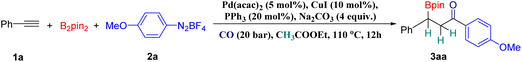Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Regioselective catalytic carbonylation and borylation of alkynes with aryldiazonium salts toward α-unsubstituted β-boryl ketones†
Fengxiang
Zhu
 *a,
Pengpeng
Yin
a and
Xiao-Feng
Wu
*a,
Pengpeng
Yin
a and
Xiao-Feng
Wu
 *bc
*bc
aDepartment School of Chemistry and Chemical Engineering, Shanxi University, Taiyuan 030006, China. E-mail: zfx201989@sxu.edu.cn
bDalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China. E-mail: xwu2020@dicp.ac.cn
cLeibniz-Institut für Katalyse e.V., Rostock 18059, Germany. E-mail: xiao-feng.wu@catalysis.de
First published on 30th September 2022
Abstract
A new Pd/Cu-catalyzed carbonylation and borylation of alkynes with aryldiazonium salts toward α-unsubstituted β-boryl ketones with complete regioselectivity has been developed. This transformation shows broad substrate scope and excellent functional-group tolerance. Moreover, the obtained 1,2-carbonylboration products provide substantial opportunities for further transformations which cannot be obtained by known carbonylation procedures. Preliminary mechanistic studies indicate that the three hydrogen atoms of the products originated from ethyl acetate.
Construction of boro-containing organic molecules remains an important and hot research field due to their wide applications in materials science,1 pharmaceuticals2 and organic chemistry.3 A multitude of methods have been developed for the synthesis of organoboron compounds over the past decades.4 Among these methods, transition-metal-catalyzed borofunctionalization of alkynes is a powerful synthetic strategy due to its high selectivity and efficiency.5 For example, the use of copper as a precatalyst for the borylation of alkynes has generated renewed interest in the area. The β-borylalkenylcopper intermediates obtained via syn addition of borylcopper to alkynes can electrophilically trap various electrophiles to form different alkenylboronates (Scheme 1, 1). The classical approach of this type of transformation is alkyne hydroboration (Scheme 1, 1a).6 Subsequently, with vinylcopper species as the proposed key intermediates, their further reactions with halogen substitutes (Scheme 1, 1b),7 CO2 (Scheme 1, 1c),8 allyl phosphates (Scheme 1, 1d),9 and tin alkoxides (Scheme 1, 1e)10 to give the corresponding alkenylboronates were reported. More recently, Mankad and Cheng reported their achievements on the direct efficient synthesis of tetrasubstituted β-borylenones using a copper-catalyzed four-component coupling reaction of simple chemical feedstocks: internal alkynes, alkyl halides, bis(pinacolato)diboron (B2pin2) and CO (Scheme 1, 1f).11 Inspired by their achievements and considering the advantage of a multicomponent borocarbonylation reaction, we developed a new Pd/Cu-catalyzed multi-component carbonylation and borylation reaction of alkynes, aryldiazonium salts, B2Pin2, ethyl acetate and CO to obtain saturated β-boryl ketones (Scheme 1, 3). In addition, this new catalyst system can catalyze the regioselective functionalization of alkynes to obtain 2,1-carbonylboration products that are different from the 1,2-products by known transition-metal-catalyzed borylacylation (Scheme 1, 2a) and borocarbonylation (Scheme 1, 2b) of alkenes.12 Nevertheless, the carbonylative and hydroborative coupling of alkynes with aryldiazonium salts to obtain saturated β-boryl ketones is still a challenge and has never been reported.
Initially, we tested various reaction conditions using phenyl acetylene (1a), 4-methoxybenzenediazonium tetrafluoroborate (2a) and bis(pinacolato)diboron as the reaction partners. To our delight, by using Pd(acac)2 and CuI as the cooperative precatalyst, PPh3 as the ligand, Na2CO3 as the base and ethyl acetate (EA) as the solvent at 110 °C under a CO atmosphere (20 bar) with 12 h reaction time, the desired borocarbonylative coupling product (3aa) was obtained in a good GC yield of 78% (Table 1, entry 1). When using Pd(OAc)2, IPrCuCl, IMesCuCl, CuCl or CuCl2 as the precatalyst, the reaction gave a reduced yield of the desired product (Table 1, entries 2–6). Similarly, reducing the pressure of CO (10 bar) led to a decreased yield of 3aa (Table 1, entry 7). Subsequently, ligands such as PCy3, DPPB, and DPEPhos and bases such as sodium tert-butoxide and cesium carbonate were found to be totally unsuitable for this transformation (Table 1, entries 8–12). With these results, we believe besides as a solvent, ethyl acetate (EA) also acts as a hydrogen source in this system. Then various solvents such as methanol, isopropanol and DMF which can provide a hydrogen source in many reduction reactions were tested but found to be ineffective for the reaction (Table 1, entries 13–15). Surprisingly, almost no reaction occurred using ethyl acetoacetate as the solvent which is more acidic than ethyl acetate (Table 1, entry 16). It is important to mention that by-products produced from hydroboration of alkyne can be detected during the optimization process.
| Entry | Variation from the standard conditions | Yield (%) |
|---|---|---|
| a Reaction conditions: 1a (0.1 mmol, 1 equiv.), B2pin2 (0.2 mmol, 2 equiv.), 2a (0.1 mmol, 1 equiv.), Pd(acac)2 (5 mol%), CuI (10 mol%), PPh3 (20 mol%), Na2CO3 (0.4 mmol, 4 equiv.), CO (20 bar), CH3COOEt (2 mL), stirred at 110 °C for 12 h, yields were determined by GC analysis using hexadecane as the internal standard. b CO (10 bar). c DPPB: 1,4-bis(diphenylphosphino)butane (10 mol%). d DPEphos: bis[2-(diphenylphosphino)phenyl] ether (10 mol%). EAA: ethyl acetoacetate. | ||
| 1 | — | 78 |
| 2 | Using Pd(OAc)2 instead of Pd(acac)2 | 44 |
| 3 | Using IPrCuCl instead of CuI | 41 |
| 4 | Using IMesCuCl instead of CuI | 38 |
| 5 | Using CuCl instead of Cul | 33 |
| 6 | Using CuCl2 instead of CuI | 31 |
| 7b | CO (10 bar) instead of CO (20 bar) | 56 |
| 8 | PCy3 instead of PPh3 | Trace |
| 9c | Using DPPB instead of PPh3 | Trace |
| 10d | Using DPEPhos instead of PPh3 | Trace |
| 11 | Using tBuONa instead of Na2CO3 | — |
| 12 | Using Cs2CO3 instead of Na2CO3 | — |
| 13 | Using MeOH instead of CH3COOEt | — |
| 14 | Using isopropanol instead of CH3COOEt | — |
| 15 | Using DMF instead of CH3COOEt | — |
| 16 | Using EAA instead of CH3COOEt | — |
With the optimal reaction conditions in hand, we initially investigated the scope of alkynes for this reaction with 4-methoxybenzenediazonium tetrafluoroborate (2a) (Scheme 2). First, a variety of aryl alkynes with electron-rich and electron-deficient groups at the para position were successfully converted to the desired products 3aa–3ga in good to excellent yields. Similarly, ortho/meta-substituted aryl alkynes could also be converted into the corresponding products in moderate to good yields (Scheme 2, 3ha–3ka). Importantly, 3-ethynylthiophene, as an example of a heterocyclic alkyne, can be successfully reacted as well, and a good yield of the targeted product was obtained (Scheme 2, 3la). Notably, aliphatic alkynes can be effectively transformed with 4-methoxybenzenediazonium tetrafluoroborate and afforded the corresponding products in good to excellent yields (Scheme 2, 3ma–3oa). However, aromatic/aliphatic diynes, internal alkynes, 3-phenyl-1-propyne and 3-methyl-1-butyne were ineffective in our procedure.
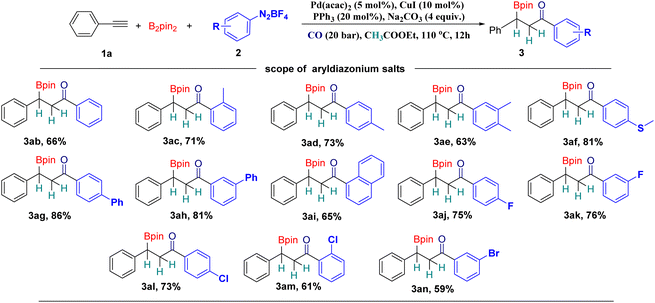
Subsequently, with phenylacetylene (1a) as the model substrate, different aryl diazonium tetrafluoroborates were tested (Scheme 3). Aryl diazonium tetrafluoroborates with electronically neutral functional groups are all suitable substrates for this methodology and good yields can be achieved in all the tested cases (Scheme 3, 3ab–3ae). Methylthiol and phenyl groups were well tolerated under our conditions (Scheme 3, 3af–3ah). A good yield of the desired product can still be achieved with 1-naphthalenyl diazonium tetrafluoroborate (Scheme 3, 3ai). Halogen substituents can be tolerated as well, including fluoride and chloride, and good yields of the corresponding products can be obtained (Scheme 3, 3aj–3am). The bromide substituent, as an important functional group in cross-coupling transformations, can be tolerated and provide 59% of the desired product, which is ready for further functionalizations (Scheme 3, 3an).
To understand the mechanism of this carbonylation process, a radical quenching experiment was designed to probe the mechanism of this reaction (Scheme 4). The reaction was fully inhibited when 3 equivalents of TEMPO were added to the model system (Scheme 4, a). The result shows that the radical intermediate may participate in the process. Next, we carried out the reaction in the absence of 4-methoxybenzenediazonium tetrafluoroborate (2a) and carbon monoxide, and alkenylboronic esters were obtained. Then 2a was added, and the reaction continued under the standard conditions but no corresponding product was produced (Scheme 4, b-1). Under identical reaction conditions, but in the absence of B2pin2, the carbonylative coupling product (4a) was obtained in an excellent GC yield of 95%. Surprisingly, the desired product 3aa could be obtained in 90% yield when B2pin2 was added (Scheme 4, b-2).
Finally, to gain insight into the hydrogen source of this reaction, alkynone (4a) was subjected to standard conditions without any catalyst and CO (Scheme 4, c-1). The results revealed that the hydrogen source cannot come from the terminal hydrogen of phenylacetylene. No reaction occurred when the experiment was performed in ultra-dry solvent and 2 equivalents of water under standard conditions (Scheme 4, c-2), which indicated that water should not be a hydrogen source for this reaction. Interestingly, when using CD3COOEt as the solvent, the deuterated product 3aa-D could be obtained in 69% yield (Scheme 4, c-3). According to the reaction results, we believe that the hydrogen came from ethyl acetate.
Based on the above control experiments and related literature,13,14 a possible reaction pathway is proposed (Scheme 5). Initially, Pd(0) precursor A will react with 2 to give the aryl Pd(II) complex along with the release of N2. Subsequent CO insertion into the C–Pd bond affords palladium carbonyl intermediate B. Terminal alkynes 1 react with CuI to produce alkynyl Cu intermediate C, which will transmetalate with Pd(II) species B. Then the produced palladium carbonyl intermediate D gives alkynone 4 and Pd(0) species by reductive elimination. Alkynone 4 together with B2pin2 in the presence of ethyl acetate will generate vinyl-boronate 5, and then another equivalent of B2pin2 will add to the carbon–carbon double bond allowing the formation of 1,1,2-tris(boronate) 6 which is not very stable under basic conditions.14 For this reason, compound 6 undergoes selective protodeboronation to generate 1,1-diboronate esters 7 which will undergo further protodeboronation to give the final product 3, and this part is most likely radical involved.
In summary, we have described a convenient procedure to synthesize saturated β-boryl ketones via cooperative Pd/Cu-catalyzed multi-component carbonylation and borylation reaction of alkynes, aryldiazonium salts, B2pin2, ethyl acetate and CO. In addition, this reaction proceeds with broad scope and functional group tolerance, and delivers β-boryl ketones in moderate to excellent yields. Mechanistic research shows that the three hydrogen atoms come from ethyl acetate.
Author contributions
FZ and XFW directed this project, prepared and revised the manuscript. PY performed all the experiments.Conflicts of interest
There is no conflict of interests to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21901148).References
- (a) C. D. Entwistle and T. B. Marder, Angew. Chem., Int. Ed., 2002, 41, 2927 CrossRef; (b) M. Elbing and G. C. Bazan, Angew. Chem., Int. Ed., 2008, 47, 834 CrossRef PubMed; (c) F. Jäkle, Chem. Rev., 2010, 110, 3985 CrossRef PubMed; (d) Y. L. Rao, H. Amarne and S. Wang, Coord. Chem. Rev., 2012, 256, 759 CrossRef; (e) L. Ji, S. Griesbeck and T. B. Marder, Chem. Sci., 2017, 8, 846 RSC.
- (a) A. Paramore and S. Frantz, Nat. Rev. Drug Discovery, 2003, 2, 611 CrossRef PubMed; (b) L. Borissenko and M. Groll, Chem. Rev., 2007, 107, 687 CrossRef PubMed; (c) M. A. Beenen, C. An and J. A. Ellman, J. Am. Chem. Soc., 2008, 130, 6910 CrossRef CAS PubMed; (d) L. J. Milo, J. H. Lai Jr, W. Wu, Y. Liu, H. Maw, Y. Li, Z. Jin, Y. Shu, S. E. Poplawski, Y. Wu, D. G. Sanford, J. L. Sudmeier and W. W. Bachovchin, J. Med. Chem., 2011, 54, 4365 CrossRef CAS PubMed.
- (a) Boronic Acids-Preparation and Applications in Organic Synthesis, Medicine and Materials, ed. D. G. Hall, Wiley-VCH, Weinheim, 2nd edn, 2011 Search PubMed; (b) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417 CrossRef CAS PubMed; (c) T. S. De Vries, A. Prokofjevs and E. Vedejs, Chem. Rev., 2012, 112, 4246 CrossRef CAS; (d) E. Dimitrijević and M. S. Taylor, ACS Catal., 2013, 3, 945 CrossRef; (e) A. J. J. Lennox and G. C. Lloyd-Jones, Chem. Soc. Rev., 2014, 43, 412 RSC; (f) L. Xu, S. Zhang and P. Li, Chem. Soc. Rev., 2015, 44, 8848 RSC; (g) E. C. Neeve, S. J. Geier, I. A. I. Mkhalid, S. A. Westcott and T. B. Marder, Chem. Rev., 2016, 116, 9091 CrossRef CAS PubMed; (h) J. W. B. Fyfe and A. J. B. Watson, Chem, 2017, 3, 31 CrossRef; (i) Y.-M. Tian, X.-N. Guo, H. Braunschweig, U. Radius and T. B. Marder, Chem. Rev., 2021, 121, 3561 CrossRef.
- (a) R. I. McDonald, G. Liu and S. S. Stahl, Chem. Rev., 2011, 111, 2981 CrossRef PubMed; (b) V. Saini, B. J. Stokes and M. S. Sigman, Angew. Chem., Int. Ed., 2013, 52, 11206 CrossRef CAS; (c) G. Yin, X. Mu and G. Liu, Acc. Chem. Res., 2016, 49, 2413 CrossRef CAS; (d) W. Wang, C. Ding, Y. Li, Z. Li, Y. Li, L. Peng and G. Yin, Angew. Chem., Int. Ed., 2019, 131, 4660 CrossRef; (e) Y. Li, H. Pang, D. Wu, Z. Li, W. Wang and G. Yin, Angew. Chem., Int. Ed., 2019, 58, 8872 CrossRef CAS PubMed; (f) H. M. Nelson, B. D. Williams, J. Miró and F. D. Toste, J. Am. Chem. Soc., 2015, 137, 3213 CrossRef CAS PubMed; (g) Z. Liu, H.-Q. Ni, T. Zeng and K. M. Engle, J. Am. Chem. Soc., 2018, 140, 3223 CrossRef CAS PubMed; (h) W. Su, T.-J. Gong, X. Lu, M.-Y. Xu, C.-G. Yu, Z.-Y. Xu, H.-Z. Yu, B. Xiao and Y. Fu, Angew. Chem., Int. Ed., 2015, 54, 12957 CrossRef CAS PubMed; (i) T. Jia, P. Cao, B. Wang, Y. Lou, X. Yin, M. Wang and J. Liao, J. Am. Chem. Soc., 2015, 137, 13760 CrossRef CAS PubMed; (j) K. B. Smith and M. K. Brown, J. Am. Chem. Soc., 2017, 139, 7721 CrossRef CAS; (k) S. R. Sardini and M. K. Brown, J. Am. Chem. Soc., 2017, 139, 9823 CrossRef CAS PubMed; (l) K. M. Logan, K. B. Smith and M. K. Brown, Angew. Chem., Int. Ed., 2015, 127, 5317 CrossRef; (m) K. M. Logan, S. R. Sardini, S. D. White and M. K. Brown, J. Am. Chem. Soc., 2018, 140, 159 CrossRef PubMed; (n) T. Fujihara, A. Sawada, T. Yamaguchi, Y. Tani, J. Terao and Y. Tsuji, Angew. Chem., Int. Ed., 2017, 56, 1539 CrossRef.
- (a) M. Suginome, A. Yamamoto and M. Murakami, J. Am. Chem. Soc., 2003, 125, 6358 CrossRef; (b) A. Yamamoto and M. Suginome, J. Am. Chem. Soc., 2005, 127, 15706 CrossRef CAS; (c) M. Suginome, M. Shirakura and A. Yamamoto, J. Am. Chem. Soc., 2006, 128, 14438 CrossRef CAS PubMed; (d) H. Yoshida, I. Kageyuki and K. Takaki, Org. Lett., 2014, 16, 3512 CrossRef CAS PubMed; (e) T. Miura, N. Oku and M. Murakami, Angew. Chem., Int. Ed., 2019, 58, 14620 CrossRef CAS.
- (a) H. Jang, A. R. Zhugralin, Y. Lee and A. H. Hoveyda, J. Am. Chem. Soc., 2011, 133, 7859–7871 CrossRef CAS PubMed; (b) H. R. Kim and J. Yun, Chem. Commun., 2011, 47, 2943 RSC; (c) Y. Sasaki, Y. Horita, C. Zhong, M. Sawamura and H. Ito, Angew. Chem., Int. Ed., 2011, 50, 2778–2782 CrossRef CAS; (d) A. L. Moure, R. N. Gómez Arrayás, D. J. Cárdenas, I. S. Alonso and J. C. Carretero, J. Am. Chem. Soc., 2012, 134, 7219 CrossRef CAS PubMed; (e) J. K. Park, B. A. Ondrusek and D. T. McQuade, Org. Lett., 2012, 14, 4790 CrossRef CAS PubMed; (f) A. L. Moure, P. Mauleón, R. G. Arrayás and J. C. Carretero, Org. Lett., 2013, 15, 2054 CrossRef CAS PubMed; (g) J. Zhao, Z. Niu, H. Fu and Y. Li, Chem. Commun., 2014, 50, 2058 RSC; (h) Q. Wang, M. Biosca, F. Himo and K. J. Szabo, Angew. Chem., Int. Ed., 2021, 60, 26327 CrossRef CAS; (i) W.-H. Li, J. Yang, H. Jing, J. Zhang, Y. Wang, J. Li, J. Zhao, D. Wang and Y. Li, J. Am. Chem. Soc., 2021, 143, 15453 CrossRef CAS; (j) M. Zhong, Y. Gagne, T. O. Hope, X. Pannecoucke, M. Frenette, P. Jubault and T. Poisson, Angew. Chem., Int. Ed., 2021, 60, 14498 CrossRef CAS.
- (a) M. Suginome, A. Yamamoto and M. Murakami, Angew. Chem., Int. Ed., 2005, 44, 2380 CrossRef CAS; (b) M. Daini, A. Yamamoto and M. Suginome, J. Am. Chem. Soc., 2008, 130, 2918 CrossRef CAS; (c) P. Liu, Y. Fukui, P. Tian, Z.-T. He, C.-Y. Sun, N.-Y. Wu and G.-Q. Lin, J. Am. Chem. Soc., 2013, 135, 11700 CrossRef; (d) H. Yoshida, I. Kageyuki and K. Takaki, Org. Lett., 2013, 15, 952 CrossRef PubMed; (e) R. Alfaro, A. Parra, J. Alemán, J. L. García Ruano and M. Tortosa, J. Am. Chem. Soc., 2012, 134, 15165 CrossRef; (f) Y. D. Bidal, F. Lazreg and C. S. J. Cazin, ACS Catal., 2014, 4, 1564 CrossRef; (g) Y. Zhou, W. You, K. B. Smith and M. K. Brown, Angew. Chem., Int. Ed., 2014, 53, 3475 CrossRef; (h) Y. Gao, N. Kim, S. D. Mendoza, S. Yazdni, A. F. Vieira, M. Liu, A. Kendrick, D. B. Grotjahn, G. Bertrand, R. Jazzar and K. M. Engle, ACS Catal., 2022, 12, 7243 CrossRef.
- L. Zhang, J. Cheng, B. Carry and Z. Hou, J. Am. Chem. Soc., 2012, 134, 14314 CrossRef PubMed.
- H.-Y. Bin, X. Wei, J. Zi, Y.-J. Zuo and T.-C. Wang, ACS Catal., 2015, 5, 6670 CrossRef CAS.
- Y. Takemoto, H. Yoshida and K. Takaki, Chem.–Eur. J., 2012, 18, 14841 CrossRef CAS PubMed.
- (a) L.-J. Cheng and N. P. Mankad, Angew. Chem., Int. Ed., 2018, 57, 10328 CrossRef CAS; (b) L.-J. Cheng and N. P. Mankad, J. Am. Chem. Soc., 2020, 142, 80 CrossRef CAS.
- (a) Y. Huang, K. B. Brown and M. K. Smith, Angew. Chem., Int. Ed., 2017, 56, 13314 CrossRef CAS PubMed; (b) Y. Yuan, F.-P. Wu, J.-X. Xu and X.-F. Wu, Angew. Chem., Int. Ed., 2020, 59, 17055 CrossRef CAS; (c) F.-P. Wu, Y. Yuan, C. Schünemann, P. C. J. Kamer and X.-F. Wu, Angew. Chem., Int. Ed., 2020, 59, 10451 CrossRef CAS; (d) Y. Yuan, F.-P. Wu, A. Spannenberg and X.-F. Wu, Sci. China: Chem., 2021, 64, 2142 CrossRef CAS; (e) F.-P. Wu, J. Holz, Y. Yuan and X.-F. Wu, CCS Chem., 2020, 2, 2643–2654 Search PubMed.
- Y. Wu, L. Zeng, H. Li, Y. Cao, J. Hu, M. Xu, R. Shi, H. Yi and A. Lei, J. Am. Chem. Soc., 2021, 143, 12460 CrossRef CAS PubMed.
- (a) A. B. Cuenca, R. Shishido, H. Ito and E. Fernandez, Chem. Soc. Rev., 2017, 46, 415 RSC; (b) G. Gao, J. Yan, K. Yang, F. Chen and Q. Song, Green Chem., 2017, 19, 3997 RSC; (c) G. Gao, Z. Kuang and Q. Song, Org. Chem. Front., 2018, 5, 2249 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2sc04867a |
| This journal is © The Royal Society of Chemistry 2022 |