Int. J. Hydrogen Energy, 1983, 8(1), 53–59 CrossRef CAS.
- B. Hagemann, M. Rasoulzadeh, M. Panfilov, L. Ganzer and V. Reitenbach, Environ. Earth Sci., 2015, 73, 6891–6898 CrossRef CAS.
- F. Feldmann, B. Hagemann, L. Ganzer and M. Panfilov, Environ. Earth Sci., 2016, 75, 1165 CrossRef.
- R. Groenenberg, J. Koornneef, J. Sijm, G. Janssen, G. Morales-Espana, J. van Stralen, R. Hernandez-Serna, K. Smekens, J. Juez-Larré, C. Goncalvez, L. Wasch, H. Dijkstra, B. Wassing, B. Orlic, L. Brunner, K. van der Valk, M. van Unen, T. H. van der Meulen, K. Kranenburg-Bruinsma, E. Winters, H. Puts, J. (GovernEUR) Van Popering-Verkerk and M. (GovernEUR) Duijn, Large-Scale Energy Storage in Salt Caverns and Depleted Fields, 2020 Search PubMed.
- J. Ennis-King, K. Michael, J. Strand, R. Sander and C. Green, Underground Storage of Hydrogen: Mapping Out the Options for Australia, 2021 Search PubMed.
- D. G. Caglayan, N. Weber, H. U. Heinrichs, J. Linßen, M. Robinius, P. A. Kukla and D. Stolten, Int. J. Hydrogen Energy, 2020, 45(11), 6793–6805 CrossRef CAS.
- R. Cammack, in Hydrogen as a Fuel, CRC Press, 1st edn, 2001 Search PubMed.
- R. R. Richard Cammack and M. Frey, Hydrogen as a Fuel Learning from Nature, ed. R. Cammack, 2001 Search PubMed.
- R. Conrad and W. Seiler, Soil Biol. Biochem., 1981, 13(1), 43–49 CrossRef CAS.
- J. A. Apps and P. C. Van De Kamp, U.S. Geol. Surv. Prof. Pap., 1993, 1570, 81–123 Search PubMed.
- N. Smith, B. G. Survey, K. D. Centre, N. Hill, N. Ng and N. Smith, NW Eur. Geol. Soc. London, Pet. Geol. Conf. Ser., 2005, 6, 481–482 Search PubMed.
- B. S. Lollar, T. C. Onstott, G. Lacrampe-Couloume and C. J. Ballentine, Nature, 2014, 516, 379–382 CrossRef CAS PubMed.
- P. L. Morrill, J. G. Kuenen, O. J. Johnson, S. Suzuki, A. Rietze, A. L. Sessions, M. L. Fogel and K. H. Nealson, Geochim. Cosmochim. Acta, 2013, 109, 222–240 CrossRef CAS.
- C. Vacqand, Doctoral thesis, L'Institut de Physique du Globe de Paris, 2011.
- G. Etiope, N. Samardžić, F. Grassa, H. Hrvatović, N. Miošić and F. Skopljak, Appl. Geochem., 2017, 84, 286–296 CrossRef CAS.
- R. M. Coveney, E. D. Goebel, E. J. Zeller, G. A. M. Dreschhoff and E. E. Angino, Am. Assoc. Pet. Geol. Bull., 1987, 71(1), 39–48 CAS.
- E. Angino, R. M. J. Coveney, E. D. Goebel, E. J. Zeller and G. Dreschhoff, Oil Gas J., 1984, 82, 146 Search PubMed.
- H. Sakai, A. Urabe and H. Wakita, Geochem. J., 1985, 19, 135–148 CrossRef.
- A. T. Huntingdon and M. Sato, Philos. Trans. R. Soc. A Math. Phys. Eng. Sci., 1973, 274(1238), 119–127 CAS.
- L. K. Ward, Trans. R. Soc. South Aust., 1933, 57, 42–47 CAS.
- B. R. B. Symonds, R. J. Poreda, W. C. Evans, C. J. Janik and B. E. Ritchie, Earth, 2003, 1–26 Search PubMed.
- H. Nakamura and K. Maéda, Bull. Geol. Surv. Jpn., 1961, 12, 489–497 CAS.
- B. M. McElduff, Doctoral thesis, Montanuniversitaet Leoben, 1991.
- J. Dubessy, M. Pagel, J. M. Beny, H. Christensen, B. Hickel, C. Kosztolanyi and B. Poty, Geochim. Cosmochim. Acta, 1988, 52(5), 1155–1167 CrossRef CAS.
- B. L. Wood, N. Z. J. Geol. Geophys., 1972, 15, 88–128 CrossRef CAS.
- В. И. Молчанов and M. A. Жарков, Hydrogen generation in lithogenesis, 1981, pp. 1–145 Search PubMed.
- V. N. Larin and C. W. Hunt, Hydridic Earth : the New Geology of Our Primordially Hydrogen-Rich Planet, Polar Publishing, Alberta, 1993 Search PubMed.
- A. V. Shcherbakov and N. D. Kozlova, Geotectonics, 1986, 20(2), 120–128 Search PubMed.
- H. Toulhoat, V. Beaumont, V. Zgonnik, N. Larin and V. N. Larin, Nat. Hydrog. Energy., 2012, 1–18 Search PubMed.
- R. Sugisaki, J. Geol., 1983, 91, 239–258 CrossRef CAS.
- E. I. Isaev, N. V. Skorodumova, R. Ahuja, Y. K. Vekilov and B. Johansson, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 9168–9171 CrossRef CAS.
- F. Freund, J. T. Dickinson and M. Cash, Astrobilogy, 2002, 2, 126–134 Search PubMed.
- N. J. P. Smith, First Break, 2002, 20(2), 246–253 Search PubMed.
- J. L. Charlou, C. Konn, J. P. Donval, V. Guyader, F. Perez, C. Mugler, P. Jeanbaptiste and E. Fourre, Goldschmidt 2012 Conf. Mineral. Mag., 2012, 1565 Search PubMed.
- K. Takai, T. Gamo, U. Tsunogai, N. Nakayama, H. Hirayama, K. H. Nealson and K. Horikoshi, Extremophiles, 2004, 8, 269–282 CrossRef CAS.
- M. Yakymchuk and I. N. Korchagin, in Geoinformatics 2020 - XIXth International Conference ‘Geoinformatics: Theoretical and Applied Aspects’, 2020 Search PubMed.
- E. A. Rogozhin, A. V. Gorbatikov, N. V. Larin and M. Y. Stepanova, Izv. Atmos. Oceanic Phys., 2010, 46, 973–981 CrossRef.
- M. Khdhiri, L. Hesse, M. E. Popa, L. Quiza, I. Lalonde, L. K. Meredith, T. Röckmann and P. Constant, Soil Biol. Biochem., 2015, 85, 1–9 CrossRef CAS.
- S. K. Johnsgard, Kansas Geological Survey, 1988, 1–112 Search PubMed.
- C. Arrouvel and A. Prinzhofer, Int. J. Hydrogen Energy, 2021, 46, 18780–18794 CrossRef CAS.
- L. H. Lin, J. Hall, J. Lippmann-Pipke, J. A. Ward, B. S. Lollar, M. DeFlaun, R. Rothmel, D. Moser, T. M. Gihring, B. Mislowack and T. C. Onstott, Geochem., Geophys., Geosyst., 2005, 6, 1–13 CrossRef.
- R. N. Greenberger, J. F. Mustard, E. A. Cloutis, L. M. Pratt, P. E. Sauer, P. Mann, K. Turner, M. D. Dyar and D. L. Bish, Earth Planet. Sci. Lett., 2015, 416, 21–34 CrossRef CAS.
- O. Warr, T. Giunta, C. J. Ballentine and B. Sherwood Lollar, Chem. Geol., 2019, 530, 119322 CrossRef CAS.
- N. G. Holm, C. Oze, O. Mousis, J. H. Waite and A. Guilbert-Lepoutre, Astrobiology, 2015, 15, 587–600 CrossRef CAS PubMed.
- S. P. Levshounova, Terra Nova, 1991, 3, 579–585 CrossRef.
- R. Rezaee, Int. J. Hydrogen Energy, 2021, 46(66), 33068–33077 CrossRef CAS.
- M. R. Rodgers and T. H. Anderson, Am. Assoc. Pet. Geol. Bull., 1984, 68(1), 92–105 CAS.
- E. I. Epelle and D. I. Gerogiorgis, AIChE J., 2020, 66(4), e16842 CrossRef CAS.
- S. R. Thiyagarajan, H. Emadi, A. Hussain, P. Patange and M. Watson, J. Energy Storage, 2022, 51 Search PubMed.
- H. B. J. Stone, I. Veldhuis and R. N. Richardson, Geol. Soc. Spec. Publ., 2009, 313, 217–226 CrossRef.
- B. Miyazaki, Geol. Soc. Spec. Publ., 2009, 313(1), 163–172 CrossRef.
- Y. Sun, S. G. Xu, P. P. Kang, Y. Z. Fu and T. X. Wang, Int. J. Environ. Res. Public Health, 2019, 16(11), 1921 CrossRef CAS PubMed.
- J. M. Ogden, Inst. Transp. Stud., 2004, 1–12 Search PubMed.
- M. Ahmed and S. Rezaei-Gomari, Resources, 2019, 8, 5 CrossRef.
- E. Acquah-Andoh, Economic Evaluation of Bowland Shale Gas Wells Development in the UK, Proc. J. World Acad. Sci. Eng. Technol., 2015, 9(7), 2550–2558 Search PubMed.
- C. Taylor, D. Lewis and D. Byles, Getting Shale Gas Working, 2013, pp. 1–188 Search PubMed.
- L. Vermaak, H. W. J. P. Neomagus and D. G. Bessarabov, Membranes, 2021, 11(4), 282 CrossRef CAS PubMed.
- A. V Milkov, Earth-Sci. Rev., 2022, 104063 CrossRef.
- J. B. Taylor, J. E. A. Alderson, K. M. Kalyanam, A. B. Lyle and L. A. Phillips, Int. J. Hydrogen Energy, 1986, 11, 5–22 CrossRef CAS.
- D. Gammer, The Role of Hydrogen Storage in a Clean Responsive Power System, Loughborough, 2015 Search PubMed.
- A. S. Lord, P. H. Kobos and D. J. Borns, Int. J. Hydrogen Energy, 2014, 39, 15570–15582 CrossRef CAS.
- H. Singh, Appl. Energy, 2022, 313, 118862 CrossRef CAS.
- A. Le Duigou, A.-G. Bader, J.-C. Lanoix and L. Nadau, Int. J. Hydrogen Energy, 2017, 42, 22987–23003 CrossRef CAS.
- J. Michalski, U. Bünger, F. Crotogino, S. Donadei, G. S. Schneider, T. Pregger, K. K. Cao and D. Heide, Int. J. Hydrogen Energy, 2017, 42, 13427–13443 CrossRef CAS.
- D. Wu, D. Wang, T. Ramachandran and J. Holladay, Energy, 2022, 249, 123638 CrossRef CAS.
- E. I. Epelle and D. I. Gerogiorgis, Optimal rate allocation for production and injection wells in an oil and gas field for enhanced profitability, AIChE J., 2019, 65(6), e16592 CrossRef.
- E. I. Epelle and D. I. Gerogiorgis, Adjoint-based well placement optimisation for Enhanced Oil Recovery (EOR) under geological uncertainty: From seismic to production, J. Pet. Sci. Eng., 2020, 190, 107091 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |