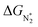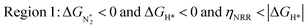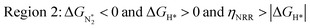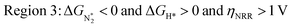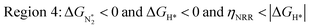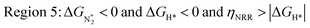Open Access Article
Open Access ArticleDescriptors and graphical construction for in silico design of efficient and selective single atom catalysts for the eNRR†
Samadhan
Kapse
 a,
Shobhana
Narasimhan
*b and
Ranjit
Thapa
a,
Shobhana
Narasimhan
*b and
Ranjit
Thapa
 *a
*a
aDepartment of Physics, SRM University – AP, Amaravati 522 240, Andhra Pradesh, India. E-mail: ranjit.t@srmap.edu.in
bTheoretical Sciences Unit and School of Advanced Materials, Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore 560 064, Karnataka, India. E-mail: shobhana@jncasr.ac.in
First published on 5th August 2022
Abstract
The electrochemical nitrogen reduction reaction (eNRR) offers the possibility of ammonia synthesis under mild conditions; however, it suffers from low yields, a competing hydrogen evolution reaction pathway, and hydrogen poisoning. We present a systematic approach toward screening single atom catalysts (SACs) for the eNRR, by focusing on key parameters computed from density functional theory and relationships between them. We illustrate this by application to 66 model catalysts of the types, TM-Pc, TM-NXCY, and TM-N3, where TM is a 3d transition metal or molybdenum. We identified the best SACs as Sc-Pc, Cr-N4, Mn-Pc, and Fe-N2C2; these show eNRR selectivity over the HER and no hydrogen poisoning. The catalysts are identified through multi-parameter optimization which includes the condition of hydrogen poisoning. We propose a new electronic descriptor Oval, the valence electron occupancy of the metal center that exhibits a volcano-type relationship with eNRR overpotential. Our multi-parameter optimization approach can be mapped onto a simple graphical construction to find the best catalyst for the eNRR over the HER and hydrogen poisoning.
1 Introduction
The Haber–Bosch process for ammonia synthesis has been described as possibly the most important scientific discovery of the twentieth century.1 However, it requires high temperatures and pressures, and results in high energy consumption and the emission of greenhouse gases.2 Alternative synthetic routes are therefore urgently needed. An environmentally friendly possibility is the electrochemical nitrogen reduction reaction (eNRR), which synthesizes ammonia from nitrogen and water under mild conditions (N2 + 6H+ + 6e− → 2NH3).3–5 However, currently available eNRR catalysts need improvement in three respects: (i) the efficiency of nitrogen fixation needs to be increased,6,7 (ii) the competing hydrogen evolution reaction (HER) needs to be suppressed,8,9 and (iii) hydrogen poisoning of active sites has to be avoided.10,11 Transition metals are popular eNRR catalysts; however they tend to favor hydrogen adsorption due to the formation of strong metal d – hydrogen σ bonds, and also tend to have low affinity for N2 adsorption.12,13 We aim to mitigate these problems by appropriately tuning the electronic structure by altering the environment surrounding metal atoms at the active site of single atom catalysts (SACs).14–20In contrast to many previously studied SACs where the metal atom was adsorbed over a suitably modified two-dimensional (2D) support,21–25 in our systems the metal atom is embedded within the 2D layer.26–29 Moreover, in previous work, typically only one criterion (usually the competing HER) was used to optimize catalyst function,30–38 whereas we will simultaneously optimize with respect to multiple criteria. Among embedded SACs, the Fe–N–C system was reported to be an efficient SAC, with a high eNRR faradaic efficiency (FE) of 56.55%, but its efficacy is limited due to the competitive HER.39 Recently, we studied Co and Fe centers in phthalocyanines,40 and concluded that Co-phthalocyanine should be the better catalyst, with a low eNRR overpotential.
We have performed density functional theory (DFT) based calculations on 66 different SACs to investigate the efficiency and selectivity of the eNRR over the HER, as well as the likelihood of H-poisoning. We show that the binding energy of the NNH intermediate and the valence electron occupancy of the metal center are descriptors for the catalytic activity toward the eNRR.
2 Results and discussion
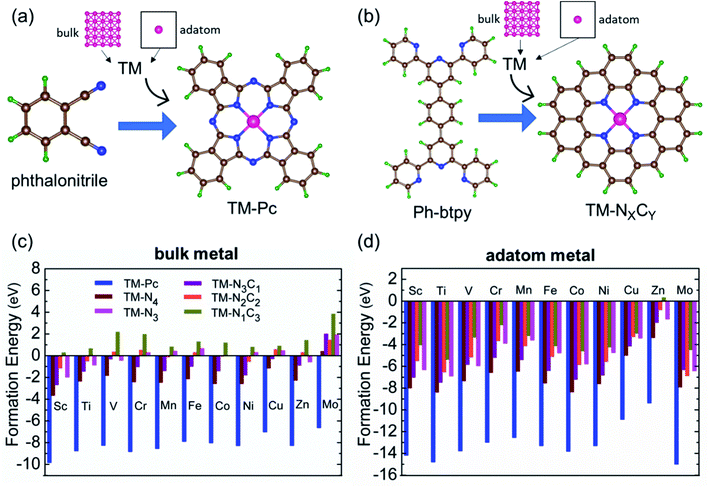
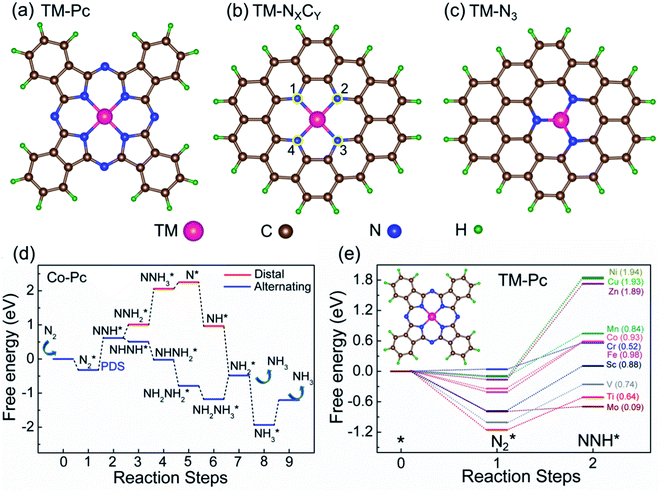
We consider six kinds of systems as model catalysts: transition metal doped phthalocyanines (TM-Pcs), four types of TM-NXCY embedded graphene systems (TM-N4, TM-N2C2, TM-N3C1, and TM-N1C3) and TM-N3 embedded graphene systems41–45 [see Fig. S1 in the ESI].† For the metal centers, we consider the ten 3d transition metals as well as molybdenum (TM- Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, and Mo).46–50 We thus construct 66 SAC systems.We first calculate the formation energies (Ef) of all the proposed SACs, see Table S1 in the ESI.† In experiments, the phthalonitrile and Ph-btpy compounds are used as sources to synthesize TM-phthalocyanine (TM-Pc) and TM-NXCY SACs respectively.51–57 Therefore, these structures are taken as reference systems when computing the Ef of the respective SACs (Fig. 1a and b). Ef is computed using the formula: Ef = ESAC + xμH + yμN − (Eref + zμC + ETM), where ESAC, Eref, and ETM are the total energies of the SAC, the phthalonitrile or Ph-btpy system, and a single transition metal (TM) atom, respectively (see SI-1 in the ESI for details).† Here, μC is the chemical potential of a single C atom in the phthalonitrile or Ph-btpy system. μH and μN are the chemical potentials of hydrogen and nitrogen, respectively, taken as the total energy of 1/2H2 and 1/2N2 in the gas phase. x is the number of hydrogen atoms that remain, y is the number of nitrogen atoms that remain, and z is the number of carbon atoms replaced in the SAC. For ETM, we have considered two possibilities: the total energy of a single metal atom in the bulk system or the energy of a metal adatom. The computed values of formation energies Ef are displayed in Fig. 1c (bulk metal as reference) and Fig. 1d (adatom as reference). The values of Ef computed using metal adatoms as the reference energy, and the trends in the values of Ef with changes in the coordination configuration, are in agreement with previous reports.58 Also, the dissolution potential (Udiss) is calculated to examine the electrochemical stability of these SACs considering both bulk and adatoms as sources of metal atoms. By employing the values of Ef in the equation, Udiss = Udiss0 − Ef/ne, we calculated the Udiss for all 66 SACs (Fig. S2 in the ESI†), where U0diss and n are the standard dissolution potential of the bulk metal and number of electrons involved in the dissolution respectively59 (SI-1 in the ESI†). We see that in most cases the value of Ef is negative, indicating thermodynamic stability and Udiss is positive, indicating electrochemical stability that shows easy formation of the systems from their sources. Also, we have performed molecular dynamics calculations to investigate the dynamic stability of one of our catalysts as an illustrative example. For this, we considered Mn-Pc as an example to find its dynamic stability at different temperatures (300 K, 500 K, 700 K, and 900 K) (Fig. S3 in the ESI†). Here, we found small fluctuations in the planarity of the Mn-Pc molecule, but a distortion or breaking of the bond is not observed, which shows the high dynamic stability of these SACs.60
To study the performance of these systems (Fig. 2a–c) as eNRR catalysts, we will plot the free energy profile of all the steps in the reaction, and then focus on three aspects (Fig. S4 in the ESI†): (a) binding of nitrogen to the catalyst, quantified by the change in the free energy of N2 adsorption 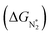 , (b) overpotential of the eNRR (ηNRR), quantified by the free energy change in the highest uphill step (potential determining step or PDS) in the free energy profile,61 and (c) the competing HER, quantified by the change in the free energy of H adsorption (ΔGH*): a low value of |ΔGH*| indicates the possibility of hydrogen evolution, and if ΔGH* < 0, one will have hydrogen poisoning.
, (b) overpotential of the eNRR (ηNRR), quantified by the free energy change in the highest uphill step (potential determining step or PDS) in the free energy profile,61 and (c) the competing HER, quantified by the change in the free energy of H adsorption (ΔGH*): a low value of |ΔGH*| indicates the possibility of hydrogen evolution, and if ΔGH* < 0, one will have hydrogen poisoning.
We first plot the free energy profile, as obtained from DFT calculations (see SI-2 of the ESI for computational details),† at electrode potential U = 0 V. Fig. 2d shows these results for the Co-Pc system and further examples are shown in Fig. S5 in the ESI.† Two possible pathways have previously been identified for the eNRR: alternating and distal (see Fig. S6 of the ESI†).62,63 We find that for the systems considered here, the alternating pathway is favored over the distal one, due to the lower free energy of the NHNH* intermediate, as compared to the 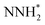 intermediate. We also observe that both pathways have the same PDS: the step from
intermediate. We also observe that both pathways have the same PDS: the step from 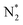 to NNH*.64,65 Furthermore, we performed the N2 adsorption calculations with a side-on configuration on all 66 SACs to investigate the presence of the enzymatic or mixing mechanism. The values of
to NNH*.64,65 Furthermore, we performed the N2 adsorption calculations with a side-on configuration on all 66 SACs to investigate the presence of the enzymatic or mixing mechanism. The values of 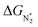 with end-on and side-on configurations are listed in Table S2 of the ESI† for a comparison. We observed that the nine TM-N3 and Mo metal center based SACs (Mo-N4, Mo-N3C1, Mo-N1C3, Mo-N3, Sc-N3, Ti-N3, V-N3, Mn-N3, and Fe-N3) show a lower free energy value of side-on N2 adsorption as compared to the end-on configuration. So, we computed the intermediate energies for these nine SACs to plot a full free energy profile of the eNRR (as shown in Fig. S7†) and compared them with the end-on pathway. It is seen that the both end-on and side-on N2 adsorption pathways have the same PDS step (
with end-on and side-on configurations are listed in Table S2 of the ESI† for a comparison. We observed that the nine TM-N3 and Mo metal center based SACs (Mo-N4, Mo-N3C1, Mo-N1C3, Mo-N3, Sc-N3, Ti-N3, V-N3, Mn-N3, and Fe-N3) show a lower free energy value of side-on N2 adsorption as compared to the end-on configuration. So, we computed the intermediate energies for these nine SACs to plot a full free energy profile of the eNRR (as shown in Fig. S7†) and compared them with the end-on pathway. It is seen that the both end-on and side-on N2 adsorption pathways have the same PDS step (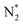 to NNH*) but a small change is observed in the value of overpotential. In the case of the other 57 SACs, the adsorption of N2 in an end-on form is more favourable than that in a side-on configuration. So, the enzymatic or mixed mechanism is not feasible in these systems.62
to NNH*) but a small change is observed in the value of overpotential. In the case of the other 57 SACs, the adsorption of N2 in an end-on form is more favourable than that in a side-on configuration. So, the enzymatic or mixed mechanism is not feasible in these systems.62
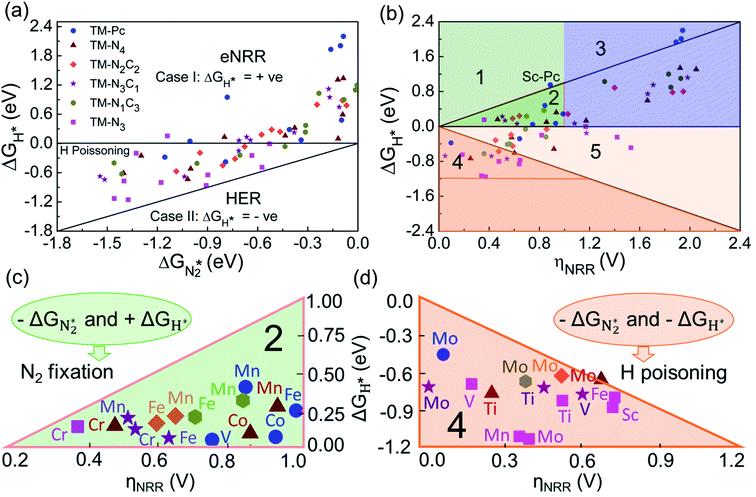
We will now screen these 66 SACs, imposing the three conditions mentioned above. As the first screening step, we demand that 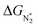 be negative, indicating a favorable exothermic reaction. As an example, in Fig. 2e, we compare the results for the free energy profile of N2 and NNH adsorption for the 11 TM-Pc systems; the values of ηNRR and the corresponding overpotentials toward the eNRR are given in parentheses in the figure. The results for the remaining systems are given in Table S1 and Fig. S8 in the ESI.† We find that
be negative, indicating a favorable exothermic reaction. As an example, in Fig. 2e, we compare the results for the free energy profile of N2 and NNH adsorption for the 11 TM-Pc systems; the values of ηNRR and the corresponding overpotentials toward the eNRR are given in parentheses in the figure. The results for the remaining systems are given in Table S1 and Fig. S8 in the ESI.† We find that 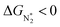 for 65 of the 66 SACs (the single exception is Cr-Pc). We also note that our values of
for 65 of the 66 SACs (the single exception is Cr-Pc). We also note that our values of 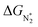 (−0.41 eV) and ηNRR (0.98 V) for the Fe-Pc system are in fairly good agreement with previously reported values of −0.39 eV and 0.85 V respectively.62 The metal centers of TM-Pc, TM-N4 and TM-N3 may influence the nearby carbon (C) and nitrogen (N) sites due to their electronic environments. We therefore also investigated N2 and NNH adsorption on the other possible sites (C and N) of Sc-Pc, Sc-N4, Sc-N3 and of pristine phthalocyanine (without metal center) systems (see Fig. S9 and S10 of the ESI†). Importantly, very high values of eNRR overpotentials are obtained on all the carbon and nitrogen sites.66 Therefore, the nitrogen and carbon sites of these SACs are unable to promote the eNRR, due to the weaker binding of the NNH intermediate. However, we see that changing the metal center in the SAC also does not succeed in enhancing the adsorption of NNH on nitrogen and carbon sites (Fig. S11 of the ESI†). Furthermore, we have considered the counter anion “Cl” for Sc-Pc and V-Pc SACs to mimic their +3 oxidation state and estimate the free energy profile of N2 and NNH adsorption (see the comparison in Fig. S12 of the ESI†). We find that the presence of the Cl anion can improve the selectivity of the catalyst for the eNRR over the HER by increasing the value of +ΔGH*.
(−0.41 eV) and ηNRR (0.98 V) for the Fe-Pc system are in fairly good agreement with previously reported values of −0.39 eV and 0.85 V respectively.62 The metal centers of TM-Pc, TM-N4 and TM-N3 may influence the nearby carbon (C) and nitrogen (N) sites due to their electronic environments. We therefore also investigated N2 and NNH adsorption on the other possible sites (C and N) of Sc-Pc, Sc-N4, Sc-N3 and of pristine phthalocyanine (without metal center) systems (see Fig. S9 and S10 of the ESI†). Importantly, very high values of eNRR overpotentials are obtained on all the carbon and nitrogen sites.66 Therefore, the nitrogen and carbon sites of these SACs are unable to promote the eNRR, due to the weaker binding of the NNH intermediate. However, we see that changing the metal center in the SAC also does not succeed in enhancing the adsorption of NNH on nitrogen and carbon sites (Fig. S11 of the ESI†). Furthermore, we have considered the counter anion “Cl” for Sc-Pc and V-Pc SACs to mimic their +3 oxidation state and estimate the free energy profile of N2 and NNH adsorption (see the comparison in Fig. S12 of the ESI†). We find that the presence of the Cl anion can improve the selectivity of the catalyst for the eNRR over the HER by increasing the value of +ΔGH*.
Earlier authors have proposed various descriptors to capture the behavior of catalysts.67–80 We demonstrate two such descriptors: an energetic one and an electronic one, to correlate with ηNRR for the SACs considered here. On plotting the data for all 66 systems, we see (Fig. 3a) that ηNRR correlates linearly with ΔGNNH*, the change in the adsorption free energy of NNH (R2 = 0.88). This underlines the key role played by the NNH* step in the eNRR and confirms that ΔGNNH* is an energy descriptor for the performance of SACs toward the eNRR. We note that there are previous reports where ΔGNNH* has been used as a descriptor to screen the eNRR performance of various catalysts.12,46,59,64,73,76 We find that Oval, the valence electron occupancy of the metal center, is an electronic descriptor which exhibits a volcano-type relationship with ηNRR for all 66 SACs (see Fig. 3b). It is clearly observed that the value of Oval on the metal center should be less than 8e to obtain ηNRR < 1 V. In the case of Ni-, Cu- and Zn- based SACs, the Oval value is more than 8e and ηNRR > 1 V. So, the descriptor Oval is an origin, which is more effective to define the eNRR activity for various SACs. In addition, we have plotted the eNRR activity as a function of other frequently considered parameters, such as the “d-band center” and “Bader charge” but find that these quantities do not show any correlation with ηNRR and they are not appropriate descriptors to define the eNRR activity of SACs with different metal centres, as has also been reported in the previous studies81,82 (Fig. S13 in the ESI†). Furthermore, we have obtained charge density difference plots to gain insight into the effect of different environments and metal centers on adsorption properties (see SI-3, Fig. S14 and S15 in the ESI†). Our results show that it is possible to modify the performance of SACs by changing the surrounding environment and Oval of the metal centers. Considering the optimum value of ηNRR as a second screening criterion, we demand that ηNRR < 1 V.59 We find that 42 of the 65 SAC systems (left after screening step 1) satisfy this criterion. Furthermore, we computed the N–N bond length after adsorption to analyse the activation of N2 by each SAC83 (see Table S3 in the ESI†). It is seen that the weak activation of N2 (N–N bond length close to 1.11 Å) obtained mostly on Ni-, Cu- and Zn- based SACs leads to a higher eNRR overpotential (>1 V).17,84 Also, we observed that the mainly TM-N3 and Mo-center based SACs show a large expansion in N–N bond length. So, we correlated the values of N–N bond length with the energy descriptor, ΔGNNH* and the linear relationship is observed with an R2 of 0.85 (Fig. S16 in the ESI†). Through charge density differences, the N2 activation can be visualized on different metal centers in the TM-Pc system (Fig. S17 in the ESI†).
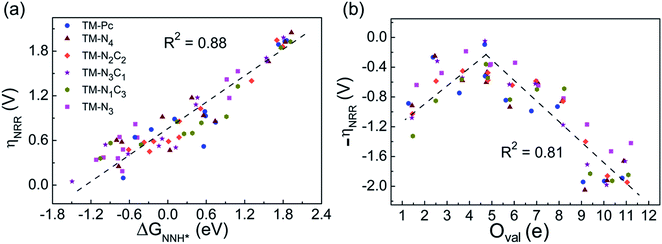 | ||
| Fig. 3 (a) Correlation between eNRR overpotential ηNRR and ΔGNNH* and (b) correlation between ηNRR and valence electron occupancy (Oval) of the metal center. | ||
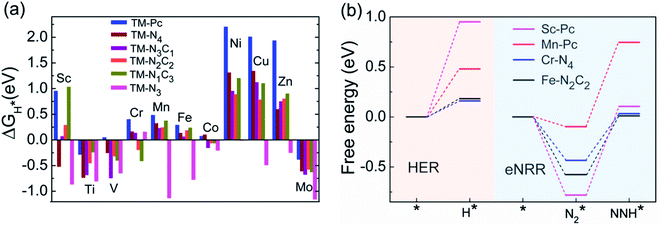
We now come to the third screening step. As mentioned above, both the FE and the NH3 yield of the eNRR are highly impacted by the competing pathway of the HER. In Fi. S18 of the ESI,† we present our results for the free energy profile of the HER for all 66 SAC systems. We will now demand that the free energy of adsorbed H, ΔGH* > 0, since this will lead to low H poisoning. To enable comparison, we plot the values of ΔGH* of all 66 SACs in Fig. 4a. We see that changing the environment of the metal centers has an impact on ΔGH*.81 Moreover, for each metal center, the trends in changes in 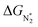 and ΔGNNH* are similar (Fig. S19 in the ESI†). We find that 36 of the 66 SACs have ΔGH* > 0, and therefore should show no H poisoning. However, of the 42 SACs left after screening step 2 (ηNRR < 1 V), only 16 SACs have ΔGH* > 0 (see Table S1†). Of these, we consider, for four different metal centers, the best SACs (Sc-Pc, Mn-Pc, Cr-N4 and Fe-N2C2) to display the computed free energy profile of H, N2 and NNH adsorption (see Fig. 4b). We emphasize again that for these four SACs, ΔGH* > 0 and
and ΔGNNH* are similar (Fig. S19 in the ESI†). We find that 36 of the 66 SACs have ΔGH* > 0, and therefore should show no H poisoning. However, of the 42 SACs left after screening step 2 (ηNRR < 1 V), only 16 SACs have ΔGH* > 0 (see Table S1†). Of these, we consider, for four different metal centers, the best SACs (Sc-Pc, Mn-Pc, Cr-N4 and Fe-N2C2) to display the computed free energy profile of H, N2 and NNH adsorption (see Fig. 4b). We emphasize again that for these four SACs, ΔGH* > 0 and 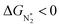 and they have high selectivity for the eNRR over the HER, as well as low ηNRR (<1 V).
and they have high selectivity for the eNRR over the HER, as well as low ηNRR (<1 V).
Thus, the optimal SAC for the eNRR should satisfy multiple criteria; we now show that this screening procedure can be visualized in a simple way that should prove very useful. We define five equations corresponding to these criteria and represent them graphically, as shown in Fig. 5. First, in Fig. 5a, for the 65 SACs that satisfy the first criterion 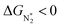 (i.e., all except Cr-Pc), we plot ΔGH*vs.
(i.e., all except Cr-Pc), we plot ΔGH*vs.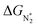 . In general, as
. In general, as 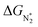 becomes more negative, so does
becomes more negative, so does 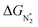 . However, 35 SACs have
. However, 35 SACs have 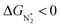 and ΔGH* > 0, making them suitable catalysts for the eNRR. For the remaining 30 SACs (mostly Mo-, Ti-, and V- based SACs and TM-N3),
and ΔGH* > 0, making them suitable catalysts for the eNRR. For the remaining 30 SACs (mostly Mo-, Ti-, and V- based SACs and TM-N3), 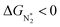 and ΔGH* < 0, favoring hydrogen poisoning as well as the possibility of the HER.
and ΔGH* < 0, favoring hydrogen poisoning as well as the possibility of the HER.
In Fig. 5b, we plot ΔGH*vs. ηNRR for the remaining 65 SACs. We separate the data in the plot into five different regions, as follows:
We predict that SACs that fall in Region 1 will have optimal performance, with very high eNRR selectivity over the HER. We can see from Fig. 5b that only Sc-Pc falls in Region 1, and the corresponding values of ΔGH*, 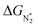 , and ηNRR are (+0.95 eV, −0.78 eV, 0.88 V). 15 SACs are found to lie in Region 2, mostly Mn, Cr, Fe and Co-based SACs (see Fig. 5c). These have high N2 adsorption and low H poisoning, making them selective toward the eNRR; however, as |ΔGH*| < ηNRR, they have a possibility, though low, of the HER. The SACs in Region 3, which mostly have Ni, Cu and Zn centers, should have the lowest H poisoning, but they have a large ηNRR > 1 V, leading to a low NH3 yield. In Region 4 (see Fig. 5d) there are 14 SACs (Mo, Ti, V centers and TM-N3), which have ηNRR < ΔGH*, so the HER should be suppressed; however, they have a high likelihood of H poisoning (ΔGH* < 0). In Table S4,† we show that, indeed, lower yield and FE are obtained for Region 4 SACs as compared to Region 2 SACs, upon extracting data from previously reported experimental studies. Finally, in Region 5, we have the 16 most undesirable SACs, where the eNRR is disfavored and the HER is likely favored. For easy understanding, a flowchart is given in Fig. S20 of the ESI† to visualize the screening process and criteria of catalysts for the eNRR.
, and ηNRR are (+0.95 eV, −0.78 eV, 0.88 V). 15 SACs are found to lie in Region 2, mostly Mn, Cr, Fe and Co-based SACs (see Fig. 5c). These have high N2 adsorption and low H poisoning, making them selective toward the eNRR; however, as |ΔGH*| < ηNRR, they have a possibility, though low, of the HER. The SACs in Region 3, which mostly have Ni, Cu and Zn centers, should have the lowest H poisoning, but they have a large ηNRR > 1 V, leading to a low NH3 yield. In Region 4 (see Fig. 5d) there are 14 SACs (Mo, Ti, V centers and TM-N3), which have ηNRR < ΔGH*, so the HER should be suppressed; however, they have a high likelihood of H poisoning (ΔGH* < 0). In Table S4,† we show that, indeed, lower yield and FE are obtained for Region 4 SACs as compared to Region 2 SACs, upon extracting data from previously reported experimental studies. Finally, in Region 5, we have the 16 most undesirable SACs, where the eNRR is disfavored and the HER is likely favored. For easy understanding, a flowchart is given in Fig. S20 of the ESI† to visualize the screening process and criteria of catalysts for the eNRR.
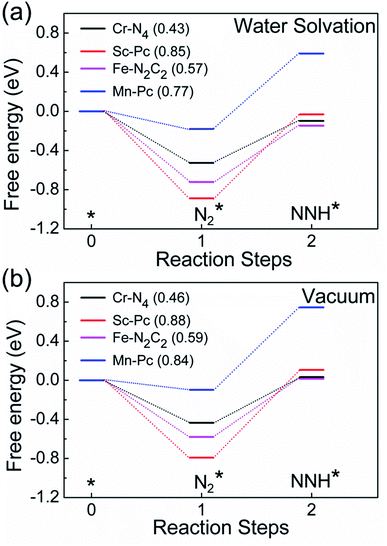
Finally, motivated by the fact that experiments are usually carried out in an aqueous environment, we have performed DFT calculations using an implicit solvation model, to understand the effect of the solvent water.85,86 First, as a benchmark, we computed the solvation energy of a water molecule using this model, obtaining a result of 0.31 eV, which is close to the reported experimental value of 0.27 eV.85 Next, we calculate the free energy profile of N2 and NNH adsorption of Cr-N4, Sc-Pc, Mn-Pc and Fe-N2C2 SACs using the implicit water solvation effect (Fig. 6a). The free energy profile of N2 and NNH adsorption without solvation is shown in Fig. 6b. On comparing the two figures, we observe that there is only a very small change in the free energy steps due to the presence of the solvent. The presence of the solvent leads to essentially negligible changes in the overpotential values, for these four SACs. In Fig. S21 of the ESI,† we have plotted a full free energy profile for the eNRR, with and without the implicit solvent, for Cr-N4 SAC. The changes in the free energy steps are very small in the whole free energy profile. Therefore, we can infer that the effect of solvent is not sufficient to change the reaction mechanism, as has also been reported in previous studies.87–89
3 Conclusions
In summary, we have screened 66 different transition metal-based SACs for possible use in the eNRR. To determine the best possible catalyst, we have considered three factors: N2 adsorption, hydrogen poisoning and the overpotential of the eNRR. Here, the valence electron occupancy (Oval) is identified as a new electronic descriptor that can predict the overpotential value. We emphasize that having a low ηNRR alone does not suffice to indicate a suitable eNRR catalyst, since if the adsorption free energy is higher for H than for N2, active sites will be poisoned, hindering the eNRR. We present a simple graphical procedure for identifying the most promising catalysts. To carry out this procedure, one has to compute only ΔGH* and ΔGNNH*, the changes in the free energies of H and NNH adsorption, respectively (note that ηNRR can be deduced if ΔGNNH* is known). The most promising candidate is identified as Sc-Pc, which we predict will have no H poisoning and will be highly selective for the eNRR over the HER. Moreover, we predict that Mn-Pc, Cr-N4, and Fe-N2C2 should also be highly efficient, with low overpotential (ηNRR < 1 V) toward the eNRR and no H poisoning.Data availability
All the data is provided in the supporting information file. Any specific data can be requested to corresponding author.Author contributions
R. T. conceived and designed the work. S. K. has performed all the DFT based calculations. R. T. & S. N. analyzed the results. S. K. and R. T. wrote the first draft. All the authors took part in writing the manuscript and commented on the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
RT thanks the Science and Engineering Research Board (SERB), India, for financial support (Grant No. CRG/2021/000620) and National Supercomputer Mission (NSM), India for financial support (Ref No: DST/NSM/R&D_HPC_Applications/2021/19). SN acknowledges the Sheikh Saqr Laboratory of ICMS, JNCASR and TUE-CMS, JNCASR. The authors thank the High-Performance Computing Center, SRM Institute of Science and Technology for providing computational facilities.References
- V. Smil, Nature, 1999, 400, 415 CrossRef CAS.
- J. Nørskov, J. Chen, R. Miranda, T. Fitzsimmons and R. Stack, United States, 2016 DOI:10.2172/1283146.
- C. J. M. van der Ham, M. T. M. Koper and D. G. H. Hetterscheid, Chem. Soc. Rev., 2014, 43, 5183–5191 RSC.
- Y. Yang, L. Zhang, Z. Hu, Y. Zheng, C. Tang, P. Chen, R. Wang, K. Qiu, J. Mao, T. Ling and S.-Z. Qiao, Angew. Chem., Int. Ed., 2020, 59, 4525–4531 CrossRef CAS PubMed.
- A. R. Singh, B. A. Rohr, J. A. Schwalbe, M. Cargnello, K. Chan, T. F. Jaramillo, I. Chorkendorff and J. K. Nørskov, ACS Catal., 2017, 7, 706–709 CrossRef CAS.
- C.-X. Huang, G. Li, L.-M. Yang and E. Ganz, ACS Appl. Mater. Interfaces, 2021, 13, 608–621 CrossRef CAS PubMed.
- W. Qiu, N. Yang, D. Luo, J. Wang, L. Zheng, Y. Zhu, E. M. Akinoglu, Q. Huang, L. Shui, R. Wang, G. Zhou, X. Wang and Z. Chen, Appl. Catal., B, 2021, 293, 120216 CrossRef CAS.
- C. Choi, S. Back, N.-Y. Kim, J. Lim, Y.-H. Kim and Y. Jung, ACS Catal., 2018, 8, 7517–7525 CrossRef CAS.
- B. H. R. Suryanto, H.-L. Du, D. Wang, J. Chen, A. N. Simonov and D. R. MacFarlane, Nat. Catal., 2019, 2, 290–296 CrossRef CAS.
- H. Li, B. Yu, Z. Zhuang, W. Sun, B. Jia and T. Ma, J. Mater. Chem. A, 2021, 9, 4184–4192 RSC.
- S. Wu, Y.-K. Peng, T.-Y. Chen, J. Mo, A. Large, I. McPherson, H.-L. Chou, I. Wilkinson, F. Venturini, D. Grinter, P. Ferrer Escorihuela, G. Held and S. C. E. Tsang, ACS Catal., 2020, 10, 5614–5622 CrossRef CAS.
- J. H. Montoya, C. Tsai, A. Vojvodic and J. K. Nørskov, ChemSusChem, 2015, 8, 2180–2186 CrossRef CAS PubMed.
- S. B. Patil and D.-Y. Wang, Small, 2020, 16, 2002885 CrossRef CAS.
- Y. Qiu, X. Peng, F. Lü, Y. Mi, L. Zhuo, J. Ren, X. Liu and J. Luo, Chem.–Asian J., 2019, 14, 2770–2779 CrossRef CAS PubMed.
- Y. Zhai, Z. Zhu, C. Zhu, K. Chen, X. Zhang, J. Tang and J. Chen, Mater. Today, 2020, 38, 99–113 CrossRef CAS.
- X. Li, Q. Zhou, S. Wang, Y. Li, Y. Liu, Q. Gao and Q. Wu, J. Phys. Chem. C, 2021, 125, 11963–11974 CrossRef CAS.
- W. Zang, T. Yang, H. Zou, S. Xi, H. Zhang, X. Liu, Z. Kou, Y. Du, Y. P. Feng, L. Shen, L. Duan, J. Wang and S. J. Pennycook, ACS Catal., 2019, 9, 10166–10173 CrossRef CAS.
- B. Singh, V. Sharma, R. P. Gaikwad, P. Fornasiero, R. Zbořil and M. B. Gawande, Small, 2021, 17, 2006473 CrossRef CAS PubMed.
- G. Chen, H. Zhong and X. Feng, Chem. Sci., 2021, 12, 15802–15820 RSC.
- X. Chen, W.-J. Ong, X. Zhao, P. Zhang and N. Li, J. Energy Chem., 2021, 58, 577–585 CrossRef.
- S. Tang, Q. Dang, T. Liu, S. Zhang, Z. Zhou, X. Li, X. Wang, E. Sharman, Y. Luo and J. Jiang, J. Am. Chem. Soc., 2020, 142, 19308–19315 CrossRef CAS PubMed.
- Z. Chen, J. Zhao, C. R. Cabrera and Z. Chen, Small Methods, 2019, 3, 1800368 CrossRef.
- Z. Wang, Z. Yu and J. Zhao, Phys. Chem. Chem. Phys., 2018, 20, 12835–12844 RSC.
- H. Zou, W. Rong, S. Wei, Y. Ji and L. Duan, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 29462–29468 CrossRef CAS.
- B. Huang, N. Li, W.-J. Ong and N. Zhou, J. Mater. Chem. A, 2019, 7, 27620–27631 RSC.
- Q. Wu, R. Peng, B. Huang, L. Kou, Y. Dai and Y. Ma, J. Mater. Chem. A, 2020, 8, 26075–26084 RSC.
- C. Ling, Y. Ouyang, Q. Li, X. Bai, X. Mao, A. Du and J. Wang, Small Methods, 2019, 3, 1800376 CrossRef.
- J. Xie, H. Dong, X. Cao and Y. Li, Mater. Chem. Phys., 2020, 243, 122622 CrossRef CAS.
- Q. Qu, S. Ji, Y. Chen, D. Wang and Y. Li, Chem. Sci., 2021, 12, 4201–4215 RSC.
- C. Liu, Q. Li, C. Wu, J. Zhang, Y. Jin, D. R. MacFarlane and C. Sun, J. Am. Chem. Soc., 2019, 141, 2884–2888 CrossRef CAS PubMed.
- S. Agarwal, R. Kumar, R. Arya and A. K. Singh, J. Phys. Chem. C, 2021, 125, 12585–12593 CrossRef CAS.
- Y. Guo, G. Wang, S. Shen, G. Wei, G. Xia and J. Zhang, Appl. Surf. Sci., 2021, 550, 149283 CrossRef CAS.
- J. Wang, L. Yu, L. Hu, G. Chen, H. Xin and X. Feng, Nat. Commun., 2018, 9, 1795 CrossRef PubMed.
- H. Tao, C. Choi, L.-X. Ding, Z. Jiang, Z. Han, M. Jia, Q. Fan, Y. Gao, H. Wang, A. W. Robertson, S. Hong, Y. Jung, S. Liu and Z. Sun, Chem, 2019, 5, 204–214 CAS.
- X. Peng, H.-X. Liu, Y. Zhang, Z.-Q. Huang, L. Yang, Y. Jiang, X. Wang, L. Zheng, C. Chang, C. Au, L. Jiang and J. Li, Chem. Sci., 2021, 12, 7125–7137 RSC.
- B. Huang, Y. Wu, B. Chen, Y. Qian, N. Zhou and N. Li, Chin. J. Catal., 2021, 42, 1160–1167 CrossRef CAS.
- M. Yao, Z. Shi, P. Zhang, W.-J. Ong, J. Jiang, W.-Y. Ching and N. Li, ACS Appl. Nano Mater., 2020, 3, 9870–9879 CrossRef CAS.
- X. Chen, X. Zhao, Z. Kong, W.-J. Ong and N. Li, J. Mater. Chem. A, 2018, 6, 21941–21948 RSC.
- M. Wang, S. Liu, T. Qian, J. Liu, J. Zhou, H. Ji, J. Xiong, J. Zhong and C. Yan, Nat. Commun., 2019, 10, 341 CrossRef CAS PubMed.
- U. K. Ghorai, S. Paul, B. Ghorai, A. Adalder, S. Kapse, R. Thapa, A. Nagendra and A. Gain, ACS Nano, 2021, 15, 5230–5239 CrossRef CAS.
- P. Song, M. Luo, X. Liu, W. Xing, W. Xu, Z. Jiang and L. Gu, Adv. Funct. Mater., 2017, 27, 1700802 CrossRef.
- F. Yang, P. Song, X. Liu, B. Mei, W. Xing, Z. Jiang, L. Gu and W. Xu, Angew. Chem., Int. Ed., 2018, 57, 12303–12307 CrossRef CAS.
- W. Song, K. Xie, Y. Guo, L. Fu and C. He, ChemPhysChem, 2021, 22, 1712–1721 CrossRef CAS PubMed.
- W. Zhao, L. Zhang, Q. Luo, Z. Hu, W. Zhang, S. Smith and J. Yang, ACS Catal., 2019, 9, 3419–3425 CrossRef CAS.
- J. Li, S. Zhao, L. Zhang, S. P. Jiang, S.-Z. Yang, S. Wang, H. Sun, B. Johannessen and S. Liu, Small, 2021, 17, 2004579 CrossRef CAS PubMed.
- J. Zhao and Z. Chen, J. Am. Chem. Soc., 2017, 139, 12480–12487 CrossRef CAS PubMed.
- Z.-Y. Wu, M. Karamad, X. Yong, Q. Huang, D. A. Cullen, P. Zhu, C. Xia, Q. Xiao, M. Shakouri, F.-Y. Chen, J. Y. T. Kim, Y. Xia, K. Heck, Y. Hu, M. S. Wong, Q. Li, I. Gates, S. Siahrostami and H. Wang, Nat. Commun., 2021, 12, 2870 CrossRef CAS PubMed.
- L. Han, X. Liu, J. Chen, R. Lin, H. Liu, F. Lü, S. Bak, Z. Liang, S. Zhao, E. Stavitski, J. Luo, R. R. Adzic and H. L. Xin, Angew. Chem., Int. Ed., 2019, 58, 2321–2325 CrossRef CAS PubMed.
- C. Ling, X. Bai, Y. Ouyang, A. Du and J. Wang, J. Phys. Chem. C, 2018, 122, 16842–16847 CrossRef CAS.
- C. Zhang, Z. Wang, J. Lei, L. Ma, B. I. Yakobson and J. M. Tour, Small, 2022, 18, 2106327 CrossRef CAS PubMed.
- S. Sarkar, A. Biswas, N. Kamboj and R. S. Dey, Inorg. Chem., 2020, 59, 13453–13464 CrossRef CAS PubMed.
- H. Yang, L. Shang, Q. Zhang, R. Shi, G. I. N. Waterhouse, L. Gu and T. Zhang, Nat. Commun., 2019, 10, 4585 CrossRef PubMed.
- Y. Pan, Y. Chen, K. Wu, Z. Chen, S. Liu, X. Cao, W.-C. Cheong, T. Meng, J. Luo, L. Zheng, C. Liu, D. Wang, Q. Peng, J. Li and C. Chen, Nat. Commun., 2019, 10, 4290 CrossRef.
- X. Wang, Z. Chen, X. Zhao, T. Yao, W. Chen, R. You, C. Zhao, G. Wu, J. Wang, W. Huang, J. Yang, X. Hong, S. Wei, Y. Wu and Y. Li, Angew. Chem., Int. Ed., 2018, 57, 1944–1948 CrossRef CAS PubMed.
- R. H. Platel, T. Teixeira Tasso, W. Zhou, T. Furuyama, N. Kobayashi and D. B. Leznoff, Chem. Commun., 2015, 51, 5986–5989 RSC.
- L. Meng, K. Wang, Y. Han, Y. Yao, P. Gao, C. Huang, W. Zhang and F. Xu, Prog. Nat. Sci.: Mater. Int., 2017, 27, 329–332 CrossRef CAS.
- M. Mukherjee, M. Samanta, P. Banerjee, K. K. Chattopadhyay and G. P. Das, Electrochim. Acta, 2019, 296, 528–534 CrossRef CAS.
- X. Guo, J. Gu, X. Hu, S. Zhang, Z. Chen and S. Huang, Catal. Today, 2020, 350, 91–99 CrossRef CAS.
- X. Guo, J. Gu, S. Lin, S. Zhang, Z. Chen and S. Huang, J. Am. Chem. Soc., 2020, 142, 5709–5721 CrossRef CAS PubMed.
- Z. Lu, G. Xu, C. He, T. Wang, L. Yang, Z. Yang and D. Ma, Carbon, 2015, 84, 500–508 CrossRef CAS.
- W. Qiu, X.-Y. Xie, J. Qiu, W.-H. Fang, R. Liang, X. Ren, X. Ji, G. Cui, A. M. Asiri, G. Cui, B. Tang and X. Sun, Nat. Commun., 2018, 9, 3485 CrossRef PubMed.
- C. He, Z.-Y. Wu, L. Zhao, M. Ming, Y. Zhang, Y. Yi and J.-S. Hu, ACS Catal., 2019, 9, 7311–7317 CrossRef CAS.
- H. Li, S. Wei, H. Wang, Q. Cai and J. Zhao, J. Colloid Interface Sci., 2021, 588, 1–8 CrossRef CAS PubMed.
- Y.-X. Lin, S.-N. Zhang, Z.-H. Xue, J.-J. Zhang, H. Su, T.-J. Zhao, G.-Y. Zhai, X.-H. Li, M. Antonietti and J.-S. Chen, Nat. Commun., 2019, 10, 4380 CrossRef PubMed.
- S. Murmu, S. Paul, S. Kapse, R. Thapa, S. Chattopadhyay, N. Abharana, S. N. Jha, D. Bhattacharyya and U. K. Ghorai, J. Mater. Chem. A, 2021, 9, 14477–14484 RSC.
- F. Wang and J. Mao, Diamond Relat. Mater., 2021, 118, 108494 CrossRef CAS.
- B. Hammer and J. K. Nørskov, in Impact of Surface Science on Catalysis, Academic Press, 2000, vol. 45, pp. 71–129 Search PubMed.
- H. Niu, X. Wang, C. Shao, Z. Zhang and Y. Guo, ACS Sustainable Chem. Eng., 2020, 8, 13749–13758 CrossRef CAS.
- M. Lokanathan, I. M. Patil, M. Navaneethan, V. Parey, R. Thapa and B. Kakade, Nano Energy, 2018, 43, 219–227 CrossRef CAS.
- C. Choi, S. Yoon and Y. Jung, Chem. Sci., 2021, 12, 3551–3557 RSC.
- L. M. Ghiringhelli, J. Vybiral, S. V. Levchenko, C. Draxl and M. Scheffler, Phys. Rev. Lett., 2015, 114, 105503 CrossRef PubMed.
- B. Huang, B. Chen, G. Zhu, J. Peng, P. Zhang, Y. Qian and N. Li, ChemPhysChem, 2022, 23, e202100785 CAS.
- X. Liu, Y. Jiao, Y. Zheng, M. Jaroniec and S.-Z. Qiao, J. Am. Chem. Soc., 2019, 141, 9664–9672 CrossRef CAS PubMed.
- S. Kapse, S. Janwari, U. V. Waghmare and R. Thapa, Appl. Catal., B, 2021, 286, 119866 CrossRef CAS.
- J. Long, X. Fu and J. Xiao, J. Mater. Chem. A, 2020, 8, 17078–17088 RSC.
- W. Nong, S. Qin, F. Huang, H. Liang, Z. Yang, C. Qi, Y. Li and C. Wang, Carbon, 2021, 182, 297–306 CrossRef CAS.
- T. Wang and F. Abild-Pedersen, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2106527118 CrossRef CAS PubMed.
- S. Sinthika and R. Thapa, RSC Adv., 2015, 5, 93215–93225 RSC.
- S. Sinthika, U. V. Waghmare and R. Thapa, Small, 2018, 14, 1703609 CrossRef PubMed.
- E. S. Erakulan and R. Thapa, Appl. Surf. Sci., 2022, 574, 151613 CrossRef.
- V. Fung, G. Hu, Z. Wu and D. Jiang, J. Phys. Chem. C, 2020, 124, 19571–19578 CrossRef CAS.
- G. Di Liberto, L. A. Cipriano and G. Pacchioni, ACS Catal., 2022, 12, 5846–5856 CrossRef CAS.
- B. Qin, Y. Li, Q. Zhang, G. Yang, H. Liang and F. Peng, Nano Energy, 2020, 68, 104374 CrossRef CAS.
- J. Mukherjee, S. Paul, A. Adalder, S. Kapse, R. Thapa, S. Mandal, B. Ghorai, S. Sarkar and U. K. Ghorai, Adv. Funct. Mater., 2022, 2200882 CrossRef CAS.
- K. Mathew, R. Sundararaman, K. Letchworth-Weaver, T. A. Arias and R. G. Hennig, J. Chem. Phys., 2014, 140, 84106 CrossRef PubMed.
- M. Garcia-Ratés and N. López, J. Chem. Theory Comput., 2016, 12, 1331–1341 CrossRef PubMed.
- Q. Zhang and A. Asthagiri, Catal. Today, 2019, 323, 35–43 CrossRef CAS.
- V. Sinha, D. Sun, E. J. Meijer, T. J. H. Vlugt and A. Bieberle-Hütter, Faraday Discuss., 2021, 229, 89–107 RSC.
- Q. Yuan, Y. Li, P. Yu, B. Ma, L. Xu, Q. Sun, H. Yang, M. Xie and T. Cheng, J. Exp. Nanosci., 2021, 16, 255–264 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2sc02625b |
| This journal is © The Royal Society of Chemistry 2022 |