Open Access Article
Open Access ArticlePhosphine-catalyzed activation of cyclopropenones: a versatile C3 synthon for (3+2) annulations with unsaturated electrophiles†
Xin
He‡
a,
Pengchen
Ma‡
ab,
Yuhai
Tang
a,
Jing
Li
a,
Shenyu
Shen
a,
Martin J.
Lear
 c,
K. N.
Houk
c,
K. N.
Houk
 *b and
Silong
Xu
*b and
Silong
Xu
 *a
*a
aSchool of Chemistry, and Xi'an Key Laboratory of Sustainable Energy Materials Chemistry, Xi'an Jiaotong University, Xi'an 710049, P. R. China
bDepartment of Chemistry and Biochemistry, University of California, Los Angeles, California 90095-1569, USA
cSchool of Chemistry, University of Lincoln, Brayford Pool, Lincoln, LN6 7TS, UK. E-mail: silongxu@mail.xjtu.edu.cn; houk@chem.ucla.edu
First published on 17th October 2022
Abstract
We herein report a phosphine-catalyzed (3 + 2) annulation of cyclopropenones with a wide variety of electrophilic π systems, including aldehydes, ketoesters, imines, isocyanates, and carbodiimides, offering products of butenolides, butyrolactams, maleimides, and iminomaleimides, respectively, in high yields with broad substrate scope. An α-ketenyl phosphorous ylide is validated as the key intermediate, which undergoes preferential catalytic cyclization with aldehydes rather than stoichiometric Wittig olefinations. This phosphine-catalyzed activation of cyclopropenones thus supplies a versatile C3 synthon for formal cycloadditon reactions.
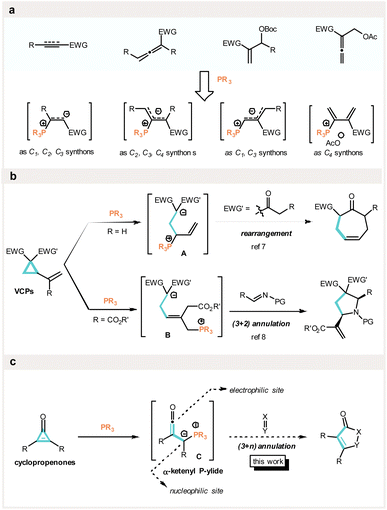
The development of effective strategies to construct cyclic molecular architectures has attracted long-standing interest from the chemistry community.1 In this regard, phosphine catalysis2 has emerged as a powerful and versatile approach for the construction of various carbo- and heterocyles. Lu's (3 + 2),3 Kwon's (4 + 2),4 and Tong's (4 + 1)5 annulations represent seminal advances in this field, from which a plethora of reactions6 and asymmetric variants2b have been inspired. Since phosphine-catalyzed reactions are usually initiated by the conjugate addition of a phosphine to a polar double or triple bond to generate reactive zwitterionic intermediates, the prevalent substrates of phosphine catalysis rely almost entirely on electron-deficient alkenes, alkynes, allenes, and their derivatives2a (Fig. 1a). These substrate entities serve as effective C1 to C4 synthons for generating various ring systems. Alternatively, we envisaged that the integration of the C–C bond activation of strained carbocycles within phosphine catalysis would significantly expand the scope. In 2018, we disclosed that electron-deficient vinylcyclopropanes (VCPs) undergo phosphine-catalyzed activation to generate zwitterions A that triggers the rearrangement of vinylcyclopropylketones to cycloheptenones (Fig. 1b, up).7 Very recently, an elegant phosphine-catalyzed enantioselective (3 + 2) annulation of electron-deficient vinylcyclopropanes with N-tosylaldimines with a zwitterion B as the key intermediate has been developed by Lu and co-workers8 (Fig. 1b, down). In the meantime, we have established that electron-deficient alkylidenecyclopropanes (ACPs) also readily undergo phosphine-catalyzed substrate-controlled rearrangements to afford polysubstituted furans and dienones.9
As part of ongoing studies, we hypothesized that cyclopropenones, as triggered by phosphines, would serve as C3 synthons for possible (3 + n) annulations (Fig. 1c). Mechanistically, the nucleophilic addition of a phosphine to cyclopropenones followed by ring cleavage would generate an α-ketenyl phosphorus ylide C.10 Prescher and co-workers11 have previously employed such ylides to react with nucleophiles, e.g. primary amines, for applications in bioorthogonal ligations. By virtue of its amphiphilic structure bearing both a nucleophilic ylide and an electrophilic ketene moiety, we proposed that it might be used as a 1,3-dipole surrogate for annulation reactions with unsaturated electrophiles (Fig. 1c).
As a subclass of “non-benzenoid aromatic compounds”, cyclopropenones12 are strained, highly unsaturated, and readily available building blocks which have drawn tremendous interest in contemporary organic synthesis due to their unique and versatile reactivities.13 The activation of these strained compounds is typically achieved through transition metal catalysis, via oxidative addition to the C–C single bond14 to bring about various transformations,13b especially annulation reactions.15 Wender and co-workers15b pioneered the Rh-catalyzed (3 + 2) cycloaddition of cyclopropenones with alkynes to build cyclopentadienones, whereas Li and co-workers15f developed a Ni-catalyzed (3 + 2) annulation of cyclopropenones with α,β-unsaturated ketones/imines to access butenolides and lactams. Gleiter and co-workers15k,l also demonstrated an interesting Co-mediated dimerization of cyclopropenones to form Co-capped benzoquinones. Other metal complexes involving Pd,15c,i Ru,15a,16 Ag,17 and so forth,18 are also known to facilitate a range of annulations with cyclopropenones. Compared to transition metal-catalyzed methods, however, the organocatalytic activation of cyclopropenones toward practical transformations remains far less explored.19 Stemming from our interest in Lewis base catalysis,7,9,20 we now report the phosphine-catalyzed activation of cyclopropenones as a new subset of C3 synthons that are capable of undergoing (3 + 2) annulations with various unsaturated electrophiles (vide infra).
Initially, we examined the phosphine-catalyzed reaction of diphenylcyclopropenone 1a with several activated alkenes such as acrylates and maleates. These attempts were unsuccessful; however, the employment of benzaldehyde 2a as the reaction partner led to the anticipated (3 + 2) annulation to afford a butenolide product 3a (Table 1). To our knowledge, the (3 + 2) annulation of cyclopropenones with simple aldehydes is unprecedented, even under transition metal catalysis.21 Another point of note is that the aforementioned α-ketenyl phosphorus ylide C does not undergo the usual Wittig reaction with aldehydes but enters into a catalytic cycloaddition pathway (see mechanism discussions below).
| Entry | catalyst | Additive | Solvent | Time | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.30 mmol), 2a (0.20 mmol), and catalyst (0.02 mmol, 10 mol%) were stirred in the solvent (2.0 mL) at r. t. under N2 atmosphere. b Yield of isolated product. c 0.20 mmol 1a was used. d 5 mol% of PMe3 was adopted. e 2 mol% of PMe3 was used. f 0.1 mol% of PMe3 was adopted. | |||||
| 1c | PPh3 | — | CH2Cl2 | 3 h | Trace |
| 2c | PBu3 | — | CH2Cl2 | 3 h | 22 |
| 3c | PMe3 | — | CH2Cl2 | 3 h | 30 |
| 4c | PMe3 | 4Å MS | CH2Cl2 | 30 min | 73 |
| 5 | PMe3 | 4Å MS | CH2Cl2 | 30 min | 99 |
| 6d | PMe3 | 4Å MS | CH2Cl2 | 2 h | 92 |
| 7e | PMe3 | 4Å MS | CH2Cl2 | 24 h | 78 |
| 8f | PMe3 | 4Å MS | CH2Cl2 | 5 d | 20 |
| 9 | PMe3 | 4Å MS | THF | 1 h | 88 |
| 10 | PMe3 | 4Å MS | CH3CN | 1 h | 35 |
| 11 | PMe3 | 4Å MS | Toluene | 1 h | 95 |
| 12 | PMe3 | 4Å MS | Cyclohexane | 1 h | 69 |
| 13 | PMe3 | 4Å MS | DMF | 1 h | 44 |
It was found that PPh3 did not promote the reaction, whereas PBu3 and PMe3 catalyzed the reaction with yields of 22% and 30% of 3a, respectively (entries 1–3). Nitrogen-containing Lewis bases such as DABCO, DMAP, and DBU were inefficient catalysts for the reaction (not shown). Interestingly, the addition of 4 Å molecular sieves (4Å MS) improved the yield to 73% in a shorter time (entry 4), suggesting the progress of the reaction to be water sensitive. Increasing the amount of 1a to 1.5 equivalents led to quantitative conversion, and halving the catalyst loading to 5 mol% still furnished an excellent yield of 92% in 2 h (entries 5 and 6). Further reducing the catalyst loading to 2 mol% gave 78% yield over 24 h, while 0.1 mol% of catalyst resulted in a substantially lower yield (entries 7 and 8). Examination of common solvents indicated dichloromethane to be optimal, although toluene gave comparable results (entries 9–13).
With optimized conditions in hand, the scope of the (3 + 2) heteroannulation of cyclopropenones with aldehydes was investigated first (Fig. 2). A series of benzaldehydes with electron-donating groups (–Me, –tBu, –OMe, –OCF3), halogens (–F, –Cl, –Br), or electron-withdrawing groups (–CO2Me, –CF3, –NO2), substituted at either para, ortho, or meta position, all proceeded smoothly producing the corresponding adducts 3b–3r in 55–96% yields. While naphthalene formaldehyde produced butenolide 3s in 88% yield, heteroaryl aldehydes such as 2-furaldehyde, 2-thienaldehyde, and 3-indole aldehyde, yielded their respective annulated products 3t–3v in 94–99% yields. The structure of 3v was confirmed by single-crystal X-ray analysis. Notably, aliphatic aldehydes, such as butyraldehyde and pentanal, were also highly efficient substrates, providing adducts 3w and 3x in 89% and 87% yields, respectively. Even paraformaldehyde was found to undergo the (3 + 2) annulation with 1a to give butenolide 3y in 82% yield. To explore the scope of cyclopropenones, fluoro- and methyl-substituted diphenylcyclopropenones (1b and 1c) were reacted with 4-methylbenzaldehyde, which produced the adducts 3z and 3aa in 91% and 93% yields, respectively. When cyclopropenones with unsymmetric substituents (R1 = aryl, R2 = methyl) were adopted, the annulated products 3ab–3ad were obtained in 89–92% yields with excellent regioselectivity, possibly due to the preferential attack of the phosphine catalyst to the less sterically hindered side of the cyclopropenone. However, when a bigger ethyl is incorporated in the cyclopropenone (R1 = phenyl, R2 = ethyl), the annulated product 3ae was obtained in 51% yield with a poor regioselectivity (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). It was then found that 1,2-dibutylcyclopropenone failed in the annulation (not shown), probably due to its less electrophilicity retarding the nucleophilic attack of the phosphine catalyst. Among aldehyde substrates, it is noteworthy that salicylic aldehyde reacted differently to form the enolate ester 4, presumably via phenolate addition to a protonated ketenyl phosphonium intermediate.22 Besides aldehydes, it was found that the ketoester 5 also underwent (3 + 2) annulation readily with representative cyclopropenones to afford fully-substituted butenolides 6a–6c in 91–98% yields (Fig. 2, bottom left). Normal ketones like acetone and benzophenone, however, were ineffective under the current reaction conditions. More intriguingly, N-tosylimine 7 was also found to be an efficient partner for (3 + 2) annulation with 1, which produced the butyrolactams 8a–8c in 71–88% yields (Fig. 2, bottom right).
1). It was then found that 1,2-dibutylcyclopropenone failed in the annulation (not shown), probably due to its less electrophilicity retarding the nucleophilic attack of the phosphine catalyst. Among aldehyde substrates, it is noteworthy that salicylic aldehyde reacted differently to form the enolate ester 4, presumably via phenolate addition to a protonated ketenyl phosphonium intermediate.22 Besides aldehydes, it was found that the ketoester 5 also underwent (3 + 2) annulation readily with representative cyclopropenones to afford fully-substituted butenolides 6a–6c in 91–98% yields (Fig. 2, bottom left). Normal ketones like acetone and benzophenone, however, were ineffective under the current reaction conditions. More intriguingly, N-tosylimine 7 was also found to be an efficient partner for (3 + 2) annulation with 1, which produced the butyrolactams 8a–8c in 71–88% yields (Fig. 2, bottom right).
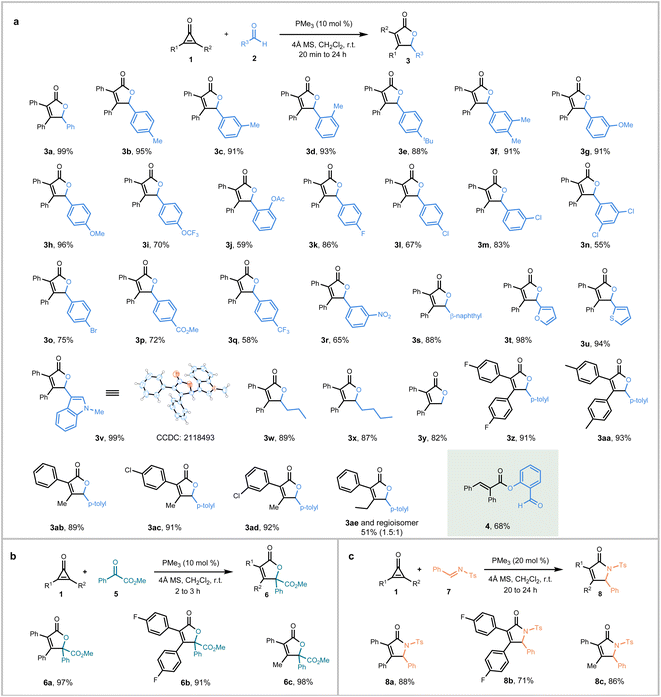 | ||
| Fig. 2 Scope of PMe3-catalyzed (3 + 2) annulation with electrophilic C = X partners. (a) Reaction with aldehydes. (b) Reaction with ketoester. (c) Reaction with imines. | ||
As C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds can be both successfully integrated into annulations, we next examined the reaction of isocyanates possessing cumulated C
N bonds can be both successfully integrated into annulations, we next examined the reaction of isocyanates possessing cumulated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds. Under optimized conditions (see ESI for details†), the phosphine-catalyzed (3 + 2) annulation of cyclopropenones with isocyanates 9 occurred exclusively at the C
N bonds. Under optimized conditions (see ESI for details†), the phosphine-catalyzed (3 + 2) annulation of cyclopropenones with isocyanates 9 occurred exclusively at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond to provide the maleimide derivatives 10 in high yield (Fig. 3). The scope of the reaction was therefore found to be broad. Aryl isocyanates with varied electron properties substituted at either para, ortho, or meta position typically reacted well to produce 10a–10k in good yields. A trend can be discerned, such that groups with increased electron-withdrawing ability on the benzene ring decreased the productivity. It was found that both alkyl and allyl isocyanates also readily coupled with cyclopropenones to provide N-substituted maleimides 10l–10q in 60–83% yields. The structure of 10e was confirmed by single-crystal X-ray analysis. Substitution of the phenyl groups of cyclopropenones was tolerated, as shown by the formation of 10r–10u in 72–81% yields. Bis-isocyanates were also found efficient, which annulated with two molecules of 1a to form adducts 10v and 10w in excellent yields. It is noteworthy that the convenient synthesis of polysubstituted maleimides by our current strategy stands in sharp contrast with transition-metal catalyzed ones, for example, as reported by Kondo and co-workers16 through ruthenium-catalyzed (2 + 2 + 1) cocyclization of isocyanates, alkynes, and CO. To further demonstrate the generality of our phosphine-catalyzed annulation method, two commercially available carbodiimides 11 were employed as annulation partners with representative cyclopropenones (Fig. 3, bottom). These reactions smoothly generated the iminomaleimides 12a–12f in excellent yields (81–91%; single-crystal X-ray structure confirming 12a unequivocally).
N bond to provide the maleimide derivatives 10 in high yield (Fig. 3). The scope of the reaction was therefore found to be broad. Aryl isocyanates with varied electron properties substituted at either para, ortho, or meta position typically reacted well to produce 10a–10k in good yields. A trend can be discerned, such that groups with increased electron-withdrawing ability on the benzene ring decreased the productivity. It was found that both alkyl and allyl isocyanates also readily coupled with cyclopropenones to provide N-substituted maleimides 10l–10q in 60–83% yields. The structure of 10e was confirmed by single-crystal X-ray analysis. Substitution of the phenyl groups of cyclopropenones was tolerated, as shown by the formation of 10r–10u in 72–81% yields. Bis-isocyanates were also found efficient, which annulated with two molecules of 1a to form adducts 10v and 10w in excellent yields. It is noteworthy that the convenient synthesis of polysubstituted maleimides by our current strategy stands in sharp contrast with transition-metal catalyzed ones, for example, as reported by Kondo and co-workers16 through ruthenium-catalyzed (2 + 2 + 1) cocyclization of isocyanates, alkynes, and CO. To further demonstrate the generality of our phosphine-catalyzed annulation method, two commercially available carbodiimides 11 were employed as annulation partners with representative cyclopropenones (Fig. 3, bottom). These reactions smoothly generated the iminomaleimides 12a–12f in excellent yields (81–91%; single-crystal X-ray structure confirming 12a unequivocally).
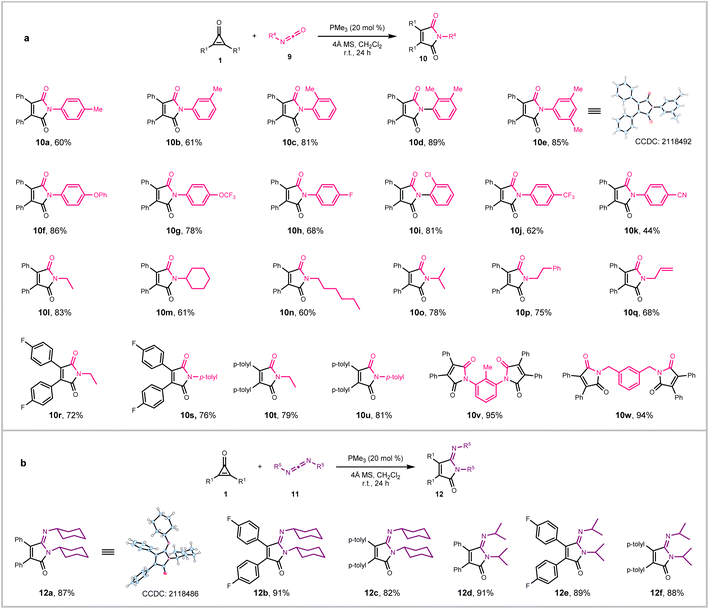 | ||
Fig. 3 Scope of PMe3-catalyzed (3 + 2) annulation with cumulated X = C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N partners. (a) Reaction with isocyanates. (b) Reaction with carbodiimides. N partners. (a) Reaction with isocyanates. (b) Reaction with carbodiimides. | ||
Collectively, our findings clearly indicate that the phosphine-catalyzed (3 + 2) heteroannulation of cyclopropenones is general for a broad range of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X substrates including aldehydes, ketoesters, imines, isocyanates and carbodiimides. Notably, the products butenolide, butyrolactam, maleimide, and iminomaleimide are of high biologically relevance23 and synthetic utility,24 which can now be readily generated in high efficiencies under mild conditions. This annulation strategy also constitutes a highly attractive alternative to transition metal-based variants.15f,i
X substrates including aldehydes, ketoesters, imines, isocyanates and carbodiimides. Notably, the products butenolide, butyrolactam, maleimide, and iminomaleimide are of high biologically relevance23 and synthetic utility,24 which can now be readily generated in high efficiencies under mild conditions. This annulation strategy also constitutes a highly attractive alternative to transition metal-based variants.15f,i
A31P NMR tracking experiment was conducted in order to detect any essential intermediates in the PMe3-catalyzed (3 + 2) annulation (See ESI for details†). When mixing cyclopropenone 1a, isocyanate 9a with PMe3 in CDCl3 for 3 h, it was found that, with the disappearance of PMe3, several new species with signals at 5.8, 15.6, 22.9, and 38.6 ppm appeared in the 31P NMR spectrum. This result supports the involvement of the phosphine in the catalysis, and implies that free phosphine is not the resting state of the catalytic cycle. In addition, when the reaction mixture was subjected to HRMS, a peak at 283.1248 (C18H19OP [M + H]+) corresponding to the adduct of 1a and PMe3 was detected, which may also support the formation of the proposed α-ketenyl ylide intermediate (See ESI for details†).
To further probe the reaction mechanism and the origins of chemoselectivity toward the formation of 3a over Wittig-based pathways to 3a*, density functional theory (DFT) calculations were performed as shown in Fig. 4 (see ESI for details†). The reaction of cyclopropenone with PMe3 has a 24.0 kcal mol−1 energy barrier to form the α-ketenyl phosphorus ylide IM1. The reaction involves concerted P–C bond formation and C–C cleavage, and no stable intermediate resulting from the phosphine addition on the cyclopropenone was found. The ketene and the phosphorus ylide are not conjugated, as the ylide C and P lie in a plane perpendicular to the plane of the ketene and its substituents. IM1 was shown to computationally undergo a concerted cycloaddition with benzaldehyde 2a to form IM2, via a five-membered ring transition state TS2 with a 24.9 kcal mol−1 barrier. This may be a pseudo-pericyclic reaction25 and does not involve a cyclic delocalized 6-electron transition state. Instead, the nucleophilic carbon of the ylide attacks the electrophilic aldehyde π system, while the oxygen of the aldehyde attacks the highly electrophilic π system of the ketene, in the plane of the forming lactone ring. The cyclization is more favorable than the Wittig-type attack of the aldehyde oxygen at the ylide phosphorus via a four-membered ring transition state TS2*, which is higher in energy than TS2 by 3.7 kcal mol−1, even though the product 3a* is more stable by 2.4 kcal mol−1. The adduct of the cycloaddition (IM2) is unstable, which readily undergoes 1,4-elimination to form product 3a. These pathway calculations are in accord with the fact that only product 3a is observed experimentally.
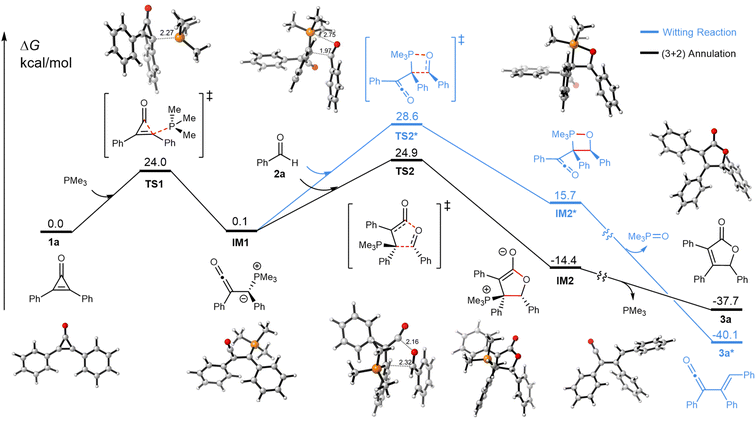 | ||
| Fig. 4 Calculated reaction profiles. The (3 + 2) annulation reaction is in black; Wittig olefination reaction is in blue. Energies are in kcal mol−1 and distances are given in Å. | ||
The frontier molecular orbitals (FMOs) of the reactants are shown in Fig. 5a. The nucleophilic carbon terminus of the phosphorus ylide, HOMO of IM1, interacts with the large LUMO coefficient at C1 of 2a. These orbitals differ in energy by 6.42 eV. Hirshfeld charges of corresponding atoms are shown in red in Fig. 5b. From the perspective of molecular charge reorganizations, these charges are very complementary to the transition state of the observed reaction. The two steps of the observed reaction have similar barriers, so that substituents that influence the rate of either step can have an effect on the overall reaction rate. Interestingly, the normally good dienophiles and dipolarophiles, acrylates and maleates, are not reactive in these cases. The low reactivity of acrylates as compared to aldehydes is likely due to the necessity for strong electrostatic interactions between the heteroatom of the electrophile and the central carbon of the ketene. In addition, it is known2a,26 that these Michael acceptors would react with PMe3 catalysts to form off cycle intermediates thereby deactivating the desired reaction mode.
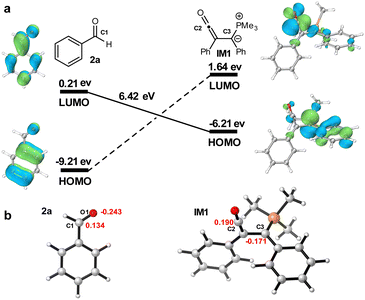 | ||
| Fig. 5 The frontier molecular orbitals (FMOs) and Hirshfeld charges. (a) FMOs interactions stabilizing TS2 (see Fig. 4). (b) Hirshfeld charges of 2a and IM1. | ||
In summary, we report the development of a phosphine-catalyzed (3 + 2) heteroannulation of cyclopropenones with an extensive range of electrophilic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X π systems including aldehydes, ketoesters, imines, isocyanates, and carbodiimides. This valuable alternative to transition metal-based methods not only provides efficient access to highly substituted sets of butenolides, butyrolactams, maleimides, and iminomaleimides, but also highlights the versatility and generality of the organocatalytic (3 + 2) annulative approach. Computational mechanistic investigations confirmed that an α-ketenyl phosphorus ylide is formed as a key intermediate. This species then undergoes a cycloaddition with aldehydes in a catalytic manner, rather than a stoichiometric Wittig olefination pathway, thus showcasing a unique and interesting reactivity. The organocatalytic activation of cyclopropenones also expands the scope of phosphine catalysis by supplying a new subset of 1,3-dipole surrogates that complements existing well-studied synthons, for example, allene substrates. Reaction development based on new modes of phosphine-catalyzed C–C bond activations is being explored in our laboratory.
X π systems including aldehydes, ketoesters, imines, isocyanates, and carbodiimides. This valuable alternative to transition metal-based methods not only provides efficient access to highly substituted sets of butenolides, butyrolactams, maleimides, and iminomaleimides, but also highlights the versatility and generality of the organocatalytic (3 + 2) annulative approach. Computational mechanistic investigations confirmed that an α-ketenyl phosphorus ylide is formed as a key intermediate. This species then undergoes a cycloaddition with aldehydes in a catalytic manner, rather than a stoichiometric Wittig olefination pathway, thus showcasing a unique and interesting reactivity. The organocatalytic activation of cyclopropenones also expands the scope of phosphine catalysis by supplying a new subset of 1,3-dipole surrogates that complements existing well-studied synthons, for example, allene substrates. Reaction development based on new modes of phosphine-catalyzed C–C bond activations is being explored in our laboratory.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and the ESI,† as well as from the authors upon request.Author contributions
S. X. conceived the original idea, supervised the project, and wrote the manuscript with support from Y. T., J. L., and M. J. L. X. H. carried out the experiment. P. M., S. S., and K. N. H. performed the computational investigations. All authors discussed the results and contributed to the final manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We are thankful for financial support from the grant of National Natural Science Foundation of China (Nos. 21871218, 22050410277, 22101223), the Fundamental Research Funds for the Central Universities, and the National Science Foundation (USA, CHE-1764328). The authors wish to acknowledge Miss Lu Bai and Miss Chao Feng at the Instrument Analysis Center of XJTU for assistance with HRMS or NMR analysis.Notes and references
- (a) M.-C. Tang, Y. Zou, K. Watanabe, C. T. Walsh and Y. Tang, Chem. Rev., 2017, 117, 5226–5333 CrossRef CAS PubMed; (b) A. Moyano and R. Rios, Chem. Rev., 2011, 111, 4703–4832 CrossRef CAS.
- (a) H. Guo, Y. C. Fan, Z. Sun, Y. Wu and O. Kwon, Chem. Rev., 2018, 118, 10049–10293 CrossRef CAS; (b) H. Ni, W.-L. Chan and Y. Lu, Chem. Rev., 2018, 118, 9344–9411 CrossRef CAS; (c) C. Xie, A. J. Smaligo, X.-R. Song and O. Kwon, ACS Cent. Sci., 2021, 7, 536–558 CrossRef CAS; (d) J. M. Lipshultz, G. Li and A. T. Radosevich, J. Am. Chem. Soc., 2021, 143, 1699–1721 CrossRef CAS.
- (a) Y. Du, X. Lu and C. Zhang, Angew. Chem., Int. Ed., 2003, 42, 1035–1037 CrossRef CAS; (b) C. Zhang and X. Lu, J. Org. Chem., 1995, 60, 2906–2908 CrossRef CAS.
- (a) Y. S. Tran and O. Kwon, J. Am. Chem. Soc., 2007, 129, 12632–12633 CrossRef CAS PubMed; (b) X.-F. Zhu, J. Lan and O. Kwon, J. Am. Chem. Soc., 2003, 125, 4716–4717 CrossRef CAS.
- Q. Zhang, L. Yang and X. Tong, J. Am. Chem. Soc., 2010, 132, 2550–2551 CrossRef CAS PubMed.
- (a) Y. K. Chung and G. C. Fu, Angew. Chem., Int. Ed., 2009, 48, 2225–2227 CrossRef CAS; (b) S. Kramer and G. C. Fu, J. Am. Chem. Soc., 2015, 137, 3803–3806 CrossRef CAS PubMed; (c) S. Y. Lee, Y. Fujiwara, A. Nishiguchi, M. Kalek and G. C. Fu, J. Am. Chem. Soc., 2015, 137, 4587–4591 CrossRef CAS; (d) H. Wang, J. Zhang, Y. Tu and J. Zhang, Angew. Chem., Int. Ed., 2019, 58, 5422–5426 CrossRef CAS; (e) L. Zhang, H. Liu, G. Qiao, Z. Hou, Y. Liu, Y. Xiao and H. Guo, J. Am. Chem. Soc., 2015, 137, 4316–4319 CrossRef CAS; (f) E. Li, H. Jin, P. Jia, X. Dong and Y. Huang, Angew. Chem., Int. Ed., 2016, 55, 11591–11594 CrossRef CAS; (g) X. Han, W. Yao, T. Wang, Y. R. Tan, Z. Yan, J. Kwiatkowski and Y. Lu, Angew. Chem., Int. Ed., 2014, 53, 5643–5647 CrossRef CAS PubMed; (h) H. Ni, X. Tang, W. Zheng, W. Yao, N. Ullah and Y. Lu, Angew. Chem., Int. Ed., 2017, 56, 14222–14226 CrossRef CAS PubMed; (i) M. Wu, Z. Han, K. Li, J. e. Wu, K. Ding and Y. Lu, J. Am. Chem. Soc., 2019, 141, 16362–16373 CrossRef CAS PubMed; (j) Z.-H. Cao, Y.-H. Wang, S. J. Kalita, U. Schneider and Y.-Y. Huang, Angew. Chem., Int. Ed., 2020, 59, 1884–1890 CrossRef CAS PubMed; (k) B. M. Trost, E. Gnanamani, C.-I. J. Hung and C. A. Kalnmals, Org. Lett., 2019, 21, 1890–1894 CrossRef CAS PubMed; (l) W. Yao, X. Dou and Y. Lu, J. Am. Chem. Soc., 2015, 137, 54–57 CrossRef CAS; (m) X. Han, Y. Wang, F. Zhong and Y. Lu, J. Am. Chem. Soc., 2011, 133, 1726–1729 CrossRef CAS PubMed; (n) F. Zhong, J. Luo, G.-Y. Chen, X. Dou and Y. Lu, J. Am. Chem. Soc., 2012, 134, 10222–10227 CrossRef CAS PubMed; (o) T. Wang, Z. Yu, D. L. Hoon, C. Y. Phee, Y. Lan and Y. Lu, J. Am. Chem. Soc., 2016, 138, 265–271 CrossRef CAS.
- J. Wu, Y. Tang, W. Wei, Y. Wu, Y. Li, J. Zhang, Y. Zheng and S. Xu, Angew. Chem., Int. Ed., 2018, 57, 6284–6288 CrossRef CAS.
- F. Zhang, X. Dai, L. Dai, W. Zheng, W.-L. Chan, X. Tang, X. Zhang and Y. Lu, Angew. Chem., Int. Ed., 2022, 61 DOI:10.1002/anie.202203212.
- X. He, Y. Tang, Y. Wang, J.-B. Chen, S. Xu, J. Dou and Y. Li, Angew. Chem., Int. Ed., 2019, 58, 10698–10702 CrossRef CAS.
- Takizawa and co-workers have documented the generation of intermediate C in 1970, see: N. Obata and T. Takizawa, Tetrahedron Lett., 1970, 11, 2231–2234 CrossRef.
- (a) H.-W. Shih and J. A. Prescher, J. Am. Chem. Soc., 2015, 137, 10036–10039 CrossRef CAS; (b) R. D. Row, H.-W. Shih, A. T. Alexander, R. A. Mehl and J. A. Prescher, J. Am. Chem. Soc., 2017, 139, 7370–7375 CrossRef CAS PubMed.
- (a) R. Breslow, R. Haynie and J. Mirra, J. Am. Chem. Soc., 1959, 81, 247–248 CrossRef CAS; (b) A. W. Krebs, Angew. Chem., Int. Ed., 1965, 4, 10–22 CrossRef.
- (a) K. Komatsu and T. Kitagawa, Chem. Rev., 2003, 103, 1371–1427 CrossRef CAS PubMed; (b) B. Prasad Raiguru, S. Nayak, D. Ranjan Mishra, T. Das, S. Mohapatra and N. Priyadarsini Mishra, Asian J. Org. Chem., 2020, 9, 1088–1132 CrossRef CAS; (c) K. T. Potts and J. S. Baum, Chem. Rev., 1974, 74, 189–213 CrossRef CAS.
- (a) D. Bai, S. Liu, J. Chen, Y. Yu, M. Wang, J. Chang, Y. Lan and X. Li, Org. Chem. Front., 2021, 8, 3023–3031 RSC; (b) Y. Luo, C. Shan, S. Liu, T. Zhang, L. Zhu, K. Zhong, R. Bai and Y. Lan, ACS Catal., 2019, 9, 10876–10886 CrossRef CAS.
- (a) T. Kondo, Y. Kaneko, Y. Taguchi, A. Nakamura, T. Okada, M. Shiotsuki, Y. Ura, K. Wada and T. Mitsudo, J. Am. Chem. Soc., 2002, 124, 6824–6825 CrossRef CAS PubMed; (b) P. A. Wender, T. J. Paxton and T. J. Williams, J. Am. Chem. Soc., 2006, 128, 14814–14815 CrossRef CAS; (c) W. T. Zhao, F. Gao and D. B. Zhao, Angew. Chem., Int. Ed., 2018, 57, 6329–6332 CrossRef CAS; (d) T. Kondo, R. Taniguchi and Y. Kimura, Synlett, 2018, 29, 717–722 CrossRef CAS; (e) A. Haito and N. Chatani, Chem. Commun., 2019, 55, 5740–5742 RSC; (f) D. Bai, Y. Yu, H. Guo, J. Chang and X. Li, Angew. Chem., Int. Ed., 2020, 59, 2740–2744 CrossRef CAS; (g) T. Nanda and P. C. Ravikumar, Org. Lett., 2020, 22, 1368–1374 CrossRef CAS PubMed; (h) J. Chen, B. Tang, X. Liu, G. Lv, Y. Shi, T. Huang, H. Xing, X. Guo, L. Hai and Y. Wu, Org. Chem. Front., 2020, 7, 2944–2949 RSC; (i) H.-Q. Zhou, X.-W. Gu, X.-H. Zhou, L. Li, F. Ye, G.-W. Yin, Z. Xu and L.-W. Xu, Chem. Sci., 2021, 12, 13737–13743 RSC; (j) F. Xie, S. Yu, Z. Qi and X. Li, Angew. Chem., Int. Ed., 2016, 55, 15351–15355 CrossRef CAS; (k) A. Schuster-Haberhauer, R. Gleiter, O. Körner, A. Leskovar, D. B. Werz, F. R. Fischer and F. Rominger, Organometallics, 2008, 27, 1361–1366 CrossRef CAS; (l) D. B. Werz, G. Klatt, J. A. Raskatov, H. Köppel and R. Gleiter, Organometallics, 2009, 28, 1675–1682 CrossRef CAS.
- T. Kondo, M. Nomura, Y. Ura, K. Wada and T.-a. Mitsudo, J. Am. Chem. Soc., 2006, 128, 14816–14817 CrossRef CAS.
- J.-T. Ren, J.-X. Wang, H. Tian, J.-L. Xu, H. Hu, M. Aslam and M. Sun, Org. Lett., 2018, 20, 6636–6639 CrossRef CAS.
- (a) J. P. Visser and J. E. Ramakers-Blom, J. Organomet. Chem., 1972, 44, C63–C65 CrossRef CAS; (b) T. Matsuda and Y. Sakurai, J. Org. Chem., 2014, 79, 2739–2745 CrossRef CAS; (c) O. Korner, R. Gleiter and F. Rominger, Synthesis, 2009, pp. 3259–3262 Search PubMed; (d) A. Schuster-Haberhauer, R. Gleiter, O. Korner, A. Leskovar, D. B. Werz, F. R. Fischer and F. Rominger, Organometallics, 2008, 27, 1361–1366 CrossRef CAS.
- (a) X. Wang, C. Yu, I. L. Atodiresei and F. W. Patureau, Org. Lett., 2022, 24, 1127–1131 CrossRef CAS; (b) Y.-L. Yang, Z. Zhang, X.-N. Zhang, D. Wang, Y. Wei and M. Shi, Chem. Commun., 2014, 50, 115–117 RSC; (c) X. Li, C. Han, H. Yao and A. Lin, Org. Lett., 2017, 19, 778–781 CrossRef CAS; (d) J. Xu, J. Cao, C. Fang, T. Lu and D. Du, Org. Chem. Front., 2017, 4, 560–564 RSC; (e) S. S. Nguyen, A. J. Ferreira, Z. G. Long, T. K. Heiss, R. S. Dorn, R. D. Row and J. A. Prescher, Org. Lett., 2019, 21, 8695–8699 CrossRef CAS.
- (a) R. Chen, S. Xu, X. Fan, H. Li, Y. Tang and Z. He, Org. Biomol. Chem., 2015, 13, 398–408 RSC; (b) T. Yan, K. R. Babu, Y. Wu, Y. Li, Y. Tang and S. Xu, Org. Lett., 2021, 23, 4570–4574 CrossRef CAS PubMed; (c) W. Wei, Y. Tang, Y. Zhou, G. Deng, Z. Liu, J. Wu, Y. Li, J. Zhang and S. Xu, Org. Lett., 2018, 20, 6559–6563 CrossRef CAS; (d) J. Zhang, Y. Tang, W. Wei, Y. Wu, Y. Li, J. Zhang, Y. Zheng and S. Xu, Org. Lett., 2017, 19, 3043–3046 CrossRef CAS PubMed; (e) D. Li, F. Cheng, Y. Tang, J. Li, Y. Li, J. Jiao and S. Xu, Org. Lett., 2021, 23, 9030–9035 CrossRef CAS; (f) S. Xu, J. Chen, J. Shang, Z. Qing, J. Zhang and Y. Tang, Tetrahedron Lett., 2015, 56, 6456–6459 CrossRef CAS; (g) K. R. Babu, X. He and S. Xu, Synlett, 2020, 31, 117–124 CrossRef CAS.
- A survey of literature shows no precedent of (3+2} annulation of simple aldehydes with cyclopropenones, except that DMF participates in a (3+2) annulation with cyclopropenones to form γ-aminobutenolides under Ag catalysis, see ref 17.
- A. Hamada and T. Takizawa, Tetrahedron Lett., 1972, 13, 1849–1850 CrossRef.
- S. Chatterjee, R. Sahoo and S. Nanda, Org. Biomol. Chem., 2021, 19, 7298–7332 RSC.
- W.-C. Wu, H.-C. Yeh, L.-H. Chan and C.-T. Chen, Adv. Mater., 2002, 14, 1072–1075 CrossRef CAS.
- D. M. Birney and P. E. Wagenseller, J. Am. Chem. Soc., 1994, 116, 6262–6270 CrossRef CAS.
- J. L. Methot and W. R. Roush, Adv. Synth. Catal., 2004, 346, 1035–1050 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2118493, 2118492 and 2118486. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2sc04092a |
| ‡ X. H. and P. M. contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |