Open Access Article
Open Access ArticleSeparation of pyrrolidine from tetrahydrofuran by using pillar[6]arene-based nonporous adaptive crystals†
Jiajun
Cao‡
 a,
Yitao
Wu‡
a,
Qi
Li
a,
Yitao
Wu‡
a,
Qi
Li
 a,
Weijie
Zhu
a,
Zeju
Wang
a,
Yang
Liu
a,
Kecheng
Jie
a,
Weijie
Zhu
a,
Zeju
Wang
a,
Yang
Liu
a,
Kecheng
Jie
 a,
Huangtianzhi
Zhu
a,
Huangtianzhi
Zhu
 *a and
Feihe
Huang
*a and
Feihe
Huang
 *abc
*abc
aState Key Laboratory of Chemical Engineering, Stoddart Institute of Molecular Science, Department of Chemistry, Zhejiang University, Hangzhou 310027, PR China. E-mail: htzzhu@zju.edu.cn; fhuang@zju.edu.cn; Fax: +86-571-8795-3189; Tel: +86-571-8795-3189
bZJU-Hangzhou Global Scientific and Technological Innovation Center, Hangzhou 311215, PR China
cGreen Catalysis Center and College of Chemistry, Zhengzhou University, Zhengzhou 450001, PR China
First published on 2nd June 2022
Abstract
Pyrrolidine, an important feedstock in the chemical industry, is commonly produced via vapor-phase catalytic ammoniation of tetrahydrofuran (THF). Obtaining pyrrolidine with high purity and low energy cost has extremely high economic and environmental values. Here we offer a rapid and energy-saving method for adsorptive separation of pyrrolidine and THF by using nonporous adaptive crystals of per-ethyl pillar[6]arene (EtP6). EtP6 crystals show a superior preference towards pyrrolidine in 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) pyrrolidine/THF mixture vapor, resulting in rapid separation. The purity of pyrrolidine reaches 95% in 15 min of separation, and after 2 h, the purity is found to be 99.9%. Single-crystal structures demonstrate that the selectivity is based on the stability difference of host–guest structures after uptake of THF or pyrrolidine and non-covalent interactions in the crystals. Besides, EtP6 crystals can be recycled efficiently after the separation process owing to reversible transformations between the guest-free and guest-loaded EtP6.
50 (v/v) pyrrolidine/THF mixture vapor, resulting in rapid separation. The purity of pyrrolidine reaches 95% in 15 min of separation, and after 2 h, the purity is found to be 99.9%. Single-crystal structures demonstrate that the selectivity is based on the stability difference of host–guest structures after uptake of THF or pyrrolidine and non-covalent interactions in the crystals. Besides, EtP6 crystals can be recycled efficiently after the separation process owing to reversible transformations between the guest-free and guest-loaded EtP6.
Pyrrolidine is an important feedstock in the chemical industry that has been widely used in the production of food, pesticides, daily chemicals, coatings, textiles, and other materials.1 Particularly, pyrrolidine is a raw material for organic synthesis of medicines such as buflomedil, pyrrocaine, and prolintane.2 Moreover, pyrrolidine is also used as a solvent in the semi-synthetic process of simvastatin, one of the best-selling cardiovascular drugs.3 In the chemical industry, there are many preparation methods for pyrrolidine. The most common way to obtain pyrrolidine is the gas-phase catalytic method using tetrahydrofuran (THF) and ammonia as raw materials;4 this is carried out at high temperature under catalysis by solid acids. However, separating pyrrolidine from the crude product is difficult because of similar molecular weights and structures between pyrrolidine (b.p. 360 K and saturated vapor pressure = 1.8 kPa at 298 K) and THF (b.p. 339 K and saturated vapor pressure = 19.3 kPa at 298 K), which result in complicated processes and large energy consumption.5 Therefore, it is worthwhile to find energy-efficient and simple methods to separate pyrrolidine from THF.
Many techniques and materials, including porous zeolites, metal–organic frameworks (MOFs), and porous polymers, have facilitated energy-efficient separations of important petrochemicals and feedstocks, including THF and pyrrolidine.6,7 However, some drawbacks of these materials cannot be ignored.8 For example, the relatively low thermal and moisture stabilities of MOFs limit their practical applications. Therefore, the development of new materials with satisfactory chemical and thermal stabilities for pyrrolidine/THF separation is of high significance.
In the past decade, pillararenes have been widely studied in supramolecular chemistry.9 Owing to their unique pillar structures and diverse host–guest recognitions, pillararenes have been used in the construction of numerous supramolecular systems.10 Recently, nonporous adaptive crystals (NACs) of macrocycles, which have shown extraordinary performance in adsorption and separation, have been developed by our group as a new type of adsorption and separation materials.11 Unlike MOFs, covalent-organic frameworks (COFs), and other materials with pre-existing pores, NACs do not have “pores“ in the guest-free form, whereas they adsorb guest vapors through cavities of macrocycles and spaces between macrocycles. NACs have been applied in separations of many significant chemicals such as alkane isomers, aromatics, and halohydrocarbon isomers.12 However, such materials have never been used to separate pyrrolidine and THF. Herein, we utilized pillararene crystals as a separation material and realized the selective separation of pyrrolidine from a mixture of pyrrolidine and THF. We found that nonporous crystals of per-ethyl pillar[6]arene (EtP6) exhibited a shape-sorting ability at the molecular level towards pyrrolidine with an excellent preference, while crystals of per-ethyl pillar[5]arene (EtP5) did not (Scheme 1). In-depth investigations revealed that the separation was driven by the host–guest complexation between pyrrolidine and EtP6, which resulted in the formation of a more stable structure upon adsorption of pyrrolidine vapor in the crystalline state. EtP6 crystals can also adsorb THF. However, when these two chemicals simultaneously exist as the vapor of a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) mixture, EtP6 prefers pyrrolidine as an adsorption target. Compared with previously reported NAC-based separation, this separation took place rapidly. 95% purity was achieved in 15 min, and the purity increased to 99.9% after 2 h of separation. Moreover, pyrrolidine was removed upon heating, along with the structural transformation of EtP6 back to its original state, endowing EtP6 with excellent recyclability.
50 (v/v) mixture, EtP6 prefers pyrrolidine as an adsorption target. Compared with previously reported NAC-based separation, this separation took place rapidly. 95% purity was achieved in 15 min, and the purity increased to 99.9% after 2 h of separation. Moreover, pyrrolidine was removed upon heating, along with the structural transformation of EtP6 back to its original state, endowing EtP6 with excellent recyclability.
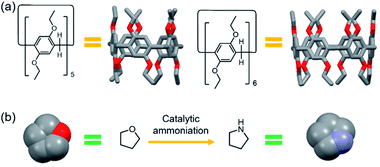 | ||
| Scheme 1 Chemical structures and cartoon representations: (a) EtP5 and EtP6; (b) THF and pyrrolidine. | ||
EtP5 and EtP6 were prepared as previously described and then a pretreatment process was carried out to obtain guest-free EtP5 and EtP6 (Fig. S1–S4†).13 According to powder X-ray diffraction (PXRD) patterns, activated EtP5 and EtP6 (denoted as EtP5α and EtP6β, respectively) were crystalline, and the patterns matched previous reports (Fig. S5 and S6†).14 Studies from our group indicated that EtP5α and EtP6β crystals were nonporous, presumably due to their dense packing modes.
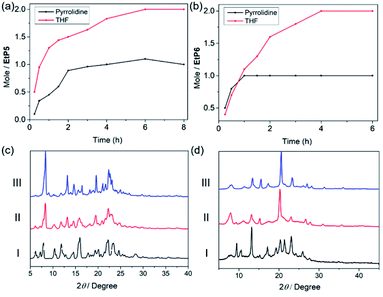
We first investigated the adsorption capabilities of EtP5α and EtP6β towards pyrrolidine and THF vapors. Based on time-dependent solid–vapor adsorption procedures, both EtP5α and EtP6β showed good ability to adsorb pyrrolidine and THF vapors. As shown in Fig. 1a, the adsorption amount of THF in EtP5α was higher than that of pyrrolidine. It took 6 hours for EtP5α to reach saturation points for adsorption of both pyrrolidine and THF vapors. The final storage of THF in EtP5α was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (molar ratio to the host), whereas the storage of pyrrolidine was 1
1 (molar ratio to the host), whereas the storage of pyrrolidine was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It seemed that the THF vapor was favored to occupy EtP5α, which was ascribed to the relatively lower boiling point of THF. A similar phenomenon was found for EtP6β. Time-dependent solid–vapor adsorption experiments for pyrrolidine demonstrated that it took just 1 hour to reach the saturation point, while it took 4 hours for the THF vapor (Fig. 1b). The adsorption amount of THF vapor was twice that of pyrrolidine. 1H NMR spectra and thermogravimetric analyses (TGA) further confirmed the adsorption and storage of THF and pyrrolidine in both hosts (Fig. S7–S16†). Meanwhile, in the desorption process, adsorbed pyrrolidine and THF in EtP6β were easily released under reduced pressure and heating. Based on these data, it was clear that pyrrolidine could be adsorbed rapidly by both EtP5α and EtP6β in molar ratios = 1
1. It seemed that the THF vapor was favored to occupy EtP5α, which was ascribed to the relatively lower boiling point of THF. A similar phenomenon was found for EtP6β. Time-dependent solid–vapor adsorption experiments for pyrrolidine demonstrated that it took just 1 hour to reach the saturation point, while it took 4 hours for the THF vapor (Fig. 1b). The adsorption amount of THF vapor was twice that of pyrrolidine. 1H NMR spectra and thermogravimetric analyses (TGA) further confirmed the adsorption and storage of THF and pyrrolidine in both hosts (Fig. S7–S16†). Meanwhile, in the desorption process, adsorbed pyrrolidine and THF in EtP6β were easily released under reduced pressure and heating. Based on these data, it was clear that pyrrolidine could be adsorbed rapidly by both EtP5α and EtP6β in molar ratios = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while THF could be captured in a relatively slow process. Structural changes after adsorption of these two vapors were analyzed via PXRD experiments, in which varying degrees of changes before and after adsorption were observed, evidencing the appearance of new crystal structures (Fig. 1c and d). Nevertheless, only slight differences were observed in the PXRD patterns after the adsorption of THF or pyrrolidine, which might be ascribed to the structural similarity of the two molecules.
1, while THF could be captured in a relatively slow process. Structural changes after adsorption of these two vapors were analyzed via PXRD experiments, in which varying degrees of changes before and after adsorption were observed, evidencing the appearance of new crystal structures (Fig. 1c and d). Nevertheless, only slight differences were observed in the PXRD patterns after the adsorption of THF or pyrrolidine, which might be ascribed to the structural similarity of the two molecules.
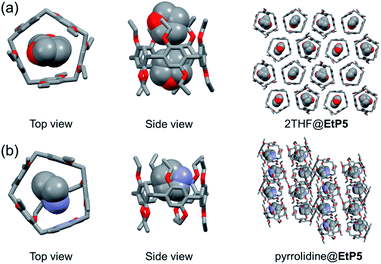
To study the mechanism of adsorption, guest-loaded single crystals were obtained by slowly evaporating either THF or pyrrolidine solutions of pillararenes (Tables S2 and S3†). In the crystal structure of THF-loaded EtP5 (2THF@EtP5, Fig. 2a and S17†),11a two THF molecules are in the cavity of one EtP5 molecule driven by multiple C–H⋯O hydrogen bonds and C–H⋯π bonds. EtP5 assembles into honeycomb-like infinite edge-to-edge 1D channels. In the crystal structure of pyrrolidine-loaded EtP5 (pyrrolidine@EtP5, Fig. 2b and S19†), one pyrrolidine molecule, stabilized by C–H⋯π interactions and C–H⋯O hydrogen bonds between hydrogen atoms on pyrrolidine and oxygen atoms on EtP5, is found in the cavity of EtP5. It's worth mentioning that a hydrogen atom which is linked with the N atom of pyrrolidine also forms a strong hydrogen bond with an oxygen atom on the ethoxy group of EtP5. EtP5 forms imperfect 1D channels because of partial distortion of orientation. The PXRD patterns simulated from these crystal structures matched well with the experimental results (Fig. S18 and S20†), which verified that the uptake of vapors transformed EtP5α into pyrrolidine-loaded EtP5.
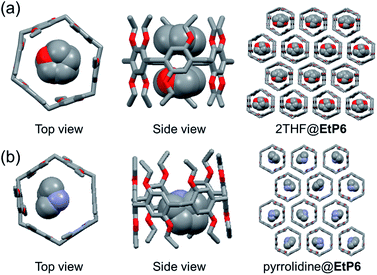
In the crystal structure of THF-loaded EtP6 (2THF@EtP6, Fig. 3a and S21†), one EtP6 molecule encapsulated two THF molecules in its cavity with C–H⋯O interactions, forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest complex. Although 1D channels are observed, EtP6 adopts a slightly different conformation, caused by the presence of THF. Moreover, the PXRD pattern of EtP6β after adsorption of THF vapor matches well with that simulated from 2THF@EtP6, which is evidence for the structural transformation upon adsorption. In the crystal structure of pyrrolidine-loaded EtP6 (pyrrolidine@EtP6, Fig. 3b and S23†), a 1
2 host–guest complex. Although 1D channels are observed, EtP6 adopts a slightly different conformation, caused by the presence of THF. Moreover, the PXRD pattern of EtP6β after adsorption of THF vapor matches well with that simulated from 2THF@EtP6, which is evidence for the structural transformation upon adsorption. In the crystal structure of pyrrolidine-loaded EtP6 (pyrrolidine@EtP6, Fig. 3b and S23†), a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex with pyrrolidine is found. Driven by C–H⋯π interactions and C–H⋯O hydrogen bonds formed by hydrogen atoms on pyrrolidine and oxygen atoms on EtP6, one pyrrolidine molecule is in the cavity of EtP6 with the nitrogen atom inside the cavity. The window-to-window packing mode of hexagonal EtP6 molecules in pyrrolidine@EtP6 contributes to the formation of honeycomb-like infinite edge-to-edge 1D channels, favorable for guest adsorption. Likewise, the PXRD result of EtP6β after adsorption of pyrrolidine is in line with the simulated pattern of pyrrolidine@EtP6, indicating that EtP6β transformed into pyrrolidine@EtP6 in the presence of pyrrolidine (Fig. S22 and S24†).
1 host–guest complex with pyrrolidine is found. Driven by C–H⋯π interactions and C–H⋯O hydrogen bonds formed by hydrogen atoms on pyrrolidine and oxygen atoms on EtP6, one pyrrolidine molecule is in the cavity of EtP6 with the nitrogen atom inside the cavity. The window-to-window packing mode of hexagonal EtP6 molecules in pyrrolidine@EtP6 contributes to the formation of honeycomb-like infinite edge-to-edge 1D channels, favorable for guest adsorption. Likewise, the PXRD result of EtP6β after adsorption of pyrrolidine is in line with the simulated pattern of pyrrolidine@EtP6, indicating that EtP6β transformed into pyrrolidine@EtP6 in the presence of pyrrolidine (Fig. S22 and S24†).
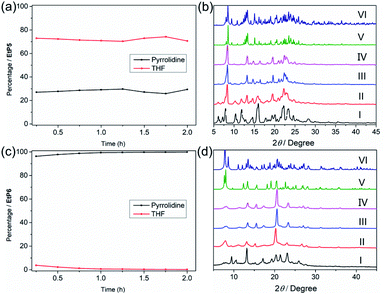
According to the adsorption ability and different crystal structures after adsorption of guest vapors, we wondered whether EtP5α or EtP6β could separate mixtures of THF and pyrrolidine. We first evaluated separation by EtP5α. GC analysis indicated that the adsorption ratios of THF and pyrrolidine were 65.7% and 34.3%, respectively, when EtP5α was exposed to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) pyrrolidine/THF mixture vapor (Fig. 4a and S25†). Such adsorption was also illustrated by 1H NMR (Fig. S26†). Although EtP5α showed a preference for THF, the selectivity is not satisfactory and cannot be applied to industrial separation. The less satisfactory selectivity may be ascribed to the similar crystal structures of EtP5 after adsorption of THF or pyrrolidine and insufficient strong stabilizing interactions. The PXRD pattern of EtP5α after adsorption of the 50
50 (v/v) pyrrolidine/THF mixture vapor (Fig. 4a and S25†). Such adsorption was also illustrated by 1H NMR (Fig. S26†). Although EtP5α showed a preference for THF, the selectivity is not satisfactory and cannot be applied to industrial separation. The less satisfactory selectivity may be ascribed to the similar crystal structures of EtP5 after adsorption of THF or pyrrolidine and insufficient strong stabilizing interactions. The PXRD pattern of EtP5α after adsorption of the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) pyrrolidine/THF mixture vapor exhibited minor differences compared with that simulated from either 2THF@EtP5 or pyrrolidine@EtP5, due to poor selectivity (Fig. 4b).
50 (v/v) pyrrolidine/THF mixture vapor exhibited minor differences compared with that simulated from either 2THF@EtP5 or pyrrolidine@EtP5, due to poor selectivity (Fig. 4b).
Nevertheless, selective separation of THF and pyrrolidine was achieved with EtP6β. As shown in Fig. 4c, time-dependent solid–vapor adsorption experiments for a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) pyrrolidine/THF mixture were conducted. Unlike the phenomenon in single-component adsorption experiments, uptake of pyrrolidine by EtP6β increased and reached the saturation point rapidly (less than 2 hours), while capture of THF was negligible. According to the NMR and GC results (Fig. S27 and S28†), the purity of pyrrolidine was determined to be 99.9% after 2 hours of adsorption, which indicates the remarkable selectivity of EtP6β for pyrrolidine. The PXRD pattern of EtP6β after adsorption of the mixture was consistent with that from single-component adsorption, indicating the structural transformation in the crystalline state upon selective capture of pyrrolidine from the mixture. Although THF and pyrrolidine have similar molecular structures, their non-covalent interactions with EtP6 are different. We assume that the hydrogen bond between N–H and the oxygen atom on EtP6 stabilizes pyrrolidine and leads to such selectivity. More importantly, compared with previous adsorption processes using NACs reported by our group, the selective separation of pyrrolidine was completed rapidly. According to the GC results, the purity of pyrrolidine reached around 95% in the initial 15 min, while it usually takes hours for selective separations of other substrates using NACs. Increasing the adsorption time to 2 h improves the purity to over 99%. The rapid separation of pyrrolidine with high purity using EtP6β shows great potential in industrial applications.
50 (v/v) pyrrolidine/THF mixture were conducted. Unlike the phenomenon in single-component adsorption experiments, uptake of pyrrolidine by EtP6β increased and reached the saturation point rapidly (less than 2 hours), while capture of THF was negligible. According to the NMR and GC results (Fig. S27 and S28†), the purity of pyrrolidine was determined to be 99.9% after 2 hours of adsorption, which indicates the remarkable selectivity of EtP6β for pyrrolidine. The PXRD pattern of EtP6β after adsorption of the mixture was consistent with that from single-component adsorption, indicating the structural transformation in the crystalline state upon selective capture of pyrrolidine from the mixture. Although THF and pyrrolidine have similar molecular structures, their non-covalent interactions with EtP6 are different. We assume that the hydrogen bond between N–H and the oxygen atom on EtP6 stabilizes pyrrolidine and leads to such selectivity. More importantly, compared with previous adsorption processes using NACs reported by our group, the selective separation of pyrrolidine was completed rapidly. According to the GC results, the purity of pyrrolidine reached around 95% in the initial 15 min, while it usually takes hours for selective separations of other substrates using NACs. Increasing the adsorption time to 2 h improves the purity to over 99%. The rapid separation of pyrrolidine with high purity using EtP6β shows great potential in industrial applications.
Apart from selectivity, recyclability is also an important parameter for an adsorbent. Consequently, recycling experiments were carried out by heating pyrrolidine@EtP6 under vacuum at 100 °C to remove adsorbed pyrrolidine. According to TGA and PXRD analysis, the recycled EtP6 solid maintained crystallinity and structural integrity that were the same as those of activated EtP6 crystals (Fig. S29 and S30†). Besides, it is worth mentioning that the recycled EtP6 solids were still capable of separating mixtures of pyrrolidine and THF without loss of performance after being recycled five times (Fig. S31†).
In conclusion, we explored the separation of pyrrolidine/THF mixtures using NACs of EtP5 and EtP6. Pyrrolidine was purified using EtP6 from a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) pyrrolidine/THF mixture with a purity of 99.9%, but EtP5 exhibited selectivity towards THF. Moreover, the separation of pyrrolidine by EtP6 was extremely fast so that over 95% purity was determined within 15 min of adsorption. The rapid separation is unique among NAC-based separations. Single-crystal structures revealed that the selectivity depended on the stability of the new structures after adsorption of the guests and the non-covalent interactions in the host–guest complexes. PXRD patterns indicated that the structures of the host crystals changed into the host–guest complexes after adsorption. Additionally, the NACs of EtP6 exhibited excellent recyclability over at least five runs; this endows EtP6 with great potential as an alternative adsorbent for rapid purification of pyrrolidine that can be applied in practical industry. The fast separation with such simple NACs in this work also reveals that minor structural differences can cause significant changes in properties, which should provide perspectives on designs of adsorbents or substrates with specifically tailored binding sites.
50 (v/v) pyrrolidine/THF mixture with a purity of 99.9%, but EtP5 exhibited selectivity towards THF. Moreover, the separation of pyrrolidine by EtP6 was extremely fast so that over 95% purity was determined within 15 min of adsorption. The rapid separation is unique among NAC-based separations. Single-crystal structures revealed that the selectivity depended on the stability of the new structures after adsorption of the guests and the non-covalent interactions in the host–guest complexes. PXRD patterns indicated that the structures of the host crystals changed into the host–guest complexes after adsorption. Additionally, the NACs of EtP6 exhibited excellent recyclability over at least five runs; this endows EtP6 with great potential as an alternative adsorbent for rapid purification of pyrrolidine that can be applied in practical industry. The fast separation with such simple NACs in this work also reveals that minor structural differences can cause significant changes in properties, which should provide perspectives on designs of adsorbents or substrates with specifically tailored binding sites.
Data availability
The crystallographic data for pyrrolidine@EtP5, 2THF@EtP6 and pyrrolidine@EtP6 have been deposited at CCDC with deposition numbers 2132121, 2132122 and 2132123, respectively.Author contributions
J. C., Z. H. and F. H. proposed the project and designed the study, J. C. and Y. W. performed the experiments, J. C., Q. L., Z. W., Y. L. and W. Z. analyzed the data. J. C. and Y. W. wrote the manuscript with inputs from all authors. F. H. directed the project with critical consultation from H. Z. and K. J.Conflicts of interest
There are no conflicts to declare.Acknowledgements
F. H. thanks the National Key Research and Development Program of China (2021YFA0910100), National Natural Science Foundation of China (22035006), Zhejiang Provincial Natural Science Foundation of China (LD21B020001) and the Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (SN-ZJU-SIAS-006) for financial support.Notes and references
- X. Wen, Appl. Chem. Ind., 2007, 36, 913 CAS.
- S. P. Clissold, S. Lynch and E. M. Sorkin, Drugs, 1987, 33, 430 CrossRef CAS PubMed.
- (a) S. Zhao, W. Xu and J. Zhao, Acta Pet. Sin., 2012, 28, 127 CAS; (b) D. Saffles, D. F. Lawson and M. Stayer, EP 0626278, 1994; (c) N. Yoshiyana, JP 04133275, 1992; (d) S. Furukawa, JP 4137362, 1992.
- (a) K. Fujita, K. Hatada and Y. Ono, J. Catal., 1974, 35, 325 CrossRef CAS; (b) K. Hatada, M. Shimada and K. Fujita, J. Catal., 1974, 35, 439 Search PubMed.
- (a) V. Yarlagadda, S. Rao, S. J. Kulkarni, M. Suvrahmanyam and A. V. Rama Rao, J. Org. Chem., 1994, 59, 3998 CrossRef; (b) C. Tsung, J. N. Kuhn, W. Huang, C. Aliaga, L.-I. Hung, G. A. Somorjai and P. Yang, J. Am. Chem. Soc., 2009, 131, 5816 CrossRef CAS PubMed.
- C.-X. Yang and X.-P. Yan, Anal. Chem., 2011, 83, 7144 CrossRef CAS PubMed.
- (a) M. Lippi and M. Cametti, Coord. Chem. Rev., 2021, 430, 213661 CrossRef CAS; (b) Q. Zhang, Y. Cui and G. Qian, Coord. Chem. Rev., 2019, 378, 310 CrossRef CAS.
- (a) M. Eddaoudi, H. Li and O. M. Yaghi, J. Am. Chem. Soc., 2000, 122, 1391 CrossRef CAS; (b) A. Kirchon, L. Feng, H. F. Drake, E. A. Joseph and H.-C. Zhou, Chem. Soc. Rev., 2018, 47, 8611 RSC; (c) O. M. Yaghi, H. Li, C. Davis, D. Richardson and T. L. Groy, Acc. Chem. Res., 1998, 31, 474 CrossRef CAS; (d) Y. Bai, Y. Dou, L. H. Xie, W. Rutledge, J.-R. Li and H.-C. Zhou, Chem. Soc. Rev., 2016, 45, 2327 RSC; (e) D. F. Sava, K. W. Chapman, M. A. Rodriguez, J. A. Greathouse, P. S. Crozier, H.-Y. Zhao, P. J. Chupas and T. M. Nenoff, Chem. Mater., 2013, 25, 2591 CrossRef CAS; (f) X. Zhao, X. Bu, E. T. Nguyen, Q.-G. Zhai, C. Mao and P. Feng, J. Am. Chem. Soc., 2016, 138, 15102 CrossRef CAS PubMed.
- (a) T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022 CrossRef CAS PubMed; (b) M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294 CrossRef CAS PubMed; (c) B. Li, Z. Meng, Q. Li, X. Huang, Z. Kang, H. Dong, J. Chen, J. Sun, Y. dong, J. Li, X. Jia, J. L. Sessler, Q. Meng and C. Li, Chem. Sci., 2017, 8, 4458 RSC; (d) T. Ogoshi, T.-a. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937 CrossRef CAS PubMed; (e) H. Zhu, Q. Li, B. Shi, H. Xing, Y. Sun, S. Lu, L. Shangguan, X. Li, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2020, 142, 17340 CrossRef CAS PubMed; (f) J.-R. Wu, A. U. Mu, B. Li, C.-Y. Wang, L. Fang and Y.-W. Yang, Angew. Chem., Int. Ed., 2018, 57, 9853 CrossRef CAS PubMed; (g) X.-N. Han, Y. Han and C.-F. Chen, J. Am. Chem. Soc., 2020, 142, 8262 CrossRef CAS PubMed; (h) L.-L. Tan, H. Li, Y. Tao, S. X.-A. Zhang, B. Wang and Y.-W. Yang, Adv. Mater., 2014, 26, 7027 CrossRef CAS PubMed; (i) N. L. Strutt, D. Fairen-Jimenez, J. Iehl, M. B. Lalonde, R. Q. Snurr, O. K. Farha, J. T. Hupp and J. F. Stoddart, J. Am. Chem. Soc., 2012, 134, 17436 CrossRef CAS PubMed; (j) W. Si, Z.-T. Li and J.-L. Hou, Angew. Chem., Int. Ed., 2014, 53, 4578 CrossRef CAS PubMed; (k) W. Si, P. Xin, Z.-T. Li and J.-L. Hou, Acc. Chem. Res., 2015, 48, 1612 CrossRef CAS PubMed; (l) M. Mastalerz, Acc. Chem. Res., 2018, 51, 2411 CrossRef CAS PubMed; (m) X.-Y. Hu, K. Jia, Y. Cao, Y. Li, S. Qin, F. Zhou, C. Lin, D. Zhang and L. Wang, Chem.–Eur. J., 2015, 21, 1208 CrossRef CAS PubMed; (n) J.-R. Wu, Z. Cai, G. Wu, D. Dai, Y.-Q. Liu and Y.-W. Yang, J. Am. Chem. Soc., 2021, 48, 20395 CrossRef PubMed.
- (a) C. Li, X. Shu, J. Li, J. Fan, Z. Chen, L. Weng and X. Jia, Org. Lett., 2012, 14, 4126 CrossRef CAS PubMed; (b) X. Shu, W. Chen, D. Hou, Q. Meng, R. Zheng and C. Li, Chem. Commun., 2014, 50, 4820 RSC; (c) M. Zhang, P.-P. Zhu, P. Xin, W. Si, Z.-T. Li and J.-L. Hou, Angew. Chem., Int. Ed., 2017, 56, 2999 CrossRef CAS PubMed; (d) Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542 CrossRef CAS PubMed; (e) Y. Cao, X.-Y. Hu, Y. Li, X. Zou, S. Xiong, C. Lin, Y.-Z. Shen and L. Wang, J. Am. Chem. Soc., 2014, 136, 10762 CrossRef CAS PubMed; (f) Q. Li, H. Zhu and F. Huang, Trends Chem., 2020, 2, 850 CrossRef CAS; (g) S.-H. Li, H.-Y. Zhang, X. Xu and Y. Liu, Nat. Commun., 2015, 6, 7590 CrossRef PubMed; (h) M. Ni, N. Zhang, W. Xia, X. Wu, C. Yao, X. Liu, X.-Y. Hu, C. Lin and L. Wang, J. Am. Chem. Soc., 2016, 138, 6643 CrossRef CAS PubMed.
- (a) K. Jie, M. Liu, Y. Zhou, M. Little, S. Bonakala, S. Chong, A. Stephenson, L. Chen, F. Huang and A. I. Cooper, J. Am. Chem. Soc., 2017, 139, 2908 CrossRef CAS PubMed; (b) T. Ogoshi, R. Sueto, K. Yoshikoshi, Y. Sakata, S. Akine and T.-a. Yamagishi, Angew. Chem., Int. Ed., 2015, 54, 9849 CrossRef CAS PubMed; (c) T. Ogoshi, K. Saito, R. Sueto, R. Kojima, Y. Hamada, S. Akine, A. M. P. Moeljadi, H. Hirao, T. Kakuta and T.-a. Yamagishi, Angew. Chem., Int. Ed., 2018, 57, 1592 CrossRef CAS PubMed; (d) Y. Wu J. Zhou, E. Li, M. Wang, K. Jie, H. Zhu and F. Huang, J. Am. Chem. Soc., 2020, 142, 19722 CrossRef PubMed; (e) A. Dey, S. Chand, B. Maity, P. M. Bhatt, M. Ghosh, L. Cavallo, M. Eddaoudi and N. M. Khashab, J. Am. Chem. Soc., 2021, 143, 4090 CrossRef CAS PubMed; (f) J.-R. Wu and Y.-W. Yang, Angew. Chem., Int. Ed., 2021, 60, 1690 CrossRef CAS PubMed; (g) J. L. Atwood, L. J. Barbour, A. Jerga and B. L. Schottel, Science, 2002, 298, 1000 CrossRef CAS PubMed; (h) P. K. Thallapally, B. P. McGrail, S. J. Dalgarno, H. T. Schaef, J. Tian and J. L. Atwood, Nat. Mater., 2008, 7, 146 CrossRef CAS PubMed; (i) K. Jie, M. Liu, Y. Zhou, M. A. Little, A. Pulido, S. Y. Chong, A. Stephenson, A. R. Hughes, F. Sakakibara, T. Ogoshi, F. Blanc, G. M. Day, F. Huang and A. I. Cooper, J. Am. Chem. Soc., 2018, 140, 6921 CrossRef CAS PubMed; (j) J.-R. Wu and Y.-W. Yang, J. Am. Chem. Soc., 2019, 141, 12280 CrossRef CAS PubMed; (k) X. Sheng, E. Li, Y. Zhou, R. Zhao, W. Zhu and F. Huang, J. Am. Chem. Soc., 2020, 142, 6360 CrossRef CAS PubMed; (l) X. Zhao, Y. Liu, Z.-Y. Zhang, Y. Wang, X. Jia and C. Li, Angew. Chem., Int. Ed., 2021, 60, 17904 CrossRef CAS PubMed; (m) J.-R. Wu, B. Li, J.-W. Zhang and Y.-W. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 998 CrossRef CAS PubMed; (n) J.-R. Wu, B. Li and Y.-W. Yang, Small, 2020, 16, 2003490 CrossRef CAS PubMed; (o) H.-Y. Zhou and C.-F. Chen, Chem. Commun., 2022, 58, 4356 RSC.
- (a) J. Zhou, G. Yu, Q. Li, M. Wang and F. Huang, J. Am. Chem. Soc., 2020, 142, 2228 CrossRef CAS PubMed; (b) M. Wang, J. Zhou, E. Li, Y. Zhou, Q. Li and F. Huang, J. Am. Chem. Soc., 2019, 141, 17102 CrossRef CAS PubMed; (c) Q. Li, K. Jie and F. Huang, Angew. Chem., Int. Ed., 2020, 59, 5355 CrossRef CAS PubMed; (d) Y. Zhao, H. Xiao, C.-H. Tung, L.-Z. Wu and H. Cong, Chem. Sci., 2021, 12, 15528 RSC; (e) K. Jie, Y. Zhou, E. Li and F. Huang, Acc. Chem. Res., 2018, 51, 2064 CrossRef CAS PubMed; (f) Y. Wang, K. Xu, B. Li, L. Cui, J. Li, X. Jia, H. Zhao, J. Fang and C. Li, Angew. Chem., Int. Ed., 2019, 58, 10281 CrossRef CAS PubMed; (g) H. Zuilhof, K. Samanta, W. Yang, X. Wan, T. U. Thikekar, Y. Chao, S. Li, K. Du, J. Xu, Y. Gao and A. C.-H. Sue, Angew. Chem., Int. Ed., 2020, 59, 3994 CrossRef PubMed; (h) H. Yao, Y.-M. Wang, M. Quan, M. U. Farooq, L.-P. Yang and W. Jiang, Angew. Chem., Int. Ed., 2020, 59, 19945 CrossRef CAS PubMed; (i) D. Luo, J. Tian, J. L. Sessler and X. Chi, J. Am. Chem. Soc., 2021, 143, 18849 CrossRef CAS PubMed; (j) Y. Zhou, K. Jie, R. Zhao, E. Li and F. Huang, J. Am. Chem. Soc., 2020, 142, 6957 CrossRef CAS PubMed; (k) J.-R. Wu, B. Li and Y.-W. Yang, Angew. Chem., Int. Ed., 2020, 59, 2251 CrossRef CAS PubMed; (l) Q. Li, H. Zhu and F. Huang, J. Am. Chem. Soc., 2019, 141, 13290 CrossRef CAS PubMed; (m) J.-R. Wu and Y.-W. Yang, CCS Chem., 2020, 2, 836 Search PubMed.
- X.-B. Hu, Z. Chen, L. Zhang, J.-L. Hou and Z.-T. Li, Chem. Commun., 2012, 48, 10999 RSC.
- K. Jie, Y. Zhou, E. Li, R. Zhao and F. Huang, Angew. Chem., Int. Ed., 2018, 57, 12845 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2132121–2132123. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc02494b |
| ‡ Jiajun Cao and Yitao Wu are first co-authors. |
| This journal is © The Royal Society of Chemistry 2022 |