Open Access Article
Open Access ArticleRapid diagnosis of egg allergy by targeting ovalbumin specific IgE and IgG4 in serum on a disposable electrochemical immunoplatform†
José M.
Gordón Pidal‡
ab,
Alejandro
Valverde‡
 a,
Sara
Benedé
*c,
Elena
Molina
c,
María
Moreno-Guzmán
a,
Sara
Benedé
*c,
Elena
Molina
c,
María
Moreno-Guzmán
 d,
Miguel Ángel
López
be,
José M.
Pingarrón
d,
Miguel Ángel
López
be,
José M.
Pingarrón
 a,
Alberto
Escarpa
a,
Alberto
Escarpa
 *be and
Susana
Campuzano
*be and
Susana
Campuzano
 *a
*a
aDepartment of Analytical Chemistry, Faculty of Chemistry, Complutense University of Madrid, 28040 Madrid, Spain. E-mail: susanacr@quim.ucm.es
bDepartment of Analytical Chemistry, Physical Chemistry and Chemical Engineering, University of Alcalá, Alcalá de Henares, E-28871, Madrid, Spain. E-mail: alberto.escarpa@uah.es
cInstitute of Food Science Research (CIAL), C/Nicolás Cabrera 9, 28049, Madrid, Spain. E-mail: s.benede@csic.es
dDepartment of Chemistry in Pharmaceutical Sciences, Analytical Chemistry, Faculty of Pharmacy, Complutense University of Madrid, Av. Complutense, s/n, 28040 Madrid, Spain
eChemical Research Institute “Andrés M. del Río”, University of Alcalá, Alcalá de Henares, E-28871, Madrid, Spain
First published on 10th December 2021
Abstract
This work reports the first electrochemical bioplatforms described to date for the single and simultaneous determination of two immunoglobulin (Ig) subtypes, IgE and IgG4, considered as reliable markers for the diagnosis and attenuation of food allergy specific to ovalbumin (OVA), one of the major egg allergenic proteins. The bioplatforms are based on the use of commercial magnetic microbeads (MBs) modified with OVA (OVA-MBs) for the selective capture of target Igs and the specific detection of IgE and IgG4 by enzymatic labelling with selective secondary antibodies conjugated to the horseradish peroxidase (HRP) enzyme. Amperometric transduction is carried out on single or dual screen-printed carbon electrodes, SP(d)CEs, in the presence of the H2O2/hydroquinone (HQ) system. The developed bioplatforms provide outstanding analytical and operational characteristics in terms of sensitivity (LOD values of 0.003 kU L−1 and 0.0002 μg L−1 for IgE and IgG4, respectively), simplicity and assay time. They were used for the determination of both Igs in serum of patients diagnosed with egg allergy upon a simple dilution (50-and 1000-times for IgE and IgG4 determination, respectively).
Introduction
The prevalence of food allergies has increased in recent decades and is recognized as a major public health burden in developed countries. However, there are many people who think they have allergies, but they really do not, so the development of new tools capable of complementing current testing would help to reduce false diagnoses.1Hen egg allergy is considered one of the most common food allergies in infants and young children,2–4 affecting 1.23 to 8.9% of child population4 and showing an increasing prevalence in recent decades, particularly in developed countries.5 It is defined as an adverse reaction of immunological nature induced by egg proteins, including ovomucoid, ovalbumin, ovotransferrin and lysozyme, and is included in the group of IgE antibody-mediated allergies.3 Ovalbumin (OVA) is the most abundant protein in egg white,6 comprising approximately 54% of the total protein and is considered one of the major allergens to evaluate and diagnose egg-allergic events closely related to IgE-mediated reactions.7
Measurement of IgE antibodies against a specific allergen (such as OVA for egg allergy) is one of the diagnostic allergy tests used in clinical practices7–9 and according to recent studies such measurement can also be used for predicting allergen immunotherapy effectiveness.1 However, manifestation of allergic symptoms can be suppressed by immunoglobulin G subclasses acting as ‘blocking antibodies’,10,11 and there is evidence that IgG4 antibody responses are associated with allergic events and should be considered as factors of allergen tolerance.12,13 In fact, IgG4 attenuates allergic responses by inhibiting the activity of IgE.14 This fact plays a key role in current clinical methods for allergy diagnosis, where elevated circulating IgE in serum and decreased IgG4 levels are directly related to allergic diseases, while larger IgG4 concentrations are characteristic of tolerance in allergic episodes, leading to decreased IgE levels in serum and allowing monitoring of allergen-specific immunotherapy efficacy by correlating increased IgG4 levels with clinical responses to allergic treatments.15,16 Therefore, IgE and IgG4 levels are considered useful biomarkers of egg allergy, and their simultaneous determination can improve the prediction of allergen tolerance providing a more effective and complete diagnosis of egg allergy and allowing monitoring of affected people to determine whether egg allergy persists, or it has been outgrown.16,17 However, available methodologies usually perform the determination of IgE and IgG4 individually, so methods allowing their simultaneous quantification and meeting the stringent requirements of current clinic in terms of simplicity, affordability, rapidity, and point-of-care (POC) performance are in demand.
The methods used to diagnose immediate allergy type I hypersensitivity reactions by targeting specific IgE include both direct (in vitro) and indirect tests (in vivo and ex vivo tests).1 Skin and oral provocation tests are the most common in vivo tests and should be performed by experienced professionals under strictly controlled conditions to avoid possible risks. The procedure of a food allergy skin test, in which a small drop of a liquid food extract is placed on the skin, lasts 15–20 minutes on average and has a sensitivity between 60% and 70%.18,19 The test is safe but should be avoided in case of pathological dermal alterations and acute allergic reactions.1,20 In addition, this test must be performed individually on each patient, which makes it laborious and time-consuming. Oral challenge testing establishes a cause–effect relationship between the ingestion of a given food or food additive and the reported reaction and requires an individual risk–benefit and protocol evaluation prior to its performance.21 It cannot be applied to pregnant women, high-risk patients due to comorbidity (e.g., acute infections) and those suffering from severe life-threatening reactions.
Although the basophil activation test (BAT) is the most widely used ex vivo test, its validation is still in progress. These drawbacks and the non-optimal performance of in vivo and ex vivo tests mean that safer, cheaper, and faster in vitro diagnostic tests, based on the determination of specific IgE against a specific allergen or its derivatives in biological fluids, are currently the most widely used. Food allergy has been classified into classes according to the IgE concentration in plasma or serum, from class 0 (<0.1 kU L−1; absent or not detectable) to class VI (>100 kU L−1, very high). Accordingly, the assay must have a high sensitivity reaching the 0.1 kU L−1 IgE level, the equivalent of 0.24 ng mL−1.1
The main commercial in vitro tests in use are ELISA kits,22,23 ImmunoCAP (PHADIA, Thermofisher Scientific),24,25 which is the “gold standard” for IgE detection in serum and the only IgE singleplex test recommended by the WHO,26 the 3gAllergy Universal Kit (IMMULITE, Siemens), and the one marketed by Dr. Fooke-Achterrath Laboratorien. These last three tests allow fully automated determination of specific IgE and total IgE in 60 to 180 minutes, provide a limit of detection (LOD) of 0.35 kU L−1 and use autoanalyzers with dual-energy X-ray absorptiometry, fluorescence, and bioluminescence detection, respectively.26,27 The price of the instrumentation ranges from 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to more than 100
000 to more than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 €, depending on the performance and working capacity. Moreover, the clinical sensitivity of in vitro blood allergy tests is lower than that of in vivo skin tests,28 and this is the main reason why these tests are not often included in routine clinical protocols. It is important to be aware that certainly, there is no marketed in vitro test able to determine IgE concentrations below 0.1 kU L−1.
000 €, depending on the performance and working capacity. Moreover, the clinical sensitivity of in vitro blood allergy tests is lower than that of in vivo skin tests,28 and this is the main reason why these tests are not often included in routine clinical protocols. It is important to be aware that certainly, there is no marketed in vitro test able to determine IgE concentrations below 0.1 kU L−1.
Within this context, unique features exhibited by electrochemical bioplatforms, such as versatility to profile multiplexed biomarkers at the POC, simplicity, affordable cost and remarkably shorter analysis times compared to conventional technologies,29 make them particularly attractive tools to assist in vitro allergy diagnosis and monitor treatment response even at the specialist's office or at home. Driven by this conviction, this work reports the first electroanalytical bioplatform reported so far for the single and simultaneous determination of OVA-specific IgE and IgG4. It is important to note that although some electrochemical biosensors for IgEs have been previously reported,1,30–33 some of them require a long preparation time32,33 or have limited sensitivity31 and none of them dealt with the determination of specific IgE against food allergens. In addition, to our knowledge, no electrochemical bioplatform has been reported so far for the determination of IgG4 or targeting the simultaneous determination of different Igs related to allergic processes. The simple and quick approach presented in this work involves the use of OVA-modified magnetic bioconjugates for the specific and efficient capture of Igs from serum, which are discriminated in a further step using the specific antibody to the Ig isotype to be detected conjugated with HRP. Single or dual amperometric transduction was carried out on disposable platforms.
Experimental
Apparatus and electrodes
A multichannel potentiostat (model 1030B, CH Instruments, Austin, TX, USA) controlled by the CHI1030B software and a Magellan V 7.1 (TECAN) ELISA plate reader were used for the amperometric and spectrophotometric measurements, respectively. Single (SPCEs, DRP-110, ϕ 4 mm, active area 12.6 mm2) and dual (SPdCEs, DRP-X1110, ϕ 2 mm, active area 6.3 mm2 each working electrode) screen-printed carbon electrodes, and the respective DRP-CAC and DRP-BICAC specific connector cables from Metrohm-DropSens S.L. (Oviedo, Asturias, Spain) were used. A Wizard IR Vortex (VELP Scientifica), a Dynamag-2 magnet magnetic separator (Invitrogen Dynal AS), a basic 20+ (Crison) pH-meter, and a thermomixer MT100 incubator shaker (Universal Labortechnik) were employed. Homemade polymethylmethacrylate (PMMA) casings with one or two embedded neodymium magnets (AIMAN GZ) were also utilized.Reagents, solutions, and serum samples
All reagents and solvents used were of the highest available analytical grade. Carboxylic acid-modified MBs (HOOC-MBs, 2.7 μm Ø, Dynabeads M-270 carboxylic acid, Cat. No. 14305D) were purchased from Invitrogen™. N-(3-Dimethyl-aminopropyl)-N′-ethylcarbodiimide (EDC), N-hydroxysulfosuccinimide (sulfo-NHS), albumin from chicken egg white (OVA, Cat. No. A5503-5G), ethanolamine, hydroquinone (HQ) and 30% w/v hydrogen peroxide (H2O2) were purchased from Sigma. NaH2PO4, Na2HPO4, NaCl, KCl, HCl and NaOH were all from Scharlab. 2-Morpholinoethanesulfonic acid (MES, from Gerbu) and Tris (from Panreac) were also employed.Biotin-anti-IgE (b-anti-IgE), biotin-anti-IgG4 (b-anti-IgG4) and avidin-HRP (Av-HRP) were acquired from ThermoFisher Scientific. Streptavidin-HRP (Strep-HRP) was purchased from Roche Diagnostics GmbH. Serum samples were obtained from the Institute of Food Science Research (CIAL) serum library, with the informed consent of the patients, and the Bioethics Committee of the CIAL approved the procedures for the correct use of the samples which were stored at −80 °C until analysis. Serum samples from egg-allergic patients with ImmunoCAP validated concentrations of 60.9 kU L−1 IgE or 32 μg L−1 IgG4 were used as standards for the optimization and characterization of the bioplatforms. It is important to note that the results for the two Ig isotypes are expressed in different units because these are the ways they are usually expressed in the clinic (1 kU L−1 is equivalent to 2.4 μg L−1 IgE1).
Buffer solutions used, all prepared in Milli-Q water from a Millipore Milli-Q purification system 18.2 MΩ cm, include: 0.1 M phosphate buffered saline solution pH 7.4 (PBS) containing 0.137 M NaCl and 0.027 M KCl; 0.05 M phosphate buffer solution pH 6.0 (PB); 0.1 M phosphate buffer solution pH 8.0; 0.025 M MES buffer solution pH 5.0 and 0.1 M Tris-HCl solution pH 7.2. Blocker™ Casein in PBS (a ready-to-use PBS solution containing 1.0% w/v purified casein, blocking buffer, BB) was purchased from Thermo Scientific.
Preparation of the magnetic bioconjugates
The bioplatforms developed involve OVA-modified MBs which were prepared according to the following procedure: a 3 μL aliquot of the HOOC-MB suspension was washed twice with 50 μL of 0.025 M MES buffer pH 5.0 for 10 min (25 °C, 950 rpm) in a 1.5 mL microcentrifuge tube. After each washing, MBs were concentrated for 3 min in the magnetic concentrator and the supernatant was removed. The MB carboxylic acid groups were activated by incubation with EDC/sulfo-NHS mixture solution for 35 min (25 °C, 950 rpm). Activated MBs were washed twice with 50 μL of 0.025 M MES buffer pH 5.0 and incubated with 25 μL of 50 μg mL−1 OVA solution prepared in 0.025 M MES buffer pH 5.0, for 30 min (25 °C, 950 rpm). Thereafter, the resulting OVA-MBs were washed twice with 50 μL of 0.025 M MES buffer pH 5.0 and incubated with 25 μL of 1 M ethanolamine solution prepared in 0.1 M phosphate buffer pH 8.0 for 60 min (25 °C, 950 rpm) to block the residual active groups. The resulting OVA–MBs were washed three times: first with 50 μL of 0.1 M Tris-HCl pH 7.2, then twice with 50 μL of BB solution and stored at 4 °C until use for the determination of IgE or IgG4.The determination of IgE involved incubation of OVA-MBs with 25 μL of a mixture solution (prepared in BB) containing the appropriate concentration of standard serum IgE (or the 1/50 diluted serum sample to analyze) supplemented with 1/1000 diluted b-anti-IgE, for 60 min (25 °C, 950 rpm). Next, the b-anti-IgE-IgE-OVA-MBs were washed twice with 50 μL of BB solution and incubated with 25 μL of 1/5000 diluted Av-HRP prepared in BB solution for 30 min (25 °C, 950 rpm). The determination of IgG4 implied the incubation of OVA-MBs for 60 min (25 °C, 950 rpm) with 25 μL of a mixture solution (prepared in BB) containing the appropriate concentration of standard serum IgG4 (or the 1/1000 diluted serum sample to analyze) supplemented with 1/1000 diluted b-anti-IgG4 and 1/5000 diluted Av-HRP.
The modified MBs (Av-HRP-b-anti-IgE-IgE-OVA-MBs and Av-HRP-b-anti-IgG4-IgG4-OVA-MBs) were washed twice with 50 μL of BB solution and re-suspended in 50 or 5 μL of 0.05 M PB pH 6.0 to carry out the single or dual amperometric determination at SPCEs or SPdCEs, respectively.
Amperometric measurements
The as-prepared 50 μL or 5 μL-aliquots of the magnetic bioconjugates suspension were deposited onto the surface of the SPCE or SPdCE (previously placed in the PMMA casing provided with neodymium magnets) working electrodes, respectively. The SP(d)CE/casing assembly was connected to the potentiostat and immersed in an electrochemical cell containing 10 mL of 0.05 M PB pH 6.0 supplemented with 1 mM fresh HQ. Amperometric measurements were performed at room temperature in stirred solutions at −0.20 V vs. the Ag pseudoreference electrode by monitoring the cathodic current variation occurring upon the addition of 50 μL of a 0.1 M H2O2 solution, recently prepared in 0.05 M PB pH 6.0. Unless otherwise specified, results shown in this work correspond to the mean value of the difference between the steady-state and the background current obtained in three replicates with the error bars estimated as triple the standard deviation of each set of replicates (α = 0.05).Analysis of serum
The determination of OVA-specific IgE and IgG4 was performed in 50-times and in 1000-times diluted serum samples prepared in BB, respectively. No significant matrix effect was observed using the diluted serum samples and, therefore, the determination of both Igs was carried out by interpolation of the measured amperometric signals into the respective calibration plot constructed with the validated serum standard solutions. The results obtained with the developed bioplatforms were compared with those provided by the ELISA methodology using the same immunoreagents and according to the following protocol. 96 well-plates were coated with 50 μg mL−1 of OVA and blocked with PBS-Tween 20 2.5%. Serum samples were diluted (1/50 and 1/100 for IgE and 1/100 to 1/1400 for IgG4 determination) in PBS-Tween 20 0.05%, added to the plate and incubated for 1 h at room temperature. After washing, detection of IgE and IgG4 was performed by adding b-anti-IgE (1/1000) and b-anti-IgG4 (1/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) followed by Av-HRP (1/10
000) followed by Av-HRP (1/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and 3,3′,5,5′-tetramethylbenzidine (TMB) (incubation times of 1 h and 30 min, respectively) as the substrate. The optical density was measured at 450 nm after stopping the reaction with H2SO4 0.5 M.
000) and 3,3′,5,5′-tetramethylbenzidine (TMB) (incubation times of 1 h and 30 min, respectively) as the substrate. The optical density was measured at 450 nm after stopping the reaction with H2SO4 0.5 M.
Results and discussion
A similar rationale was involved in the determination of both Igs (Fig. 1). It relied on direct immunoassays implemented on the surface of HOOC-MBs covalently modified with OVA using EDC/sulfo-NHS chemistry. The developed strategies profited the benefits offered by MBs to provide a highly accessible functionalized surface allowing an efficient target/ligand interaction and their easy and rapid separation from solution thus minimizing mass transfer barriers and biofouling problems that often occur when the sample matrix is exposed directly to the electrode surface.34–36 Igs from egg-allergic serum were selectively captured onto OVA-MBs and the target ones (IgE or IgG4) were recognized with specific biotinylated antibodies (b-anti-IgE or b-anti-IgG4) that were enzymatically labelled with Avidin-HRP (Av-HRP). The MBs carrying the HRP labelled immunocomplexes were magnetically captured on the surface of disposable screen-printed electrodes for single or dual determination and amperometric transduction was performed at −0.20 V vs. the Ag pseudo-reference electrode in the presence of the H2O2/HQ system.37 The resulting cathodic current variation, attributed to the enzymatic reduction of H2O2 mediated by HQ, was directly proportional to the concentration of the target Igs in the analyzed sample.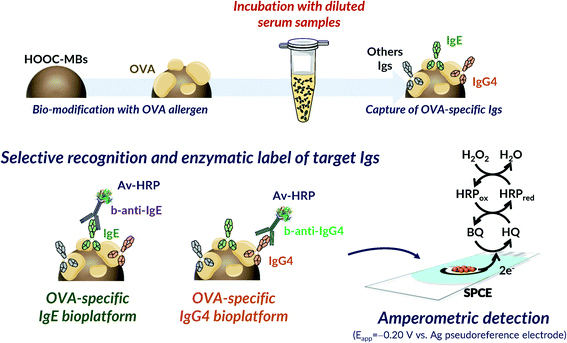 | ||
| Fig. 1 Schematic display of the fundamentals involved in the bioplatforms developed for the amperometric determination of the OVA-specific IgE and IgG4. | ||
It is important to mention at this point that calibration is a matter of concern in allergy biosensing due to the non-availability of allergen-specific standards. The WHO standard, which is a mixture of non-food specific human IgEs, is commonly used as a calibrator. However, since from an analytical point of view a better calibrator should be a food-specific IgE with the same affinity for the antigen as the IgE produced by patients who have suffered an allergic event,1 we have used in this work serum samples from egg-allergic patients with validated concentrations of 60.9 kU L−1 IgE and 32 μg L−1 IgG4 as standards for the optimization and characterization of the bioplatforms.
Evaluation of key experimental variables
The influence of key experimental variables involved in the immunoassays for the single determination of each target Ig was evaluated. For this purpose, the amperometric responses obtained in the absence (blank, B) and in the presence (signal, S) of 0.30 kU L−1 IgE and 0.036 μg L−1 IgG4 serum standard solutions were compared. Larger signal/blank (S/B) ratio values were adopted as the criterion to select the experimental variables. In addition, experimental variables such as the amount of magnetic microcarriers and those involved in the amperometric transduction (detection potential, pH, composition of the supporting electrolyte and H2O2 and HQ concentrations) were taken from previous works.38–40 The results obtained in the optimization studies are shown in Fig. S1 and S2 and summarized in Table S1 (in the ESI†).A key variable is the modification of activated MBs by incubation with an OVA solution. As can be seen in Fig. S1 and S2a) and b),† the use of concentrations larger than 50 μg mL−1 and incubation times longer than 30 min gave rise to smaller S/B ratios. This effect can be attributed to a worse recognition of the target Igs due to steric hindrance when a large OVA loading is immobilized on the MB surface.41 It is important to note the low S/B values in the absence of immobilized OVA for both target Igs compared to those obtained when the selected OVA amount is used (0 vs. 50 bars in Fig. S1a and S2a†), confirming that only OVA-specific Igs were detected. Another important variable is the number of performed incubation steps from the preparation of the OVA-MBs. The following protocols were tested: 1) a single incubation step with a mixed solution containing the serum standard, b-anti-IgE or b-anti-IgG4 and Av-HRP; 2) two successive incubation steps: 2A) a first incubation with a mixture solution containing the serum standard and b-anti-IgE or b-anti-IgG4, followed by a further incubation with Av-HRP, 2B) an initial incubation with the standard serum and a subsequent incubation with a mixture solution containing b-anti-IgE or b-anti-IgG4 and Av-HRP; 3) three successive incubation steps in the serum standard, b-anti-IgE or b-anti-IgG4 and Av-HRP solutions. According to the results shown in panels c of Fig. S1 and S2,† while protocol 2A was found to be favourable for the determination of both Ig isotypes, the S/B ratio was slightly larger using protocol 1 for the determination of IgG4. Since batches of MBs were individually prepared for the determination of each Ig isotype, different protocols, 2A for IgE and 1 for IgG4, were selected. Nevertheless, protocol 2A can be used for the determination of both Igs, if considered convenient, without a big loss in sensitivity for the IgG4 determination.
Moreover, Fig. S1 and S2† show that 1/1000 and 1/5000 dilutions of secondary antibody (panel d) and Av-HRP (panels f and e, respectively) provided larger S/B ratios for both Igs. Furthermore, the S/B ratios obtained by performing enzyme labelling with streptavidin-HRP (Strep-HRP) or Av-HRP were compared. Fig. S3 in the ESI† shows that a significant improvement in the measured ratio was found using Av-HRP for both target Igs. Although streptavidin is less prone to non-specific binding because it is not glycosylated, the avidin–biotin binding is the strongest known non-covalent interaction between a protein and a ligand. Therefore, the obtained results (similar to those observed using the same immunoreagents in a conventional ELISA strategy) can be attributed to the use of conjugates from different companies and/or to the different affinities of both proteins for biotin (Kd): Av: ∼1.3 × 10−15 M and Strep: ∼0.04 × 10−15 M.
Table S1 (in the ESI†) summarizes the results obtained in the evaluation of the variables optimized for the single determination of both types of Igs, including the checked ranges and the values selected.
Analytical characteristics
The calibration plots constructed with the bioplatforms prepared under the optimized experimental conditions for the amperometric determination of each target Ig, making serial dilutions of the serum samples from egg-allergic patients with validated concentrations of IgE (60.9 kU L−1) and IgG4 (32 μg L−1) used as standards, are displayed in Fig. 2. According to the employed bioassay format, in both cases the variation of the measured cathodic current increases with the respective Ig concentration. The corresponding analytical characteristics are summarized in Table 1.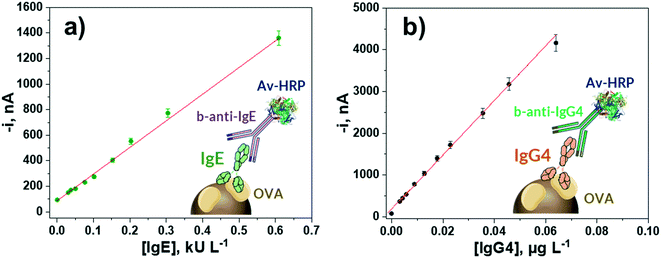 | ||
| Fig. 2 Calibration plots constructed with the developed amperometric bioplatforms for the determination of OVA-specific IgE (a) and IgG4 (b) serum standard solutions. | ||
| Parameters | IgE bioplatform | IgG4 bioplatform |
|---|---|---|
| Linear range | (0.01–0.60) kU L−1 | (0.0006–0.0640) μg L−1 |
| R 2 | 0.994 | 0.998 |
| Slope | (2100 ± 100) nA L kU−1 | (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 2000) nA L μg−1 000 ± 2000) nA L μg−1 |
| Intercept | (90 ± 5) nA | (170 ± 10) nA |
| LOD | 0.003 kU L−1 (0.0072 ng mL−1) | 0.0002 μg L−1 |
| LQ | 0.01 kU L−1 | 0.0006 μg L−1 |
| RSD, % (n = 10) | 4.1 | 4.8 |
The limit of detection (LOD) and quantification (LQ) values were estimated according to 3 × sb/slope and 10 × sb/slope, respectively, where sb is the standard deviation of 10 amperometric measurements in the absence of the target Ig. The wide linear ranges and low LOD values provided by the developed bioplatforms allow the detection and follow-up of egg allergic patients. Moreover, the relative standard deviation (RSD) values, calculated from the amperometric responses for 0.30 kU L−1 IgE and 0.036 μg L−1 IgG4 serum standard solutions, provided by 10 different bioplatforms prepared in the same way on the same day, were 4.1 and 4.8%, respectively, thus showing the robustness of the procedures used for the preparation of magnetic bioconjugates and the amperometric detection at SPCEs.
The lack of electrochemical biosensors reported for the determination of these Ig isotypes precludes a comprehensive comparison of the obtained analytical characteristics. However, the achieved LOD values are in between those reported for the total content of IgE, i.e., not allergen-specific IgE (42 fg mL−1–23 ng mL−1).31,32 Nevertheless, the prepared bioplatforms provide important competitive advantages in terms of simplicity and assay time.
Compared with other methodologies, Feyzkhanova et al.42 developed a method for the simultaneous quantitative analysis of IgE and IgG4 through a microarray-based method. The good sensitivity achieved is overshadowed by the 20 h of serum incubation to perform the fluorescence determination. In the case of ELISA methodologies, very recently Zhang et al.23 used an indirect ELISA for the detection of allergen specific IgE in the serum of cow's milk allergic children in a 3 h assay time, while Su et al.22 reported an ELISA test method for detecting serum IgG4 levels in 4 h. Similarly, the ELISA methodology developed in our laboratory using the same immunoreagents as those for the electrochemical bioplatforms took 4 h. Importantly, compared to ELISA methodologies, the developed bioplatforms are similar or competitive for IgG4 detection in terms of sensitivity (linear ranges of (0.03–0.3) kU L−1 and (0.0057–0.0457) μg L−1 for IgE and IgG4, respectively, with the same immunoreagents), affordability, compatibility with multiplexed determinations and POC operation. Regarding the ImmunoCAP technology, the achieved LOD values for IgE24 and IgG425 in human serum or plasma are 0.1 kU L−1 and 0.0007 μg L−1, respectively, in a similar assay time to that needed with the developed bioplatforms (100 min vs. 90 min). Therefore, although the ImmunoCAP is extensively used as the ‘gold standard’ for the determination of IgE or IgG4, the electrochemical bioplatforms reported in this work exhibit remarkable advantages in terms of sensitivity for IgE (LOD values of 0.003 vs. 0.1 kU L−1), cost-effective instrumentation and volume of serum required per determination (1 μL vs. 40 μL), which make them useful tools for the rapid and accurate determination of IgE and IgG4 levels in serum in any setting.
It is worth noting that the analytical characteristics of the developed bioplatforms, with a LQ of 0.01 kU L−1 for IgE, would allow placing on the market in vitro tests capable of determining IgE concentrations below 0.1 kU L−1, which is currently lacking.
With the aim of shortening the whole preparation of the bioplatforms, the storage stability of OVA-MBs was tested (Fig. S4 in the ESI†). OVA-MBs were stored after preparation (day 0) at 4 °C in 50 μL of filtered PBS pH 7.4, and the amperometric responses provided by the bioplatforms prepared using the stored OVA-MBs for 0 and 0.30 kU L−1 IgE (Fig. S4a†) and 0.036 μg L−1 IgG4 (Fig. S4b†) were recorded during several control days. The results obtained showed that no significant differences between the S/B values provided by both immunoconjugates were observed for at least 25 days after the OVA-MB preparation, showing that the determination can be completed in less than 90 minutes.
In addition, the selectivity of the biosensing platforms against other coexisting proteins in serum was tested (Fig. S5 in the ESI†). The amperometric responses measured for 0 and 0.30 kU L−1 IgE (Fig. S5a†), and 0.036 μg L−1 IgG4 (Fig. S5b†) serum standard solutions were compared with those obtained in the presence of human serum albumin (HSA) and human IgG (HIgG) at the concentration levels expected in healthy individuals. Fig. S5† shows significant interference in the presence of HSA and HIgG for both Igs. These interferences are probably due to the presence of human antibodies reactive with animal proteins (human anti-animal antibodies, HAAA), which can coexist with HIgG and not purified HSA.43 However, Fig. S5† shows that a 50-fold dilution of HSA and HIgG allowed the interferences to be avoided, thus ensuring that they will not be a limitation when 50-fold or more diluted serum samples are analyzed.
Application to the analysis of serum samples from egg-allergic and non-allergic individuals
The developed bioplatforms were applied for the single determination of the target Igs in serum from non-egg allergic individuals and from patients diagnosed with egg allergy. The existence of a possible matrix effect was first evaluated. Calibration plots constructed for IgE and IgG4 serum standard solutions prepared in buffered solution (Fig. 2a and b) were compared with those constructed in a representative 50-(IgE) or 1000-(IgG4) times diluted sample from a control volunteer. The texp values calculated (n = 5; α = 0.05) when the slope values of both calibration plots were compared were 0.351 and 0.932 for IgE and IgG4, respectively, smaller than the ttab value of 4.303. Therefore, no significant matrix effect was observed under the mentioned conditions and, accordingly, the determination of the two target Igs could be performed by simple interpolation of the amperometric responses obtained for the diluted samples into the calibration graphs prepared with the OVA-specific IgE and IgG4 serum standard solutions.The results obtained for the quantification of the endogenous content of OVA-specific IgE and IgG4 in the serum samples of the 7 healthy individuals and the 16 egg-allergic patients analyzed are summarized in Table 2. In addition, the results provided by the ELISA methodology involving the same immunoreagents are given. As expected, a significantly higher expression of OVA-specific IgE was found in egg-allergic patients compared with the control group (Fig. 3), thus confirming the usefulness of the developed IgE bioplatform to perform reliable diagnosis of egg-allergy using minimal volumes of serum (0.5 μL sample/determination).
| Subject | Sample | Gender | Age (years) | IgE,a kU L−1 (RSD,b %) | t exp | IgG4,a μg L−1 (RSD,b %) | t exp | ||
|---|---|---|---|---|---|---|---|---|---|
| Bioplatform | ELISA | Bioplatform | ELISA | ||||||
| a Mean value ± ts√n; n = 3; α = 0.05. b n = 3 replicates. | |||||||||
| CT | 1 | M | 4 | 0.03 ± 0.01 (9.6) | 0.01 ± 0.04 (173) | 1.979 | 0.07 ± 0.02 (9.6) | 0.12 ± 0.09 (30.7) | 1.149 |
| 2 | M | 5 | 0.022 ± 0.004 (8.0) | 0.02 ± 0.05 (100) | 0.055 | 0.12 ± 0.02 (7.7) | 0.10 ± 0.04 (15.8) | 1.917 | |
| 3 | F | 2 | 0.015 ± 0.003 (8.9) | 0.02 ± 0.05 (89.2) | 0.138 | 0.11 ± 0.03 (9.6) | 0.10 ± 0.08 (31.1) | 0.513 | |
| 4 | F | 6 | 0.05 ± 0.01 (8.3) | 0.1 ± 0.2 (90.8) | 0.432 | 0.15 ± 0.04 (9.6) | 0.2 ± 0.1 (21.0) | 2.532 | |
| 5 | M | 5 | 0.03 ± 0.01 (8.3) | 0.01 ± 0.04 (173) | 0.864 | 0.51 ± 0.07 (5.6) | 0.49 ± 0.09 (7.3) | 0.746 | |
| 6 | F | 43 | 0.021 ± 0.004 (7.7) | 0.00 ± 0.01 (173) | 2.605 | 0.04 ± 0.01 (9.2) | 0.0 ± 0.1 (9.3) | 1.707 | |
| 7 | M | 8 | 0.05 ± 0.01 (8.9) | 0.1 ± 0.1 (86.6) | 0.862 | 0.12 ± 0.02 (5.8) | 0.1 ± 0.2 (61.3) | 0.456 | |
| EA | 8 | F | 7 | 5.9 ± 0.5 (3.3) | 5 ± 1 (7.6) | 2.458 | — | — | — |
| 9 | F | 5 | 19 ± 3 (7.4) | 20 ± 8 (16.0) | 0.488 | — | — | — | |
| 10 | M | 6 | 11 ± 2 (7.7) | 13 ± 3 (7.6) | 2.574 | — | — | — | |
| 11 | F | 11 | 10 ± 2 (6.8) | 9 ± 3 (11.6) | 1.326 | — | — | — | |
| 12 | F | 5 | 12 ± 2 (5.5) | 11 ± 2 (7.5) | 1.621 | — | — | — | |
| 13 | M | 2 | 12 ± 3 (8.7) | 13 ± 4 (11.5) | 0.959 | — | — | — | |
| 14 | M | 4 | 6 ± 1 (6.1) | 6 ± 2 (11.1) | 0.229 | — | — | — | |
| 15 | M | 3 | 7 ± 2 (8.5) | 7 ± 5 (28.8) | 0.083 | — | — | — | |
| 16 | F | 1 | 8 ± 1 (7.0) | 7 ± 2 (13.5) | 1.495 | — | — | — | |
| 17 | M | 10 | — | — | — | 23 ± 1 (2.2) | 22 ± 4 (7.8) | 0.984 | |
| 18 | M | 3 | — | — | — | 23 ± 1 (1.6) | 21 ± 2 (4.5) | 2.665 | |
| 19 | M | 13 | — | — | — | 3.5 ± 0.5 (6.2) | 3.4 ± 0.3 (3.9) | 0.681 | |
| 20 | M | 3 | — | — | — | 11 ± 1 (3.7) | 10 ± 1 (5.4) | 2.513 | |
| 21 | F | 4 | — | — | — | 4.7 ± 0.8 (7.2) | 4 ± 2 (17.2) | 1.451 | |
| 22 | M | 9 | — | — | — | 1.1 ± 0.3 (9.6) | 1.2 ± 0.3 (8.5) | 1.145 | |
| 23 | F | 42 | — | — | — | 4.2 ± 0.7 (7.2) | 5 ± 1 (10.4) | 2.331 | |
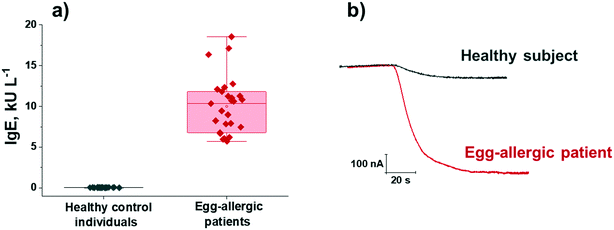 | ||
| Fig. 3 Serum OVA-specific IgE concentrations measured with the developed bioplatform in 7 serum samples from control individuals and 9 from egg-allergic patients (three replicates per determination included) (a); real amperometric traces recorded for representative serum samples from a healthy subject (sample 1 in Table 2) and an egg-allergic patient (sample 9 in Table 2) (b). Boxplots in a) express range, median and interquartile range (IQR). | ||
In addition, the results in Table 2 show that no significant differences were apparent between the results provided by the bioplatforms and the ELISA method (in all cases texp values were lower than the ttab at the α = 0.05 significance level, 2.776).44 Such agreement is confirmed by the excellent correlation plots displayed in Fig. S6 (in the ESI†).
Moreover, the possibility of using the bioplatforms in POC devices was checked. To do that, the amperometric responses provided by the bioplatforms prepared from the magnetic bioconjugates resulting from incubating the OVA-MBs in a single 10 min-step in mixtures containing the serum sample supplemented with the detection antibody and the enzymatic tracer were compared for representative control volunteers and egg-allergic patients. Fig. 4 shows the obtained results. Importantly, even under these demanding conditions, it was possible to clearly identify patients with egg allergy, confirming the suitability of the developed methodology for the diagnosis/monitoring of these patients during routine consultation.
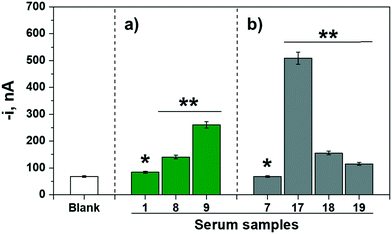 | ||
| Fig. 4 Amperometric responses measured for the determination of a) IgE (green bars) and b) IgG4 (grey bars) with bioplatforms prepared from magnetic bioconjugates resulting from incubation of the OVA-MBs in a single 10 min-step in mixtures containing the serum sample supplemented with the detection antibody and the enzymatic tracer for representative control volunteers (*) and egg-allergic patients (**). For comparison purposes the response provided by the bioplatforms in the absence of the target Ig (blank, similar with both bioplatforms) is shown. The reference for the tested samples corresponds to those given in Table 2. | ||
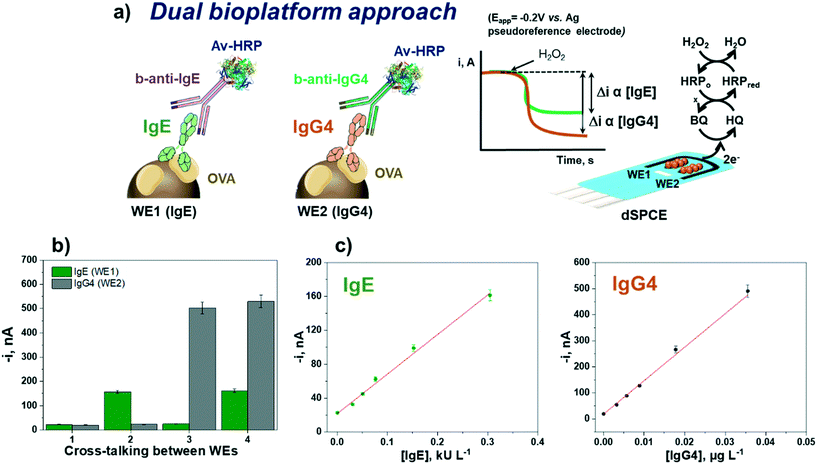
Dual bioplatform for the simultaneous determination of IgE and IgG4
Once the suitability and applicability of the developed electrochemical bioplatforms for the single determination of OVA-specific IgE and IgG4 were demonstrated, the simultaneous determination of IgE and IgG4 was implemented on a dual transduction disposable platform (Fig. 5) according to the procedure described in section “Analysis of serum”.The reliability of the methodology was verified by checking the possible crosstalk between adjacent working electrodes45 through the measurement of serum standard mixture solutions with different concentrations of both Igs. Fig. 5b shows that no apparent cross-reactivity occurred, thus demonstrating the feasibility of the bioplatform for the dual determination of OVA-specific IgE and IgG4.
The calibration plots for IgE and IgG4 serum standard solutions constructed with the dual electrochemical bioplatform (Fig. 5c) provided linear ranges over 0.02–0.30 kU L−1 (R2 = 0.99) for IgE, fitting the equation i (nA) = (470 ± 30) nA L kU−1 × [IgE] kU L−1 + (22 ± 2) nA, and over 0.003–0.036 μg L−1 (R2 = 0.992) for IgG4, according to the equation i (nA) = (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 ± 600) nA L μg−1 × [IgG4] μg L−1 + (19 ± 2) nA. Since the conjugates used were prepared in the same way, the lower sensitivity found at SPdCEs (SlopeIgE = 470 nA L kU−1 and SlopeIgG4 = 12
900 ± 600) nA L μg−1 × [IgG4] μg L−1 + (19 ± 2) nA. Since the conjugates used were prepared in the same way, the lower sensitivity found at SPdCEs (SlopeIgE = 470 nA L kU−1 and SlopeIgG4 = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 nA L μg−1) compared with that achieved at single SPCEs (SlopeIgE = 2100 nA L kU−1 and SlopeIgG4 = 65
900 nA L μg−1) compared with that achieved at single SPCEs (SlopeIgE = 2100 nA L kU−1 and SlopeIgG4 = 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nA L μg−1) should be attributed solely to the smaller working electrode surface area in SPdCEs compared to SPCEs (6.3 vs. 12.6 mm2).
000 nA L μg−1) should be attributed solely to the smaller working electrode surface area in SPdCEs compared to SPCEs (6.3 vs. 12.6 mm2).
The dual bioplatform was applied for the simultaneous determination of both Igs in 9 serum samples, 3 from control volunteers and 6 from egg-allergy individuals, following the same protocol described in section “Application to the analysis of serum samples from egg-allergic and non-allergic individuals”. The obtained results are summarized in Table 3 together with those provided by the single bioplatforms. Despite the loss of sensitivity inherent to the use of the dual electrochemical bioplatform, no significant differences were found for the endogenous concentrations of OVA-specific IgE and IgG4 measured either by the single or dual bioplatforms (ttab = 2.776), thus demonstrating the feasibility of dual biosensing methodology for the reliable diagnosis and monitoring of egg-allergy through the determination of the serological level of the target Igs.
| Subject | Sample | IgE,a kU L−1 | IgG4,a μg L−1 | ||||
|---|---|---|---|---|---|---|---|
| Dual | Single | t exp | Dual | Single | t exp | ||
| a Mean value ± ts√n; n = 3; α = 0.05. | |||||||
| CT | 1 | 0.02 ± 0.01 | 0.03 ± 0.01 | 2.597 | 0.13 ± 0.05 | 0.07 ± 0.02 | 1.531 |
| 2 | 0.03 ± 0.02 | 0.022 ± 0.004 | 1.818 | 0.16 ± 0.04 | 0.12 ± 0.02 | 2.585 | |
| 3 | 0.02 ± 0.01 | 0.015 ± 0.003 | 1.578 | 0.10 ± 0.04 | 0.11 ± 0.03 | 0.870 | |
| EA | 8 | 5.9 ± 0.4 | 5.9 ± 0.5 | 0.071 | — | — | — |
| 9 | 19 ± 4 | 19 ± 3 | 0.016 | — | — | — | |
| 12 | 11 ± 3 | 12 ± 2 | 1.408 | — | — | — | |
| 17 | — | — | — | 24 ± 3 | 23 ± 1 | 1.188 | |
| 20 | — | — | — | 11 ± 2 | 11 ± 1 | 0.225 | |
| 21 | — | — | — | 5.1 ± 0.6 | 4.7 ± 0.8 | 1.706 | |
Conclusions
This work reports for the first time electrochemical bioplatforms for the single or simultaneous determination of allergen specific IgE and IgG4, two of the most relevant biomarkers for diagnosis and monitoring of allergies. The proposed strategy involves the use of micromagnetic particles modified with the protein allergen responsible for the food allergic reaction, for the efficient capture of the serum specific Igs, as well as the labelling of the target isotypes with secondary antibodies conjugated with the enzyme and amperometric transduction, upon their magnetic capture on the working electrodes of the disposable platforms.The concept, developed for the detection of egg allergy in patients through the determination of OVA-specific IgE and IgG4, allows achievement of excellent analytical characteristics in terms of sensitivity and selectivity, and suitability for application in serum samples from patients diagnosed with this common food allergy after a simple dilution and without a matrix effect. An important advantage is the short test time (optimized at 90 min but with the possibility to be reduced to 10 min if necessary). The simplicity of the protocol and the use of cost-effective instrumentation, easy to handle and interpret by any user and at the POC, are other inherent advantages of the developed methodology. In addition, its versatility and the availability of electrochemical transducers invites the transfer, in a simple way, to multiplexed disposable electrode substrates with 2-, 4-, 8- and up to 96-working sensor units. These devices would allow the simultaneous investigation, globally or individually, of different Ig isotypes against different allergenic antigens in a single run and device. These features make the developed electrochemical bioplatforms ideal, once exhaustively validated, for easy implementation as POC testing devices in clinical practice in different settings of interest (inpatient, outpatient and even at home), thus improving the reliability of egg allergy diagnosis and allowing longitudinal trials to monitor whether the applied treatment is working to overcome it.
Author contributions
Alejandro Valverde: methodology, investigation, writing – review & editing-original draft. José Gordon Pidal: methodology, investigation, writing – review & editing-original draft. Sara Benedé: conceptualization, investigation, supervision, resources, review & editing-original draft. Elena Molina: conceptualization, investigation, supervision, resources, review & editing-original draft, funding acquisition. María Moreno-Guzmán: supervision, review & editing-original draft. Miguel Ángel López: supervision, review & editing-original draft. José M. Pingarrón: supervision, resources, review & editing-original draft. Alberto Escarpa: supervision, review & editing-original draft, funding acquisition. Susana Campuzano: conceptualization, supervision, resources, writing, review & editing-original draft, funding acquisition.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The financial support of PID2019-103899RB-I00 to S. C., AGL2018-88964-R to E. M. (Spanish Ministerio de Ciencia e Innovación), Grant PID2020-118154GB-I00 funded by MCIN/AEI/10.13039/501100011033 to A. E. Research Projects, and TRANSNANOAVANSENS-CM Program from the Comunidad de Madrid (Grant S2018/NMT-4349) to A. E. and S. C. is gratefully acknowledged. J. G. acknowledges a TRANSNANOAVANSENS-CM Program, A. V. a predoctoral contract from Complutense University of Madrid and S. B. a Juan de la Cierva Incorporación contract (Ref.: IJCI-2017-31345) from the Ministerio de Economía, Industria y Competitividad.References
- S. Morais, L. A. Tortajada-Genaro, Á. Maquieira and M. Á. Gonzalez Martinez, TrAC, Trends Anal. Chem., 2020, 127, 115904 CrossRef CAS.
- C. C. Roehr, G. Edenharter, S. Reimann, I. Ehlers, M. Worm, T. Zuberbier and B. Niggemann, Clin. Exp. Allergy, 2004, 34, 1534 CrossRef CAS PubMed.
- J. C. Caubet and J. Wang, Pediatr. Clin. North Am., 2011, 58, 427 CrossRef PubMed.
- S. H. Sicherer and H. A. Sampson, J. Allergy Clin. Immunol., 2018, 141, 41 CrossRef CAS PubMed.
- M. Moghtaderi, S. H. Nabavizadeh and S. H. Teshnizi, Allergol. Immunopathol., 2020, 48, 265 CrossRef PubMed.
- J. A. Huntington and P. E. Stein, J. Chromatogr. B: Biomed. Sci. Appl., 2001, 756, 189 CrossRef CAS.
- Y. T. Lin, C. T. Wu, J. L. Huang, J. H. Cheng and K. W. Yeh, J. Microbiol., Immunol. Infect., 2016, 49, 112 CrossRef CAS PubMed.
- R. G. Heine, N. Laske and D. J. Hill, Curr. Allergy Asthma Rep., 2006, 6, 145 CrossRef CAS PubMed.
- J. Xu, Y. Ye, J. Ji, J. Sun and X. Sun, Crit. Rev. Food Sci. Nutr., 2021 DOI:10.1080/10408398.2021.1907736.
- C. Kanagaratham, Y. S. El Ansari, O. L. Lewis and H. C. Oettgen, Front. Immunol., 2020, 11, 1 CrossRef PubMed.
- R. X. Foong and A. F. Santos, Pediatr. Allergy Immunol., 2021, 32, 223 CrossRef CAS PubMed.
- W. van de Veen and M. Akdis, J. Allergy Clin. Immunol., 2016, 138, 1434 CrossRef CAS PubMed.
- T. A. E. Platts-Mills, B. Keshavarz, J. M. Wilson, R. Li, P. W. Heymann, D. R. Gold, E. C. McGowan and E. A. Erwin, Children, 2021, 8, 1 CrossRef PubMed.
- L. K. James and S. J. Till, Curr. Allergy Asthma Rep., 2016, 16, 1 CrossRef PubMed.
- J. C. Caubet, R. Bencharitiwong, E. Moshier, J. H. Godbold, H. A. Sampson and A. N. Wegrzyn, J. Allergy Clin. Immunol., 2012, 129, 739 CrossRef CAS PubMed.
- M. Vazquez-Ortiz, M. Pascal, R. Jiménez-Feijoo, J. Lozano, M. T. Giner, L. Alsina, M. A. Martín-Mateos and A. M. Plaza, Clin. Exp. Allergy, 2014, 44, 579 CrossRef CAS PubMed.
- S. Okamoto, S. Taniuchi, K. Sudo, Y. Hatano, K. Nakano, T. Shimo and K. Kaneko, Allergy, Asthma, Clin. Immunol., 2012, 8, 1 CrossRef PubMed.
- L. Heinzerling, A. Mari, K. C. Bergmann, M. Bresciani, G. Burbach, U. Darsow, S. Durham, W. Fokkens, M. Gjomarkaj, T. Haahtela, A. T. Bom, S. Wöhrl, H. Maibach and R. Lockey, Clin. Transl. Allergy, 2013, 3, 1 CrossRef PubMed.
- T. A. Popov, G. Passalacqua, S. N. González-Díaz, D. Plavec, F. Braido, J. L. García-Abujeta, L. Dubuske, P. Rouadi, M. Morais-Almeida, S. Bonini, L. Cheng and I. J. Ansotegui, World Allergy Organ. J., 2020, 13, 100466 CrossRef PubMed.
- A. Cianferoni and J. M. Spergel, Allergol. Int., 2009, 58, 457 CrossRef CAS PubMed.
- P. Demoly, N. F. Adkinson, K. Brockow, M. Castells, A. M. Chiriac, P. A. Greenberger, D. A. Khan, D. M. Lang, H. S. Park, W. Pichler, M. Sanchez-Borges, T. Shiohara and B. Y. H. Thong, Allergy, 2014, 69, 420 CrossRef CAS PubMed.
- Y. Su, W. Sun, C. Wang, X. Wu, Y. Miao, H. Xiong, L. Bai and L. Dong, PLoS One, 2015, 10, 1 Search PubMed.
- B. Zhang, D. Liu, L. Zheng, X. Tan, Y. Yu, J. Zhang, X. Li and H. Li, J. Immunol. Methods, 2021, 497, 113110 CrossRef CAS PubMed.
- K. H. Park, J. Lee, D. W. Sim and S. C. Lee, Ann. Lab. Med., 2018, 38, 23 CrossRef CAS PubMed.
- S. R. Kim, K. H. Park, J. E. Lee, B. J. Kim, K. J. Lim and J. W. Park, Yonsei Med. J., 2020, 61, 524 CrossRef CAS PubMed.
- H. J. C. Neto and N. A. Rosario, Allergol. Immunopathol., 2009, 37, 31 CrossRef.
- M. van Hage, C. Hamsten and R. Valenta, J. Allergy Clin. Immunol., 2017, 140, 974 CrossRef CAS PubMed.
- M. Rubio, P. J. Bousquet and P. Demoly, Curr. Opin. Allergy Clin. Immunol., 2010, 10, 457 CrossRef CAS PubMed.
- P. Yáñez-Sedeño, S. Campuzano and J. M. Pingarrón, Chem. Commun., 2019, 55, 2563 RSC.
- M. P. Kreuzer, C. K. O'Sullivan, M. Pravda and G. G. Guilbault, Anal. Chim. Acta, 2001, 442, 45 CrossRef CAS.
- K. I. Papamichael, M. P. Kreuzer and G. G. Guilbault, Sens. Actuators, B, 2007, 121, 178 CrossRef CAS.
- Y. Wang, M. Cui, M. Jiao and X. Luo, Anal. Bioanal. Chem., 2018, 410, 5871 CrossRef CAS PubMed.
- S. J. Oh, J. K. Ahn, H. Park, Y. Song, S. J. Kwon and H. B. Shin, Anal. Methods, 2019, 11, 4410 RSC.
- M. E. Cortina, L. J. Melli, M. Roberti, M. Mass, G. Longinotti, S. Tropea, P. Lloret, D. A. Rey Serantes, F. Salomon, M. Lloret, A. J. Caillava, S. Restuccia, J. Altcheh, C. A. Buscaglia, L. Malatto, J. E. Ugalde, L. Fraigi, C. Moina, G. Ybarra, A. E. Ciocchini and D. J. Comerci, Biosens. Bioelectron., 2016, 80, 24 CrossRef CAS PubMed.
- J. Kudr, B. Klejdus, V. Adam and O. Zitka, TrAC, Trends Anal. Chem., 2018, 98, 104 CrossRef CAS.
- M. Pastucha, Z. Farka, K. Lacina, Z. Mikŭsova and P. Skladal, Microchim. Acta, 2019, 186, 312 CrossRef PubMed.
- C. Camacho, J. C. Matías, B. Chico, R. Cao, L. Gómez, B. Simpson and R. Villalonga, Electroanalysis, 2007, 19, 2538 CrossRef CAS.
- M. Eguílaz, M. Moreno-Guzmán, S. Campuzano, A. González-Cortés, P. Yáñez-Sedeño and J. M. Pingarrón, Biosens. Bioelectron., 2010, 26, 517 CrossRef PubMed.
- F. Conzuelo, M. Gamella, S. Campuzano, D. G. Pinacho, A. J. Reviejo, M. P. Marco and J. M. Pingarrón, Biosens. Bioelectron., 2012, 36, 81 CrossRef CAS PubMed.
- B. Esteban-Fernández de Ávila, V. Escamilla-Gómez, S. Campuzano, M. Pedrero, J. P. Salvador, M. P. Marco and J. M. Pingarrón, Sens. Actuators, B, 2013, 188, 212 CrossRef.
- A. Valverde, A. Montero-Calle, R. Barderas, M. Calero, P. Yáñez-Sedeño, S. Campuzano and J. M. Pingarrón, Electrochim. Acta, 2021, 371, 137815 CrossRef CAS.
- G. Feyzkhanova, S. Voloshin, O. Smoldovskaya, A. Arefieva, M. Filippova, V. Barsky, L. Pavlushkina, V. Butvilovskaya, A. Tikhonov, Y. Reznikow and A. Rubina, Clin. Proteomics, 2017, 14, 1 CrossRef PubMed.
- S. E. F. Melanson, M. J. Tanasijevic and P. Jarolim, Circulation, 2007, 116, 501 CrossRef PubMed.
- J. N. Miller and J. C. Miller, Statistics and chemometrics for analytical chemistry, 6th edn, Pe. 2010 Search PubMed.
- V. Escamilla-Gómez, D. Hernández-Santos, M. B. González-García, J. M. Pingarrón-Carrazón and A. Costa-García, Biosens. Bioelectron., 2009, 24, 2678 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sd00040c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |