Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tuning iron spin states in single-atom nanozymes enables efficient peroxidase mimicking†
Xiaoqian
Wei‡
abc,
Shaojia
Song‡
d,
Weiyu
Song
 d,
Yating
Wen
a,
Weiqing
Xu
a,
Yifeng
Chen
a,
Zhichao
Wu
a,
Ying
Qin
a,
Lei
Jiao
a,
Yu
Wu
a,
Meng
Sha
a,
Jiajia
Huang
a,
Xiaoli
Cai
a,
Lirong
Zheng
d,
Yating
Wen
a,
Weiqing
Xu
a,
Yifeng
Chen
a,
Zhichao
Wu
a,
Ying
Qin
a,
Lei
Jiao
a,
Yu
Wu
a,
Meng
Sha
a,
Jiajia
Huang
a,
Xiaoli
Cai
a,
Lirong
Zheng
 e,
Liuyong
Hu
f,
Wenling
Gu
a,
Miharu
Eguchi
cg,
Toru
Asahi
b,
Yusuke
Yamauchi
e,
Liuyong
Hu
f,
Wenling
Gu
a,
Miharu
Eguchi
cg,
Toru
Asahi
b,
Yusuke
Yamauchi
 *bcg and
Chengzhou
Zhu
*bcg and
Chengzhou
Zhu
 *a
*a
aKey Laboratory of Pesticide and Chemical Biology of Ministry of Education, College of Chemistry, Central China Normal University, Wuhan, 430079, PR China. E-mail: czzhu@ccnu.edu.cn
bFaculty of Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo 169-8555, Japan
cJST-ERATO Yamauchi Materials Space-Tectonics Project, International Center for Materials Nanoarchitechtonics (WPI-MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan
dState Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing, 102249, P. R. China
eInstitute of High Energy Physics, Chinese Academy of Sciences, Beijing Synchrotron Radiation Facility, Beijing, 100049, P. R. China
fSchool of Materials Science and Engineering, Wuhan Institute of Technology, Wuhan 430205, P. R. China
gAustralian Institute for Bioengineering and Nanotechnology (AIBN), The University of Queensland, Brisbane, QLD 4072, Australia. E-mail: y.yamauchi@uq.edu.au
First published on 26th October 2022
Abstract
The large-scale application of nanozymes remains a significant challenge owing to their unsatisfactory catalytic performances. Featuring a unique electronic structure and coordination environment, single-atom nanozymes provide great opportunities to vividly mimic the specific metal catalytic center of natural enzymes and achieve superior enzyme-like activity. In this study, the spin state engineering of Fe single-atom nanozymes (FeNC) is employed to enhance their peroxidase-like activity. Pd nanoclusters (PdNC) are introduced into FeNC, whose electron-withdrawing properties rearrange the spin electron occupation in Fe(II) of FeNC–PdNC from low spin to medium spin, facilitating the heterolysis of H2O2 and timely desorption of H2O. The spin-rearranged FeNC–PdNC exhibits greater H2O2 activation activity and rapid reaction kinetics compared to those of FeNC. As a proof of concept, FeNC–PdNC is used in the immunosorbent assay for the colorimetric detection of prostate-specific antigen and achieves an ultralow detection limit of 0.38 pg mL−1. Our spin-state engineering strategy provides a fundamental understanding of the catalytic mechanism of nanozymes and facilitates the design of advanced enzyme mimics.
Introduction
Peroxidases (POD), a family of enzymes that catalytically oxidize certain compounds in the presence of peroxides (H2O2 in most cases), play a significant role in biological systems, such as H2O2 detoxification in the human body.1,2 They can also be isolated from organisms and applied to various applications such as biosensing, therapy, and environmental protection.3–5 However, their large-scale commercialization remains an ongoing issue because of the disadvantages of high cost, difficult recovery and variability.6 Nanozymes, nanomaterials with enzyme-like characteristics, have been discovered as substitutes for natural enzymes with the merits of excellent stability, facile storage and easy production.6–10 Nevertheless, one obstacle lying in nanozymes emanates from their low catalytic efficiency, which is mainly attributed to their insufficient intrinsic activity.7 Overcoming these limitations to acquire highly active POD-like nanozymes is, therefore, a subject of interest and priority.Compared with simulating functionally, mimicking natural enzymes from the perspective of biological structure may provide a more reasonable strategy to achieve high catalytic performance.11–14 Single-atom nanozymes with well-defined electronic and geometric structures are appealing alternatives to natural enzymes as they can mimic the highly evolved catalytic center of natural enzymes at the atomic scale.12,15,16 Given the similar heme structure of horseradish peroxidase (HRP), Fe single-atom nanozymes are promising to catalyze H2O2 activation.15,17–21 More importantly, tuning the local environment of single atoms is expected to optimize their electronic structure for enhanced POD-like catalytic performance. For example, heteroatoms such as B, S and P have been introduced as “modulators” to decrease the energy barrier during the reduction of H2O2.11,22,23 FeN5, in which the Fe single atom is coordinated by axial N atoms, emulates penta-coordinated heme systems and shows enhanced POD-like performance compared to its FeN4 counterpart.14 Furthermore, according to the frontier molecular orbital theory, the catalysis process depends on the electron donation and back-donation steps between the reactants/intermediates and metal centers.24,25 During the reaction process involving the production of multistep intermediates, the number of unpaired electrons cannot always be conserved, resulting in the spin-related electron transfer and thus the d orbital occupation and spintronic configuration sensitive reaction kinetics and thermodynamics.26 Inspired by this, tuning the spin state of metal atoms in single-atom nanozymes is expected to enhance their POD-like activity, realizing the efficient activation of H2O2 and revealing the underlying relationships between spin states and performance.
In this study, we develop spin-dependent Fe single-atom nanozymes by employing Pd nanoclusters (PdNC) as “modulators” (named FeNC–PdNC) for the first time to achieve enhanced POD-like activity. The synergistic effect between PdNC and Fe single atoms results in the outstanding POD-like activity of FeNC–PdNC in comparison to FeNC. Studies show that PdNC promotes the formation of a greater amount of medium spin (MS) Fe(II) atoms, which are more active than low spin (LS) Fe(II) owing to the weakened binding strength towards oxygen-containing intermediates and accelerated H2O2 activation. The spin-dependent H2O2 reduction opens an avenue for the rational design of highly active POD mimicking.
Results and discussion
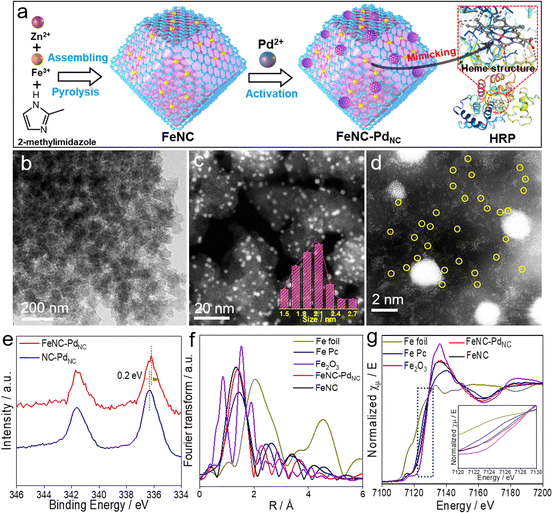
As displayed in Fig. 1a, FeNC–PdNC was prepared via the confinement effect of metal–organic frameworks (MOFs). Briefly, FeNC was prepared by pyrolysis of Fe-doped zeolitic imidazolate frameworks (ZIF-8) (Fig. S1†). Then, Pd2+ ions were incorporated into FeNC through a micropore adsorption strategy and in situ reduced to form PdNC.27 The rich metal nodes of ZIF-8 and the pores after annealing act as two “claws”, anchoring Fe single atoms and PdNC.28 To explore the role of PdNC, NC–PdNC without Fe single atoms was also prepared using ZIF-8 as the precursor. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of FeNC–PdNC display its dodecahedral shape inherited from ZIF-8 (Fig. 1b and S2†). The high-angle annular dark-field scanning TEM (HAADF-STEM) image confirms the highly dense and uniformly distributed PdNC confined in the carbon with an average size of 2.13 nm (Fig. 1c). The Brunauer–Emmett–Teller (BET) area of FeNC–PdNC, FeNC and NC–PdNC is 547.0, 554.9 and 550.5 m2 g−1, respectively (Fig. S3 and Table S1†), which guarantees the accessibility of active sites.29 The aberration-corrected HAADF-STEM (AC-HAADF-STEM) image shown in Fig. 1d reveals that numerous Fe single atoms surround PdNC. The enlarged AC-HAADF-STEM and corresponding energy dispersive spectroscopy (EDS) mapping confirm the existence of C, N, O, Fe, and Pd elements and the uniformly distributed Pd species in PdNC (Fig. S4†). X-ray diffraction (XRD) patterns exhibit Pd(111) characteristic peaks in both FeNC–PdNC and NC–PdNC, confirming the successful introduction of PdNC into FeNC–PdNC (Fig. S5†). Inductively coupled plasma optical emission spectrometry (ICP-OES) analysis shows that the contents of Pd and Fe in FeNC–PdNC were ∼5.6 and ∼0.36 wt%, respectively. Raman spectra (Fig. S6†) indicate that both FeNC–PdNC and FeNC have rich disorientated graphene structures with a high ratio of D and G band intensities (ID/IG).30X-ray photoelectron spectroscopy (XPS) analysis was performed to understand the chemical states of various elements in the nanozymes. The C 1s spectra shown in Fig. S7a† exhibit four peaks corresponding to sp2-C, sp3-C, C–Nx, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C–O.31 The N 1s spectra can be deconvoluted into four peaks at 398.5, 399.5, 400.4, and 401.1 eV, which are attributed to pyridinic N, FeN, pyrrolic N, and graphitic N, respectively (Fig. S7b†).32 The high ratios of FeN content in FeNC–PdNC (23.1%) and FeNC (21.6%) indicate the presence of numerous Fe single-atom species (Table S2†). In particular, the binding energy of Fe 2p in FeNC–PdNC is higher than that in FeNC, whereas Pd shows a decreased binding energy compared to NC–PdNC (Fig. 1e and S8†). This suggests that electron transfer occurs from Fe single atoms to PdNC, owing to the difference in the electronegativities of Pd and Fe2+.33 Moreover, the decreased electron density on the Fe center in FeNC–PdNC may significantly regulate its catalytic behavior.
O/C–O.31 The N 1s spectra can be deconvoluted into four peaks at 398.5, 399.5, 400.4, and 401.1 eV, which are attributed to pyridinic N, FeN, pyrrolic N, and graphitic N, respectively (Fig. S7b†).32 The high ratios of FeN content in FeNC–PdNC (23.1%) and FeNC (21.6%) indicate the presence of numerous Fe single-atom species (Table S2†). In particular, the binding energy of Fe 2p in FeNC–PdNC is higher than that in FeNC, whereas Pd shows a decreased binding energy compared to NC–PdNC (Fig. 1e and S8†). This suggests that electron transfer occurs from Fe single atoms to PdNC, owing to the difference in the electronegativities of Pd and Fe2+.33 Moreover, the decreased electron density on the Fe center in FeNC–PdNC may significantly regulate its catalytic behavior.
To further elucidate the changes in the local chemical configuration of Fe species after the introduction of PdNC, the Fe K-edge X-ray absorption fine structure (XAFS) was obtained. As depicted in Fig. 1f, the Fe K-edge extended-XAFS (EXAFS) Fourier-transformed magnitudes of FeNC–PdNC and FeNC show major peaks at ∼1.4 Å, which is consistent with that of iron-porphyrin (Fe Pc) and attributed to the backscattering between Fe and N atoms.11,34 The FeN coordination numbers in FeNC–PdNC and FeNC obtained via the quantitative EXAFS fitting analysis were 4.1 and 4.2, respectively (Fig. S9 and Table S3†). X-ray absorption near edges structure (XANES) spectra were obtained to identify the valence state of Fe species. As displayed in Fig. 1g, the absorption edges of Fe K-edge XANES curves of FeNC–PdNC and FeNC are between Fe Pc and Fe2O3. In addition, the valence state of FeNC–PdNC is more positive than that of FeNC, which indicates the decreased electron density and is consistent with the XPS results.35,36 First derivative XANES spectra were obtained to reveal the oxidation state of Fe (Fig. S10†). Overall, the characteristic Fe2+ peak (∼7122.6 eV), similar to that of Fe Pc, appears for both FeNC–PdNC and FeNC, while the peak of FeNC–PdNC shows a slight positive shift compared to that of FeNC, indicating its relatively positive charge upon the integration of PdNC.
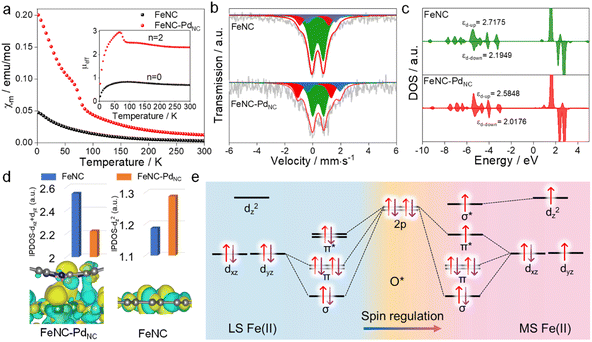
The charge redistribution primarily dominates the electronic configuration of Fe(II), i.e., the spin state, and thus regulates the catalytic activity. N coordinated Fe(II) exhibits multiple states, such as LS (dxy2 dxz2 dyz2), MS (dxy2 dxz2 dyz1 dz21) and high spin (HS; dxy2 dxz1 dyz1 dz21 dx2−y21) owing to the variation of the coordination environment (Fig. S11†).37 In the case of LS Fe(II), the Fe atom splits into fully occupied low-level orbitals whereas in MS Fe(II), one electron from the dyz orbital will hop to the dz2 orbital, resulting in unpaired electrons. To verify the spin redistribution experimentally, the zero-field cooling temperature-dependent (ZFC-T) magnetic susceptibility (χm) study was carried out. As shown in Fig. 2a, the total effective magnetic moment (μeff) was obtained according to the equation, 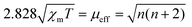 to calculate the number of single spin electrons (n).38 The n of Fe(II) increases from ∼0.52 in FeNC to ∼2.18 in FeNC–PdNC, confirming the increase in spin state.
to calculate the number of single spin electrons (n).38 The n of Fe(II) increases from ∼0.52 in FeNC to ∼2.18 in FeNC–PdNC, confirming the increase in spin state.
For quantification, Mössbauer spectroscopy analysis, which is a powerful tool for identifying the spin polarization configuration, was conducted. Three different doublets (D1–D3) are fitted for FeNC–PdNC and FeNC, corresponding to LS Fe(II), MS Fe(II), and HS Fe(II), respectively.39 FeNC–PdNC possesses a higher content of MS Fe(II) (33.4%) than FeNC (17.1%) (Fig. 2b and Table S4†). This could be attributed to the strong interactions between the Fe single atoms and PdNC in FeNC–PdNC that effectively reshaped the electronic structure of Fe, achieving the Fe 3d electron-spin configuration transition from LS to MS. Based on this, the electronic spin information of Fe single atoms was further investigated through density functional theory (DFT) calculations. FeN4 embedded in graphene was used to simulate FeNC, while the FeNC coupling with Pd (111) facets was used to simulate FeNC–PdNC. As shown in Fig. 2c, the projected density of states (DOS) diagrams reveal that the distance between the d-band center of spin up and spin down (|εd-up-εd-down|) increases with the introduction of PdNC, indicating a higher spin state for the Fe center. The integral projected density of states analysis of Fe 3d orbital splitting reveals that the increasing spin state mainly originated from the increased Fe dz2 contributions (Fig. 2d), which is also visualized by the spin density diagram (Fig. S12†). Compared to FeNC, the highest occupied molecular orbital (HOMO) of Fe(II) in FeNC–PdNC shows one dz2 orbital perpendicular to the plane within the introduction of PdNC compared to FeNC (Fig. 2d below).
Typically, Fe-based catalysts strongly bind to oxygen, circumscribing proton–electron transfer and catalytic activity. During the H2O2 reduction procedure, the 2p orbital of O in H2O2/intermediates and 3d orbital of Fe sites hybridize, making the 3d electronic state act as spin-dependent gates to regulate electron transfer and orbital interaction in the catalytic reaction.24 To confirm this phenomenon, the H2O2 reduction process in both LS and MS Fe(II) was theoretically predicted. Due to the scaling relationships of different intermediates, O* is chosen as the model to study its interaction with Fe sites.40 Besides, the interaction between Fe-dxy/dx2−y2 orbitals and adsorbed O* is negligible because they cannot mix based on symmetry conservation.41 As shown in Fig. 2e, the interaction between the Fe center and adsorbed O* changes along with the change in the spin state of Fe(II). In particular, a half-filled dz2 orbital in MS Fe(II) can accept fewer valence electrons from *O with the generation of one σ* orbital, favorable for the desorption of H2O2/intermediates to trigger a catalytic cycle (Fig. S13†). In this case, the local microenvironment disturbance is exerted to modulate the electronic hopping and occupation of the 3d orbital, achieving the spin reconfiguration and regulating the enzyme-like activity.
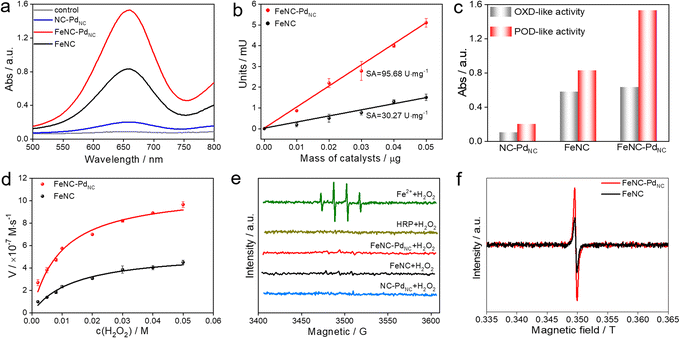
The POD-like catalytic activity of FeNC–PdNC was quantitively assessed using a chromogenic biochemical reaction of 3,3′,5,5′-tetramethylbenzidine (TMB), which can be oxidized by H2O2 to ox-TMB in blue color (at 652 nm).42,43 As expected, FeNC–PdNC exhibits two times the catalytic activity of FeNC (Fig. 3a). Besides, the specific activity (SA) value of FeNC–PdNC was 95.68 U mg−1, which is more than three-fold higher than that of FeNC (30.27 U mg−1) (Fig. 3b). Meanwhile, the injection of SCN− prominently impairs the POD-like performance of FeNC–PdNC, confirming the main role of Fe single atoms in the POD-like property (Fig. S14†).13 Thus, the above results illustrate that Fe single atoms play a critical role in POD-like activity and the existence of PdNC can significantly boost their activity, despite the sluggish kinetics of H2O2 reduction at PdNC. Generally, the unsatisfactory specificity of nanomaterials has been a major constraint in the development of nanozymes.44 Therefore, the oxidase (OXD)-like activity, the major interfering reaction to POD-like activity was also evaluated (Fig. 3c and S15†). Unlike the POD-like activity of FeNC–PdNC, the OXD-like activity shows negligible enhancement after the integration of PdNC. Moreover, the POD-like activity of FeNC–PdNC is much higher than that of the addition of FeNC and NC–PdNC activities, which demonstrates the synergistic effect between PdNC and Fe single atoms. The Michaelis–Menten equation was applied to assess the POD-like activity of nanozymes (Fig. 3d, S16 and S17†). The Michaelis–Menten constant (Km), maximum velocity (Vmax), and Kcat (Kcat = Vmax/[S]) are listed in Table S5†. FeNC–PdNC exhibits higher Kcat (5.10 s−1) than FeNC (2.56 s−1) towards H2O2, indicating its higher POD-like activity. More importantly, FeNC–PdNC also has competitive catalytic activity compared with other reported nanozymes. Both FeNC–PdNC and FeNC exhibit high pH-dependent properties similar to natural HRP and higher temperature tolerance than HRP (Fig. S18†). The as-prepared nanozymes maintain their POD-like activity after long-term storage and the treatment of strong acidic and alkaline solutions compared to HRP, suggesting that they can perfectly surmount the disadvantage of the inferior stability of natural enzymes (Fig. S19 and S20†). Therefore, FeNC–PdNC is a promising alternative to natural enzymes.
To understand the potential catalytic intermediates, electron paramagnetic resonance (EPR) spectroscopy was performed by using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as an ˙OH trapping agent. No signal of ˙OH was detected in HRP/H2O2 and all nanozymes/H2O2 systems except for the Fe2+/H2O2 system (Fig. 3e). The existence of ˙OH radical scavenger t-butyl alcohol has little effect on the activity of FeNC–PdNC and FeNC, indicating that ˙OH is not a critical active intermediate in the catalytic reaction (Fig. S21†). Inspired by the catalytic mechanism of natural HRP, the Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate is considered as the active intermediate.45,46 Then a low-temperature (77 K) EPR experiment was performed. As expected, rhombic signals at g = 2 were detected after the reaction of FeNC–PdNC and FeNC with excess H2O2, suggesting the formation of compound oxoferryl Fe(IV)
O intermediate is considered as the active intermediate.45,46 Then a low-temperature (77 K) EPR experiment was performed. As expected, rhombic signals at g = 2 were detected after the reaction of FeNC–PdNC and FeNC with excess H2O2, suggesting the formation of compound oxoferryl Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and the porphyrin cation radical (named as the oxo-iron porphyrin π-cation radical [Fe(IV)
O and the porphyrin cation radical (named as the oxo-iron porphyrin π-cation radical [Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O Por+˙]) during the reaction process (Fig. 3f).47 Notably, the intensity of FeNC–PdNC is higher than that of FeNC. Considering the heme-like structure of FeNC similar to natural HRP, the essential active intermediate Fe(IV)
O Por+˙]) during the reaction process (Fig. 3f).47 Notably, the intensity of FeNC–PdNC is higher than that of FeNC. Considering the heme-like structure of FeNC similar to natural HRP, the essential active intermediate Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O might have formed via the bound reactive oxygen species (ROS) pathway while PdNC is expected to enhance the production of Fe(IV)
O might have formed via the bound reactive oxygen species (ROS) pathway while PdNC is expected to enhance the production of Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species.
O species.
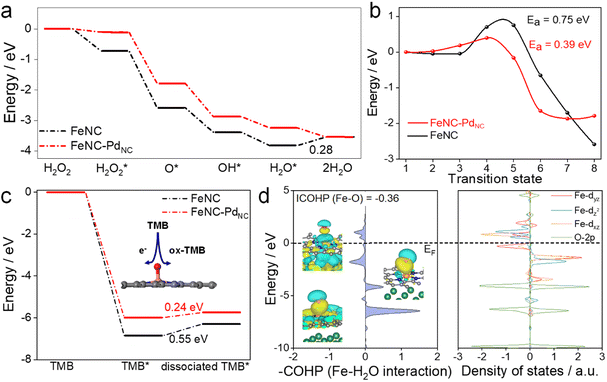
DFT calculations were performed to theoretically understand the effect of the spin state on the POD-like activity. As shown in Fig. 4a and S22,† the H2O2 heterolytic dissociation route is energetically more favorable for FeNC and FeNC–PdNC. Moreover, the H2O* desorption on FeNC is the rate-determining step (RDS) with an energy barrier of 0.28 eV due to the strong binding between Fe single atoms and oxygen-containing species. Encouragingly, this step is exothermic for FeNC–PdNC, indicating that PdNC integration facilitates the timely H2O desorption. Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, a critical intermediate for TMB oxidation similar to that observed for natural HRP, is produced by the dehydration of the adsorbed H2O2 as follows: H2O2 → O* + H2O.48 As shown in Fig. 4b, the H2O2 dehydration process is not thermodynamically favorable for FeNC with an energy barrier of 0.75 eV. After the integration of PdNC, this energy barrier is decreased to 0.36 eV on FeNC–PdNC, promoting the formation of Fe(IV)
O, a critical intermediate for TMB oxidation similar to that observed for natural HRP, is produced by the dehydration of the adsorbed H2O2 as follows: H2O2 → O* + H2O.48 As shown in Fig. 4b, the H2O2 dehydration process is not thermodynamically favorable for FeNC with an energy barrier of 0.75 eV. After the integration of PdNC, this energy barrier is decreased to 0.36 eV on FeNC–PdNC, promoting the formation of Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate sites. As mentioned previously, the spin state of Fe(II) fluctuates the interaction between Fe sites and *O, which further improves its oxidation capacity towards TMB. Next, the effect of PdNC integration on TMB oxidation was evaluated. The oxidation of TMB proceeded through the N–H bond cleavage of TMB and transformation H to Fe(IV)
O intermediate sites. As mentioned previously, the spin state of Fe(II) fluctuates the interaction between Fe sites and *O, which further improves its oxidation capacity towards TMB. Next, the effect of PdNC integration on TMB oxidation was evaluated. The oxidation of TMB proceeded through the N–H bond cleavage of TMB and transformation H to Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O along with transfer of an electron from the substrate to the nanozymes. As a result, the dissociation energy of TMB decreases from 0.55 eV in FeNC to 0.24 eV in FeNC–PdNC, thus rendering it more favorable to oxidize TMB (Fig. 4c).
O along with transfer of an electron from the substrate to the nanozymes. As a result, the dissociation energy of TMB decreases from 0.55 eV in FeNC to 0.24 eV in FeNC–PdNC, thus rendering it more favorable to oxidize TMB (Fig. 4c).
The orbital interactions between H2O* and Fe sites were explored. The COHP and DOS analysis reveals that the energy level of the bonding molecular orbital of FeNC–PdNC shifts to lower energy, whereas that of the antibonding molecular orbital shifts to higher energy, which weakens the Fe–O bond strength in the H2O* adsorption configuration (Fig. 4d and S23†). Furthermore, an axial σ* antibonding molecular orbital is observed for H2O* on FeNC–PdNC owing to the electrons in the higher level dz2 orbital, while the antibonding contribution is mainly from π* antibonding orbitals for FeNC. This unique σ* antibonding orbital contributes to the timely H2O desorption from FeNC–PdNC. These DFT results confirm that FeNC–PdNC with a higher ratio of MS Fe(II) exhibits highly efficient POD-like activity.
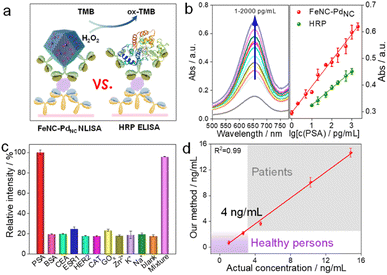
Subsequently, we performed a proof-of-principle demonstration. FeNC–PdNC was applied as a label in the colorimetric nanozyme-linked immunosorbent assay (NLISA), and prostate-specific antigen (PSA) was chosen as the model analyte (Fig. 5a).49–51 Typically, the surface of FeNC–PdNC is positively charged, which can conjugate with the labeling antibody of PSA (Ab2) as a signal tag through electrostatic interaction (Fig. S24†). The red fluorescence emission in the confocal laser scanning microscope demonstrates the successful immobilization of Ab2 (labeled by rhodamine B) on FeNC–PdNC (Fig. S25†). The absorption spectra and corresponding calibration curves were obtained, where FeNC–PdNC NLISA exhibits a wide detection range of 1–2000 pg mL−1 with a lower variable coefficient of 0.18–5.18% (Fig. 5b and S26†). The limit of detection (LOD) defined by the 3SD method is 0.38 pg mL−1. In contrast, the LOD of the traditional HRP-based enzyme-linked immunosorbent assay (ELISA) is 5.38 pg mL−1 with a detection range of 10–1000 pg mL−1 (Fig. S27†). The sensitivity of FeNC–PdNC towards PSA is also higher than those in previous reports, suggesting that FeNC–PdNC has potential immunoassay applications (Table S6†).
In terms of specificity, an obvious characteristic intensity was observed for PSA, while there are negligible changes in the signal intensity for other biomarkers and metal ions (BSA, CEA, ESR1, HER2, CAT, GOx, Zn2+, K+, and Na+) compared to the blank samples (Fig. 5c). In the presence of all of the above interferents, the FeNC–PdNC NLISA maintains its high-intensity signal for PSA, indicating its good selectivity and anti-interference ability. Furthermore, FeNC–PdNC NLISA exhibits outstanding repeatability and reproducibility with low calculated relative standard deviations (RSD; 4.23% and 1.92%, respectively) (Fig. S28†). Stability tests were conducted for ten days, where the RSD was 3.29% (Fig. S29†). By taking advantage of its high activity, selectivity, and stability, FeNC–PdNC NLISA was used to detect PSA in real serum samples. As expected, the results of FeNC–PdNC NLISA are consistent with those of chemiluminescence analysis, indicating its remarkable feasibility and accuracy for practical application in the proposed assay (Fig. 5d). Thus, FeNC–PdNC NLISA has potential applications in clinical diagnosis and can be extended for the detection of other biomarkers.
Conclusions
Spin-dependent Fe single-atom nanozymes have been developed to achieve enhanced POD-like activity. Experimental and theoretical calculations reveal that the electron-withdrawing effect of PdNC induces the LS to MS transition of the Fe single atom with the increased electron occupation in the dz2 orbital. The charge transfer and orbital interactions between the Fe sites and intermediates display spin-dependent features, where the higher electron occupation in the dz2 is beneficial for the generation of the essential Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediates and promotes the timely desorption of H2O*. Thanks to the superior catalytic activity, FeNC–PdNC can be used to construct NLISA for sensitive detection of PSA, exhibiting satisfactory selectivity and sensitivity. This spin-state engineering strategy for the design of nanozymes not only provides a fundamental understanding of their catalytic mechanism but also promotes the design and development of enzyme-like catalysts for the future.
O intermediates and promotes the timely desorption of H2O*. Thanks to the superior catalytic activity, FeNC–PdNC can be used to construct NLISA for sensitive detection of PSA, exhibiting satisfactory selectivity and sensitivity. This spin-state engineering strategy for the design of nanozymes not only provides a fundamental understanding of their catalytic mechanism but also promotes the design and development of enzyme-like catalysts for the future.
Data availability
The data supporting the findings of this study are available within the article and in the ESI.†Author contributions
C. Z. and Y. Y. conceptualized and supervised this study. X. W. designed the experiments and wrote the paper. S. S. and W. S. performed the theoretical calculations. Y. W., W. X., Y. C., Z. W., Y. Q., Y. W., M. S., J. H., and X. C. performed the experiments and reviewed the manuscript. L. Z. conducted X-ray absorption experiments and L. J. contributed to XAS data analysis. L. H., W. G., and M. E. provided helpful discussions. All authors discussed and revised the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge the financial support of the National Natural Science Foundation of China (no. 22074049, 22004042 and 22104114), the Fundamental Research Funds for the Central Universities (no. CCNU22JC006), the Program of Introducing Talents of Discipline to Universities of China (111 program, B17019) and the JST-ERATO Yamauchi Materials Space-Tectonics Project (JPMJER2003). This work was performed in part at the Queensland node of the Australian National Fabrication Facility, a company established under the National Collaborative Research Infrastructure Strategy to provide nano and microfabrication facilities for Australia's researchers. X. W. thanks the China Scholarship Council for financial support (no. 202106770020). The authors thank the 1W1B station in Beijing Synchrotron Radiation Facility (BSRF) for X-ray absorption spectroscopy measurements.Notes and references
- M. A. Komkova, E. E. Karyakina and A. A. Karyakin, J. Am. Chem. Soc., 2018, 140, 11302–11307 CrossRef CAS PubMed.
- J. Yao, Y. Cheng, M. Zhou, S. Zhao, S. Lin, X. Wang, J. Wu, S. Lia and H. Wei, Chem. Sci., 2018, 9, 2927–2933 RSC.
- B. Liu, Z. Sun, P.-J. J. Huang and J. Liu, J. Am. Chem. Soc., 2015, 137, 1290–1295 CrossRef CAS PubMed.
- W. Wu, L. Huang, E. Wang and S. Dong, Chem. Sci., 2020, 11, 9741–9756 RSC.
- K. Herget, P. Hubach, S. Pusch, P. Deglmann, H. Götz, T. E. Gorelik, I. y. A. Gural'skiy, F. Pfitzner, T. Link and S. Schenk, Adv. Mater., 2017, 29, 1603823 CrossRef PubMed.
- M. Liang and X. Yan, Acc. Chem. Res., 2019, 52, 2190–2200 CrossRef CAS PubMed.
- H. Wei and E. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- Y. Wang, G. Jia, X. Cui, X. Zhao, Q. Zhang, L. Gu, L. Zheng, L. H. Li, Q. Wu and D. J. Singh, Chem, 2021, 7, 436–449 CAS.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang and S. Perrett, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- S. Ji, B. Jiang, H. Hao, Y. Chen, J. Dong, Y. Mao, Z. Zhang, R. Gao, W. Chen and R. Zhang, Nat. Catal., 2021, 4, 407–417 CrossRef CAS.
- L. Huang, J. Chen, L. Gan, J. Wang and S. Dong, Sci. Adv., 2019, 5, eaav5490 CrossRef CAS PubMed.
- Y. Chen, L. Jiao, H. Yan, W. Xu, Y. Wu, L. Zheng, W. Gu and C. Zhu, Anal. Chem., 2021, 93, 12353–12359 CrossRef CAS PubMed.
- W. Xu, W. Song, Y. Kang, L. Jiao, Y. Wu, Y. Chen, X. Cai, L. Zheng, W. Gu and C. Zhu, Anal. Chem., 2021, 93, 12758–12766 CrossRef CAS PubMed.
- L. Jiao, H. Yan, Y. Wu, W. Gu, C. Zhu, D. Du and Y. Lin, Angew. Chem., Int. Ed., 2020, 132, 2585–2596 CrossRef.
- S. Lin and H. Wei, Sci. China: Life Sci., 2019, 62, 710–712 CrossRef PubMed.
- X. Wei, X. Luo, N. Wu, W. Gu, Y. Lin and C. Zhu, Nano Energy, 2021, 84, 105817 CrossRef CAS.
- Y. Chen, P. Wang, H. Hao, J. Hong, H. Li, S. Ji, A. Li, R. Gao, J. Dong and X. Han, J. Am. Chem. Soc., 2021, 143, 18643–18651 CrossRef CAS PubMed.
- W. Wu, L. Huang, X. Zhu, J. Chen, D. Chao, M. Li, S. Wu and S. Dong, Chem. Sci., 2022, 13, 4566–4572 RSC.
- M. Huo, L. Wang, H. Zhang, L. Zhang, Y. Chen and J. Shi, Small, 2019, 15, 1901834 CrossRef PubMed.
- M. S. Kim, J. Lee, H. S. Kim, A. Cho, K. H. Shim, T. N. Le, S. S. A. An, J. W. Han, M. I. Kim and J. Lee, Adv. Funct. Mater., 2020, 30, 1905410 CrossRef CAS.
- L. Jiao, W. Xu, Y. Zhang, Y. Wu, W. Gu, X. Ge, B. Chen, C. Zhu and S. Guo, Nano Today, 2020, 35, 100971 CrossRef CAS.
- L. Jiao, Y. Kang, Y. Chen, N. Wu, Y. Wu, W. Xu, X. Wei, H. Wang, W. Gu and L. Zheng, Nano Today, 2021, 40, 101261 CrossRef CAS.
- G. Zhou, P. Wang, H. Li, B. Hu, Y. Sun, R. Huang and L. Liu, Nat. Commun., 2021, 12, 1–9 CrossRef PubMed.
- L. Foppa, C. Copéret and A. Comas-Vives, J. Am. Chem. Soc., 2016, 138, 16655–16668 CrossRef CAS PubMed.
- R. Gao, J. Wang, Z.-F. Huang, R. Zhang, W. Wang, L. Pan, J. Zhang, W. Zhu, X. Zhang and C. Shi, Nat. Energy, 2021, 6, 614–623 CrossRef CAS.
- M. Hu, S. Zhao, S. Liu, C. Chen, W. Chen, W. Zhu, C. Liang, W. C. Cheong, Y. Wang and Y. Yu, Adv. Mater., 2018, 30, 1801878 CrossRef PubMed.
- L. Jiao, G. Wan, R. Zhang, H. Zhou, S. H. Yu and H. L. Jiang, Angew. Chem., Int. Ed., 2018, 57, 8525–8529 CrossRef CAS PubMed.
- X. Wei, X. Luo, H. Wang, W. Gu, W. Cai, Y. Lin and C. Zhu, Appl. Catal., B, 2020, 263, 118347 CrossRef CAS.
- X. Wei, S. Song, N. Wu, X. Luo, L. Zheng, L. Jiao, H. Wang, Q. Fang, L. Hu and W. Gu, Nano Energy, 2021, 84, 105840 CrossRef CAS.
- G. Chen, H. Zhong and X. Feng, Chem. Sci., 2021, 12, 15802–15820 RSC.
- N. Zhang, T. Zhou, M. Chen, H. Feng, R. Yuan, W. Yan, Y. Tian, X. Wu, W. Chu and C. Wu, Energy Environ. Sci., 2020, 13, 111–118 RSC.
- K. Li and D. Xue, J. Phys. Chem. A, 2006, 110, 11332–11337 CrossRef CAS PubMed.
- G. Gan, X. Li, L. Wang, S. Fan, J. Mu, P. Wang and G. Chen, ACS Nano, 2020, 14, 9929–9937 CrossRef CAS PubMed.
- Y. Han, Y. Wang, R. Xu, W. Chen, L. Zheng, A. Han, Y. Zhu, J. Zhang, H. Zhang and J. Luo, Energy Environ. Sci., 2018, 11, 2348–2352 RSC.
- Z. Li, Z. Zhuang, F. Lv, H. Zhu, L. Zhou, M. Luo, J. Zhu, Z. Lang, S. Feng and W. Chen, Adv. Mater., 2018, 30, 1803220 CrossRef PubMed.
- X. Li, C.-S. Cao, S.-F. Hung, Y.-R. Lu, W. Cai, A. I. Rykov, S. Miao, S. Xi, H. Yang and Z. Hu, Chem, 2020, 6, 3440–3454 CAS.
- W. Cheng, P. Yuan, Z. Lv, Y. Guo, Y. Qiao, X. Xue, X. Liu, W. Bai, K. Wang and Q. Xu, Appl. Catal., B, 2020, 260, 118198 CrossRef CAS.
- G. Yang, J. Zhu, P. Yuan, Y. Hu, G. Qu, B.-A. Lu, X. Xue, H. Yin, W. Cheng and J. Cheng, Nat. Commun., 2021, 12, 1–10 CrossRef PubMed.
- H. Xu, D. Cheng, D. Cao and X. C. Zeng, Nat. Catal., 2018, 1, 339–348 CrossRef CAS.
- Z. Chen, H. Niu, J. Ding, H. Liu, P. H. Chen, Y. H. Lu, Y. R. Lu, W. Zuo, L. Han and Y. Guo, Angew. Chem., Int. Ed., 2021, 133, 25608–25614 CrossRef.
- Y. Wu, J. Wen, W. Xu, J. Huang, L. Jiao, Y. Tang, Y. Chen, H. Yan, S. Cao and L. Zheng, Small, 2021, 17, 2101907 CrossRef CAS PubMed.
- X. Xia, J. Zhang, N. Lu, M. J. Kim, K. Ghale, Y. Xu, E. McKenzie, J. Liu and H. Ye, ACS Nano, 2015, 9, 9994–10004 CrossRef CAS PubMed.
- Z. Xi, X. Cheng, Z. Gao, M. Wang, T. Cai, M. Muzzio, E. Davidson, O. Chen, Y. Jung and S. Sun, Nano Lett., 2019, 20, 272–277 CrossRef PubMed.
- P. Travascio, P. K. Witting, A. G. Mauk and D. Sen, J. Am. Chem. Soc., 2001, 123, 1337–1348 CrossRef CAS PubMed.
- A. Ivancich, P. Dorlet, D. B. Goodin and S. Un, J. Am. Chem. Soc., 2001, 123, 5050–5058 CrossRef CAS PubMed.
- K. C. Christoforidis, M. Louloudi, E. R. Milaeva, Y. Sanakis and Y. Deligiannakis, Mol. Phys., 2007, 105, 2185–2194 CrossRef CAS.
- X. Chen, L. Zhao, K. Wu, H. Yang, Q. Zhou, Y. Xu, Y. Zheng, Y. Shen, S. Liu and Y. Zhang, Chem. Sci., 2021, 12, 8865–8871 RSC.
- Y.-Q. Yang, Y.-C. Yang, M.-H. Liu and Y.-H. Chan, Anal. Chem., 2019, 92, 1493–1501 CrossRef PubMed.
- A. Díaz-Fernández, R. Miranda-Castro, N. de-Los-Santos-Álvarez, M. J. Lobo-Castañón and P. Estrela, Biosens. Bioelectron., 2021, 175, 112872 CrossRef PubMed.
- Z. Lyu, S. Ding, N. Zhang, Y. Zhou, N. Cheng, M. Wang, M. Xu, Z. Feng, X. Niu and Y. Cheng, Research, 2020, 2020, 4724505 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2sc05679h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |