Nanosized high entropy spinel oxide (FeCoNiCrMn)3O4 as a highly active and ultra-stable electrocatalyst for the oxygen evolution reaction†
Abstract
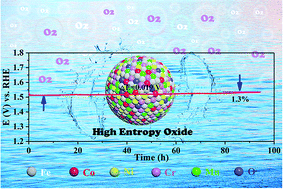
High-entropy oxides (HEOs) with a multi-component single-phase structure are considered as promising electrocatalysts for the oxygen evolution reaction due to their good catalytic activity and tailorable electrochemical properties. Herein, a series of small-sized non-noble metal-based HEO nanoparticles composed of five transition metal elements of Fe, Co, Ni, Cr, and Mn are synthesized via a simple solvothermal strategy followed by different-temperature heat treatment. The HEO treated at 400 °C exhibits the best catalytic activity with an overpotential of 288 mV at a current density of 10 mA cm−2 and outstanding stability (potential change of only 1.3% after a 95 h OER test at 10 mA cm−2) in 1 M KOH electrolyte, outperforming the commercial RuO2 electrocatalysts. The excellent catalytic performance, including the low overpotential, fast dynamics, and superb long-term durability, is mainly attributed to the advantages of the large number of active sites provided by ultra-small nanoparticles, the synergistic effect of various catalytically active metal elements, and the entropy stabilization effect. Therefore, this work enriches the selection of high-entropy oxide elements, provides an idea for solving the current problems of traditional methods, and develops a prospective material for OER catalysts.
- This article is part of the themed collection: Energy Frontiers: Electrochemistry and Electrochemical Engineering


 Please wait while we load your content...
Please wait while we load your content...