Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Organic ionic fluid-based wearable sensors for healthcare
Zhiwu
Chen
and
Yapei
Wang
 *
*
Key Laboratory of Advanced Light Conversion Materials and Biophotonics, Department of Chemistry, Renmin University of China, Beijing, 100872, China. E-mail: yapeiwang@ruc.edu.cn
First published on 30th May 2022
Abstract
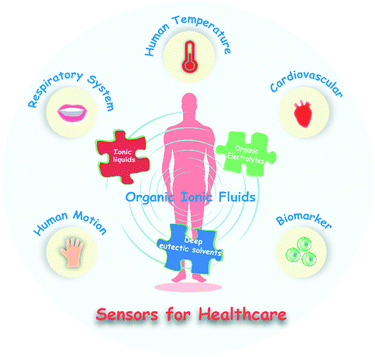
Wearable sensors for healthcare, as an offshoot of prospective medical devices, have been invested in with a great deal of expectations and efforts. Encouragingly, the past decades have witnessed an increased number of flexible sensing materials and some satisfactory milestones have been achieved. Among the explosive emergence of flexible materials, organic ionic fluids rank as a new dazzling star and have been widely applied in wearable sensors for healthcare due to their attractive merits, particularly their high flexibility, wide electrochemical window, design diversity, negligible saturated vapor pressure and excellent solubility. This review article systematically summarizes the categories of organic ionic fluids and the fabrication strategies of electrical sensors based on them. At the same time, the roles that organic ionic fluids play in sensors for healthcare are also interpreted in detail from the aspect of their operating principles. More importantly, combined with specific disease models, the advances and accessibility of these sensors towards various healthcare objects have been fully summarized, including body temperature, human movement, the respiratory system, the cardiovascular system and biomarkers in sweat. Finally, constructive perspectives are put forward for the future development of this exciting field.
1. Introduction
Throughout the development of medicine in human civilization, from the traditional Chinese disease diagnostic techniques, namely, “observation, auscultation and olfaction, inquiry, pulse feeling and palpation”, to the springing up of modern diagnosis and treatment instruments, convenience and accuracy have always been eternal pursuits of healthcare. The progress of human society has put forward greater and newer requirements for medical and health systems. Particularly in the era of a worldwide pandemic, realizing the real-time and accurate monitoring of human health status is an urgent need of contemporary society. In addition, the health problems of local people are particularly acute in remote areas where health resources are scarce. While advances in the Internet of Things technology promise remote health monitoring for people living in remote areas, current expensive and bulky medical tools still make the vision of peer-to-peer personalized medicine a dream. Therefore, the development of portable, low-cost, remotely controlled, real-time monitoring medical equipment is also imperative. To this end, wearable sensors, which are regarded as the next generation of medical diagnostic platforms due to their portability and lightweight characteristics, are gradually stepping into the spotlight and have received increasing appreciation.1–9Physiological parameters can directly reflect the status of human health.10 The real-time monitoring and evaluation of physiological parameters can not only provide immediate early warning and treatment for diseases but also offer valuable information for rehabilitation evaluation. These physiological parameters often include vital signals of the human body (such as body motion, skin temperature, heart rate, blood pressure, and respiration rate), physiological parameters related to activity levels (such as body motion), and biomarkers in vivo or secreted (such as sweat and expired gas). Thus, an ideal wearable sensor for healthcare should possess the ability to specifically identify physiological parameters of the human body. Most importantly, the sensor should have sufficient flexibility and compliance. In other words, such sensors should comply with the cambered human tissues and organs, while fully maintaining their functionality during routine human activities. At the same time, the sensing materials should also maintain long-term stability to achieve lasting conversion and output of these signals. The recent years have witnessed an explosive increase in research on flexible sensing materials.11 Carbon,6 reduced-dimensional metals,12 liquid metals13 and conductive polymers14 have been integrated into wearable sensors that attempt to monitor vital signals for healthcare. Besides, organic ionic fluids, including ionic liquids, organic electrolytes, and deep eutectic solvents, have gradually become one of the most promising candidates for flexible sensing materials due to their inherent deformability, easy workability, adequate stability and high ionic conductivity (Fig. 1).7,15–18 In particular, organic ionic fluids exhibit excellent affinity and solubility towards polar molecules, which are widely regarded as desired recognition and sensing platforms for biomarkers.19,20 It should not be ignored that organic ionic fluids also potentially confer unique device manufacturing processes due to their fluidity. Thus, deepening the understanding of the unique properties of organic ionic fluids is helpful to expand the scope of their application in the field of healthcare, and mastering the preparation process of the corresponding devices is conducive to the advancement of liquid conductor materials from basic theoretical research to application. To date, there has been no overview of organic ionic fluids for healthcare, especially for basic materials, device processing technology, and disease diagnostic models.
In this review, we summarize the latest research on organic ionic fluids in wearable sensors for healthcare with focus on device processing technology. At the same time, we also focus on the possible diseases reflected by physiological parameters and discuss the role of organic ionic fluids in monitoring these signals. Finally, we share constructive perspectives for the future development of this field.
2. Organic ionic fluids for wearable sensors
Wearable sensors usually consist of three key components: the substrate material, the active material and the interface layer between them.21 As the carrier of active materials, the substrate is the cornerstone of the whole device. The active material, offering the sensing function, is the core component of the device. However, in special cases, there is no obvious distinction between them because the active material itself can play the role of the substrate material. Typically, flexible materials such as polymers and fabrics are usually selected as substrate materials in wearable sensors.21–23 The active materials also require flexibility to adapt to the deformation of the substrate. The intrinsic fluid property of organic ionic fluids enables them to have incomparable deformation ability, which can satisfactorily meet the basic requirements of wearable materials. This section summarizes the roles of these organic ionic fluids in wearable sensors according to their physical and chemical properties.2.1 Categories of organic ionic fluids
In general, organic ionic fluids can be divided into three typical categories, namely organic electrolytes, ionic liquids, and deep eutectic solvents. The physiological environment of the human body is a typical electrolyte atmosphere. Various ionic species in the aqueous phase, such as potassium ions, sodium ions and calcium ions, play a pivotal role in the generation, conduction and feedback of various sensory signals that control the normal operation of various physiological functions of the human body.24–26 Thus, it can be concluded that ion sensing is the sensing form closest to life. In recent years, many ionic hydrogels have been developed for human–computer interaction, in which an aqueous electrolyte selected as the active material acts as a medium for ionic sensing.27 However, a narrow electrochemical window and the environmental evaporation of aqueous electrolytes can be encountered, which will restrict the performance of the sensor. As an emerging alternative, organic electrolytes exhibit excellent stability and are thus gaining popularity. Strictly speaking, ionic liquids, as well as some eutectic solvents, belong to the subclass of organic electrolytes; however, there are obvious differences in the composition and preparation of these species, showing their own unique attributes. Thus, in this review, we distinguish them from organic electrolytes. Organic electrolytes mentioned in this review are the typical ionic fluids that are prepared by dissolving solid electrolytes in organic solvents, such as calcium chloride dissolved in ethylene glycol.28Ionic liquids are the most studied and widely used organic ionic fluids in the field of sensing. They are molten salts composed of organic cations and organic or inorganic anions. The increasing size of the cation and anion weakens the coulombic interaction between them, resulting in their melting point being below 100 °C, thereby showing fluidic characteristics at room temperature.29,30 Deep eutectic solvents are room-temperature fluid compounds related to ionic liquids, but the difference is that they are eutectic compositions of hydrogen bond donors (HBDs) and acceptors (HADs) in a certain proportion, exhibiting a reduced melting point.31 According to the types of HBDs and HADs, deep eutectic solvents can be further divided into ionic and non-ionic species. Among them, ionic deep eutectic solvents undoubtedly belong to organic ionic fluids. Some often-used organic ionic fluids in wearable sensors are exemplified in Fig. 2, separately from organic electrolytes, ionic liquids and ionic deep eutectic solvents.
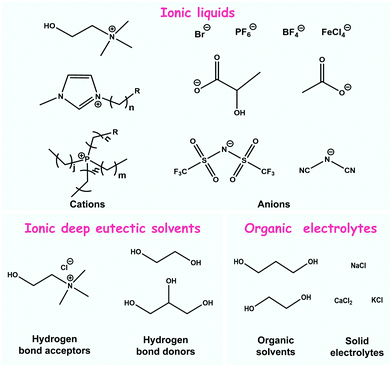 | ||
| Fig. 2 Typically used organic ionic fluids in wearable sensors for healthcare: ionic liquids, ionic deep eutectic solvents and organic electrolytes. | ||
2.2 The role of organic ionic fluids in electrical sensors for healthcare
Signal recognition and transformation are the typical basic processes of health monitoring sensors.8 As shown in Fig. 3, depending on the sensing object of healthcare, sensors can be further divided into physical sensors and chemical sensors. Physical sensors generally involve sensing objects such as strain and pressure related to human activities, human temperature and electrocardiogram signals related to electrophysiology (ECG). Of course, some studies also categorize sensors related to the ECG signal recording as electrophysiological sensors.32–34 To realize the sensing of these physical signals, the preferred sensing media rely on electronic devices. In the past decade, ionic fluids with high ionic conductivity, non-volatility and wide electrochemical windows have been shown to be attractive ionic electronic sensing media. From the point of view of the transformation mechanism, we can further broadly classify the physical sensors into resistive and capacitive types.35 The strain and stress stimuli applied to ionic sensing devices can cause a shape change of the material, which is accompanied by variations in resistance. Subsequently, real-time monitoring of strain and pressure can be realized by recording the change in resistance. Similarly, the variation in temperature can have a sharp influence on the coulombic interaction between cations and anions, resulting in sensitive resistance changes. Therefore, organic ionic fluids have been proven to be ultra-thermal materials.7,17 Capacitive ionic fluidic sensors are also widely adopted in strain and pressure sensing, and their main operating mechanism is that the physical distance changes between the ionic electrodes due to strain and pressure stimulation, leading to a change in capacitance value.36 In addition, highly flexible sensors based on organic ionic fluids can also be applied as epidermal electrodes attached to human skin to record electrophysiological signals similar to an ECG.37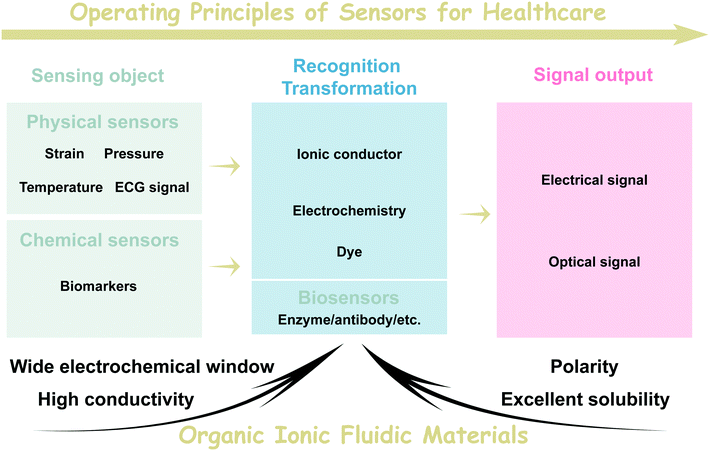 | ||
| Fig. 3 Schematic representation of the operating mechanism of sensors for healthcare and the main advantages of organic ionic fluids in such sensors. | ||
Organic ionic fluids can not only be used as a platform to convert mechanical and other physical stimuli into electrical signals but can also realize chemical recognition or sensing. Most organic ionic fluids based on ionic bonds are humidity-sensitive, especially ionic liquids, which have been widely applied in human respiratory-related humidity sensors.38–40 When water molecules are absorbed into the sensing medium, the ionic interaction is weakened, promoting ion migration to a certain extent, which is reflected in the corresponding electrical output signal as a reduction in the resistance value.
It is worth mentioning that ionic fluids are characterized by polarity and wide electrochemical windows, which make them highly stable electrolytes used to mediate the occurrence of electrochemical reactions involving polar biomarkers, so as to realize the highly sensitive identification and monitoring of biomarkers efficiently.41–44 Besides, the outstanding solubility of organic ionic fluids for organic dyes and biomolecules is also a point of focus, especially their tendency to stabilize bio-macromolecules such as enzymes and antibodies, making them attractive vehicles in the field of chemical sensing.20,44,45 In particular, these kinds of sensors are also classified as an important branch of chemical sensors, namely biosensors, which refers to sensors that utilize bio-macromolecular enzymes, antibodies or cells to realize the specific recognition and conversion of sensing objects.8 With the assistance of organic ionic fluids to dyes and bio-macromolecules, these sensors are also being zealously explored for the recognition of health-related biomarkers, which can then be converted into corresponding usable electrical or optical signals.
Generally, organic ionic fluids are considered emerging ionic conductors for the monitoring of health-related strain, pressure and electrophysiological signals due to their good ionic conductivity, high ion density and low volatility. Meanwhile, they have also become indispensable materials in chemical sensors due to the advantages of strong polarity and the ability to dissolve and stabilize dyes or bio-macromolecules. Subsequently, in the fourth section, we will further elaborate on the application potential and performance points of organic ionic fluids in healthcare based on specific disease diagnosis models.
3. Fabrication of organic ionic fluid-based sensors
The liquid properties of organic ionic fluids also potentially endow the corresponding sensors with special processing techniques. From the viewpoint of preventing device leakage, it is natural to think of a rigid container as a substrate material to encapsulate active liquids. However, this will greatly reduce the flexibility of the device and make it difficult to be adapted in health monitoring sensors attached to the human body. Meanwhile, the sensors will lose their function if the device is damaged in the duration of its service life. Therefore, flexibility is the most basic performance parameter of a substrate material. As shown in Fig. 4, at present, the fabrication strategies of organic ionic fluid-based sensors can be roughly divided into two categories, namely the polymer composite strategy and fluidic channel confine strategy.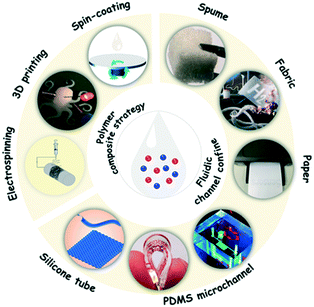 | ||
| Fig. 4 The fabrication strategies of organic ionic fluid-based sensors include two categories: the polymer composite strategy and fluidic channel confine strategy. The materials used in the fluidic channel confine are usually paper, fabric, spume, PDMS microchannels and silicone tubes. The images of these materials have been reprinted with permission from ref. 46, copyright 2017, American Chemical Society; ref. 23, copyright 2020, American Chemical Society; ref. 17, copyright 2015, Wiley-VCH; ref. 47, copyright 2011, Royal Society of Chemistry; and ref. 48, copyright 2020, Wiley-VCH, respectively. The processing techniques typically used in the polymer composite strategy are 3D printing, electrospinning and spin-coating. The image of 3D printing is reproduced with permission from ref. 49, copyright 2020, American Chemical Society. | ||
In recent years, the polymer composite strategy of gelation by mixing elastic polymers with organic ionic fluids has attracted great attention in the field of ionic conductors and has been widely studied. In general, these sensing materials are prepared by virtue of their compatibility, which comes from hydrogen bonds, electrostatic interactions or ion–dipole interactions between the polymer and organic ionic fluids.50–53 Importantly, the organic ionic fluid plays a plasticizing role, endowing the materials with excellent flexible properties. However, with an increase in ionic fluid content, the mechanical properties of the materials are also weakened along with an increase in the risk of leakage.50 Therefore, in order to improve the mechanical properties of these materials, many feasible strategies and theories have been derived, such as chemical cross-linking, double network structures, the polymer crystallization strategy, dissipation-induced toughening theory, and phase separation. For instance, Fan and co-workers developed a tough double network ionic conductor involving chemically cross-linked poly(furfuryl methacrylate-co-methyl methacrylate) and physically cross-linked poly(vinylidene fluoride-co-hexafluoropropylene) networks, wherein 80 wt% ionic liquids were effectively immobilized (Fig. 5a).54 In addition, the well-established dissipation-induced toughening theory is also universally adapted to improve the stretchability and toughness of ionic conducting materials. This toughening effect comes from the energy dissipation caused by weak interactions between the polymer chains. Therefore, strengthening the weak interaction between polymer chains is of great help in improving the material's mechanical properties. Wang's group cleverly took advantage of the high polarity of fluoropolymers and promoted the physical cross-linking degree of polymer chains using the ion–dipole interaction between polar groups in the polymer and ionic salt, effectively improving the tensile properties of the materials (Fig. 5b).52 Similarly, Wu and co-workers also preferred utilizing the ion–dipole interaction between ionic liquids and polyzwitterions, and additionally introduced poly(acrylic acid) to generate hydrogen bonds, which enriched the dynamic environment of the system and extended the elongation-at-break of the resulting ionic conductors beyond 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000% (Fig. 5c).50 However, polymer chains solvated by ionic motifs tend to show large physical spacing, which makes it difficult to form effective interactions between polymers, and it is for this reason that ionic fluid materials are considered plasticizers. Therefore, weakening the shielding of ionic fluids to the interactions between polymer chains will significantly improve the energy dissipation effect.55 Based on this principle, Moon and Hu both reported application cases utilizing phase separation induced by incompatible sectional polymer chains with organic ionic fluid materials to enhance the interactions between polymer chains, achieving excellent toughened effects (Fig. 5d).55,56 The crystallization behavior of the polymer can also enhance the mechanical properties of ionic conductor materials. Sun et al. introduced the easily crystallized PVA segment that served as a nano-filler in ionic conductors to reinforce their mechanical properties (Fig. 5e).51 In addition, their research group also broadened the crystallization strategy into polyurethane elastomers and achieved exceptional strain-adaptive stiffening and damage tolerance through strain-induced crystallization (Fig. 5f).57 From the aspect of device processing, the most common technologies are spin-coating or the solvent-assisted natural air drying of the precursor solution mixed with the polymer and ionic fluid, which are convenient for the testing and characterization of material properties.51,58 However, this is not optimal from both an aesthetic point of view and the functional considerations of wearable sensors. In order to prepare sensors with a controllable structure for wearable devices, a novel method that draws support from 3D printing technology to construct complex structure morphologies has been established.49,59,60 It is worth mentioning that the air permeability of the sensors also affects their comfort while being worn. A successful application case is to prepare a film with good air permeability by electrospinning a composite solution of a polymer and ionic fluid materials.58,61 Broadly speaking, the existing device processing technology still cannot perfectly meet the actual application requirements. Particularly, device miniaturization processing is currently facing a bottleneck.
000% (Fig. 5c).50 However, polymer chains solvated by ionic motifs tend to show large physical spacing, which makes it difficult to form effective interactions between polymers, and it is for this reason that ionic fluid materials are considered plasticizers. Therefore, weakening the shielding of ionic fluids to the interactions between polymer chains will significantly improve the energy dissipation effect.55 Based on this principle, Moon and Hu both reported application cases utilizing phase separation induced by incompatible sectional polymer chains with organic ionic fluid materials to enhance the interactions between polymer chains, achieving excellent toughened effects (Fig. 5d).55,56 The crystallization behavior of the polymer can also enhance the mechanical properties of ionic conductor materials. Sun et al. introduced the easily crystallized PVA segment that served as a nano-filler in ionic conductors to reinforce their mechanical properties (Fig. 5e).51 In addition, their research group also broadened the crystallization strategy into polyurethane elastomers and achieved exceptional strain-adaptive stiffening and damage tolerance through strain-induced crystallization (Fig. 5f).57 From the aspect of device processing, the most common technologies are spin-coating or the solvent-assisted natural air drying of the precursor solution mixed with the polymer and ionic fluid, which are convenient for the testing and characterization of material properties.51,58 However, this is not optimal from both an aesthetic point of view and the functional considerations of wearable sensors. In order to prepare sensors with a controllable structure for wearable devices, a novel method that draws support from 3D printing technology to construct complex structure morphologies has been established.49,59,60 It is worth mentioning that the air permeability of the sensors also affects their comfort while being worn. A successful application case is to prepare a film with good air permeability by electrospinning a composite solution of a polymer and ionic fluid materials.58,61 Broadly speaking, the existing device processing technology still cannot perfectly meet the actual application requirements. Particularly, device miniaturization processing is currently facing a bottleneck.
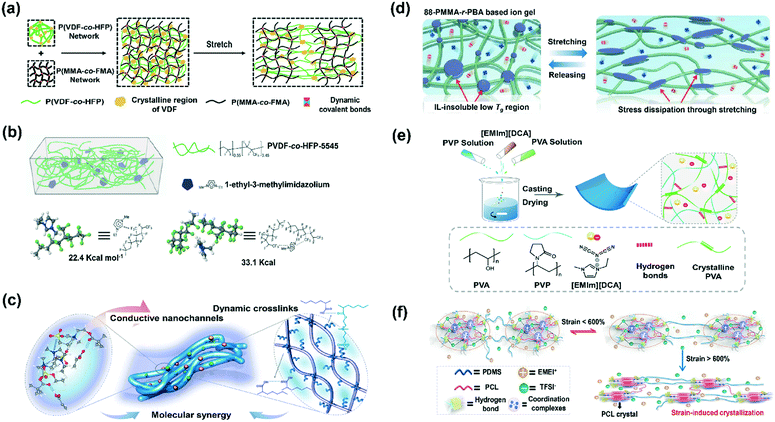 | ||
| Fig. 5 (a) Schematic illustration of the double network ionic conductor involving chemically cross-linked and physically cross-linked networks. Reproduced with permission from ref. 54, copyright 2018, American Chemical Society. (b) The design concept of an ionic conductor using ion–dipole interaction. Reproduced with permission from ref. 52, copyright 2017, Wiley-VCH. (c) Synergistic design of the multiple weak interactions for the ultra-stretchable ionic conductor. Reproduced with permission from ref. 50, copyright 2019, Nature Publishing Group. (d) Phase separation of poorly ion-solvated polymer chains for the toughness of ionic conductors. Reproduced with permission from ref. 56, copyright 2020, Wiley-VCH. (e) Crystallized PVA segments served as nano-fillers for the reinforcement of ionic gels. Reproduced with permission from ref. 51, copyright 2020, American Chemical Society. (f) Schematic diagram of strain-induced crystallization in polyurethane elastomer-based ionic conductors. Reproduced with permission from ref. 57, copyright 2021, American Chemical Society. | ||
Microfluidic technology is exploited to manipulate and store liquid materials due to its characteristics of miniaturization, also known as a “lab on a chip”.62 The microstructure pattern in the flexible substrate can confine ionic fluids to construct the ion signal pathway of sensing, which makes microfluidic sensors a significant branch of wearable ionic sensors. Many fluid channels based on flexible materials, such as silicone tubes, PDMS and PLA, have been combined with ionic fluids for sensing.17,28,47,48 The moduli of these substrates match well with that of the human body, and are therefore excellent substrate materials for wearable sensors. Meanwhile, self-healing characteristics of the sensors can be achieved by exploiting a self-healing flexible substrate. Our group has pioneered the development of a supramolecular polymer substrate for self-healing fluidic chips (Fig. 6a). On account of the abundant hydrogen bonds between the polymer chains, the material has excellent self-healing properties, and therein the microchannels with a diameter of 0.709 mm can cover the height of the 22.82 mm ionic fluid column without any leakage through capillary force.63 In addition, paper-based sensors are derived from the development of the microfluidic concept, and the micro-gaps between paper fibers become natural channels for capillary action to control fluids.46,64–67 Paper chips are cheap and widely available, and there are specific products for commercial sensors. Fabrics, sponges, and yarns, which have similar structures to paper, are considered ideal substrates because of their excellent absorbability and flexibility towards ionic fluids.17,23,68,69 Cheng and co-workers systematically studied the wetting behavior of ionic liquids on cotton fabric, sponges, rubber bands and rubber film, and defined this preparation method as the “liquid-wetting-solid strategy”, which is universal and applicable in various ionic liquids with different hydrophilic and hydrophobic properties (Fig. 6b).70
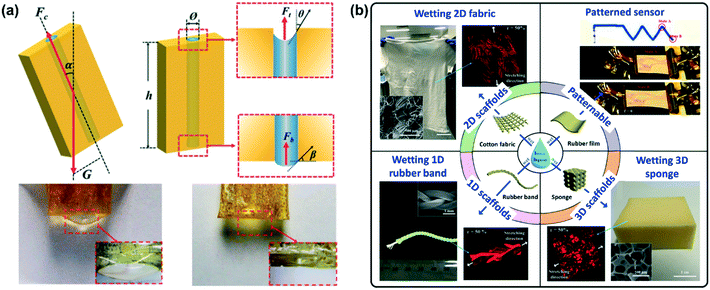 | ||
| Fig. 6 (a) Microchannel encapsulation of ionic liquids based on flexible self-healing polymers. Reproduced with permission from ref. 63, copyright 2015, Wiley-VCH. (b) Commonly used materials of the liquid-wetting-solid strategy for the encapsulation of ionic liquids. Reproduced with permission from ref. 70, copyright 2016, American Chemical Society. | ||
4. Organic ionic fluid-based sensors for healthcare
Essentially, real-time and accurate healthcare requires wearable materials to specifically recognize and transform vital signals that can reflect human health status. Human activity level, body temperature, respiratory rate, the electrocardiogram, type of exhaled air, sweat composition and other physiological parameters are closely related to human health status.10 Accurate and quantitative monitoring of these physiological parameters can help people know their own physical conditions in real-time and give early warning for certain diseases, which is beneficial to formulating and implementing effective intervention measures as soon as possible (Table 1). The inflexibility of traditional inorganic and metal materials has always been the primary problem facing their use as wearable materials. Their flexible processing and preparation processes are usually complicated, which also causes an increase in production costs. At the same time, large-scale processing is more difficult. These factors limit the marketization and popularization of wearable electronic devices based on such materials. However, the intrinsic fluid property of organic ionic fluids makes them possess incomparable advantages in terms of flexibility. In addition, the preparation of ionic fluids is usually relatively simple, and large-scale industrial production has been established, which greatly reduces the production cost of wearable sensor materials and helps promote the low-cost production and commercialization of wearable sensing devices. More importantly, organic ionic fluids have outstanding designability, which can endow materials with various sensing functions through functional molecular design, giving researchers great inspiration and a wide space for expansion. After decades of rapid development, organic ionic fluids have been exploited in a variety of sensors for healthcare and have shown promising application prospects in the diagnosis and treatment of many diseases. Based on the possible health applications and disease models, this section will introduce organic ionic fluid-based wearable sensors related to human movement, body temperature, respiration, the cardiovascular system and biomarkers in sweat, and analyze the key scientific points of sensor design and preparation. At the same time, we will also put forward constructive expectations for the future development of corresponding sensing systems.| Sensing object | Locations of the sensor | Working principles | Signal output | Possible health applications or disease models |
|---|---|---|---|---|
| Limb movement | Finger, forearm, leg | Mechano-electric | Current or resistance | Step monitoring, activity levels |
| Body temperature | Skin, armpit | Thermo-electric | Current or resistance | Fever, COVID-19, menstrual cycle, inflammation |
| Respiration rate | Mouth, nose, abdomen | Mechano-electric | Current or resistance | Sleep apnea, asthma, chronic obstructive pulmonary disease |
| Chemical–electric | ||||
| Expired volatile organic compounds | Mouth, nose, | Electrochemical | Current or resistance, chromaticity | Halitosis, liver and kidney diseases, lung cancer |
| Chemical–electric | ||||
| Photochemical | ||||
| Heart rate | Wrist, feet | Bio-electric | Current or resistance, ECG signal | Arrhythmic disorders, cardiovascular diseases |
| Mechano-electric | ||||
| Glucose | — | Electrochemical | Current | Diabetes |
| Sweat pH | Wrist | Photochemical | Chromaticity | Acne, irritant contact dermatitis, metabolism |
| Lactate | Forearm | Electrochemical | Current | Anaerobic metabolism |
4.1 Human motion sensors
As people pay increasing attention to sub-health, proper physical exercise has become an indispensable daily activity for modern people. It has become the most effective way for people to maintain a vibrant state of health. Through the monitoring of the human movement level, the first-hand movement level data can help people make a reasonable exercise plan to improve their health. In addition, accidental falls have become the main cause of accidental injuries among the elderly (over 60 years old) at home, increasingly and seriously threatening their safety. Wearable motion sensors can monitor the step length and frequency of the elderly in real-time, and alarm signals can be triggered once falling occurs. These are the most urgent needs of wearable motion sensors.14As ionic conductors, organic ionic fluids have also been widely developed as an ideal platform for wearable motion sensors, which can transform the mechanical strain stimuli generated by motion into highly accessible electrical signals under both resistive and capacitive sensing modes. Both sensing modes are easily accessible to the reported elastic conductors based on organic ionic fluids. For instance, Daoud and co-workers have presented a solvent-free ionic elastomer (IE) network, as shown in Fig. 7a, which can be designed as motion sensors in both the capacitive and resistive modes.71 The relative resistance change (ΔR/R0) and the relative capacitance change (ΔC/C0) of the IE under different strains are legible, which validates its excellent motion-sensing functionality. Although organic ionic fluids have fast reversible mechanical strain behavior, the hysteretic response of electrical signals caused by the viscoelastic behavior of the substrate materials still affects the sensing performance of the strain sensor, especially in the case of large strain sensing. In reality, hysteresis-free strain sensing can be guaranteed by regulating the viscoelastic behavior of the elastic substrate through reasonable molecular design. Besides, it is also an effective way to inhibit the viscoelastic effect of the elastic substrate by constructing a special ion pathway structure. Fig. 7b shows the ionic-liquid-based wavy (ILBW) strain sensor designed by Lee and co-workers.72 They found that the ILBW sensor showed lower energy dissipation behavior compared to the normal ionic liquid-based flat (ILBF) strain sensors. The resistance response of the ILBW sensor was fairly deterministic with no hysteresis under strain, showing excellent potential for accurate and quantitative motion detection.
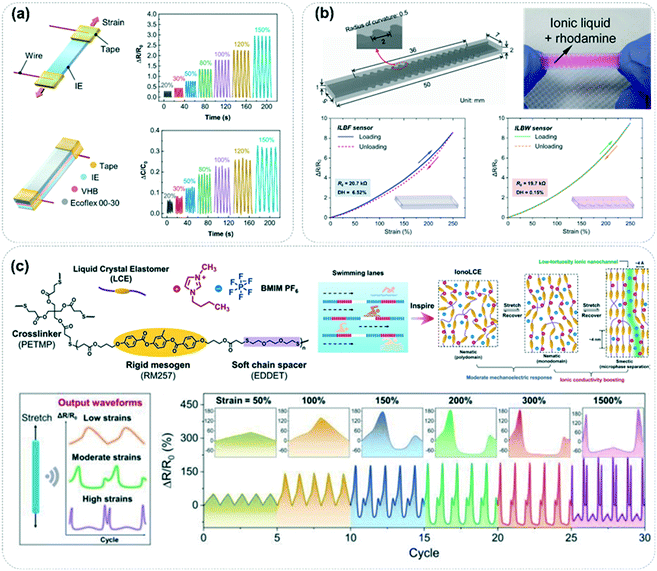 | ||
| Fig. 7 (a) The capacitive and resistive modes of ionic elastomers based on ionic liquids. Reproduced with permission from ref. 71, copyright 2022, Elsevier. (b) The ionic-liquid-based wavy (ILBW) strain sensor for hysteresis-free motion monitoring. Reproduced with permission from ref. 72, copyright 2016, American Chemical Society. (c) Schematic diagram of ionotronic fibers compounded by a liquid crystal elastomer and ionic liquid, and the output electric signals of the strain-regulated mobility of the ionic species. Reproduced with permission from ref. 74, copyright 2021, Wiley-VCH. | ||
Besides the hysteresis of the electric signal response, the gauge factor (GF) is the most critical parameter to evaluate the sensitivity of a strain sensor. GF is defined as the relative change in the resistance value per unit strain.56,73 Although organic ionic fluid-based conductors have better deformability than electronic conductors, their GF value under small strain rarely exceeds 10, which is hardly comparable with that of metal strain sensors.56 Therefore, the development of motion sensors should pursue the improvement of strain response sensitivity. Here we point out that the introduction of the strain response molecular mechanism into the sensing system to regulate the mobility of ionic species may realize the preparation of strain sensors with high GF values. Moreover, the regulation of ion mobility has become the most powerful design concept to improve the performance of ion strain sensors. Wu and co-workers designed a highly robust ionotronic fiber, which was beneficial to the synergy between the ionic liquid and liquid crystal elastomer with alternate rigid mesogen units and soft chain spacers (Fig. 7c).74 The ionotronic fiber was induced to generate a micro-phase separated ionic conductive channel under strain stimulation, which altered the mobility of the ionic species, leading to an unprecedented increase in ionic conductivity. More interestingly, this ionotronic fiber showed a unique output waveform of electrical signals under different degrees of strain, which laid a foundation for the discernible recognition of strain through the waveform.
Motion monitoring of the simple limb bending process has been a commonplace application scenario, and the research focus in this field considers specific sports items or people. For example, the monitoring of swimming movement requires the development of water-resistant motion sensors, which have made breakthrough progress. For example, fluoropolymers and fluorine-rich ionic liquids have been explored for underwater applications.75,76 In addition, personalized motion sensors have been developed to assist athletes, such as ice athletes, adjust their motion posture through the feedback of motion information, so as to improve their performance and avoid accidental injuries caused by non-standard movements.
4.2 Human temperature sensors
Body temperature is the most basic vital sign of the human body and is often related to influenza and inflammation. Whether it is normal or not directly reflects our physical conditions.10 Particularly in the contemporary era of the COVID-19 pandemic, body temperature monitoring has become a necessary primary procedure for disease screening. Traditional mercury thermometers have disadvantages such as their complicated measurement operation, being difficult to carry and easy to damage and their toxicity, and infrared thermal imagers are expensive and bulky. Therefore, the development of wearable flexible thermometers has become more urgent in today's severe anti-epidemic situation. Our group has paid special attention to the ultra-high thermal sensitivity of ionic liquids, and a series of flexible temperature sensors have thus been developed. As shown in Fig. 8a, as a typical ionic liquid, 1-octyl-3-methyl imidazolium acetate ([OMIm][Ac]) has an intrinsic thermal response value of up to 1000% at a ΔT of 40 °C, which is unmatched by other thermally sensitive materials.17 It can accurately detect the temperature of the back of the hand, palm and forehead, and even the temperature changes before and after drinking alcohol can be clearly monitored. In addition to ionic liquids, organic electrolytes also have excellent thermal sensitivity. Our group also explored the sensing performance of temperature sensors based on green electrolyte fluids, which can sensitively detect temperature changes before and after meals, as well as female menstrual periods (Fig. 8b).28 The ultra-high thermal sensitivity of organic ionic fluids derives from the thermo-sensitive coulombic interaction between the cation and anion, which leads to a significant change in ion migration under the changed thermal field. However, it is worth noting that the device structure and block size directly affect the response time of the sensor. Therefore, our research group anchored our hope on low-cost thin paper sensors to shorten the temperature response time as far as possible (Fig. 8c).46 We found that it takes only 8 s for the paper thermometer to reach an electrical equilibrium at a given temperature due to the shortened heat exchange distance between the ionic liquid and the heat source. Meanwhile, because of their advantages of large-scale fabrication, paper thermometers can integrate multiple temperature-sensing elements to realize the multidimensional imaging of thermal fields.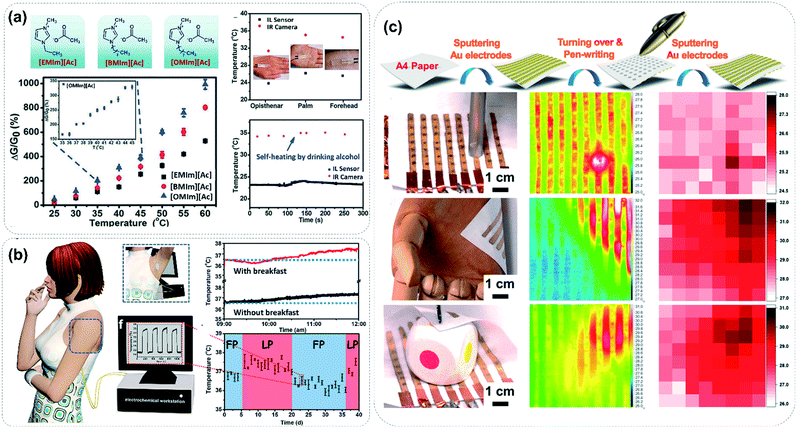 | ||
| Fig. 8 (a) Temperature response values of ultra-thermal ionic liquids and their application in temperature monitoring. Reproduced with permission from ref. 17, copyright 2015, Wiley-VCH. (b) Green electrolytes used for temperature monitoring before and after meals and during female menstrual periods; FP and LP refer to the follicular phase and luteal phase, respectively. Reproduced with permission from ref. 28, copyright 2018, American Chemical Society. (c) Paper thermometer for ultrafast thermal response and thermal imaging. Reproduced with permission from ref. 46, copyright 2017, American Chemical Society. | ||
In practical application, an unavoidable dilemma faced by wearable temperature sensors is the interference of skin strain on the temperature response signal of ionic fluid-based materials, which will seriously affect the accuracy of real-time temperature monitoring. In order to eliminate the interference of skin deformation, our research group also actively explored the design strategy of strain-insensitive temperature sensors. As shown in Fig. 9a, we designed a serpentine microchannel based on PDMS.17 The temperature sensor based on this structure showed strain insensitivity in both biaxial directions and maintained a consistent electrical signal level under the applied strain. Similarly, the temperature sensor based on a coiled thread can also maintain stable temperature response behavior under different tensile strains due to its strain tolerance (Fig. 9b).77 In addition to these structural design strategies, numerical operation analysis can also be utilized to distinguish the strain response signals and temperature response signals. Particularly, more than three groups of sensor arrays can be used to construct three linear ternary equations in the mathematical sense, and the accurate monitoring of human body temperature can be realized by analyzing these linear equations.78 Despite the deep development of temperature sensors based on organic ionic fluids, due to the durability and accuracy of these sensors, there are still challenges in developing durable temperature-sensing materials that can reversibly adhere to the skin and avoid interference from epidermal secretions.
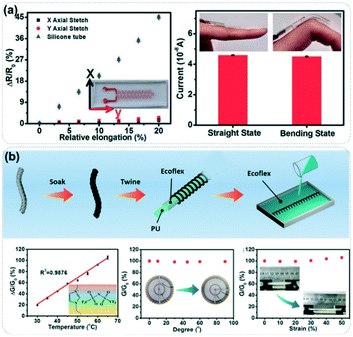 | ||
| Fig. 9 (a) Strain response behavior of the temperature sensor based on serpentine micro-channels. Reproduced with permission from ref. 17, copyright 2015, Wiley-VCH. (b) Strain-insensitive temperature sensor constructed by coiled thread adsorption ionic liquid. Reproduced with permission from ref. 77, copyright 2017, Wiley-VCH. | ||
4.3 Breathing-related sensors
The respiratory system is a natural window for matter exchange between the human body and the outside world. The respiratory rate and volatile organic compounds exhaled are extremely critical vital signs and biomarkers as well as significant warning signals of human diseases. For example, patients with sleep apnea or chronic obstructive pulmonary disease have extremely unstable breathing rates during sleep.79 The breathing status of the patient can be monitored in real-time by wearing a comfortable breathing rate sensor, which could instantly wake up patients with prolonged apnea to avoid suffocation. Typically, the respiratory process of the human body is always accompanied by contraction and relaxation of the abdominal cavity; therefore, ionic conductors that monitor abdominal strain can also be utilized for sensing respiratory rate. Zhang and co-workers encapsulated organic electrolytes in Ecoflex microchannels to prepare a flexible strain sensor.80 By attaching it to the abdomen, it could not only detect different types of breathing events but also identify dangerous processes for apnea (Fig. 10a). Besides, moisture is the main component of exhaled gas, so humidity sensing of exhaled gas is also an effective way to monitor respiratory rate.38,39,58 The varied ionic conductivities of typical organic ionic fluids associated with moisture absorption make them ideal materials for humidity sensing. However, the difficulty of separating ionic fluids from water molecules causes the serious problem of long-time response.58,81 Recent studies have shown that solid materials based on ionic liquids have shorter humidity response times due to the weakened interaction between water molecules and solid materials.38 However, there is still a significant gap between these kinds of sensors and the strain sensors mentioned above in terms of monitoring sensitivity. In order to further weaken the interaction between water molecules and solid materials, Zhu et al. combined hydrophobic ionic liquids ([EMI][TFSI]) with hydrophobic polymers (P(VDF-HFP)) to produce an ultrafast-responsive humidity sensing film using electrostatic spinning technology, which could recognize up to 120 Hz humidity change, comparable to commercial humidity sensors.58 This ultrafast response to humidity is mainly due to the lower humidity hysteresis of the hydrophobic structure and the high specific surface area of the fiber structure. This kind of humidity sensing material with a special structure can undoubtedly become an ideal material for monitoring respiratory rate.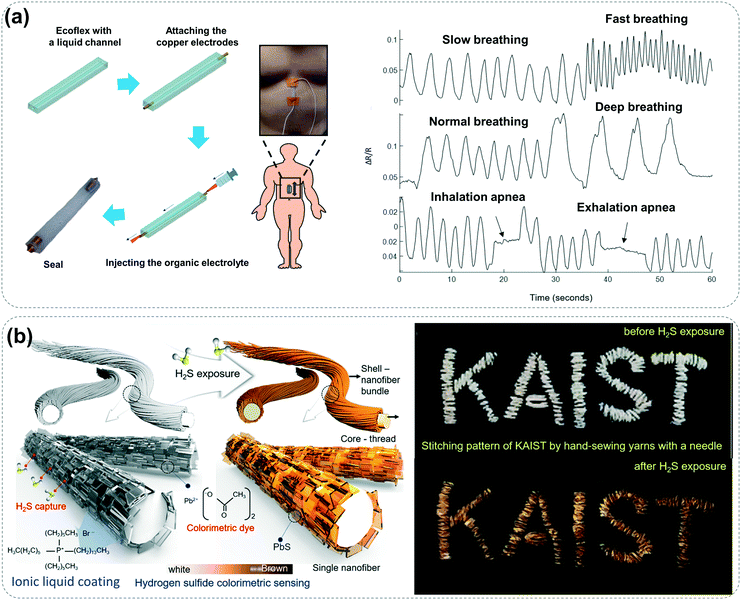 | ||
| Fig. 10 (a) Flexible strain sensor based on organic electrolytes for respiratory rate monitoring. Reproduced with permission from ref. 80, copyright 2021, MDPI. (b) The eye-readable hydrogen sulfide gas sensing platform woven by yarn adsorbed with an ionic liquid and lead acetate dye. Reproduced with permission from ref. 23, copyright 2020, American Chemical Society. | ||
Human exhaled gas contains not only conventional substances such as water vapor, carbon dioxide, and oxygen but also toxic gases such as ammonia, hydrogen sulfide and acetone in some special cases, which are generally used as biomarkers to diagnose human diseases.23,40,43,82 People with oral diseases often exhale hydrogen sulfide gas, which always corresponds to halitosis and can seriously affect an individual's social image.23 Ammonia has also been linked to liver and kidney disease, and toluene is often present in the breath of lung cancer patients.43,83 Therefore, the monitoring of these volatile organic compounds can provide reference information for the diagnosis and prevention of relevant diseases. In recent years, ionic liquids have been selected as ideal electrolytes for wearable flexible gas sensors based on electrochemical analysis due to their strong polarity, excellent gas adsorption and stability.42,65 In addition, ionic liquids with these merits are also utilized in the manufacture of visual gas-sensing materials. Kim and co-workers reported an eye-readable hydrogen sulfide gas sensing platform, which is woven with yarn adsorbed with trihexyl(tetradecyl)phosphonium bromide (C32H68PBr) and lead acetate, wherein the ionic liquid acts as an effective hydrogen sulfide adsorbent and lead acetate acts as a colorimetric dye (Fig. 10b).23 The porous gas-sensing yarn can respond to 1 ppm hydrogen sulfide, and can thus be regarded as a sensitive gas-sensing system with visual output. More importantly, realization of the selective recognition and sensing of various volatile organic compounds relevant to human breath has been one of the toughest challenges in the field. Swager's group developed pastes of an ionic liquid and carbon nanotubes as a platform for gas sensing, and systematically studied the chemical affinities of the sensor arrays consisting of imidazolium-based ionic liquids with different substituents and counterions towards various volatile organic compounds as well as the swelling properties of the corresponding carbon nanotube network.83 The results show that the selective detection of volatile organic compounds can be realized by selecting different ionic liquids. In the future, it will be imperative to study the interaction between ionic liquids and gas molecules at the molecular level and understand the rules therein, which will be helpful for researchers and businesspersons to actively try to fabricate highly sensitive and highly selective gas sensors to serve human health.
4.4 Cardiovascular-related sensors
Unexpected cardiovascular disease has always been a deadly killer threatening human life and health.32,84 Therefore, monitoring cardiovascular vital signs in real-time and taking corresponding early warning measures in time are effective means to reduce mortality. Cardiovascular-related clinical signs generally include heart rate, blood pressure, blood oxygen saturation, and blood glucose.32 The sensor object of a non-invasive organic ionic fluid-based sensor usually focuses on the heart rate, blood pressure and blood glucose, among which heart rate detection is the most widespread. The sensing modes of heart rate include mechano-electric mechanisms and bio-electric mechanisms. The mechano-electric mechanism is to transform the strain or pressure stimulation caused by pulse vibration into corresponding electrical signals by resistive or capacitive sensors.78,85 Compared with resistive sensors, capacitive sensors show higher sensitivity in detecting weak vibrations caused by pulse. Pan's group developed a capacitive ionic sensor array called “FootBeat” that attaches to the dorsal foot in a comfortable manner to accurately collect the pulse waveform, which can be analyzed to acquire a heart rate comparable to that obtained from commercial electrocardiography equipment.85 Bio-electric sensors are commonly used in the acquisition of ECG signals, which utilize electrodes attached to the skin to pick up signals of electrical activities from different heart muscles.86 Tight fitting of the electrodes to the skin is essential for obtaining a complete and accurate ECG signal. For this purpose, Ouyang et al. prepared a self-adhesive deep eutectic solvent-based flexible electrode for ECG signal acquisition.37 The self-adhesive property of the electrode derived from the presence of tannic acid. Compared with a tannin-free electrode, the self-adhesive electrode can record clear ECG signals without noise (Fig. 11a). Although the real-time monitoring of ECG signals in air is well established, the failure of the electrode interface underwater is a major limiting factor for the recording of ECG signals in aqueous environments. Wu and co-workers explored the possibility of detecting ECG signals underwater using hydrophobic ionogel materials, as previously reported.87 The hydrophobic ionic electrode demonstrates superior performance to commercial electrode devices in accurately recording complete ECG signals underwater for long periods of time (Fig. 11b). Since the electrode is in direct contact with the human epidermis, more attention should be paid to the long-term biocompatibility of organic ionic fluids and the evaluation of skin irritation in the future.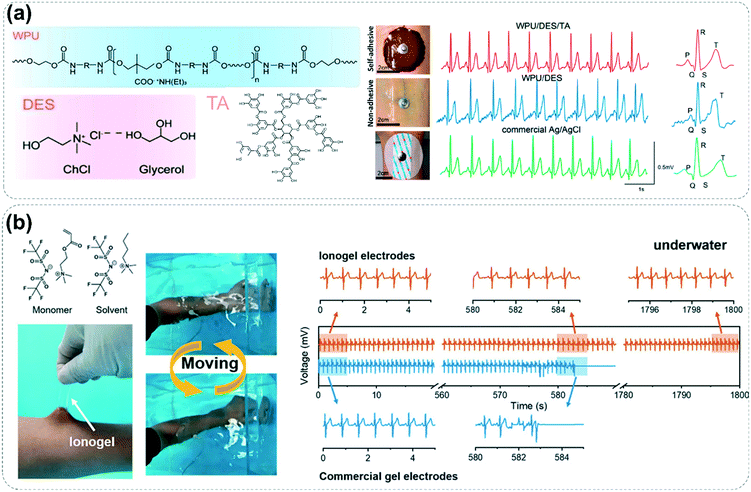 | ||
| Fig. 11 (a) Composition of a self-adhesive ionic electrode material and the corresponding recorded data of the ECG signal. Reproduced with permission from ref. 37, copyright 2021, American Chemical Society. (b) Hydrophobic ionogel for underwater ECG signal monitoring. Reproduced with permission from ref. 87, copyright 2021, Wiley-VCH. | ||
4.5 Sweat-related sensors
Sweat is a biological fluid secreted by sweat glands located in the dermis of the skin and contains numerous biomarkers with clinical application potential. Typically, real-time monitoring of glucose levels in sweat, which reflect blood glucose levels in the body, can help diabetics understand their blood glucose levels to achieve optimal management of the disease.88–90 In addition, lactate associated with anaerobic exercise can also be traced in sweat, and the realization of its real-time detection is helpful for professional athletes to grasp the relevant parameters of their physical health and enhance the effectiveness of exercise.91 Sweat-related wearable sensors are therefore the ideal platform for non-invasive healthcare and disease diagnosis.Due to the high specificity of enzymes in the recognition of biomarkers, enzyme-based biosensors have high application value in the monitoring of biomarkers and the diagnosis of related diseases. It is worth mentioning that the vigorous application of organic electrochemical transistors (OECTs) with signal amplification effects in wearable biosensors has been met with success in the highly sensitive monitoring of biomarkers at very low concentrations in sweat.5 A typical OECT consists of three electrodes (gate, source and drain), an organic semiconductor layer, and an electrolyte layer, where the gate and the organic semiconductor active layer are connected by the electrolyte (Fig. 12a).5,92 When the electrolyte layer is exposed to biomarkers, the doping status of the active layer is affected, thus changing the channel current and achieving quantitative detection of the analytes. Compared with aqueous electrolytes, ionic liquids have wide electrochemical windows and high ionic conductivity. At the same time, ionic liquids also have good dispersity and stability towards biological macromolecules such as enzymes and can even improve enzyme activity to a certain extent. Therefore, ionic liquids are gradually considered a possible substitute for electrolytes in OECTs. In a pioneering work reported by Malliaras et al., triisobutyl(methyl)phosphonium tosylate [P1,4,4,4][Tos] was selected as the supporting electrolyte of the OECTs, in which glucose oxidase was dispersed into the electrolyte.93 The detection range for glucose concentration of the resulting OECTs is shown to be at least 10−7 to 10−2 M (Fig. 12b). Furthermore, the sensor has an effective service life of up to 30 days due to the stabilization of enzyme activity by the ionic liquid. In order to ensure the flexibility and stability of this kind of transistor sensor, this group further prepared an ionogel immobilized with the LOx enzyme and a ferrocene mediator, which not only realized the sensitive electrochemical sensing of lactate but also endowed the sensor with wearability (Fig. 12c).91
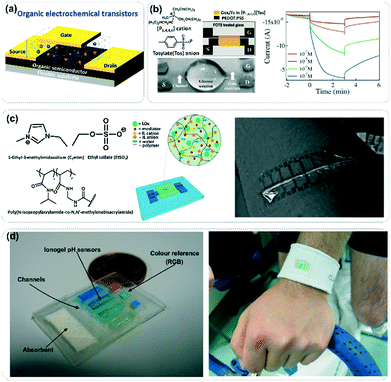 | ||
| Fig. 12 (a) Schematic illustration of typical organic electrochemical transistors (OECTs) for wearable biosensors, wherein organic ionic fluids can be compounded with enzymes to act as selective supporting electrolytes. (b) Chemical structure of [P1,4,4,4][Tos] and organic electrochemical transistors based on glucose oxidase (GOx) for glucose detection. The diagram on the right shows the response current of different concentrations of glucose solution. Reproduced with permission from ref. 93, copyright 2010, Royal Society of Chemistry. (c) Ionogel as an electrolyte of OECTs in a wearable lactate sensor. Reproduced with permission from ref. 91, copyright 2012, Royal Society of Chemistry. (d) Ionic liquid-based microfluidic sensing platform for the visual monitoring of sweat pH. Reproduced with permission from ref. 88, copyright 2012, Elsevier. | ||
Ionic liquids not only have a strong ability to stabilize and disperse enzymes but also are outstanding carrier materials for dyes. Benito-Lopez et al. immobilized four kinds of dyes in an ionogel to fabricate a wearable chromogenic barcode that could monitor the pH value of sweat (Fig. 12d).88 With the participation of microfluidic technology, fresh sweat can be transported to the indicative dye region, and the real-time monitoring and analysis of fresh sweat can be realized. However, the color contrast of the sensing material is not significant, and it therefore needs a specific algorithm to map the hue saturation value to quantify color variation. In the future, great efforts should be devoted to the improvement of chromatic sensitivity; then such a visual sensor will undoubtedly become the ideal platform for non-invasive, low-cost and efficient wearable sweat pH monitoring.
5. Conclusion and perspectives
The last decade has witnessed significant progress in organic ionic fluids in the field of healthcare. They have become a class of competitive conductors for flexible wearable sensors due to their excellent electrochemical stability, ultra-high compliance, negligible saturated vapor pressure and high ionic conductivity. There are a great number of examples of formulating ionic fluids as electrical sensors for mechanical stimulus response. In addition, many organic ionic fluids have outstanding thermal stability and exhibit sensitive electrical response to temperature changes, which can be exploited in flexible thermometers for the precise detection of temperature variation. Noteworthily, organic ionic fluids can also dissolve and stabilize polar molecules, bio-macromolecules and organic dye molecules, extending them as host materials for chemical sensors with expanded sensing abilities. Herein, we systematically summarize the types of organic ionic fluids along with their preparation techniques and the strategies of related sensors. Combined with the sensing mechanisms and possible disease diagnostic models, we also review the latest research on organic ionic fluids in healthcare, including the aspects of human movement, body temperature, the respiratory system, the cardiovascular system and biomarkers in sweat. While there have been tremendous advances in such wearable sensors for healthcare, onerous challenges still remain.The complexity of the realistic sensing atmosphere and the overall structure of the device greatly limit the detection performance of the sensor, and so the pursuit of excellent performance of the sensing material is always a popular topic in the field of sensing. For instance, the gradient distribution of human epidermal temperature and the interference of ambient temperature are notable factors affecting the accuracy of temperature detection. Therefore, in order to accurately measure the temperature of the human body directly, sensors with ultra-thin nature should be guaranteed to be tightly attached to the skin. The curve of electrical response signal with temperature of ionic liquids typically obeys VTF equation, which limits its thermal sensitivity near the range of human body temperature as opposed to its sensitivity in the high-temperature region. Herein, we propose a strategy of tunable thermal activation to improve the thermal sensitivity of ionic liquids in the low-temperature region. In the field of chemical sensing, the adsorption of conventional ionic fluids towards polar molecules is broad-spectrum. Although the specificity of sensing materials has been improved by using highly specific enzymes or selective chemical reactions, the adsorbed non-target molecules still cause irreversible damage to the service life and sensing performance of devices. In the future, ionic fluids with molecular recognition ability can be developed to enhance the overall selectivity of sensing devices.
Toxicity and biocompatibility are unavoidable issues of wearable sensors for healthcare. It is a fundamental and critical requirement for qualified sensors to ensure that sensors in direct contact with the skin do not cause allergic and inflammatory reactions during long-term service. Although the existing preparation strategies for organic ionic fluids have been able to avoid the risk of leakage, the problem of microscopic molecular diffusion still exists. The development of biocompatible ionic fluids with good sensing performance and the long-term safety evaluation of sensors are important basic research topics urgently needed at present.
From the perspective of user experience, wearable sensors are expected to be insensitive and unobtrusive and not have an unsatisfactory influence on the daily lives of people. Therefore, it is necessary to realize the miniaturization of flexible sensing devices by using more sophisticated fluid-processing technology. Moreover, the miniaturization of devices is beneficial in improving the detection performance of sensors. In summary, the miniaturization of devices is another valuable research direction in this field. Driven by these foundations, the systematic integration of multifunctional sensing materials will eventually become a reality.
The existing sensor system, which takes an electrical signal as the output mode, still extensively relies on electronic circuit-based signal-reading equipment. However, the introduction of intrinsically rigid electronic circuits will break the originally intended flexibility of the sensing system. The development of an all-liquid sensing and signal output system will enable the modulus matching of the sensing system and signal reading system. Despite its awful oxidation, liquid metal is undoubtedly the best choice at present due to its comparable conductivity with conventional metals. The signal conversion mechanism between liquid metal and ionic systems needs to be studied in depth.
The integration of healthcare and feedback therapy is a potential development trend of wearable intelligent medical treatment. Since ionic liquids can be used as drug precursors and drug delivery carriers, and some ionic liquids also have antibacterial and antiviral effects, wearable medical devices based on ionic liquids that integrate biomedical uses and sensing functions is another attractive perspective.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21825503).References
- V. Amoli, J. S. Kim, S. Y. Kim, J. Koo, Y. S. Chung, H. Choi and D. H. Kim, Adv. Funct. Mater., 2020, 30, 1904532 CrossRef CAS.
- C. Zhao, Y. Wang, G. Tang, J. Ru, Z. Zhu, B. Li, C. F. Guo, L. Li and D. Zhu, Adv. Funct. Mater., 2022, 32, 2110417 CrossRef CAS.
- R. Scaffaro, A. Maio and M. C. Citarrella, Eur. Polym. J., 2021, 151, 110421 CrossRef CAS.
- Y. Lee, J. Park, A. Choe, S. Cho, J. Kim and H. Ko, Adv. Funct. Mater., 2020, 30, 1904523 CrossRef CAS.
- M. Y. Lee, H. R. Lee, C. H. Park, S. G. Han and J. H. Oh, Acc. Chem. Res., 2018, 51, 2829 CrossRef CAS PubMed.
- F. Sun, M. Tian, X. Sun, T. Xu, X. Liu, S. Zhu, X. Zhang and L. Qu, Nano Lett., 2019, 19, 6592 CrossRef CAS PubMed.
- Q. Gui, Y. He and Y. Wang, Adv. Electron. Mater., 2021, 7, 2000780 CrossRef CAS.
- J. Kim, A. S. Campbell, B. E.-F. de Ávila and J. Wang, Nat. Biotechnol., 2019, 37, 389 CrossRef CAS PubMed.
- A. Sharma, A. Singh, V. Gupta and S. Arya, Sens. Diagn., 2022, 1, 387 RSC.
- H. C. Koydemir and A. Ozcan, Annu. Rev. Anal. Chem., 2018, 11, 127 CrossRef CAS PubMed.
- Q. Lyu, S. Gong, J. Yin, J. M. Dyson and W. Cheng, Adv. Healthcare Mater., 2021, 10, 2100577 CrossRef CAS PubMed.
- J. Yi and Y. Xianyu, Adv. Funct. Mater., 2022, 32, 2113012 CrossRef CAS.
- Z. Ma, Q. Huang, Q. Xu, Q. Zhuang, X. Zhao, Y. Yang, H. Qiu, Z. Yang, C. Wang, Y. Chai and Z. Zheng, Nat. Mater., 2021, 20, 859 CrossRef CAS PubMed.
- Y. He, Q. Gui, Y. Wang, Z. Wang, S. Liao and Y. Wang, Small, 2018, 14, 1800394 CrossRef PubMed.
- N. Gao, Q. Ma, Y. He and Y. Wang, Chem. J. Chin. Univ., 2020, 41, 901 CAS.
- Y. He, X.-Q. Xu, S. Lv, H. Liao and Y. Wang, Langmuir, 2019, 35, 1192 CrossRef CAS PubMed.
- H. Jia, Y. He, X. Zhang, W. Du and Y. Wang, Adv. Electron. Mater., 2015, 1, 1500029 CrossRef.
- S. Li, Y. Li, Y. Wang, H. Pan and J. Sun, CCS Chem., 2021, 3, 3360 CrossRef.
- R. Patel, M. Kumari and A. B. Khan, Appl. Biochem. Biotechnol., 2014, 172, 3701 CrossRef CAS PubMed.
- R. D. Munje, S. Muthukumar, B. Jagannath and S. Prasad, Sci. Rep., 2017, 7, 1950 CrossRef PubMed.
- W. Gao, H. Ota, D. Kiriya, K. Takei and A. Javey, Acc. Chem. Res., 2019, 52, 523 CrossRef CAS PubMed.
- C.-C. Kim, H.-H. Lee, H. Oh Kyu and J.-Y. Sun, Science, 2016, 353, 682 CrossRef CAS PubMed.
- D.-H. Kim, J.-H. Cha, J. Y. Lim, J. Bae, W. Lee, K. R. Yoon, C. Kim, J.-S. Jang, W. Hwang and I.-D. Kim, ACS Nano, 2020, 14, 16907 CrossRef CAS PubMed.
- J. P. Adelman, J. Maylie and P. Sah, Annu. Rev. Physiol., 2012, 74, 245 CrossRef CAS PubMed.
- A. Dhaka, V. Viswanath and A. Patapoutian, Annu. Rev. Neurosci., 2006, 29, 135 CrossRef CAS PubMed.
- D. Julius, Annu. Rev. Cell Dev. Biol., 2013, 29, 355 CrossRef CAS PubMed.
- C. Yang and Z. Suo, Nat. Rev. Mater., 2018, 3, 125 CrossRef CAS.
- X. Tao, S. Liao, S. Wang, D. Wu and Y. Wang, ACS Sens., 2018, 3, 1338 CrossRef CAS PubMed.
- B. Wang, L. Qin, T. Mu, Z. Xue and G. Gao, Chem. Rev., 2017, 117, 7113 CrossRef CAS PubMed.
- S. K. Singh and A. W. Savoy, J. Mol. Liq., 2020, 297, 112038 CrossRef CAS.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232 CrossRef CAS PubMed.
- S. Chen, J. Qi, S. Fan, Z. Qiao, J. C. Yeo and C. T. Lim, Adv. Healthcare Mater., 2021, 10, 2100116 CrossRef CAS PubMed.
- W.-H. Yeo, Y.-S. Kim, J. Lee, A. Ameen, L. Shi, M. Li, S. Wang, R. Ma, S. H. Jin, Z. Kang, Y. Huang and J. A. Rogers, Adv. Mater., 2013, 25, 2773 CrossRef CAS PubMed.
- W. B. Han, G.-J. Ko, T.-M. Jang and S.-W. Hwang, ACS Appl. Electron. Mater., 2021, 3, 485 CrossRef CAS.
- C. F. Guo and T. Pan, Adv. Mater., 2021, 33, 2003464 CrossRef PubMed.
- S. G. Yoon and S. T. Chang, J. Mater. Chem. C, 2017, 5, 1910 RSC.
- S. Wang, H. Cheng, B. Yao, H. He, L. Zhang, S. Yue, Z. Wang and J. Ouyang, ACS Appl. Mater. Interfaces, 2021, 13, 20735 CrossRef CAS PubMed.
- S. Xiao, J. Nie, R. Tan, X. Duan, J. Ma, Q. Li and T. Wang, Mater. Chem. Front., 2019, 3, 484 RSC.
- L. C. Fernandes, D. M. Correia, N. Pereira, C. R. Tubio and S. Lanceros-Méndez, ACS Appl. Polym. Mater., 2019, 1, 2723 CrossRef CAS.
- C. Esteves, S. I. C. J. Palma, H. M. A. Costa, C. Alves, G. M. C. Santos, E. Ramou, A. L. Carvalho, V. Alves and A. C. A. Roque, Adv. Mater., 2022, 34, 2107205 CrossRef CAS PubMed.
- P. Manusha and S. Senthilkumar, J. Mater. Sci.: Mater. Electron., 2022, 33, 8576 CrossRef CAS.
- G. Hussain and D. S. Silvester, Anal. Chem., 2016, 88, 12453 CrossRef CAS PubMed.
- L. Gao, X. Yang, Y. Shu, X. Chen and J. Wang, J. Colloid Interface Sci., 2018, 512, 819 CrossRef CAS PubMed.
- M. J. A. Shiddiky and A. A. J. Torriero, Biosens. Bioelectron., 2011, 26, 1775 CrossRef CAS PubMed.
- M. Czugala, R. Gorkin Iii, T. Phelan, J. Gaughran, V. F. Curto, J. Ducrée, D. Diamond and F. Benito-Lopez, Lab Chip, 2012, 12, 5069 RSC.
- X. Tao, H. Jia, Y. He, S. Liao and Y. Wang, ACS Sens., 2017, 2, 449 CrossRef CAS PubMed.
- C.-Y. Wu, W.-H. Liao and Y.-C. Tung, Lab Chip, 2011, 11, 1740 RSC.
- N. Gao, X. Wu, Y. He, Q. Ma and Y. Wang, Adv. Electron. Mater., 2020, 6, 1901388 CrossRef CAS.
- C.-W. Lai and S.-S. Yu, ACS Appl. Mater. Interfaces, 2020, 12, 34235 CrossRef CAS PubMed.
- Z. Lei and P. Wu, Nat. Commun., 2019, 10, 3429 CrossRef PubMed.
- D. Weng, F. Xu, X. Li, S. Li, Y. Li and J. Sun, ACS Appl. Mater. Interfaces, 2020, 12, 57477 CrossRef CAS PubMed.
- Y. Cao, T. G. Morrissey, E. Acome, S. I. Allec, B. M. Wong, C. Keplinger and C. Wang, Adv. Mater., 2017, 29, 1605099 CrossRef PubMed.
- Z. Chen, Q. Gui and Y. Wang, Green Chem. Eng., 2021, 2, 346 CrossRef.
- Z. Tang, X. Lyu, A. Xiao, Z. Shen and X. Fan, Chem. Mater., 2018, 30, 7752 CrossRef CAS.
- M. Wang, P. Zhang, M. Shamsi, J. L. Thelen, W. Qian, V. K. Truong, J. Ma, J. Hu and M. D. Dickey, Nat. Mater., 2022, 21, 359 CrossRef CAS PubMed.
- Y. M. Kim and H. C. Moon, Adv. Funct. Mater., 2020, 30, 1907290 CrossRef CAS.
- X. Wang, Y. Wang, X. Yang, Z. Lu, Y. Men and J. Sun, Macromolecules, 2021, 54, 10767 CrossRef CAS.
- X. Zhao, K. Zhou, Y. Zhong, P. Liu, Z. Li, J. Pan, Y. Long, M. Huang, A. Brakat and H. Zhu, Nano Res., 2021, 14, 1202 CrossRef CAS.
- G. C. Luque, M. L. Picchio, A. P. S. Martins, A. Dominguez-Alfaro, N. Ramos, I. del Agua, B. Marchiori, D. Mecerreyes, R. J. Minari and L. C. Tomé, Adv. Electron. Mater., 2021, 7, 2100178 CrossRef CAS.
- L. Cai, G. Chen, B. Su and M. He, Chem. Eng. J., 2021, 426, 130545 CrossRef CAS.
- J. Chen, F. Wang, G. Zhu, C. Wang, X. Cui, M. Xi, X. Chang and Y. Zhu, ACS Appl. Mater. Interfaces, 2021, 13, 51567 CrossRef CAS PubMed.
- J. C. Yeo and C. T. Lim, Lab Chip, 2016, 16, 4082 RSC.
- Y. He, S. Liao, H. Jia, Y. Cao, Z. Wang and Y. Wang, Adv. Mater., 2015, 27, 4622 CrossRef CAS PubMed.
- A. W. Martinez, S. T. Phillips, E. Carrilho, S. W. Thomas, H. Sindi and G. M. Whitesides, Anal. Chem., 2008, 80, 3699 CrossRef CAS PubMed.
- N. Dossi, R. Toniolo, A. Pizzariello, E. Carrilho, E. Piccin, S. Battiston and G. Bontempelli, Lab Chip, 2012, 12, 153 RSC.
- Z. Yao, P. Coatsworth, X. Shi, J. Zhi, L. Hu, R. Yan, F. Güder and H.-D. Yu, Sens. Diagn., 2022, 1, 312 RSC.
- S. Li, N. Pan, Z. Zhu, R. Li, B. Li, J. Chu, G. Li, Y. Chang and T. Pan, Adv. Funct. Mater., 2019, 29, 1807343 CrossRef.
- N. Jiang, H. Li, D. Hu, Y. Xu, Y. Hu, Y. Zhu, X. Han, G. Zhao, J. Chen, X. Chang, M. Xi and Q. Yuan, Compos. Commun., 2021, 27, 100845 CrossRef.
- X. Zheng, Y. Jia and A. Chen, Nat. Commun., 2021, 12, 4875 CrossRef CAS PubMed.
- Z. Ma, B. Su, S. Gong, Y. Wang, L. W. Yap, G. P. Simon and W. Cheng, ACS Sens., 2016, 1, 303 CrossRef CAS.
- L. Wang, Y. Wang, S. Yang, X. Tao, Y. Zi and W. A. Daoud, Nano Energy, 2022, 91, 106611 CrossRef CAS.
- D. Y. Choi, M. H. Kim, Y. S. Oh, S.-H. Jung, J. H. Jung, H. J. Sung, H. W. Lee and H. M. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 1770 CrossRef CAS PubMed.
- S. G. Yoon, H.-J. Koo and S. T. Chang, ACS Appl. Mater. Interfaces, 2015, 7, 27562 CrossRef CAS PubMed.
- M. Yao, B. Wu, X. Feng, S. Sun and P. Wu, Adv. Mater., 2021, 33, 2103755 CrossRef CAS PubMed.
- Y. Cao, Y. J. Tan, S. Li, W. W. Lee, H. Guo, Y. Cai, C. Wang and B. C. K. Tee, Nat. Electron., 2019, 2, 75 CrossRef.
- Z. Yu and P. Wu, Adv. Mater., 2021, 33, 2008479 CrossRef CAS PubMed.
- Q. Gui, Y. He, N. Gao, X. Tao and Y. Wang, Adv. Funct. Mater., 2017, 27, 1702050 CrossRef.
- Z. Chen, N. Gao, Y. Chu, Y. He and Y. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 33557 CrossRef CAS PubMed.
- X. Peng, K. Dong, C. Ning, R. Cheng, J. Yi, Y. Zhang, F. Sheng, Z. Wu and Z. L. Wang, Adv. Funct. Mater., 2021, 31, 2103559 CrossRef CAS.
- H. Zhang, A. Lowe, A. Kalra and Y. Yu, Sensors, 2021, 21, 5760 CrossRef CAS PubMed.
- H. Ota, K. Chen, Y. Lin, D. Kiriya, H. Shiraki, Z. Yu, T.-J. Ha and A. Javey, Nat. Commun., 2014, 5, 5032 CrossRef CAS PubMed.
- E. Fernandez, P. G. Saiz, N. Peřinka, S. Wuttke and R. Fernández de Luis, Adv. Funct. Mater., 2021, 31, 2010703 CrossRef CAS.
- C. H. Park, V. Schroeder, B. J. Kim and T. M. Swager, ACS Sens., 2018, 3, 2432 CrossRef CAS PubMed.
- Y. J. Hong, H. Jeong, K. W. Cho, N. Lu and D.-H. Kim, Adv. Funct. Mater., 2019, 29, 1808247 CrossRef.
- Z. Zhang, Z. Zhu, B. Bazor, S. Lee, Z. Ding and T. Pan, IEEE Trans. Biomed. Eng., 2019, 66, 3072 Search PubMed.
- M. Hammad, A. Maher, K. Wang, F. Jiang and M. Amrani, Measurement, 2018, 125, 634 CrossRef.
- Z. Yu and P. Wu, Adv. Funct. Mater., 2021, 31, 2107226 CrossRef CAS.
- V. F. Curto, C. Fay, S. Coyle, R. Byrne, C. O'Toole, C. Barry, S. Hughes, N. Moyna, D. Diamond and F. Benito-Lopez, Sens. Actuators, B, 2012, 171–172, 1327 CrossRef CAS.
- J. Kim, A. S. Campbell and J. Wang, Talanta, 2018, 177, 163 CrossRef CAS PubMed.
- G. E. Fenoy, W. A. Marmisollé, W. Knoll and O. Azzaroni, Sens. Diagn., 2022, 1, 139 RSC.
- D. Khodagholy, V. F. Curto, K. J. Fraser, M. Gurfinkel, R. Byrne, D. Diamond, G. G. Malliaras, F. Benito-Lopez and R. M. Owens, J. Mater. Chem., 2012, 22, 4440 RSC.
- N. Wang, A. Yang, Y. Fu, Y. Li and F. Yan, Acc. Chem. Res., 2019, 52, 277 CrossRef CAS PubMed.
- S. Y. Yang, F. Cicoira, R. Byrne, F. Benito-Lopez, D. Diamond, R. M. Owens and G. G. Malliaras, Chem. Commun., 2010, 46, 7972 RSC.
| This journal is © The Royal Society of Chemistry 2022 |