Open Access Article
Open Access ArticleHeterometallic nanomaterials: activity modulation, sensing, imaging and therapy
Shan-Shan
Li
a,
Ai-Jun
Wang
 b,
Pei-Xin
Yuan
b,
Pei-Xin
Yuan
 b,
Li-Ping
Mei
b,
Li-Ping
Mei
 b,
Lu
Zhang
b,
Lu
Zhang
 b and
Jiu-Ju
Feng
b and
Jiu-Ju
Feng
 *b
*b
aInstitute for Chemical Biology & Biosensing, College of Life Sciences, Qingdao University, 308 Ningxia Road, Qingdao, 266071, China
bKey Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Life Sciences, College of Geography and Environmental Sciences, Zhejiang Normal University, Jinhua 321004, China. E-mail: jjfeng@zjnu.cn
First published on 12th April 2022
Abstract
Heterometallic nanomaterials (HMNMs) display superior physicochemical properties and stability to monometallic counterparts, accompanied by wider applications in the fields of catalysis, sensing, imaging, and therapy due to synergistic effects between multi-metals in HMNMs. So far, most reviews have mainly concentrated on introduction of their preparation approaches, morphology control and applications in catalysis, assay of heavy metal ions, and antimicrobial activity. Therefore, it is very important to summarize the latest investigations of activity modulation of HMNMs and their recent applications in sensing, imaging and therapy. Taking the above into consideration, we briefly underline appealing chemical/physical properties of HMNMs chiefly tailored through the sizes, shapes, compositions, structures and surface modification. Then, we particularly emphasize their widespread applications in sensing of targets (e.g. metal ions, small molecules, proteins, nucleic acids, and cancer cells), imaging (frequently involving photoluminescence, fluorescence, Raman, electrochemiluminescence, magnetic resonance, X-ray computed tomography, photoacoustic imaging, etc.), and therapy (e.g. radiotherapy, chemotherapy, photothermal therapy, photodynamic therapy, and chemodynamic therapy). Finally, we present an outlook on their forthcoming directions. This timely review would be of great significance for attracting researchers from different disciplines in developing novel HMNMs.
1. Introduction
In recent years, monometallic nanomaterials (MMNMs) have found widespread applications in optics, catalysis, energy and life sciences, thanks to their intrinsic advantages (e.g. good stability and biocompatibility, high catalytic properties, and superior electronic conductivity) over bulk counterparts.1–4 As demonstrated by previous studies, their unique chemical and physical properties (e.g. chemical stability, and electrical and optical properties) mainly originate from tunneling effect, quantum size effect, and surface effect. Such properties can be finely modulated by controlling the size, composition, morphology, architecture, surface functionalization, and crystal structure/phase.4,5To achieve their scalable design and synthesis, a series of synthesis methods have been developed, including electrodeposition, wet-chemical synthesis, hydrothermal treatment, and sonochemical methods.4–7 Accordingly, diverse micro-/nano-structures have been constructed (e.g. sheets, wires, dendrites, tubes and particles), showing wide applications in the aforementioned fields,1,4,7 in spite of their limited resources and high price, along with instability. For their potential application, it is ideal yet challenging to simultaneously combine low cost, superior optical/electrical properties, and good stability, although this point is difficult to satisfy only with MMNMs.
To meet the requirements, substantial efforts have been made towards synthesis of advanced heterometallic nanomaterials (HMNMs) in the past several decades. Generally, doping with a second or even third metal can greatly change spatial arrangement patterns, local bonding geometry (structural effects), and distributions of active sites (ensemble effects) to enable easy availability of more active sites, coupled with revival of activity of surface atoms (electronic effects), which in turn improves the physicochemical properties,8,9 owing to synergistic impacts of multiple metals in HMNMs.10,11 As a result, HMNMs have wider applications than MMNMs in the fields of sensing, imaging and therapy.
For preparation of HMNMs, reduction of metal precursors and disassembly of a larger object are two common routes, termed as bottom-up and top-down methods, respectively.7,12 The former requires suitable metal salts, reducing agent(s) and solvents. Notably, some solvents such as polyols and amines can simultaneously work as reducing agents.13–15 Also, some surfactants, polymers and polyelectrolytes are usually required for metal crystal growth and colloidal stability, coupled with regulation of synthesis parameters (i.e. reaction temperature, reaction time, reagent concentration, etc.). In short, the bottom-up approach commonly involves co-reduction, seeded-growth, and anodic dissolution.7,12 Alternatively, the top-down method is an effective way to prepare HMNMs, although it is less used than the bottom-up counterpart.12 Particularly, laser ablation is a highly controllable top-down method, where well-dispersed HMNMs are harvested upon applying a laser beam on a solid target.16
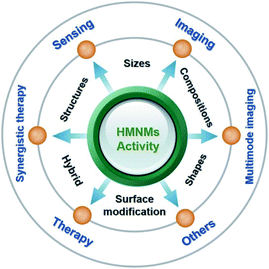
Up to date, early reviews have mainly focused on introducing preparation methods, morphology control, and applications of metal nanomaterials in catalysis,1,2,7 determination of heavy metal ions17 and antimicrobial activity.18 Nevertheless, most of the outlined applications correlate to MMNMs,17,18 while scarcely involving HMNMs. To this end, this review mainly introduces the latest developments in activity modulation of HMNMs by finely regulating the shape, size, composition, configuration and surface modification, coupled with outlining their main applications in sensing, imaging and therapy within past five years due to the article length limitation (Fig. 1). Finally, an outlook on their forthcoming directions is provided. This timely review would be instructive to deeply illustrate the correlations between the physicochemical properties and synthetic parameters of HMNMs, and provide some valuable insights for researchers from different disciplines (e.g. chemistry, materials science, biology and nanotechnology).
2. Tailoring the physicochemical properties of HMNMs
To our knowledge, the chemical and physical properties of HMNMs as well as their advanced applications correlate with their architecture, surface atomic arrangement, and coordination. The properties have close correlation with their size, morphology, and compositions.19–21 Lin et al. constructed bimetallic Au–Bi nanoparticles (NPs) with a size of about 5 nm, which exhibited a significantly improved thermal effect under the same light radiation.19 Hou and co-workers prepared Au3Cu tetrapod nanocrystals (TPNCs) for multi-modal image-guided photothermal therapy (PTT) within the second near-infrared (NIR-II) region.20 In addition to size and morphology, we also investigated the influences of compositions, structures, and surface modification on the properties of HMNMs and the hybrid composites.2.1 Sizes
Size of HMNMs is crucial to their properties, due to the well-known size effect.9,19,21 Plainly, reducing their size can greatly improve their utilization with the purpose to increase the occupancy ratio of surface atoms, ultimately improving their physical/chemical properties.22 For instance, Quan's group synthesized ultra-small FePd nanodots (around 3.4 nm), exhibiting good photothermal conversion efficiency (PCE) in the NIR-II region.9 In another case, a trimetallic (triM) PdCuFe alloy was prepared with a diameter of 5.5 nm, which worked as a stimulus to activate Fenton reactors for chemodynamic therapy (CDT).21 In addition, the size of the nanodots can be finely tailored using different precursor types and other synthesis conditions (i.e. reaction temperature, reaction speed, duration, etc.).23 The resultant nanodots exhibited good biocompatibility, and can be easily removed because of their small size.Besides, Au–Ag bimetallic nanoclusters (BNCs) displaying efficient NIR-II aqueous electrochemiluminescence (ECL) were synthesized by combining Au nanoclusters with Ag, which were applied for selective analysis of a carbohydrate antigen (CA125).24 Jia and co-workers reported ultra-small bovine serum albumin-directed Au–Ag (Au–Ag@BSA) NPs with a size range of 2–4 nm, which showed great promise as a contrast agent in X-ray computed tomography (CT).25
2.2 Morphology
For HMNMs, their physicochemical properties not only are related to the particle sizes, but also closely depend on their shapes.26–28 Among them, there appear many Au/Pt-based nanostructures with a variety of shapes (e.g. particles, sheets, dendrites, flowers, multipods and stars) used in analysis, imaging and therapy (Fig. 2), mainly attributing to their good biocompatibility, high stability, easy modification, excellent catalytic properties, and tunable plasmon properties.20,26,27,29–34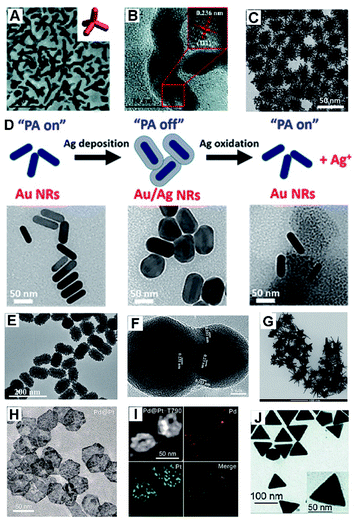 | ||
| Fig. 2 (A) TEM image of Au3Cu TPNCs. Inset shows the geometric model. (B) HR-TEM image of Au3Cu TPNCs. Reproduced with permission.20 Copyright 2018, The Royal Society of Chemistry. (C) TEM image of Au@Pt nanodendrites. Reproduced with permission.26 Copyright 2017, American Chemical Society. (D) Schematic diagram of the experiment and TEM images of Au NRs, Au/Ag NRs, and etched Au/Ag NRs. Reproduced with permission.27 Copyright 2018, American Chemical Society. (E) TEM images of mulberry-like Au@PtPd porous nanorods. Reproduced with permission.30 Copyright 2020, Elsevier. (F) HR-TEM image of a Au/Ag alloy nanopeanut. Reproduced with permission.31 Copyright 2019, Elsevier. (G) TEM image of Au/Pt stars. Reproduced with permission.32 Copyright 2020, Springer. (H) TEM image of Pd@Pt nanoplates. (I) HAADF-STEM image and elemental mapping of Pd@Pt-T790. Reproduced with permission.33 Copyright 2020, American Chemical Society. (J) TEM image of the Au@Ag triangular NPs. Reproduced with permission.34 Copyright 2018, The Royal Society of Chemistry. | ||
Recently, dendrite-like architectures of HMNMs have attracted substantial attention because of their appealing properties, owing to more steps, edges and corners that would create more active sites facilely available.26,35,36 For example, Pan and co-workers developed PEGylated Au@Pt nanodendrites as an enhanced theranostic agent for CT imaging and photothermal/radiation therapy (Fig. 2C).26 Clearly, the absorption of Au@Pt nanodendrites positively shifts to the NIR region upon gradual growth of Pt nanobranches, thereby enhancing efficacy of PTT and radiation therapy within cancer cells.
Meanwhile, core/shell structures usually have specific core/shell configurations, and attract tremendous interest owing to their magnified surface area and low density. Jokerst's group developed Au nanorods with a Ag shell (Au/Ag NRs), and the Ag shell can be further etched away with ferricyanide, ultimately achieving photoacoustic (PA) imaging and a good antibacterial effect (Fig. 2D).27
Hierarchical porous nanostructures have large specific surface areas and low density, which offer more active sites due to rich atomic steps, edges and corner atoms on the interconnected structures compared to solid counterparts.37–39 Besides, such unique configurations can efficiently suppress Ostwald ripening effects, consequently improving operation stability. Lately, porous Au@Pt NPs were physically absorbed with doxorubicin (DOX) and then chemically conjugated with cRGD (a cell penetrating peptide), which displayed strong NIR absorbance and high photo-conversion efficiency for PA image-guided enhanced PTT of MDA-MB-231 tumors.29 Similarly, mulberry-like Au@PtPd porous nanorods were prepared, which worked as signal amplifiers for sensitive detection of carcinoembryonic antigen (CEA) with satisfactory results even in human serum samples (Fig. 2E).30
Also, porous Au@Rh nanostructures displayed catalase-like activity, and was further coated with tumor cell membrane (CM) and photosensitizer indocyanine green (ICG). As a result, in vitro and in vivo observations of the Au@Rh-ICG-CM certify effective transformation of endogenous hydrogen peroxide (H2O2) to oxygen and a boost in production of tumor-toxic singlet oxygen, thereby significantly enhancing oxygen-dependent cancer photodynamic therapy (PDT).28
Aside from the above, Au-, Pt-, and/or Pd-based nanoplates or nanotriangles demonstrate intense NIR absorption and large specific surface area, showing extensive applications in antibacterial and therapy fields.33,34,40,41 For example, Pd@Pt nanoplates were successfully bridged with meso-tetra(4-carboxyphenyl)porphine (T790) for catalysis-enhanced ultrasound (US)-driven sonodynamic therapy (SDT) to treat methicillin-resistant Staphylococcus aureus (MRSA)-infected myositis (Fig. 2H and I).33 Notably, T790 anchored on Pd@Pt can effectively inhibit the catalase-like activity, while their enzyme-like activity was recovered under irradiation by catalytic decomposition of endogenous H2O2 to O2 in bacterial infections. A similar observation was found when using two-dimensional (2D) Pd@Au core–shell nanostructures with the purpose to alleviate tumor hypoxia, which in turn significantly improved the cancer radiotherapy (RT) outcomes.40
2.3 Compositions
In general, HMNMs receive greater interest than MMNMs from both technological and scientific perspectives, and display superior physiochemical properties and stability to monometallic counterparts.1,7,42,43 Their improved physical and chemical performances mainly stem from geometric (ensemble effect), electronic (ligand effect), and synergistic effects associated with different metals as reported in the literature.21,44,45 As earlier certified, doping noble metal(s) with nonprecious transition metal(s) (M = Fe, Co, Cu, Ni, etc.) is feasible for constructing advanced HMNMs with desired compositions, particularly accompanied by less usage of noble metal(s), improved physicochemical properties and operation durability.9,21,46,47 For example, ultra-small cysteine (Cys)-functionalized FePd nanodots were synthesized, which exhibited highly effective hyperthermia upon irradiation in the NIR-II region, and eventually showed a largely enhanced radiation effect for triple-modal imaging and thermo-RT.9It is known that Pd is commonly recognized as a photothermal agent with high efficiency, having great potential in PTT.48 Besides, Fe is an important element in human body for its magnetism, and often behaves as a contrast agent for magnetic resonance (MR) scanning.23 Recently, an ultra-small PdCuFe alloy nanozyme was prepared, which showed cascade glutathione peroxidase (GSH-Px) and peroxidase (POD) mimicking activities in circumneutral pH with a high PCE (62%) for synergistic tumor cell apoptosis, coupled with US-promoted tumor-specific CDT in the NIR window, exemplifying a multi-functional nanoenzyme design towards tumor inhibition.21 In addition, the incorporated Fe well retained catalytic activity of Pd and displayed a lower cost to some extent.
Also, porous PdPtCoNi@Pt-skin nanopolyhedra were fabricated in our laboratory to build a sandwich-like electrochemical sensor for ultrasensitive assay of creatine kinase-MB (CK-MB).46 Similarly, PtCoCuPd hierarchical branch-like tripods were constructed to develope an immunosensor for bioanalysis of cardiac troponin I (cTnI).47 These examples demonstrate that the optimized compositions in HMNMs provide a widespread prospect in establishment of ultra-sensitive electrochemical immunosensors.46,47,49–52
Interestingly, metallic Janus nanoparticles (JNPs) represent effective combination of two or even more chemically discrepant metals into one single system.53,54 They attract tremendous interest as they integrate multi-functional properties, and simultaneously perform more synergistic functions particularly in analytical chemistry.55–58 Lately, Au–Pt JNPs were fabricated and their catalytic oxidation of a luminophore was examined by ECL microscopy, where single Au and Pt counterparts acted as references.55 As seen from elemental mapping images, Au–Pt JNPs exhibited asymmetrical structures formed with equivalent Au and Pt (Fig. 3A), and showed distinctly boosted ECL intensity and stability, manifesting a superior catalytic effect compared to individual Au and Pt NPs.
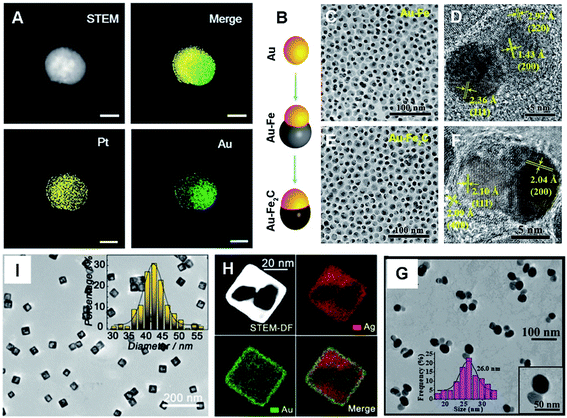 | ||
| Fig. 3 (A) HAADF-STEM image and elemental mapping of a Au–Pt JNP, scale bar: 25 nm. Reproduced with permission.55 Copyright 2018, Wiley. (B) Schematic diagram of the synthesis process of Au–Fe2C JNPs. TEM images of Au–Fe heterostructures (C and D) and Au–Fe2C JNPs (E and F). Reproduced with permission.56 Copyright 2017, American Chemical Society. (G) TEM image of Au–Ag(3) JNPs and the corresponding size distribution. Reproduced with permission.57 Copyright 2019, American Chemical Society. (I) TEM image of Ag–Au nanocages. The inset shows the size distribution. (H) HAADF-STEM image and elemental mapping of Ag–Au nanocages. Reproduced with permission.58 Copyright 2019, American Chemical Society. | ||
Similarly, monodisperse Au–Fe2C JNPs were prepared via a three-step strategy: Au seeds, Au–Fe heterostructures, and Au–Fe2C JNPs (Fig. 3B–F).56 The resulting Au–Fe2C JNPs exhibited significant PCE and prominent magnetic properties, thanks to the associated absorption peak located in the NIR window coupled with incorporated Fe as an essential composition. As well, Au–Ag JNPs were prepared with stable and enhanced SERS activity (Fig. 3G), and further explored for building a ratiometric surface enhanced Raman scattering (SERS) sensor for quantitative assay of ochratoxin A (OTA).57 What's more, a hollow Janus hybrid nanozyme was designed by bifacial regulation of Ag–Au nanocages (Fig. 3H and I), which effectively worked as a SERS liquid biopsy platform for determination of tumor-related biomarkers.58 These successful examples certify the critical roles of compositions in fine modulation of the activity.
2.4 Structures
As we know, noble metal-based nanostructures have attracted significant attention in drug delivery, therapeutics and biosensing fields; however, their high cost is a main problem.17,18,59 To circumvent this, it is attractive to employ HMNMs rather than MMNMs. In general, HMNMs mainly contain alloyed, porous and hollow structures. Plainly, alloyed HMNMs show interesting chemical and physical properties by virtue of the synergistic effect derived from incorporated metals, outperforming individual ones.7 Alternatively, porous structures frequently have larger specific surface areas and create more active sites due to the abundant atomic steps, edges and corner atoms on such interconnected structures, accompanied by effective suppression of Ostwald ripening effects, consequently showing scalable optical/electrochemical properties and improved durability in contrast with solid counterparts.28,29,37,60For instance, porous Au@Rh nanostructures were fabricated as a therapeutic reagent upon loading the pores with a photosensitizer, combined with parcelling with CM (Fig. 4A–E).28 The nanocomposite improved the loading and simultaneously stabilized the activity of the photosensitizer during the biotransportation process without any premature release. The unique core–shell structure and porous shell confirmed ready availability of the two metals exposed to the reactants, modulating the core/shell boundary interactions via electronic and surface strain effects, in turn achieving the best catalytic performance.61 Additionally, the porous structure can effectively trap therapeutic drugs.62 Other representative examples include porous Au@Pt NPs for relieving oxidative stress damage (OSD) and chemo-photothermal co-therapy,29 as well as porous Au–Ag nanorods with sufficient internal hotspots for highly sensitive SERS detection.60
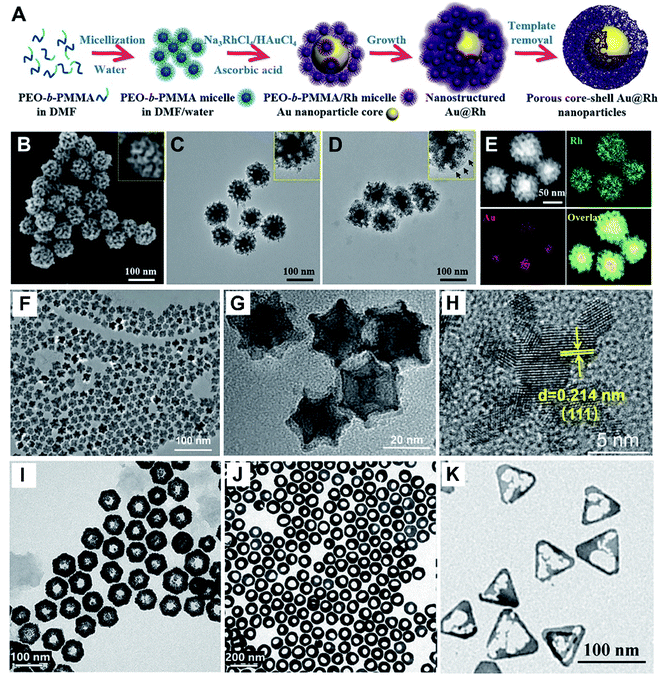 | ||
| Fig. 4 (A) Schematic diagram of the preparation process of porous Au@Rh nanomaterials. (B) SEM image of Au@Rh nanomaterials. (C) TEM image of Au@Rh nanomaterials. (D) TEM image of Au@Rh-CM nanomaterials. (E) HAADF-STEM image and elemental mapping of Au and Rh. Reproduced with permission.28 Copyright 2020, Wiley. TEM (F and G) and HR-TEM (H) images of PtCu3 nanocages. Reproduced with permission.63 Copyright 2019, Wiley. TEM images of Au@Pt nanodisks (I) and Pt@Au nanorings (J). Reproduced with permission.66 Copyright 2019, American Chemical Society. (K) TEM image of Au–Ag HTNs. Reproduced with permission.67 Copyright 2020, Springer. | ||
Three-dimensional (3D) hollow architectures have highly open structures and molecular surfaces with high density of active sites, which increase the loading capacity of target molecules and promote interfacial mass/electron transport. Lately, PtCu3 nanocages were synthesized, which worked as a horseradish peroxidase (HRP)-like nanozyme and GSH-Px, and both of them are beneficial to cancer therapy (Fig. 4F–H).63 Also, GSH-depleted PtCu3 nanocages performed as a sonosensitizer, displaying high production rate of reactive oxygen species (ROS) upon US irradiation. Owing to the fantastic properties of metallic nanoframes, our research group also prepared 3D open-structured PtCu nanoframes and rhombic dodecahedral Cu3Pt nanoframes, and explored their applications in electrochemical immunosensing systems.64,65
It is known that hollow Au-based nanomaterials show strong interactions with light and surrounding media, on account of their larger surface area than that of solid particles, showing great promise for establishing sensitive NIR sensors. Recently, dual Pt@Au nanoring@DNA probes were developed for fluorescence (FL) imaging and PTT of tumor cells (Fig. 4I and J).66 Also, Au–Ag hollow nanotriangles (Au–Ag HTNs) have great surface-to-volume ratio, sharp edges and vertices, along with a unique local curvature relative to other Au–Ag NPs, thus displaying strong absorption in the NIR-II region (Fig. 4K).67 As the Au–Ag HTNs were further immobilized with glucose oxidase (GOx), they effectively promoted a plasmon-accelerated cascade reaction for high-efficiency tumor therapy.
2.5 Surface modification
Apart from the shape, size, composition and structure, the properties of HMNMs also correlate with surface properties. Generally, surface modification strategies include surface engineering (e.g. coating with a polymer, silane, dendrimers and/or other metal(s)), attachment of functional groups to block surface access, and regulation of electronic structure and surface acidity, which in turn improve the activity, stability, specificity and biocompatibility.44,63,68Coating HMNM surfaces with a polymer makes them less toxic and more biocompatible. Notably, modification of HMNMs with hydrophilic polymers is instructive to improve their dispersibility, combined with their efficient conjugation with biomolecules, which is the key step in sensing, drug delivery, and therapeutic applications.20,29,69,70 Nevertheless, it is difficult to achieve such conjugations in many cases, except by initially coating with an appropriate linker. For example, thiolated polyethylene glycol (SH-PEG) acted as a linker for porous Au@Pt NPs to effectively load anticancer drug DOX, and further functionalization with the cRGD peptide, eventually showing significant improvement in colloidal stability and targeting property.29
By scalable functionalization with more specific ligands, selectivity can be greatly improved for different target molecules in such nanosystems.29,71 Plainly, folic acid (FA) is a feasible endocytic ligand for folate receptors that are overexpressed in diverse human cancer cells, and hence displays high affinity towards cancer cells.20,32,71 For instance, Au eyeball-like NPs with open-mouthed Pd shells (Au@Pd) were prepared and further modified with FA for cell-targeting and Chlorin e6 (Ce6), which led to their selective accumulation at the tumor site and effective triggering of cell apoptosis as a photodynamic agent.71 Similarly, Au3Cu tetrapod nanocrystals (TPNCs) were synthesized and immobilized with PEG, Cy5 and FA, endowing the Au3Cu TPNCs with superior physical stability, FL imaging effect, and accurate targeting ability for multi-modal image-guided PTT, by adopting multispectral photoacoustic tomography (MSOT) imaging.20 In addition, an Au/Pt star-shaped core (Au/Pt star) was successively combined with L-cysteine by covalently linking cystamine dihydrochloride and targeted ligand rHSA-FA, followed by physical adsorption of a NIR fluorophore (IR780) and GOx.32 The resulting composite accelerates GSH-triggered sequential catalysis for tumor imaging and eradication based on the star-like Au/Pt enzyme carrier system.
Also, BSA is a commonly used protective molecule in various probes and sensor designs, which can prevent non-specific adsorption and improve specificity and biocompatibility accordingly.49,64,72,73 For example, Au nanobipyramid cores with external silver nanorods were prepared, and subsequently coated with 4-mercaptobenzoicacid (4-MBA), FA and reduced bovine serum albumin (rBSA).74 The hybrid nanoprobes had appealing specificity and biocompatibility with living gastric cancer cells (MGC-803) due to the conjugation of FA and modification of rBSA that can effectively avoid non-specific attachment of non-targeted cells. In the same way, single-signal and dual-signal ratio immunosensors were separately built based on reduced graphene oxide (rGO)-polydopamine-grafted-ferrocene/Au@Ag nanoshuttles and hollow Ni@PtNi yolk–shell nanocages-thionine (Ni@PtNi HNCs)37 as well as PtCu hollow nanoframes,55 where BSA performed as a sealing agent in the construction of the biosensors.
By virtue of high specificity of the A–T and G–C hydrogen-bonded Watson–Crick interactions, coupled with superior biocompatibility and scalable designability, DNA has achieved success in construction of diverse nanostructures and its practical application in biological analysis.75 In this regard, NIR light-activated Pt@Au nanoring@DNA probes were designed for FL imaging and targeted PTT of cancer cells, owing to fine regulation of specific recognition and imaging by dehybridization of double-stranded DNA (dsDNA), combined with temperature variation upon NIR light irradiation.66 Also, amino-terminated AS1411 formed G-quadruplex structures on Pt-based nanostructures via Hoogsteen hydrogen bonds. To this end, an enzyme-free electrochemical biosensor was developed based on PdRu/Pt heterostructures, integrated with hemin/G-quadruplex as a signal magnification probe, which finally showed efficient determination of circulating tumor cells (CTCs).76
Small molecules (e.g. Cys and GSH) have hydrophilic groups such as amine and carboxyl groups, which improve solubility of HMNMs in aqueous media, ultimately realizing good dispersibility of HMNMs.9,77 Lately, ultra-small Cys-coated FePd bimetallic nanodots showed a negative zeta potential of −45.4 mV, and still remained uniform in phosphate buffer solution (PBS) and serum samples even after immersion for 24 h, reflecting their good biocompatibility and excellent stability, unlike pure FePd counterpart which already deposited at the bottle bottom. Both the in vitro and in vivo results certify Cys–FePd nanodots as a feasible contrast agent for tri-modal CT/MR/PA imaging and hypoxia-resistant thermo-RT in a NIR-II scope.8
By the same token, GSH capped Au–Bi NPs were prepared and further immobilized with IR808 dye through sulfate and carboxylic groups of GSH (termed Au–Bi-GSH@IR808 for clarity). Such a hybrid composite showed good-dispersion in an aqueous environment (due to GSH ligands) and prominent CT imaging property, and noticeably enhanced therapeutic effect in accurate diagnosis and treatment of cancer.77
Last but not least, photosensitizer ICG was efficiently loaded and retained in the cavity of a porous Au@Rh biphasic core–shell nanostructure (denoted as Au@Rh-ICG). The Au@Rh-ICG was further grafted with the tumor CM via homologous binding. The resulting nanocomposite behaved as an H2O2-driven oxygenerator to alleviate tumor hypoxia for simultaneous bimodal imaging and enhanced PDT, and showed large tumor accumulation and high biocompatibility, along with superior FL and PA imaging properties.28
2.6 Construction of hybrid nanomaterials
In general, HMNMs exhibit superior catalytic activity due to the synergistic effects between different metals and unique structures.7,42 Alternatively, single particles tend to agglomerate together due to large surface energy, and consequently reduce the effective surface area and weaken the catalytic properties.78–82 To address this issue, many materials such as carbon materials,78,79,83–93 metal oxides,80–82,94–107 and sulfides,108–111 as well as metal organic frameworks (MOFs, consisting of organic ligands and metal ions)112–114 have been explored as supports or wrapping materials recently. Among them, there are many carbon- and metal-oxide based hybrid nanomaterials, showing broad applications in sensing and analysis (Table 1).78–107 For example, a seed-mediated approach was developed for aqueous synthesis of heterodimeric structures such as AgPtalloy–Fe3O4 and Aucore@Pdshell–Fe3O4,115 and they were adopted as excellent contrast agents in imaging modalities such as optical coherence tomography (OCT) and PA with excellent performances.| Hybrid nanomaterials | Applications | Ref. |
|---|---|---|
| AgPt–rGO | CEA | 83 |
| Au@Pt/GO | H2O2 | 84 |
| PtCu@rGO/g-C3N4 | PSA | 85 |
| FePt/GO | MR/CT imaging | 86 |
| Cu–Ag/rGO | Glucose and ascorbic acid | 87 |
| PtPd/N-GQDs@Au | CEA | 88 |
| Pd@Au@Pt/COOH-rGO | CEA and PSA | 89 |
| 3D graphene/AuPtPd | ctDNA | 78 |
| Au–Pd/GO | H2O2 | 90 |
| HAC-AuPt | ctDNA | 91 |
| PdCu/CB | H2O2 | 92 |
| PdFe/GDY | GSH detection and antibacterial | 93 |
| SWCNT/Ag/Au | SERS imaging of hypoxia | 79 |
| AuAg@p-SiO2 | H2O2 | 94 |
| Au–Pt/SiO2 | H2O2 | 95 |
| Au/Gd@SiO2 | MSCs | 80 |
| Fe3O4@SiO2–Au@Pd0.30 | Glucose | 96 |
| FePt/SiO2/Au | PTT and MRI imaging | 97 |
| Au@Ag@SiO2 | AFP | 98 |
| Co/Fe–MSNs | Cysteine | 99 |
| SiO2@Au@Ag | PSA | 81 |
| AuPd@FexOy | CDT–PTT | 100 |
| Fe3O4@Au@Ag | IgG | 101 |
| Au@Pd/NH2–MoO2 | HBsAg | 102 |
| MnO2@PtCo | Hypoxic tumors | 82 |
| MnO2/Au@Pd^Pt | NSE | 103 |
| Ag–Au–TiO2 | Photocatalytic and antimicrobial | 104 |
| Cu2O@PtPd | Early cell apoptosis | 105 |
| Au–Ag/Co3O4 | H2O2 | 106 |
| Co3O4@CeO2–Au@Pt | SCCA | 107 |
Recently, graphene116 and other 2D transition metal dichalcogenides (TMDCs) have found widespread applications in versatile electronic and optoelectronic applications, owing to their unique optical, electrical and mechanical properties.117 Particularly, TMDCs such as MoS2 display a mono-layered unique structure, large specific surface area, high catalytic activity, good electrical and optical properties, integrated with high mechanical flexibility.108,109 Generally, they worked as ideal alternatives to replace the state-of-the-art silicon-based technology.118 Incorporation of HMNMs such as PtPd nanocubes in MoS2 nanosheets (PtPd NCs@MoS2) largely improved electronic conductivity and produced more reaction sites, showing accelerated POD-like activity by contrast with PtPd NCs and MoS2 alone.108 Zhao and co-workers prepared Au–Pd–Pt nanoflower modified MoS2 nanosheets (Au–Pd–Pt/MoS2) as a co-reaction accelerator for building an ECL immunosensor to detect cystatin C (CYSC), and they showed a wide linear range and low limit of detection (LOD).109
It is noteworthy that HMNMs are certified to show distinct enhancement in the photocurrents of photoactive materials, due to the localized surface plasmon resonance (LSPR) effect.119 Besides, typical Schottky junctions are formed by fine integration of noble metals and photoactive materials, showing robust improvement in the transport and separation of photoinduced carriers.111 For instance, ultrasensitive split-type and GOx-mediated photoelectrochemical (PEC) immunoanalysis was developed for CEA assay by employing photoactive CdS nanorods combined with PdPt nanozyme.110 Also, Jin et al. chose GOx and alkaline phosphatase (ALP) to prepare core–shell CdSe/ZnS@Au hybrid nanostructures.111
As already reported, MOFs show a remarkable increase in loading capacity, thanks to the large specific surface area, scalable porosity, facile surface modification, good stability, and mild synthesis conditions.120 MOFs have wide applications in biosensing, analysis and therapy.121 For instance, iron-porphyrinic MOFs (PCN-223-Fe) were synthesized with Zr4+ and iron-porphyrin (as a linker), and then in situ located with spindle-shaped PtAg NPs, followed by exploring them to build an electrochemical aptasensor for ultrasensitive assay of OTA.112
3. Applications of HMNMs in analytical chemistry
3.1 Sensing
By contrast with MMNMs, HMNMs have gained tremendous research focus due to their intrinsic properties such as superior biocompatibility, good electrical conductivity, and extremely high catalytic activity.7,8 To date, HMNM-based biosensing devices are continuously built and applied in determination of metal ions, small molecules, proteins, nucleic acids, and cancer cells.24,76,78,96,122–124Specifically, Hg2+ is one of heavy metal ions with wide applications in industry and agriculture, whose strong toxicity and bioaccumulation even at very low concentrations can cause tumor, neural disorder, kidney damage, and epithermal tissue damage.125 As a result, various methods have been developed for its assay in the last decades.126,127 Recently, Au@Pt NPs122 and Ag–Cu NPs128 were prepared for sensitive detection of Hg2+. Later on, a Ag–Fe bimetallic-3-(trimethoxysilyl) propyl methacrylate framework was synthesized as a colorimetric probe for determination of Hg2+, and further applied for efficient analysis of Hg2+ in different environmental aqueous media.129
As an extremely toxic metal, Cd2+ frequently exists in agriculture and industry, and can induce different lesions and diseases (e.g. pulmonary edema, emphysema, pneumonitis and even cancer).130 Therefore, many economical and effective approaches have been developed for its qualitative and quantitative analysis.123,131 For instance, a femtosecond laser processing technique was developed for selective formation of layered Cu–Ag nanodots within glass microfluidic channels, combined with electroless plating.123 In the presence of crystal violet, Rhodamine 6G was detected on the resulting SERS microfluidic chip with an enhancement factor of 7.3 × 108 and a relative standard deviation of 8.88%, followed by achieving real-time assay of Cd2+ down to 10 ppb.
In addition, Pb2+ is recognized as a major, ubiquitous and bioaccumulative heavy metal pollutant in environment and food.132 It is harmful to kidneys and brain even at a very low concentration, and hence many strategies have been designed for its detection recently.132,133 For example, Au@Pt NPs were prepared by deposition of Pt onto Au NPs, and they served as a nanozyme for colorimetric determination of Pb2+ leaching from a Pb2+–S2O32− system with a linear range of 20 ∼ 800 nM, as well as high sensitivity and selectivity.133
To date, a variety of HMNM-associated sensors have been constructed for H2O2 detection, including SERS,94,124 colorimetric detection,84,95,137 and electrochemical sensing.92,106 For example, ultra-thin porous silica shell-coated Au–Ag alloy NPs (AuAg@p-SiO2) were synthesized and immobilized with 4-mercaptophenylboronic acid (MPBA) and 4-mercaptophenylacetylene (MPAE, 1986 cm−1) as internal standard. After incubation with dopamine (DA), the bridging molecules were grafted on the particle surface via a borate bond between DA and MPBA, followed by conjugating 3-(4-(phenylethynyl) benzylthio) propanoic acid (PEB, 2214 cm−1) as signaling alkyne molecules via an amide bond between the carboxyl group on the PEB and the amino group on the DA, eventually forming a ratiometric SERS nanoprobe for Raman imaging of H2O2 in living cells.94 The Raman signals at 2214 cm−1 were significantly decayed with H2O2 when the alkynyl on the PEB was released from the particle surface, while that of MPAE at 1986 cm−1 remained unchanged. Thus, quantitative analysis of H2O2 concentration was realized according to the ratiometric value of I1986/I2214 in a linear scope of 0.12 ∼ 8 μM, with a LOD as low as 52 nM (S/N = 3).
In another case, FePt–Au NPs were prepared by a seed-mediated hydrothermal approach, and they can oxidize 3,3′,5,5′-tetramethylbenzidine (TMB) to a blue product, eventually developing a colorimetric sensor for fast detection of H2O2.137 What's more, a PdCu NP/carbon black (CB) based electrochemical biosensor was fabricated for sensitively detecting H2O2 released from live cells.92
Similarly, glucose is an important chemical substance correlated to nervous system, so its determination is essential138 and rational when HMNMs are integrated with GOx by utilizing the intrinsic POD-like ability.96,139 For example, Au@Pd NPs were efficiently decorated on a nanomagnet-silica shell (Fe3O4@SiO2), which showed superior POD-like activity for TMB oxidation in the presence of H2O2.96 Besides, a simple colorimetric sensor was established after physical adsorption of GOx, showing high selectivity and sensitivity for detecting glucose. In another instance, Pd nanosheet-supported Au NPs were synthesized by galvanic replacement, and further applied for sensitive glucose sensing based on their high catalysis towards TMB oxidation.139
Aside from H2O2 and glucose, HMNMs were also utilized for analysis of other molecules, including ascorbic acid,87 biothiol,93,99 hydrazine,140 dibutyl phthalate,141 adenosine,142,143 ractopamine,144 chloramphenicol,145 hydroquinone,146 malathion,147 nitrite,148 Sudan I,149 and tetrabromobisphenol A.150
| Nanomaterials | Protein markers | Linear range | LOD | Ref. |
|---|---|---|---|---|
| Au@Pt/Au | p53 peptide | 50–1000 nM | 66 nM | 154 |
| Cu3Pt | AFP | 0.1–104 pg mL−1 | 0.033 pg mL−1 | 65 |
| Au@Pt/MoSe2 | AFP | 10–2 × 108 fg mL−1 | 3.3 fg mL−1 | 155 |
| Au@Ag | AFP | 0.5–100 pg mL−1 | 0.081 pg mL−1 | 156 |
| AuNS@Ag@SiO2 | AFP | 3–3 × 106 pg mL−1 | 0.72 pg mL−1 | 98 |
| PtCo | CA153 | 0.1–200 U mL−1 | 0.0114 U mL−1 | 36 |
| AuPt | CA199 | 0.05–50 U mL−1 | 0.03 U mL−1 | 157 |
| Au@Pt | CA153 | 0.5–200 U mL−1 | 0.17 U mL−1 | 158 |
| AuAg | CA199 | 1–30 U mL−1 | 0.228 U mL−1 | 159 |
| Au–Ag | CA125 | 5 × 10−4–1 U mL−1 | 5 × 10−5 U mL−1 | 24 |
| AgPt | CEA | 5–5 × 107 fg mL−1 | 1.43 fg mL−1 | 84 |
| PtPd/N-GQDs@Au | CEA | 5–5 × 107 fg mL−1 | 2 fg mL−1 | 88 |
| PdAuCu | CEA | 0.001–100 ng mL−1 | 0.23 pg mL−1 | 52 |
| MoS2/Au@AgPt | CEA | 10–108 fg mL−1 | 3.09 fg mL−1 | 160 |
| Au@PtPd | CEA | 50–108 fg mL−1 | 16.7 fg mL−1 | 30 |
| CdS/PdPt | CEA | 1–5 × 103 pg mL−1 | 0.21 pg mL−1 | 110 |
| Au@Pt | CEA | 0.025–1.6 ng mL−1 | 0.021 ng mL−1 | 122 |
| Ni–Pt | CEA | 5−500 pg mL−1 | 1.1 pg mL−1 | 161 |
| PtCu@rGO/g-C3N4 | PSA | 50−4 × 107 fg mL−1 | 16.6 fg mL−1 | 85 |
| AuPd@Au | PSA | 0.1–50 ng mL−1 | 0.078 ng mL−1 | 162 |
| Au@Pt | PSA | 0.1–50 ng mL−1 | 0.018 ng mL−1 | 163 |
| AuPtAg | PSA | 0.05–50 ng mL−1 | 0.017 ng mL−1 | 164 |
| PtCu | PSA | 0.01–100 ng mL−1 | 0.003 ng mL−1 | 64 |
| Au/Ag | PSA | 10–200 ng mL−1 | 1.53 ng mL−1 | 165 |
| Au@Ag | PSA | 5–120 fg mL−1 | 0.94 fg mL−1 | 166 |
| SiO2@Au@Ag | PSA | 2.5–25 ng mL−1 | 0.006 ng mL−1 | 81 |
| rGO/Thi/AuPt and Au@Pd | NSE | 0.0001–50 ng mL−1 | 0.03 pg mL−1 | 167 |
| MnO2/Au@Pd^Pt | NSE | 10–108 fg mL−1 | 4.17 fg mL−1 | 103 |
| Au@Pd | CFP-10 | 5 × 10−13−5 × 10−4 g mL−1 | 5.6 × 10−12 g mL−1 | 168 |
| PdPtCoNi@Pt | CK-MB | 0.001–2500 ng mL−1 | 0.62 pg mL−1 | 46 |
| AuPdCu | CK-MB | 0.001–2000 ng mL−1 | 0.88 pg mL−1 | 50 |
| 12–8.5 × 104 pg mL−1 | 8 pg mL−1 | |||
| Pd@Au@Pt/COOH–rGO | CEA/PSA | 3–6 × 104 pg mL−1 | 2 pg mL−1 | 89 |
| PtCoCuPd | cTnI | 0.001–100 ng mL−1 | 0.2 pg mL−1 | 47 |
| NH2-MIL-88B(Fe2Co)-MOF | cTnI | 0.1–10 × 107 fg mL−1 | 13 fg mL−1 | 169 |
| Au–Pd–Pt/MoS2 | CYSC | 1–5 × 106 fg mL−1 | 0.35 fg mL−1 | 109 |
| Au/Ag | DNase I | 0−80 unit mL−1 | 0.056 unit mL−1 | 170 |
| PtPd@MoS2 | HBs Ag | 32−108 fg mL−1 | 10.2 fg mL−1 | 108 |
| Au@Pd–MoO2 | HBs Ag | 10−108 fg mL−1 | 3.3 fg mL−1 | 102 |
| AgPtCo | HE4 | 0.001–50 ng mL−1 | 0.487 pg mL−1 | 35 |
| Au@Ag and Ni@PtNi | HER2 | 0.01–100 ng mL−1 | 3.3 pg mL−1 | 49 |
| Au–Ag | IgG | 0.89–1000 pM | 0.89 pM | 171 |
| Fe3O4@Au@Ag | IgG | 1–106 pg mL−1 | 1 pg mL−1 | 101 |
| Au@Ag | Albumin | 10–300 mg L−1 | 0.2 mg L−1 | 172 |
| MOFs/AuPt | LAG-3 protein | 0.01–103 ng mL−1 | 1.1 pg mL−1 | 114 |
| Au@Pt | MMP-2 | 0.5–100 ng mL−1 | 0.18 ng mL−1 | 173 |
| PtCoNi | NT-proBNP | 0.001–10 ng mL−1 | 0.35 pg mL−1 | 51 |
| Pd@PtRh | PCT | 20−108 fg mL−1 | 6.74 fg mL−1 | 174 |
| PtCoIr | PCT | 0.001–100 ng mL−1 | 0.46 pg mL−1 | 175 |
| Au@Pt | SARS-CoV-2 | 10−100 ng mL−1 | 11 ng mL−1 | 176 |
| Co–Fe@hemin | SARS-CoV-2 | 0.2–100 ng mL−1 | 0.1 ng mL−1 | 177 |
| Au@Ag | SCCA | 2.5–115 ng mL−1 | 0.85 ng mL−1 | 178 |
| Co3O4@CeO2–Au@Pt | SCCA | 0.1–8 × 104 pg mL−1 | 33 fg mL−1 | 107 |
MicroRNAs (miRNAs), as a group of small molecules, consist of 18–22 nucleotide long noncoding RNA, and play a decisive role in RNA silencing and regulation of gene expression upon reverse transcription.180 Their varied expression would correlate with abnormal conditions such as suboptimal growth and diseases (e.g. cardiovascular disease, Alzheimer's disease, skin disease, and cancer), and they work as biomarkers in pathophysiological environments for determining the onset and prognosis of diseases in clinical diagnosis.181
For instance, mesoporous Au–Ag alloy films were prepared via electrochemical micelle assembly, and they showed high catalytic activity towards redox reaction of [Fe(CN)6]3−/4- and were used to build a miRNA sensor with a low LOD.182 By bifacial regulation of Ag–Au nanocages and subsequent deposition of DNAzyme-motif nanobrushes on the external cage surface, a hollow Janus hybrid nanozyme was designed towards SERS liquid biopsy at nano-/micro-scales, due to improved POD-like activity.58 Likewise, PtCuCo triM alloys were fabricated, which showed good catalytic activity in a luminol-H2O2 system towards determination of miRNA-21.183
Hemin/G-quadruplex DNAzyme was subtly integrated with Pt NPs decorated hyperbranched PdRu nanospines (PdRu NSs/Pt), and they displayed simultaneous catalysis of H2O2 to achieve multiplexed signal magnification, confirming feasibility for analysis of model CTCs with a LOD as low as 2 cell mL−1.76 Meanwhile, BSA coated Bi/Pt NPs (BSA-Bi/Pt) exhibited highly boosted POD mimicking activity in comparison with BSA-Pt NPs, combined with largely improved stability in harsh surroundings (e.g. high temperature, extreme pH environment, and high ionic strength), and a complicated biological matrix, realizing efficient assay of cancer cells (e.g. MCF-7) through the target recognition function of FA.186
Apart from the above, other representative examples include inner Au nanobipyramids and external Ag nanorods as a substrate for building a SERS biosensor,74 and Cu2O@PtPd for constructing an amperometric cytosensor.105 These examples certify their promising potential for early evaluation of cancers and even therapeutic effect of anticancer drugs in clinic.
3.2 Imaging
HMNMs demonstrate substantial potential as radiosensitizers for enhancement of RT in biomedical applications.187,188 They have been widely adopted in imaging to promote development of sensing, catalysis, diagnosis and therapy, in which many techniques such as photoluminescence (PL), ECL, FL, Raman, MR, CT and PA microscopy are involved.25,55,63,170,189,190 This section only gives some typical examples of different imaging applications.Combined with near-field modeling, far-field spectroscopy is frequently adopted to investigate optical properties of the plasmonic structures based on the near-field interactions.194,195 By contrast, far- and near-field spectral responses are certified to have lots of important applications.196,197 For instance, Halas et al. exhibited spatial and spectral mapping of optical forces between nanotips and Au–Al nanodisk heterodimers by photoinduced force microscopy (Fig. 5A–F).197 They found that near-field coupling derived from nanoscale gaps accounts for inter-particle interactions.
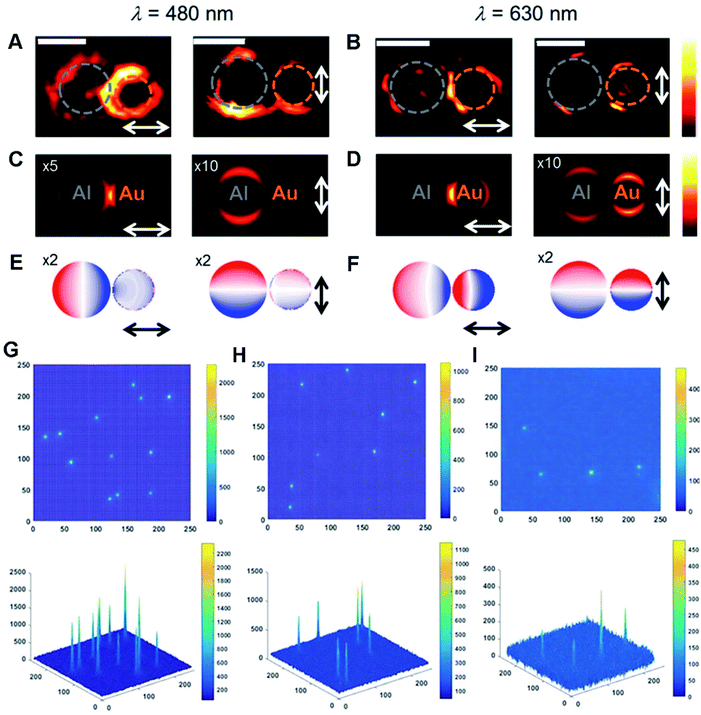 | ||
| Fig. 5 (A and B) Photoinduced force maps of Al–Au heterodimer structures using Si tips (gap size = 5 nm). (C and D) Photoinduced force maps of the heterodimer structures (Al disc d = 92 nm, Au disc d = 66 nm, and gap size = 5 nm). (E and F) Corresponding surface charge plots in the heterodimer structures. The arrows display the direction of in-plane polarization. Scale bars: 100 nm. Reproduced with permission.197 Copyright 2018, American Chemical Society. 2D and 3D ECL intensity diagrams of (G) Au–Pt JNPs, (H) Pt NPs, and (I) Au NPs. Reproduced with permission.55 Copyright 2018, Wiley. | ||
Beyond the above, ECL is a potential initiated form of chemiluminescence, and offers opportunity for an optical readout in catalysis, sensing and analysis, where luminophores are oxidized or reduced at the electrode surface and yield emissive excited-state species.198–200 For instance, ECL microscopy was applied to characterize electro–catalytic activity of Au–Pt JNPs (Fig. 5G–I).55 In comparison with single particles, Au–Pt JNPs largely boosted the osmotic gradient during electrocatalytic oxidation of Ru(bpy)32+ due to their directionality and asymmetry, and effectively prevented the formation of individual Au and Pt oxides, ultimately improving the ECL stability of Au–Pt JNPs compared to single counterparts.201
To overcome this problem, a Au–Se nanoplatform was developed by combining Se-modified peptides with Au NPs, and it exhibits excellent anti-interference property and high-fidelity fluorescence signals even in physiological systems, enabling the monitoring of changes of caspase-9 in MCF-7 cells by treating with staurosporine.190 After that, an Au–Se-bonded nanoprobe was developed for monitoring urokinase-type plasminogen activator (uPA) and matrix metalloproteinase-9 (MMP-9) in MCF cells, achieving real-time in situ imaging of their dynamic changes and regulatory correlations.204 Specifically, Au NPs were conjugated with two Se-modified peptide chains initially grafted with 5-aminomethyl Rhodamine (5-TAMRA) and fluorescein isothiocyanate (FITC). The anchored peptide chains are selectively cleaved after contacting with uPA and MMP-9, finally recovering the FL. Further, the functional Au–Se probe was utilized to monitor lipopolysaccharide (LPS)-treated cancer cell imaging, and the FL for uPA emerged early, reflecting that uPA worked upstream of MMP-9. Overall, the aforementioned research provides visual determination methods of the biomarkers to monitor invasive potential of breast cancer cells by in situ FL imaging of uPA and/or MMP-2 proteins and evaluate degree of breast cancer malignancy, combined with illustrating correlations among signal molecules of other signaling pathways in future.205
With continuous research advancement, HMNM-based FL imaging displays potential for application in clinical practice to monitor treatment procedures of cancers.32,66,77,206 For example, core–shell Au/Pt star based multifunctional nanoprobes were successfully constructed upon conjugation with a GSH-sensitive S–S bond, a targeting ligand (rHSA-FA), a NIR fluorophore (IR780) and GOx. The IR780 molecules were released via cleavage of disulfide linker by GSH in cells, followed by sequential GSH-triggered catalysis for real-time tumor imaging and PTT&PDT.32
Also, Au@Pt NPs were successively modified with a mitochondrion-targeting triphenylphosphine (TPP) group, a cell-targeting ligand (FA), and a photosensitizer (Ce6), and they further worked as a platform for rapid uptake of multifunctional mesopores by mitochondria in cells.206 As confirmed by FL imaging, the as-formed Au@Pt NPs showed substantial enhancement in the PDT effect and conversion of laser radiation into heat, and thermally induced MCF-7 cell damage, in turn boosting therapeutic efficiency. Overall, the above nanosystems behaved as scalable phototherapeutic agents for significantly enhanced cancer therapy and molecular targets associated with disease progression.
As known, Au- and Ag-based HMNMs are most frequently used as SERS-active substrates, owing to their superior plasmonic properties, high chemical stability, and low cytotoxicity.170,209,211 For instance, alloyed Au/Ag NPs acted as a SERS substrate for quantitative analysis of endonuclease via modification with 4-thiophenylacetylene (4-TPA, as the internal standard molecule) and single-stranded DNA (ssDNA) carrying 3-[4-(phenylethynyl)benzylthio]propanoic acid (PEB, as the reporter molecule), as revealed in Fig. 6A–C.170 Briefly, PEB molecules were released upon exposure to endonuclease, and thus the associated Raman peak at 2215 cm−1 became weak, on account of breakage of the ssDNA. Simultaneously, Raman peak at 1983 cm−1 from 4-TPA almost remained constant, and thereby determination of endonuclease was achieved with a LOD of 0.056 unit mL−1, according to the ratio variations of the two peak intensities (I1983/I2215). Finally, SERS imaging was explored to monitor HeLa cells incubated with Au/Ag NPs during apoptosis, certifying the overexpression of endonucleases during the cell apoptotic process. In another case, Ag/Au alloy NPs were supported on SWCNTs initially assembled with azoalkynes, which were adopted for ratiometric SERS imaging of hypoxia with assistance of the Raman band from the alkyne and the 2D-band of SWCNTs.79
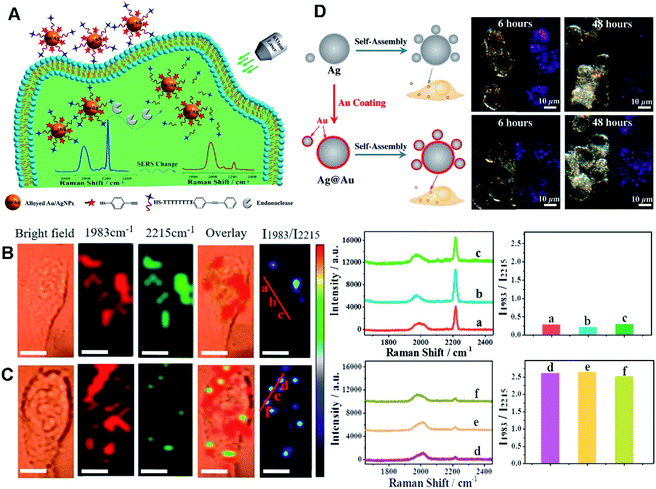 | ||
| Fig. 6 (A) Schematic diagram of DNA-alkyne-modified Au/Ag NPs for ratiometric SERS detection of endonuclease. SERS imaging of HeLa cells incubated with the nanoprobe before (B) and after (C) incubation with PEITC. I1983/I2215 Raman ratiometric images are presented in pseudocolor. Scale bar: 10 μm. SERS spectra and ratiometric peak were obtained from points (a–c) and (d–f). Reproduced with permission.170 Copyright 2018, American Chemical Society. (D) Schematic diagram of the assembly process for Ag core/satellites and Ag@Au core/satellites. The corresponding dark field image and SERS mapping image of different probes at different times. Reproduced with permission.209 Copyright 2017, American Chemical Society. | ||
In order to improve chemical stability, Ag nanospheres were efficiently coated with Au shells by a stoichiometric method, and their chemical stability was evaluated with NaSH, H2O2, and H2S gas.209 The Ag@Au core/satellites exhibited 3-fold enhancement in SERS intensity relative to Ag core/satellites, and highly improved stability for SERS imaging (Fig. 6D). Besides, Au NPs behaved as the core, bridging Raman reporter molecule 4-MBA and copper(II) carboxylate MOFs (Cu3(BTC)2) used as the shell for synthesis of core–shell Au@Cu3(BTC)2 NPs, showing feasible application in SERS imaging and chemo-phototherapy.211 Specifically, inner Au NPs worked as photothermal agents and SERS-active substrates, and the external Cu3(BTC)2 shell was further linked with a specific aptamer for cell-targeting and loaded with an anticancer drug such as DOX, finally showing an effective theranostic effect in chemo-PTT by a series of experiments.
For example, ultrasmall Au–Ag@BSA NPs were obtained by utilizing BSA as a template, and they showed high stability, good dispersity and biocompatibility, as well as low cytotoxicity in A549 and MCF-7 cells.25 Notably, the hybrid NPs with an Au/Ag molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 showed improved CT performance as a contrast agent in CT imaging of early-stage zebrafish embryos, surpassing Au NPs and iohexol. Inspired by the formation mechanism of gallstones, Au–Ag, Pt–Ag and Pd–Ag 3D supra-nanostructures were developed as contrast agents for medical CT imaging and radiosensitizers for image-guided radiation therapy.214 In another example, PEGylated Au@Pt nanodendrites were synthesized as CT contrast with significant enhancement in CT imaging signals and an improved theranostic agent for tumor therapy using PTT/RT.26
2 showed improved CT performance as a contrast agent in CT imaging of early-stage zebrafish embryos, surpassing Au NPs and iohexol. Inspired by the formation mechanism of gallstones, Au–Ag, Pt–Ag and Pd–Ag 3D supra-nanostructures were developed as contrast agents for medical CT imaging and radiosensitizers for image-guided radiation therapy.214 In another example, PEGylated Au@Pt nanodendrites were synthesized as CT contrast with significant enhancement in CT imaging signals and an improved theranostic agent for tumor therapy using PTT/RT.26
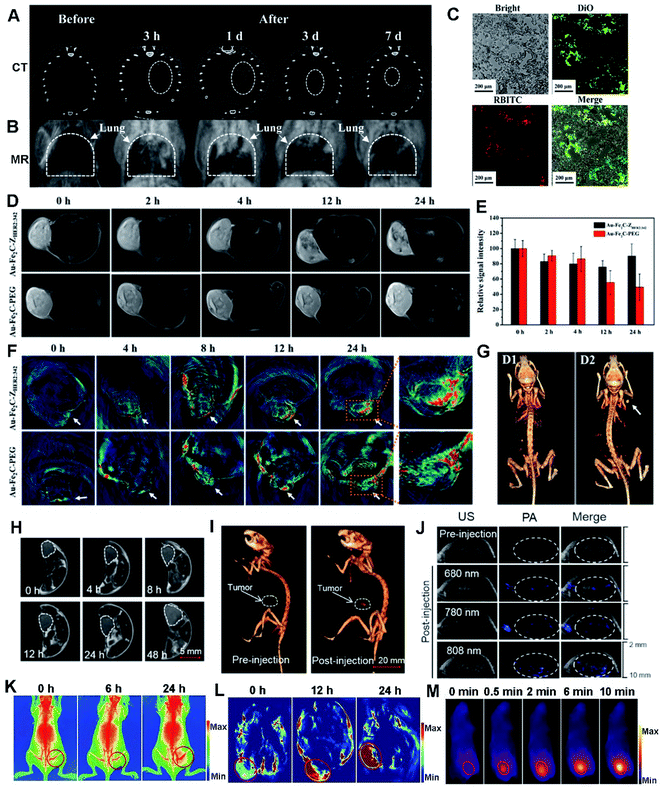 | ||
| Fig. 7 CT (A) and MR (B) images of the Au/GdNC@SiO2-labeled hMSCs in the lung of a pulmonary fibrosis mouse. (C) Fluorescence images of pulmonary frozen sections from the mouse. Reproduced with permission.80 Copyright 2020, American Chemical Society. (D) Real-time T2-weighted MR images of MDA-MB-231 tumor-bearing mice at different time points. (E) Relative MR intensity at different time points. (F) In vivo MSOT images of tumors in mice taken at different times. (G) 3D reconstructed CT images before (D1) and after (D2) the intratumor injection of Au–Fe2C–PEG JNPs. Reproduced with permission.56 Copyright 2017, American Chemical Society. (H) Tumoral accumulation of SeAuFe-EpC NPs within 48 h. (I) The 3D in vivo CT images of MCF-7 tumor-bearing mice before and after injection with SeAuFe-EpC NPs. (J) Representative photos of PA images of a mouse model before and after intravenous injection under different NIR wavelengths. Reproduced with permission.188 Copyright 2020, Elsevier. Triple-modal imaging of mice after injection with TPAN at indicated time points. X-ray images (K), PA images (L), and IR thermal images (M) of the marked tumor areas. Reproduced with permission.40 Copyright 2019, Wiley. | ||
Besides, PtCu3 nanocages first performed as a HRP-like enzyme for catalytic decomposition of H2O2 in acidic media for CDT, and behaved as a GSH-POD mimic to promote GSH depletion via oxidation and further inhibited GSH-induced ROS scavenging in human umbilical vein endothelial cells and 4T1 cells. Furthermore, the dual-functional PtCu3 nanocages worked as a sonosensitizer for cancer treatment guided by PA/CT dual-modal imaging under ultrasonication, together with minimal toxicity to normal tissues at therapeutic doses.63 In addition, pH-responsive FePt-based NPs were reported as a treatment agent for real-time dual-modality MR/CT imaging both in vivo and in vitro to monitor tumour therapy.86
To make the therapy process more accurate, subtle integration of PTT with multiple imaging techniques is regarded as an encouraging strategy to achieve precise cancer therapy by acquiring more comprehensive and accurate information, certifying the feasibility to examine accumulated photothermal agents in tumors, assess size and location of tumors, monitor the PTT treatment process and evaluate treatment efficiency.213,217 Accordingly, a large variety of theranostic HMNMs with multiple functions have been developed so far.20,56,218
Recently, Au–Fe2C JNPs were developed and they showed an excellent photothermal effect due to their broad absorption in the NIR region.56 By virtue of their superior magnetic and optical properties, Au–Fe2C JNPs are demonstrated to be an ideal reagent for triple-modal MR/MSOT/CT imaging (Fig. 7D–G). Later on, Au3Cu tetrapod nanocrystals (TPNCs) were synthesized and continuously functionalized with PEG, Cy5 and FA, termed as Au3Cu@PEG–Cy5–FA. The resultant composite displayed high photostability, penetrability of deep tissue and desired PCE, finally exhibiting excellent MSOT in the NIR-II range and FL imaging potential towards selective treatment of tumors.20
Meanwhile, magnetic/plasmonic hybrid HMNMs are very promising for multi-modal imaging. To prove it, a ternary heteronanostructure (SeAuFe-EpC) was synthesized and specifically targeted to a tumor area, achieving multi-modal imaging-guided breast tumor RT (Fig. 7H–J).188 The doped Se in SeAuFe-EpC NPs distinctly reduced the energy barrier, improved the electronic conductivity, facilitated the conversion of 3O2 to singlet oxygen (1O2), induced irreversible death of tumor cells and ultimately realized a synergistic anti-cancer effect, as visibly identified by three-modality imaging of in vivo CT, PA and MR. Likewise, AgPtalloy–Fe3O4 and Aucore@Pdshell–Fe3O4 were designed with good hydrophilicity, making them ideal for MR imaging, PA imaging, CT imaging, and OCT in biomedical applications.115
As a feature of tumors, hypoxia has severe adverse effects on the outcomes of tumor chemotherapy, RT or PTT.9,28,40,41 To conquer tumor hypoxia-induced RT tolerance, two-dimensional Pd@Au core–shell nanostructures (TPAN) were applied to stably produce O2 for a long time by catalysis of endogenous H2O2, and their catalytic properties can be improved by surface plasmon resonance (SPR) effect.40 By virtue of good photothermal efficiency and high NIR absorption, TPAN as a multi-modal imaging contrast gradually accumulated in tumor sites along with time variation due to enhanced permeability and retention (EPR) effect (Fig. 7K–M), as illustrated by X-ray, PA and NIR-II laser derived photothermal modality imaging, eventually achieving satisfactory therapeutic synergistic efficacy in vivo upon integration of radio and PTT. Overall, the above examples associated with precise cancer therapy are rational and feasible for promising clinic translation.
3.3 Therapy
Cancer is a group of diseases, and correlates to almost any part of human body, combined with inducing severe health problems and heavy social burdens.219 In general, most clinicians still depend on conventional RT, chemotherapy and other surgery-mediated techniques for high-efficiency treatment of malignancies.220 To date, many nanomaterial based therapeutic approaches have been developed for treatment of malignancies including PTT, PDT and CDT.19,28,63,77 Among them, a series of HMNM-based studies have turned from single-therapy to subtly integrate the above techniques, as the advantages of synergistic therapy strategies are certified.71,77,100,188,218 Notably, two types of lasers are usually used to visibly optimize PTT and PDT effects in combined PTT/PDT, as well as suitable HMNMs with high PCE.71,77For example, porous Au@Pt NPs were constructed and they showed strong absorption in the NIR section and high PCE. After further functionalization with cRGD peptide and DOX, the nanocomposite exhibited observable reduction in OSD and seriously inhibited tumor growth by chemo-photothermal co-therapy, integrated with alleviation of DOX-induced oxidative damage.29
Lately, a Pt@Au nanoring@DNA (denoted as PAD for simplicity) probe was developed for FL imaging guided targeting PTT, and it behaved as an excellent photosensitizer upon NIR light irradiation in tumor cells, due to strong absorption of Pt@Au nanorings.66 The dual PAD probe holds great promise towards in vitro diagnosis and therapy of cancer cells (activated by NIR light such as the activation of specific recognition and FL imaging along with targeted PTT). Likewise, hybrid FePt/SiO2/Au NPs were applied as a theranostic material for PTT and MR imaging of cancer urothelial (RT4) cells, by taking advantage of both magnetic and optical properties, which confirmed them as high efficiency and selective photo-thermal and MRI contrast agents, achieving highly selective and safe treatment of cancerous cells without any damage to healthy tissue.97
For example, biomimetic MnO2@PtCo nanoflowers were fabricated with catalase-like and oxidase-like properties, which effectively relieved hypoxic conditions and simultaneously induced cell death via the ROS-mediated mechanism, showing significant improvement in tumor therapy.82 Similarly, a bimetallic AuPt nanozyme was synthesized with help of silk fibroin, which effectively transformed adsorbed O2 and endogenous H2O2 into ˙O2− and ˙OH, respectively,216 finally inducing irreversible damage to tumor cells in tumor-bearing mice.
To increase the PDT efficacy, a photosensitizer-Pd@Pt nanoplates with catalase-like activity was developed by subsequent immobilization of PEG and Ce6 (termed Pd@Pt–PEG–Ce6), which delivered Ce6 to the cancer cells and efficiently converted H2O2 into O2 for the Pd@Pt based theranostic nanosystem, hence remarkably increasing the efficacy of PDT (Fig. 8A–C).41 In another example, a nanoplatform was built by loading ICG in cavity of porous Au@Rh–CM (Au@Rh–ICG–CM), which exhibited high-quality FL and PA imaging effects, in turn revealing extremely efficient PDT efficacy in tumor treatment (Fig. 8D–F).28 The successful examples not only facilitate advance of HMNMs in hypoxic tumor theranostics, but also offer some valuable guidelines for establishing other nanosystems in cancer therapy.
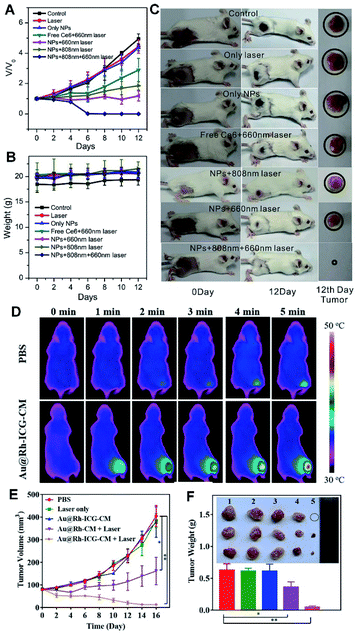 | ||
| Fig. 8 (A) Changes in the tumor volumes of mice after different treatments. (B) Changes in the weights of mice in different groups. (C) Tumor changes of mice before and after treatment. Reproduced with permission.41 Copyright 2018, Wiley. (D) Photothermal images of MDA-MB-231-tumor-bearing mice exposed to an 808 nm laser (0.3 W cm−2) for 5 min. (E) Size changes of MDA-MB-231 tumors in nude mice under different treatments. (F) Average weights of tumors and photos of tumors harvested at 16 d from treatment of PBS: Laser only, Au@Rh–ICG–CM, Au@Rh-CM + Laser, and Au@Rh–ICG–CM + Laser (*p < 0.05, **p < 0.01). Reproduced with permission.28 Copyright 2018, Wiley. | ||
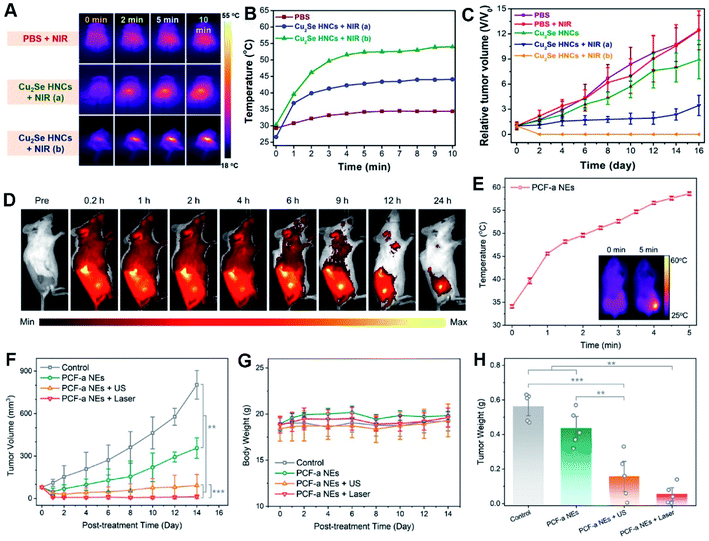 | ||
| Fig. 9 In vivo photothermal-enhanced CDT with PEG-Cu2Se HNCs. (A) IR thermal images and (B) temperature changes at the tumor sites of 4T1 tumor-bearing mice. (C) Relative tumor growth curves. Reproduced with permission.44 Copyright 2018, Wiley. (D) Fluorescence imaging of 4T1 tumor-bearing mice after intravenous injection of Cy5.5-labeled PCF-a NEs. (E) Temperature growth curve of the tumor site with PCF-a NEs under 808 nm laser irradiation. The inset shows the infrared thermal images. (F) Tumor growth curves. (G) Average body weights of mice. (H) Average weights of tumors (*p < 0.05, **p < 0.01, and ***p < 0.001). Reproduced with permission.21 Copyright 2021, American Chemical Society. | ||
As known, US can create highly concentrated shock waves by intense local vibration and yield more ROS due to acoustic cavitation effect, accompanied by boosting mass transfer of a nanozyme, in turn significantly accelerating a Fenton or Fenton-like reaction for CDT.21,232 Thereby, subtle integration of US with CDT onto the nanozyme can effectively prevent tumor growth and recurrence.21,235,236 For example, ultra-small PdCuFe alloy nanozymes (PCF-a NEs) were developed, which showed high PCE and competent GSH-Px-like and POD-like activity in circumneutral pH for highly enhanced synergistic CDT and PTT treatment within the NIR scope, exemplifying the high-efficiency tumor inhibition by the as-built artificial enzyme (Fig. 9D–H).21
The above examples demonstrate feasibility of utilizing HMNM-based nanozymes for highly specific tumor therapy enhanced by external stimuli, and then illustrate the correlations of respective compositions and structures with the properties of such nanozymes.
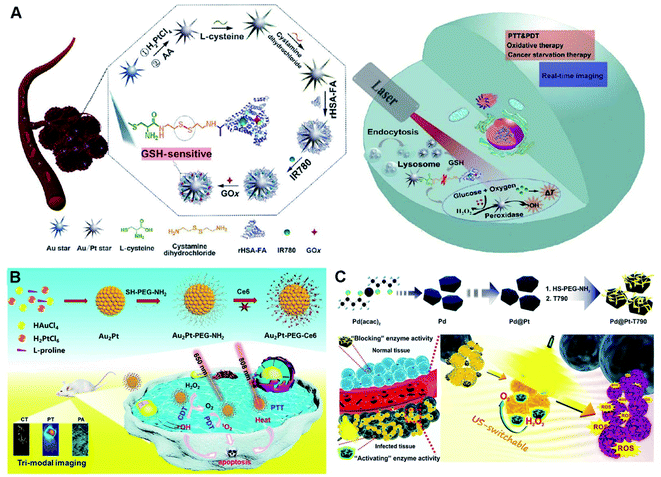 | ||
| Fig. 10 (A) Schematic diagram of the synthesis process and multifunctional application in vivo of the probes. Reproduced with permission.32 Copyright 2020, Springer. (B) Schematic diagram of the preparation of Au2Pt-PEG-Ce6 and multi-modal imaging-guided synergistic PTT/PDT/CDT. Reproduced with permission.218 Copyright 2020, Elsevier. (C) Schematic illustration of the synthesis process of the Pd@Pt-T790 nanoplatform and its US-switchable nanozyme catalytic enhanced SDT for bacterial infection. Reproduced with permission.33 Copyright 2020, American Chemical Society. | ||
Likewise, Au eyeball-like yolk–shell Au@Pd NPs were prepared with open-mouthed Pd shells and then immobilized with FA and Ce6, which displayed excellent photodynamic efficacy of Ce6 upon irradiation and high specificity of conjugated FA to target MCF-7 cancer cells in broad NIR window, eventually harvesting synergistic PDT&PTT inducing cell apoptosis.71
Although synergistic therapy demonstrates great promise for highly efficient cancer treatment, it is still a huge challenge to build a multi-functional nanosystem by a simple and high-efficiency strategy. For example, Au2Pt NPs with multiple functions were facilely synthesized at room temperature, followed by successive covalent linkage of SH–PEG–NH2 and Ce6 to transform O2 to 1O2, and they exhibited catalase-like ability to convert H2O2 to O2 for relaxation of tumor hypoxia and improvement in PDT efficiency, combined with the POD-like activity to produce ˙OH for CDT (Fig. 10B).218 Furthermore, the Au2Pt-PEG-Ce6 with dual enzyme-like properties provided possibility of PA and photothermal guided NIR-responsive PTT as a photothermal transition agent, owing to high PCE in the NIR region. In addition, the Au2Pt-PEG-Ce6 nanoformulation acted as a contrast agent for CT imaging. Overall, the multifunctional platform presented great potential in multimodal imaging-guided synergistic PTT/PDT/CDT with remarkable tumor specificity and enhanced therapy of tumors.
To date, NIR laser-mediated PTT has received tremendous attention as a non-invasive therapy to directly kill tumor cells and improve the tumor hypoxic microenvironment, where many HMNMs have been applied as high-efficiency photothermal agents in the PTT of cancers.66,97 However, such agents hardly completely ablate tumors, particularly deep-seated cancers, owing to insufficient absorption efficiency of natural tissue absorbents.9,26 For effective cancer theranostics, several examples confirm effectiveness of combining PTT with other therapeutic techniques such as RT in a single platform.9,26
Quan et al. reported Cys-coated FePd nanodots with a high absorption located at 1064 nm, which produced effective hyperthermia (35.4%) with an improved radiation effect, combined by working as a contrast agent for CT/MR/PA imaging.9 Besides, PEGylated Au@Pt nanodendrites were constructed as an improved theranostic agent for sharply enhanced synergistic PTT/RT therapy and CT imaging in the NIR scope relative to single RT or PTT.26 As a result, the integration of Au@Pt nanodendrites improved RT with PTT, and more effectively suppressed the growth of cancer cells in this research.
However, the limited penetration depth severely restricts their extensive PDT application, especially in deeply located cancers.33 Fortunately, recently emerged SDT, as a type of ROS-based noninvasive therapeutic modality, shows effective treatment of deep-seated tumors triggered by US.63,238 For example, an US-switchable nanozyme is desirable to strengthen SDT against deep-seated bacterial infection by producing catalytic oxygen (Fig. 10C).33 As shown, a hybrid nanoplatform was built by modifying Pd@Pt nanoplates with organic sonosensitizer T790 (Pd@Pt-T790). Such a modification severely inhibited the catalase-like activity of the as-formed nanoenzyme, which was recovered upon US irradiation. By virtue of this US-switchable enzyme, the Pd@Pt-T790-based nanotherapeutic bioplatform completely eradicated the myositis caused by MRSA in vitro and in vivo upon US irradiation, accompanied by supervising the SDT progress through PA and MR imaging.
Also, CDT appears as another kind of ROS-derived therapeutic technique for effective treatment of deep-seated tumors, by subtly exploiting two characteristics, namely acidity and overexpressed H2O2 in internal complicated tumor microenvironment without any external stimulus.63 To improve the effect of CDT-enhanced SDT, PtCu3 nanocages were developed as a sonosensitizer to yield ROS under US irradiation in 4T1-bearing mice (Fig. 11).63 Importantly, PtCu3 nanocages functioned as a HRP-like nanozyme to catalyze H2O2 by forming ˙OH for CDT, and simultaneously worked as a GSH-Px like enzyme to accelerate GSH depletion, thus weakening the ability of GSH to eliminate ROS. More importantly, the PtCu3 cages have the capacity to realize effective CDT-enhanced SDT via GSH elimination, along with PA/CT multi-modal imaging of tumor-bearing mice.
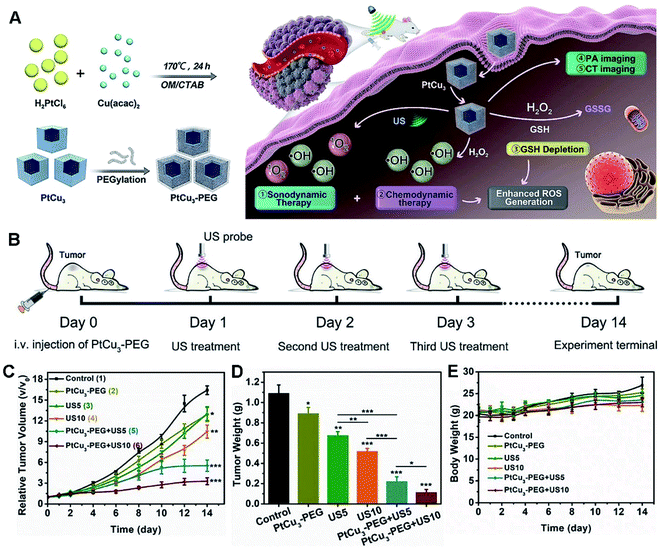 | ||
| Fig. 11 (A) Schematic illustration of PA/CT dual-modal imaging guided cancer CDT and GSH depletion enhanced SDT by PtCu3 nanocages. (B) Schematic diagram of the treatment process for SDT mediated by PtCu3-PEG in a 4T1 tumor model. (C) Tumor growth curves of mice treated with different ways. (D) Tumor weights at the end (*p < 0.05, **p < 0.01, ***p < 0.001). (E) Body weight changes during the treatment. Reproduced with permission.63 Copyright 2019, Wiley. | ||
3.4 Others
For instance, a triM nanozyme was developed with high multiple catalytic activities and environmental selectivity, showing significant preference for effective elimination of ROS and reactive nitrogen species (RNS) in neutral media (Fig. 12).244 As shown by in vitro experiments, the triM nanozyme clearly boosted viability of destructed nerve cells. Meanwhile, lipid peroxidation and superoxide dismutase (SOD) activity were greatly recovered upon treatment with the triM nanozyme in an in vivo test.
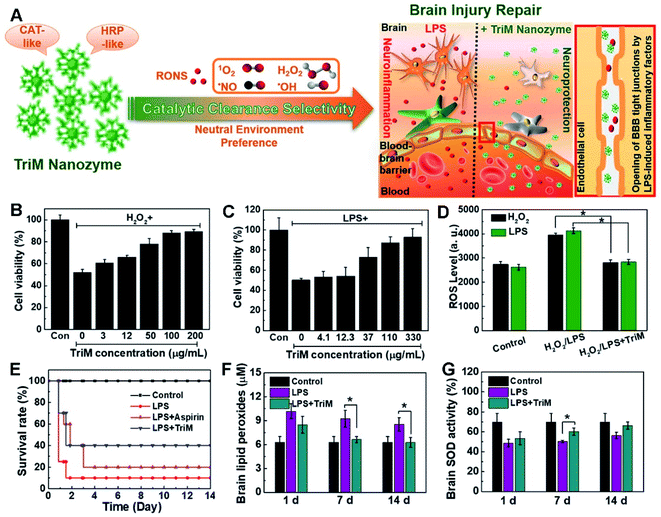 | ||
| Fig. 12 (A) Schematic illustration of the brain injury repair mechanism of the triM nanozyme. Cell viability of (B) H2O2- or (C) LPS-treated N2a cells in the presence of trim nanozymes. (D) Quantitative analyses of ROS levels with the triM nanozyme treatment in H2O2- or LPS-stimulated N2a cells. (E) Survival curves of mice with or without treatment of triM nanozymes. (F) Lipid peroxides and (G) SOD activity assays in the brain. Reproduced with permission.244 Copyright 2019, American Chemical Society. | ||
Among them, Ag NPs have been extensively investigated due to their high antibacterial efficacy.18,245 However, their poor stability and serious cytotoxicity for mammalian cells seriously confine their further applications.247 To overcome the present bottlenecks, HMNMs are regarded as good alternatives owing to the superior bio-compatibility and antibacterial properties, making them suitable for therapeutic applications.27,34,189,247 Importantly, carbohydrate-coated Au–Ag NPs were minimally toxic to mammalian cells, but showed more toxicity to MDR Enterobacter cloacae and Escherichia coli than most potent antibiotics.247 Interesting, in vivo results showed enhanced efficiency of elimination of MDR MRSA in mice skin wounds by Au–Ag NPs compared to gentamicin, eventually promoting fast healing of the infected wounds.
Similarly, Ag+ ions were efficiently released by oxidative etching from the external Ag shell in Au/Ag NRs, which were in situ noninvasively monitored by PA imaging.27 Moreover, the hybrid particles showed strong bactericidal efficacy by killing over 99.99% of both methicillin-resistant MRSA (32 μM Ag+ equivalent) and Escherichia coli (8 μM Ag+ equivalent) for high-efficiency coalescence of MRSA skin infections. These nanosystems provide promising tools with on-demand antimicrobial and self-reporting abilities for imaging and therapy of infectious diseases in vivo.
Beyond the above, Fe–Cu NPs were prepared and subsequently impregnated onto cotton cloth, which showed strong antimicrobial activity for MDR MRSA, Gram positive/negative bacteria, and fungus in humans, coupled with good biocompatibility and broad-spectrum antimicrobial properties.248 Importantly, the hybrid composite displayed good wound healing ability by inhibiting microbial growth of several types of microbes and found feasibility for treatment of Wistar albino rats with infected diabetic wounds (DW), as evidenced by in vivo tests (Fig. 13).
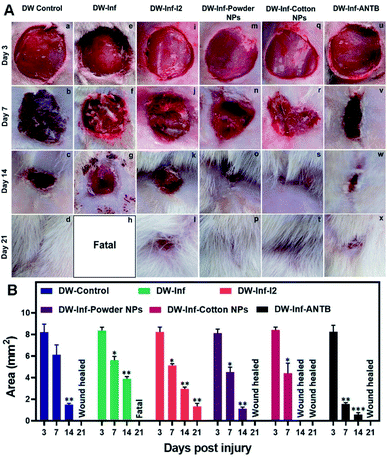 | ||
| Fig. 13 (A) Photographs representing the in vivo study on the effects of treatment with the prepared wound healing materials on MRSA infection-induced DW in rats. (a–d) Untreated simple DW. (e–h) Untreated infected DW. (i–l) Infected DW treated with iodine. (m–p) Infected DW treated with the powder nanocomposite. (q–t) Infected DW treated with a nanocomposite impregnated cotton swab. (u–x) Infected DW treated with a topical antibiotic. (B) Results of wound area measurement done at 3, 7, 14, and 21 days after infliction of injury. Reproduced with permission.248 Copyright 2019, American Chemical Society. | ||
4. Conclusions and prospects
In summary, HMNMs and derived nanomaterials show high improvement in physicochemical properties by finely modulating microstructural parameters (e.g. size, shape, composition, structure, and surface modification) to regulate the spatial arrangement patterns and local electronic structures in scalable synthesis, combined with exploring their potential applications in clinical practice, as supported by the collected research. The attractive activities of HMNMs endow them with wider biomedical applications than that of MMNMs in the above summaries, which is mainly attributed to the aforementioned synergistic effects between different metals.Although substantial developments have been already made in the aforementioned HMNMs (frequently prepared by trial-and-error methods), their theoretical design and controllable synthesis even at the atomic level are always required for building reliable clinical devices to overcome new issues emerging in practice. Besides, the long-term biological safety, large-scale production, quality control, and insufficient knowledge related to pharmacokinetics are still issues in potential biomedical applications. Further, theoretical studies based on density functional theory are urgent yet challenging to have in-depth cognition of the interfacial interactions between HMNMs and biomolecules. There is no doubt that the era of controlled synthesis and biomedical applications of HMNMs is coming.
Author contributions
Shan–Shan Li: investigation, methodology, writing – original draft, funding acquisition. Ai-Jun Wang: investigation, methodology. Pei-Xin Yuan, Li-Ping Mei, and Lu Zhang: investigation. Jiu-Ju Feng: writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Abbreviations
| HMNMs | Heterometallic nanomaterials |
| MMNMs | Monometallic nanomaterials |
| NPs | Nanoparticles |
| TPNCs | Tetrapod nanocrystals |
| PTT | Photothermal therapy |
| NIR | Near-infrared |
| NIR-II | Second near-infrared |
| CDT | Chemodynamic therapy |
| BNCs | Bimetallic nanoclusters |
| ECL | Electrochemiluminescence |
| CA | Carbohydrate antigen |
| BSA | Bovine serum albumin |
| rBSA | Reduced bovine serum albumin |
| CT | Computed tomography |
| PA | Photoacoustic |
| T790 | meso-Tetra(4-carboxyphenyl)porphine |
| US | Ultrasound |
| SDT | Sonodynamic therapy |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| TPAN | Two-dimensional Pd@Au core–shell nanostructure |
| SPR | Surface plasmon resonance |
| RT | Radiotherapy |
| HNCs | Hollow nanocubes |
| PCE | Photothermal conversion efficiency |
| PCF-a NEs | PdCuFe alloy nanozymes |
| POD | Peroxidase |
| cTnI | Cardiac troponin I |
| JNPs | Janus nanoparticles |
| SERS | Surface enhanced Raman scattering |
| OTA | Ochratoxin A |
| TEM | Transmission electron microscope |
| HR-TEM | High-resolution TEM |
| HAADF | High-angle annular dark field |
| STEM | Scanning transmission electron microscope |
| SEM | Scanning electron microscope |
| CM | Cell membrane |
| DOX | Doxorubicin |
| OSD | Oxidative stress damage |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ROS | Reactive oxygen species |
| GOx | Glucose oxidase |
| HTNs | Hollow nanotriangles |
| PAD | Pt@Au nanoring@DNA |
| SH-PEG | Thiolated polyethylene glycol |
| HRP | Horseradish peroxidase |
| GSH-Px | Glutathione peroxidase |
| FA | Folic acid |
| Ce6 | Chlorin e6 |
| MSOT | Multispectral photoacoustic tomography |
| 4-MBA | 4-Mercaptobenzoicacid |
| dsDNA | Double-stranded DNA |
| CTCs | Circulating tumor cells |
| GSH | Glutathione |
| Cys | Cysteine |
| PBS | Phosphate buffer solution |
| PDT | Photodynamic therapy |
| MOFs | Metal organic frameworks |
| CEA | Carcinoembryonic antigen |
| PSA | Prostate antigen |
| AFP | Alpha fetoprotein |
| rGO | Reduced graphene oxide |
| GO | Graphene oxide |
| C3N4 | Graphitic carbon nitride |
| GQDs | Nitrogen-doped graphene quantum dots |
| OMC | Ordered mesoporous carbon |
| HAC | High-active carbon |
| CB | Carbon black |
| GDY | Graphdiyne |
| SWCNT | Single-walled carbon nanotube |
| Thi | Thionine |
| MSNs | Mesoporous silica nanoparticles |
| MR | Magnetic resonance |
| ctDNA | Circulating tumor DNA |
| hMSCs | Human mesenchymal stem cells |
| IgG | Immunoglobulin G |
| NSE | Neuron-specific enolase |
| SCCA | Squamous cell carcinoma antigen |
| CYSC | Cystatin C |
| PEC | Photoelectrochemical |
| ALP | Alkaline phosphatase |
| PCN-223-Fe | Iron-porphyrinic metal–organic framework |
| PRL | Prolactin |
| LAG-3 | Lymphocyte activation gene-3 |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
| CFP-10 | Anticulture filtrate protein-10 |
| CK-MB | Creatine kinase-MB |
| HE4 | Human epididymis protein 4 |
| HER2 | Human epidermal growth factor receptor 2 |
| NT-proBNP | N-Terminal pro-B-type natriuretic peptide |
| PCT | Procalcitonin |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| miRNAs | MicroRNAs |
| LOD | Limit of detection |
| FL | Fluorescence |
| LSPR | Localized surface plasmon resonance |
| SPR | Surface plasmon resonance |
| 2PPL | Two-photon photoluminescence |
| AFM | Atomic force microscopy |
| uPA | Urokinase-type plasminogen activator |
| MMP | Matrix metalloproteinase |
| 5-TAMRA | 5-Aminomethyl Rhodamine |
| FITC | Fluorescein isothiocyanate |
| LPS | Lipopolysaccharide |
| TPP | Triphenylphosphine |
| 4-TPA | 4-Thiophenylacetylene |
| ssDNA | Single-stranded DNA |
| PEB | 3-[4-(Phenylethynyl)benzylthio]propanoic acid |
| PEITC | 2-Phenethyl isothiocyanate |
| Cu3(BTC)2 | Copper(II) carboxylate MOFs |
| ICG | Indocyanine green |
| OCT | Optical coherence tomography |
| EPR | Enhanced permeability and retention |
| RNS | Reactive nitrogen species |
| triM | Trimetallic |
| SOD | Superoxide dismutase |
| MDR | Multidrug resistant |
| DW | Diabetic wounds |
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22004076) and the Natural Science Foundation of Shandong Province (ZR2020QB100).Notes and references
- C. Gao, F. Lyu and Y. Yin, Chem. Rev., 2021, 121, 834–881 CrossRef CAS PubMed.
- U. B. Kim, D. J. Jung, H. J. Jeon, K. Rathwell and S.-g. Lee, Chem. Rev., 2020, 120, 13382–13433 CrossRef CAS PubMed.
- Y. Liu, J. Li, M. Chen, X. Chen and N. Zheng, Theranostics, 2020, 10, 10057–10074 CrossRef CAS PubMed.
- Z. Fan and H. Zhang, Chem. Soc. Rev., 2016, 45, 63–82 RSC.
- K. D. Gilroy, A. Ruditskiy, H.-C. Peng, D. Qin and Y. Xia, Chem. Rev., 2016, 116, 10414–10472 CrossRef CAS PubMed.
- C.-L. Yang, L.-N. Wang, P. Yin, J. Liu, M.-X. Chen, Q.-Q. Yan, Z.-S. Wang, S.-L. Xu, S.-Q. Chu, C. Cui, H. Ju, J. Zhu, Y. Lin, J. Shui and H.-W. Liang, Science, 2021, 374, 459–464 CrossRef CAS PubMed.
- M. Zhou, C. Li and J. Fang, Chem. Rev., 2021, 121, 736–795 CrossRef CAS PubMed.
- Y. Wang, L. Cao, N. J. Libretto, X. Li, C. Li, Y. Wan, C. He, J. Lee, J. Gregg, H. Zong, D. Su, J. T. Miller, T. Mueller and C. Wang, J. Am. Chem. Soc., 2019, 141, 16635–16642 CrossRef CAS PubMed.
- M. Lyu, D. Zhu, Y. Duo, Y. Li and H. Quan, Biomaterials, 2020, 233, 119656 CrossRef PubMed.
- L. Wang, A. Holewinski and C. Wang, ACS Catal., 2018, 8, 9388–9398 CrossRef CAS.
- M. Wang, L. Wang, H. Li, W. Du, M. U. Khan, S. Zhao, C. Ma, Z. Li and J. Zeng, J. Am. Chem. Soc., 2015, 137, 14027–14030 CrossRef CAS PubMed.
- K. Loza, M. Heggen and M. Epple, Adv. Funct. Mater., 2020, 30, 1909260 CrossRef CAS.
- M. Zhao, Z. D. Hood, M. Vara, K. D. Gilroy, M. Chi and Y. Xia, ACS Nano, 2019, 13, 7241–7251 CrossRef CAS PubMed.
- Z. Lyu, S. Zhu, L. Xu, Z. Chen, Y. Zhang, M. Xie, T. Li, S. Zhou, J. Liu, M. Chi, M. Shao, M. Mavrikakis and Y. Xia, J. Am. Chem. Soc., 2021, 143, 149–162 CrossRef CAS PubMed.
- W. Niu, Y. A. A. Chua, W. Zhang, H. Huang and X. Lu, J. Am. Chem. Soc., 2015, 137, 10460–10463 CrossRef CAS PubMed.
- D. Zhang, B. Gökce and S. Barcikowski, Chem. Rev., 2017, 117, 3990–4103 CrossRef CAS PubMed.
- B. Unnikrishnan, C.-W. Lien, H.-W. Chu and C.-C. Huang, J. Hazard. Mater., 2021, 401, 123397 CrossRef CAS PubMed.
- P. Makvandi, C.-y. Wang, E. N. Zare, A. Borzacchiello, L.-n. Niu and F. R. Tay, Adv. Funct. Mater., 2020, 30, 1910021 CrossRef CAS.
- F. He, H. Ji, L. Feng, Z. Wang, Q. Sun, C. Zhong, D. Yang, S. Gai, P. Yang and J. Lin, Biomaterials, 2021, 264, 120453 CrossRef CAS PubMed.
- Z. Wang, Y. Ju, S. Tong, H. Zhang, J. Lin, B. Wang and Y. Hou, Nanoscale Horiz., 2018, 3, 624–631 RSC.
- D. Jana, D. Wang, A. K. Bindra, Y. Guo, J. Liu and Y. Zhao, ACS Nano, 2021, 15, 7774–7782 CrossRef CAS PubMed.
- L. Zhang, L. T. Roling, X. Wang, M. Vara, M. Chi, J. Liu, S.-I. Choi, J. Park, J. A. Herron, Z. Xie, M. Mavrikakis and Y. Xia, Science, 2015, 349, 412 CrossRef CAS PubMed.
- M. Chen, J. P. Liu and S. Sun, J. Am. Chem. Soc., 2004, 126, 8394–8395 CrossRef CAS PubMed.
- L. Fu, X. Gao, S. Dong, H.-Y. Hsu and G. Zou, Anal. Chem., 2021, 93, 4909–4915 CrossRef CAS PubMed.
- Z. Chu, L. Chen, X. Wang, Q. Yang, Q. Zhao, C. Huang, Y. Huang, D.-P. Yang and N. Jia, ACS Biomater. Sci. Eng., 2019, 5, 1005–1015 CrossRef CAS PubMed.
- X. Liu, X. Zhang, M. Zhu, G. Lin, J. Liu, Z. Zhou, X. Tian and Y. Pan, ACS Appl. Mater. Interfaces, 2017, 9, 279–285 CrossRef CAS PubMed.
- T. Kim, Q. Zhang, J. Li, L. Zhang and J. V. Jokerst, ACS Nano, 2018, 12, 5615–5625 CrossRef CAS PubMed.
- J. Wang, J. Sun, W. Hu, Y. Wang, T. Chou, B. Zhang, Q. Zhang, L. Ren and H. Wang, Adv. Mater., 2020, 32, 2001862 CrossRef CAS PubMed.
- Q. Yang, J. Peng, Y. Xiao, W. Li, L. Tan, X. Xu and Z. Qian, ACS Appl. Mater. Interfaces, 2018, 10, 150–164 CrossRef CAS PubMed.
- Y. Jia, Y. Li, S. Zhang, P. Wang, Q. Liu and Y. Dong, Biosens. Bioelectron., 2020, 149, 111842 CrossRef CAS PubMed.
- W. Fang, Z. Cheng, Y. Yang, A. Shen and J. Hu, Appl. Mater. Today, 2019, 15, 252–262 CrossRef.
- A. Zhang, Q. Zhang, G. Alfranca, S. Pan, Z. Huang, J. Cheng, Q. Ma, J. Song, Y. Pan, J. Ni, L. Ma and D. Cui, Nano Res., 2020, 13, 160–172 CrossRef CAS.
- D. Sun, X. Pang, Y. Cheng, J. Ming, S. Xiang, C. Zhang, P. Lv, C. Chu, X. Chen, G. Liu and N. Zheng, ACS Nano, 2020, 14, 2063–2076 CrossRef CAS PubMed.
- Y. Peng, Y. Liu, X. Lu, S. Wang, M. Chen, W. Huang, Z. Wu, G. Lu and L. Nie, J. Mater. Chem. B, 2018, 6, 2813–2820 RSC.
- L. Fan, Y. Yan, B. Guo, M. Zhao, J. Li, X. Bian, H. Wu, W. Cheng and S. Ding, Sens. Actuators, B, 2019, 296, 126697 CrossRef CAS.
- X.-Y. Ge, Y.-G. Feng, S.-Y. Cen, A.-J. Wang, L.-P. Mei, X. Luo and J.-J. Feng, Anal. Chim. Acta, 2021, 1176, 338750 CrossRef CAS PubMed.
- P. Song, L.-L. He, A.-J. Wang, L.-P. Mei, S.-X. Zhong, J.-R. Chen and J.-J. Feng, J. Mater. Chem. A, 2015, 3, 5321–5327 RSC.
- S.-S. Li, A.-J. Wang, Y.-Y. Hu, K.-M. Fang, J.-R. Chen and J.-J. Feng, J. Mater. Chem. A, 2014, 2, 18177–18183 RSC.
- S.-S. Li, J.-J. Lv, Y.-Y. Hu, J.-N. Zheng, J.-R. Chen, A.-J. Wang and J.-J. Feng, J. Power Sources, 2014, 247, 213–218 CrossRef CAS.
- Y. Yang, M. Chen, B. Wang, P. Wang, Y. Liu, Y. Zhao, K. Li, G. Song, X.-B. Zhang and W. Tan, Angew. Chem., Int. Ed., 2019, 58, 15069–15075 CrossRef CAS PubMed.
- J. Wei, J. Li, D. Sun, Q. Li, J. Ma, X. Chen, X. Zhu and N. Zheng, Adv. Funct. Mater., 2018, 28, 1706310 CrossRef.
- Y. Shi, Z. Lyu, M. Zhao, R. Chen, Q. N. Nguyen and Y. Xia, Chem. Rev., 2021, 121, 649–735 CrossRef CAS PubMed.
- T.-H. Yang, J. Ahn, S. Shi, P. Wang, R. Gao and D. Qin, Chem. Rev., 2021, 121, 796–833 CrossRef CAS PubMed.
- X. Wang, X. Zhong, H. Lei, Y. Geng, Q. Zhao, F. Gong, Z. Yang, Z. Dong, Z. Liu and L. Cheng, Chem. Mater., 2019, 31, 6174–6186 CrossRef CAS.
- Z.-P. Wu, S. Shan, S.-Q. Zang and C.-J. Zhong, Acc. Chem. Res., 2020, 53, 2913–2924 CrossRef CAS PubMed.
- X.-Y. Wang, Y. Chen, L.-P. Mei, A.-J. Wang, P.-X. Yuan and J.-J. Feng, Sens. Actuators, B, 2020, 315, 128088 CrossRef CAS.
- Y. Chen, L.-P. Mei, J.-J. Feng, P.-X. Yuan, X. Luo and A.-J. Wang, Biosens. Bioelectron., 2019, 145, 111638 CrossRef CAS PubMed.
- A. J. McGrath, Y.-H. Chien, S. Cheong, D. A. J. Herman, J. Watt, A. M. Henning, L. Gloag, C.-S. Yeh and R. D. Tilley, ACS Nano, 2015, 9, 12283–12291 CrossRef CAS PubMed.
- X.-Y. Wang, Y.-G. Feng, A.-J. Wang, L.-P. Mei, P.-X. Yuan, X. Luo and J.-J. Feng, Sens. Actuators, B, 2021, 331, 129460 CrossRef CAS.
- S.-Y. Cen, Y.-G. Feng, J.-H. Zhu, X.-Y. Wang, A.-J. Wang, X. Luo and J.-J. Feng, Sens. Actuators, B, 2021, 333, 129573 CrossRef CAS.
- Y. Chen, X.-Y. Wang, A.-J. Wang, L.-P. Mei, P.-X. Yuan, X. Luo and J.-J. Feng, Sens. Actuators, B, 2021, 326, 128794 CrossRef CAS.
- Y. Chen, A.-J. Wang, P.-X. Yuan, X. Luo, Y. Xue and J.-J. Feng, Biosens. Bioelectron., 2019, 132, 294–301 CrossRef CAS PubMed.
- G. Loget, D. Zigah, L. Bouffier, N. Sojic and A. Kuhn, Acc. Chem. Res., 2013, 46, 2513–2523 CrossRef CAS PubMed.
- X. Zhang, Q. Fu, H. Duan, J. Song and H. Yang, ACS Nano, 2021, 15, 6147–6191 CrossRef CAS PubMed.
- M.-J. Zhu, J.-B. Pan, Z.-Q. Wu, X.-Y. Gao, W. Zhao, X.-H. Xia, J.-J. Xu and H.-Y. Chen, Angew. Chem., Int. Ed., 2018, 57, 4074–4078 CrossRef.
- Y. Ju, H. Zhang, J. Yu, S. Tong, N. Tian, Z. Wang, X. Wang, X. Su, X. Chu, J. Lin, Y. Ding, G. Li, F. Sheng and Y. Hou, ACS Nano, 2017, 11, 9239–9248 CrossRef CAS PubMed.
- F. Zheng, W. Ke, L. Shi, H. Liu and Y. Zhao, Anal. Chem., 2019, 91, 11812–11820 CrossRef CAS PubMed.
- Y. Su, Q. Zhang, X. Miao, S. Wen, S. Yu, Y. Chu, X. Lu, L.-P. Jiang and J.-J. Zhu, ACS Appl. Mater. Interfaces, 2019, 11, 41979–41987 CrossRef CAS PubMed.
- Y. Feng, Y. Cheng, Y. Chang, H. Jian, R. Zheng, X. Wu, K. Xu, L. Wang, X. Ma, X. Li and H. Zhang, Biomaterials, 2019, 217, 119327 CrossRef CAS PubMed.
- S. Ke, C. Kan, X. Zhu, C. Wang, X. Wang, Y. Chen, X. Zhu, Z. Li and D. Shi, CrystEngComm, 2021, 23, 3467–3476 RSC.
- F. Tao, M. E. Grass, Y. Zhang, D. R. Butcher, J. R. Renzas, Z. Liu, J. Y. Chung, B. S. Mun, M. Salmeron and G. A. Somorjai, Science, 2008, 322, 932 CrossRef CAS PubMed.
- J. Kang, J. Joo, E. J. Kwon, M. Skalak, S. Hussain, Z.-G. She, E. Ruoslahti, S. N. Bhatia and M. J. Sailor, Adv. Mater., 2016, 28, 7962–7969 CrossRef CAS PubMed.
- X. Zhong, X. Wang, L. Cheng, Y. a. Tang, G. Zhan, F. Gong, R. Zhang, J. Hu, Z. Liu and X. Yang, Adv. Funct. Mater., 2020, 30, 1907954 CrossRef CAS.
- Y. Chen, P.-X. Yuan, A.-J. Wang, X. Luo, Y. Xue, L. Zhang and J.-J. Feng, Biosens. Bioelectron., 2019, 126, 187–192 CrossRef CAS PubMed.
- A.-J. Wang, X.-Y. Zhu, Y. Chen, P.-X. Yuan, X. Luo and J.-J. Feng, Sens. Actuators, B, 2019, 288, 721–727 CrossRef CAS.
- H. Zhang, Y. Wang, H. Zhong, J. Li and C. Ding, ACS Appl. Bio Mater., 2019, 2, 5012–5020 CrossRef CAS PubMed.
- M. Xu, Q. Lu, Y. Song, L. Yang, C. Ren, W. Li, P. Liu, Y. Wang, Y. Zhu and N. Li, Nano Res., 2020, 13, 2118–2129 CrossRef CAS.
- B. Liu and J. J. N. R. Liu, Nano Res., 2017, 10, 1125–1148 CrossRef CAS.
- S.-S. Li, Q.-Y. Guan, G. Meng, X.-F. Chang, J.-W. Wei, P. Wang, B. Kang, J.-J. Xu and H.-Y. Chen, Sci. Rep., 2017, 7, 2296 CrossRef PubMed.
- S. S. Li, Q. Y. Guan, M. M. Zheng, Y. Q. Wang, D. J. Ye, B. Kang, J. J. Xu and H. Y. Chen, Chem. Sci., 2017, 8, 7582–7587 RSC.
- X. Cai, S. Ding, Q. Shi, Z. Lyu, D. Liu, W.-j. Dong, M. Du, P. Dutta, Y. Song, D. Du and Y. Lin, ACS Appl. Bio Mater., 2020, 3, 5922–5929 CrossRef CAS PubMed.
- S.-S. Li, M. Zhang, J.-H. Wang, F. Yang, B. Kang, J.-J. Xu and H.-Y. Chen, Anal. Chem., 2019, 91, 8398–8405 CrossRef CAS PubMed.
- S.-S. Li, Y.-Y. Tan, Y. Zhang, M. Liu and A. Liu, Bioelectrochemistry, 2021, 140, 107804 CrossRef CAS PubMed.
- J. Chang, A. Zhang, Z. Huang, Y. Chen, Q. Zhang and D. Cui, Talanta, 2019, 198, 45–54 CrossRef CAS PubMed.
- Y. Dong, C. Yao, Y. Zhu, L. Yang, D. Luo and D. Yang, Chem. Rev., 2020, 120, 9420–9481 CrossRef CAS PubMed.
- X. Zhou, Q. Pu, H. Yu, Y. Peng, J. Li, Y. Yang, H. Chen, Y. Weng and G. Xie, J. Colloid Interface Sci., 2021, 599, 752–761 CrossRef CAS PubMed.
- P. P. Jia, H. J. Ji, S. K. Liu, R. Zhang, F. He, L. Zhong and P. P. Yang, J. Mater. Chem. B, 2021, 9, 101–111 RSC.
- M. Chen, D. Wu, S. Tu, C. Yang, D. Chen and Y. Xu, Biosens. Bioelectron., 2021, 173, 112821 CrossRef CAS PubMed.
- X. Qin, Y. Si, D. Wang, Z. Wu, J. Li and Y. Yin, Anal. Chem., 2019, 91, 4529–4536 CrossRef CAS PubMed.
- J. Huang, J. H. Huang, H. Bao, X. Ning, C. Yu, Z. Chen, J. Chao and Z. Zhang, ACS Appl. Bio Mater., 2020, 3, 2489–2498 CrossRef CAS PubMed.
- X.-H. Pham, E. Hahm, T. H. Kim, H.-M. Kim, S. H. Lee, S. C. Lee, H. Kang, H.-Y. Lee, D. H. Jeong, H. S. Choi and B.-H. Jun, Nano Res., 2020, 13, 3338–3346 CrossRef CAS.
- Z. Wang, Y. Zhang, E. Ju, Z. Liu, F. Cao, Z. Chen, J. Ren and X. Qu, Nat. Commun., 2018, 9, 3334 CrossRef PubMed.
- R. Wang, J.-J. Feng, Y. Xue, L. Wu and A.-J. Wang, Sens. Actuators, B, 2018, 254, 1174–1181 CrossRef CAS.
- E. Ko, V.-K. Tran, S. E. Son, W. Hur, H. Choi and G. H. Seong, Sens. Actuators, B, 2019, 294, 166–176 CrossRef CAS.
- J. Feng, Y. Li, M. Li, F. Li, J. Han, Y. Dong, Z. Chen, P. Wang, H. Liu and Q. Wei, Biosens. Bioelectron., 2017, 91, 441–448 CrossRef CAS PubMed.
- L. Yue, J. Wang, Z. Dai, Z. Hu, X. Chen, Y. Qi, X. Zheng and D. Yu, Bioconjugate Chem., 2017, 28, 400–409 CrossRef CAS PubMed.
- G. Darabdhara, B. Sharma, M. R. Das, R. Boukherroub and S. Szunerits, Sens. Actuators, B, 2017, 238, 842–851 CrossRef CAS.
- Y. Yang, Q. Liu, Y. Liu, J. Cui, H. Liu, P. Wang, Y. Li, L. Chen, Z. Zhao and Y. Dong, Biosens. Bioelectron., 2017, 90, 31–38 CrossRef CAS PubMed.
- S. C. Barman, M. F. Hossain, H. Yoon and J. Y. Park, Biosens. Bioelectron., 2018, 100, 16–22 CrossRef CAS PubMed.
- W. Dong, Y. Ren, Z. Bai, Y. Yang and Q. Chen, Bioelectrochemistry, 2019, 128, 274–282 CrossRef CAS PubMed.
- K. Chen, H. Zhao, Z. Wang and M. Lan, Anal. Chim. Acta, 2021, 1169, 338628 CrossRef CAS PubMed.
- Y. Liu, H. Li, S. Gong, Y. Chen, R. Xie, Q. Wu, J. Tao, F. Meng and P. Zhao, Sens. Actuators, B, 2019, 290, 249–257 CrossRef CAS.
- T. Wang, Q. Bai, Z. Zhu, H. Xiao, F. Jiang, F. Du, W. W. Yu, M. Liu and N. Sui, Chem. Eng. J., 2021, 413, 127537 CrossRef CAS.
- Y. Si, L. Li, X. Qin, Y. Bai, J. Li and Y. Yin, Anal. Chim. Acta, 2019, 1057, 1–10 CAS.
- L. Wu, W. Yin, X. Tan, P. Wang, F. Ding, H. Zhang, B. Wang, W. Zhang and H. Han, Sens. Actuators, B, 2017, 248, 367–373 CrossRef CAS.
- O. Adeniyi, S. Sicwetsha and P. Mashazi, ACS Appl. Mater. Interfaces, 2020, 12, 1973–1987 CrossRef CAS PubMed.
- N. Kostevšek, I. Abramovič, S. Hudoklin, M. E. Kreft, I. Serša, A. Sepe, J. Vidmar, S. Šturm, M. Spreitzer, J. Ščančar, S. Kobe and K. Žužek Rožman, Nanoscale, 2018, 10, 1308–1321 RSC.
- J. Zhao, C. Wu, L. Zhai, X. Shi, X. Li, G. Weng, J. Zhu, J. Li and J.-W. Zhao, J. Mater. Chem. C, 2019, 7, 8432–8441 RSC.
- M. Aghayan, A. Mahmoudi, M. R. Sazegar and F. Adhami, J. Mater. Chem. B, 2021, 9, 3716–3726 RSC.
- Y. Sun, H. Chen, Y. Huang, F. Xu, G. Liu, L. Ma and Z. Wang, Biomaterials, 2021, 274, 120821 CrossRef CAS PubMed.
- L. W. Yap, H. Chen, Y. Gao, K. Petkovic, Y. Liang, K. J. Si, H. Wang, Z. Tang, Y. Zhu and W. Cheng, Nanoscale, 2017, 9, 7822–7829 RSC.
- Q. Yang, P. Wang, E. Ma, H. Yu, K. Zhou, C. Tang, J. Ren, Y. Li, Q. Liu and Y. Dong, Bioelectrochemistry, 2021, 138, 107713 CrossRef CAS PubMed.
- E. Ma, P. Wang, Q. Yang, H. Yu, F. Pei, Y. Zheng, Q. Liu, Y. Dong and Y. Li, ACS Biomater. Sci. Eng., 2020, 6, 1418–1427 CrossRef CAS PubMed.
- K. Jagajjanani Rao and S. Paria, J. Cleaner Prod., 2017, 165, 360–368 CrossRef CAS.
- J.-X. Liu, X.-L. Liang, F. Chen and S.-N. Ding, Sens. Actuators, B, 2019, 300, 127046 CrossRef CAS.
- Y. Zhang, D. Deng, X. Zhu, S. Liu, Y. Zhu, L. Han and L. Luo, Anal. Chim. Acta, 2018, 1042, 20–28 CrossRef CAS PubMed.
- Y. Li, Y. Zhang, F. Li, J. Feng, M. Li, L. Chen and Y. Dong, Biosens. Bioelectron., 2017, 92, 33–39 CrossRef CAS PubMed.
- Z. Tan, H. Dong, Q. Liu, H. Liu, P. Zhao, P. Wang, Y. Li, D. Zhang, Z. Zhao and Y. Dong, Biosens. Bioelectron., 2019, 142, 111556 CrossRef CAS PubMed.
- Y. Li, Y. Wang, L. Bai, H. Lv, W. Huang, S. Liu, S. Ding and M. Zhao, Anal. Chim. Acta, 2020, 1125, 86–93 CrossRef CAS PubMed.
- G. Chen, Y. Qin, L. Jiao, J. Huang, Y. Wu, L. Hu, W. Gu, D. Xu and C. Zhu, Anal. Chem., 2021, 93, 6881–6888 CrossRef CAS PubMed.
- Z. Jie, G. Qi, C. Xu and Y. Jin, Anal. Chem., 2019, 91, 14074–14079 CrossRef PubMed.
- J. Zhang, X. Xu and Y. Qiang, Sens. Actuators, B, 2020, 312, 127964 CrossRef CAS.
- F. Zhang, F. Huang, W. Gong, F. Tian, H. Wu, S. Ding, S. Li and R. Luo, J. Electroanal. Chem., 2021, 882, 115032 CrossRef CAS.
- W. Xu, Z. Qin, Y. Hao, Q. He, S. Chen, Z. Zhang, D. Peng, H. Wen, J. Chen, J. Qiu and C. Li, Biosens. Bioelectron., 2018, 113, 148–156 CrossRef CAS PubMed.
- J. Zeng, M. Gong, D. Wang, M. Li, W. Xu, Z. Li, S. Li, D. Zhang, Z. Yan and Y. Yin, Nano Lett., 2019, 19, 3011–3018 CrossRef CAS PubMed.
- Z. Sun, S. Fang and Y. H. Hu, Chem. Rev., 2020, 120, 10336–10453 CrossRef CAS PubMed.
- S. Manzeli, D. Ovchinnikov, D. Pasquier, O. V. Yazyev and A. Kis, Nat. Rev. Mater., 2017, 2, 17033 CrossRef CAS.
- A. K. Singh, P. Kumar, D. J. Late, A. Kumar, S. Patel and J. Singh, Appl. Mater. Today, 2018, 13, 242–270 CrossRef.
- L. Cheng, C. Yuan, S. Shen, X. Yi, H. Gong, K. Yang and Z. Liu, ACS Nano, 2015, 9, 11090–11101 CrossRef CAS PubMed.
- S. Lv, Y. Tang, K. Zhang and D. Tang, Anal. Chem., 2018, 90, 14121–14125 CrossRef CAS PubMed.
- J. Yang and Y.-W. Yang, Small, 2020, 16, 1906846 CrossRef CAS PubMed.
- Q. Fu, Z. Wu, D. Du, C. Zhu, Y. Lin and Y. Tang, ACS Sens., 2017, 2, 789–795 CrossRef CAS PubMed.
- S. Bai, D. Serien, A. Hu and K. Sugioka, Adv. Funct. Mater., 2018, 28, 1706262 CrossRef.
- J. Wu, K. Qin, D. Yuan, J. Tan, L. Qin, X. Zhang and H. Wei, ACS Appl. Mater. Interfaces, 2018, 10, 12954–12959 CrossRef CAS PubMed.
- G. Panthi and M. Park, J. Hazard. Mater., 2022, 424, 127565 CrossRef CAS PubMed.
- X. Yang, X. Han, Y. Zhang, J. Liu, J. Tang, D. Zhang, Y. Zhao and Y. Ye, Anal. Chem., 2020, 92, 12002–12009 CrossRef CAS PubMed.
- L. Liu, K. Ye, C. Lin, Z. Jia, T. Xue, A. Nie, Y. Cheng, J. Xiang, C. Mu, B. Wang, F. Wen, K. Zhai, Z. Zhao, Y. Gong, Z. Liu and Y. Tian, Nat. Commun., 2021, 12, 3870 CrossRef CAS PubMed.
- S. Li, T. Wei, M. Tang, F. Chai, F. Qu and C. Wang, Sens. Actuators, B, 2018, 255, 1471–1481 CrossRef CAS.
- S. Balasurya, D. A. Al Farraj, A. M. Thomas, N. A. Alkubaisi, L. L. Raju, A. Das and S. Sudheer Khan, J. Environ. Chem. Eng., 2020, 8, 104305 CrossRef CAS.
- H. Zhu, X. Tan, L. Tan, H. Zhang, H. Liu, M. Fang, T. Hayat and X. Wang, ACS Sustainable Chem. Eng., 2018, 6, 5206–5213 CrossRef CAS.
- Z. Lu, F. He, C. Y. Hsieh, X. Wu, M. Song, X. Liu, Y. Liu, S. Yuan, H. Dong, S. Han, P. Du and G. Xing, ACS Appl. Nano Mater., 2019, 2, 1664–1674 CrossRef CAS.
- Y. J. Yang, W. X. Li and J. W. Liu, Anal. Chim. Acta, 2021, 1147, 124–143 CrossRef CAS PubMed.
- Z.-J. Xie, M.-R. Shi, L.-Y. Wang, C.-F. Peng and X.-L. Wei, Microchim. Acta, 2020, 187, 255 CrossRef CAS PubMed.
- E. W. Miller, A. E. Albers, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2005, 127, 16652–16659 CrossRef CAS PubMed.
- B. Perillo, M. Di Donato, A. Pezone, E. Di Zazzo, P. Giovannelli, G. Galasso, G. Castoria and A. Migliaccio, Exp. Mol. Med., 2020, 52, 192–203 CrossRef CAS PubMed.
- T. Konno, E. P. Melo, J. E. Chambers and E. Avezov, Cells, 2021, 10, 233 CrossRef CAS PubMed.
- Y. Ding, B. Yang, H. Liu, Z. Liu, X. Zhang, X. Zheng and Q. Liu, Sens. Actuators, B, 2018, 259, 775–783 CrossRef CAS.
- L. Jansson and C. Hellerstrom, Am. J. Physiol., 1986, 251, E644–E647 CAS.
- S. Cai, Z. Fu, W. Xiao, Y. Xiong, C. Wang and R. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 11616–11624 CrossRef CAS PubMed.
- Z. Yang, X. Zheng and J. Zheng, Chem. Eng. J., 2017, 327, 431–440 CrossRef CAS.
- J. Li, H. Jin, M. Wei, W. Ren, J. Wang, Y. Zhang, L. Wu and B. He, Sens. Actuators, B, 2021, 331, 129401 CrossRef CAS.
- K. Tian, Y. Ma, Y. Liu, M. Wang, C. Guo, L. He, Y. Song, Z. Zhang and M. Du, Sens. Actuators, B, 2020, 303, 127199 CrossRef CAS.
- Q. Zhu, B. Liang, Y. Liang, L. Ji, Y. Cai, K. Wu, T. Tu, H. Ren, B. Huang, J. Wei, L. Fang, X. Liang and X. Ye, Biosens. Bioelectron., 2020, 153, 112019 CrossRef CAS PubMed.
- J. Chen, G. Cheng, K. Wu, A. Deng and J. Li, Electrochim. Acta, 2020, 361, 137061 CrossRef CAS.
- X. Zhu, L. Gao, L. Tang, B. Peng, H. Huang, J. Wang, J. Yu, X. Ouyang and J. Tan, Biosens. Bioelectron., 2019, 146, 111756 CrossRef PubMed.
- T. Chen, J. Xu, M. Arsalan, Q. Sheng, J. Zheng, W. Cao and T. Yue, Talanta, 2019, 198, 78–85 CrossRef CAS PubMed.
- S. Singh, P. Tripathi, N. Kumar and S. Nara, Biosens. Bioelectron., 2017, 92, 280–286 CrossRef CAS PubMed.
- S.-S. Li, Y.-Y. Hu, A.-J. Wang, X. Weng, J.-R. Chen and J.-J. Feng, Sens. Actuators, B, 2015, 208, 468–474 CrossRef CAS.
- C. Wang, L. Hu, K. Zhao, A. Deng and J. Li, Electrochim. Acta, 2018, 278, 352–362 CrossRef CAS.
- S. Yakubu, J. Xiao, J. Gu, J. Cheng, J. Wang, X. Li and Z. Zhang, Sens. Actuators, B, 2020, 325, 128909 CrossRef CAS.
- L. Wu and X. Qu, Chem. Soc. Rev., 2015, 44, 2963–2997 RSC.
- M. Labib, E. H. Sargent and S. O. Kelley, Chem. Rev., 2016, 116, 9001–9090 CrossRef CAS PubMed.
- S. M. Hanash, S. J. Pitteri and V. M. Faca, Nature, 2008, 452, 571–579 CrossRef CAS PubMed.
- A. Iglesias-Mayor, O. Amor-Gutiérrez, A. Novelli, M.-T. Fernández-Sánchez, A. Costa-García and A. de la Escosura-Muñiz, Anal. Chem., 2020, 92, 7209–7217 CrossRef CAS PubMed.
- S. Zhang, C. Zhang, Y. Jia, X. Zhang, Y. Dong, X. Li, Q. Liu, Y. Li and Z. Zhao, Bioelectrochemistry, 2019, 128, 140–147 CrossRef CAS PubMed.
- L. Yang, M. X. Gao, L. Zhan, M. Gong, S. J. Zhen and C. Z. Huang, Nanoscale, 2017, 9, 2640–2645 RSC.
- X. Weng, Y. Liu, Y. Xue, A.-J. Wang, L. Wu and J.-J. Feng, Sens. Actuators, B, 2017, 250, 61–68 CrossRef CAS.
- A.-J. Wang, X.-Y. Zhu, Y. Chen, X. Luo, Y. Xue and J.-J. Feng, Sens. Actuators, B, 2019, 292, 164–170 CrossRef CAS.
- R. Wang, J.-J. Feng, W.-D. Liu, L.-Y. Jiang and A.-J. Wang, Biosens. Bioelectron., 2017, 96, 152–158 CrossRef CAS PubMed.
- E. Ma, P. Wang, Q. Yang, H. Yu, F. Pei, Y. Li, Q. Liu and Y. Dong, Biosens. Bioelectron., 2019, 142, 111580 CrossRef CAS PubMed.
- Z. Xi, K. Wei, Q. Wang, M. J. Kim, S. Sun, V. Fung and X. Xia, J. Am. Chem. Soc., 2021, 143, 2660–2664 CrossRef CAS PubMed.
- R. Wang, W.-D. Liu, A.-J. Wang, Y. Xue, L. Wu and J.-J. Feng, Biosens. Bioelectron., 2018, 99, 458–463 CrossRef CAS PubMed.
- R. Wang, A.-J. Wang, W.-D. Liu, P.-X. Yuan, Y. Xue, X. Luo and J.-J. Feng, Biosens. Bioelectron., 2018, 102, 276–281 CrossRef CAS PubMed.
- Y.-C. Shi, A.-J. Wang, P.-X. Yuan, L. Zhang, X. Luo and J.-J. Feng, Biosens. Bioelectron., 2018, 111, 47–51 CrossRef CAS PubMed.
- Y. Liu, M. Pan, W. Wang, Q. Jiang, F. Wang, D.-W. Pang and X. Liu, Anal. Chem., 2019, 91, 2086–2092 CrossRef CAS PubMed.
- J. R. Wang, C. Xia, L. Yang, Y. F. Li, C. M. Li and C. Z. Huang, Anal. Chem., 2020, 92, 4046–4052 CrossRef CAS PubMed.
- Y. Chen, X.-Y. Ge, S.-Y. Cen, A.-J. Wang, X. Luo and J.-J. Feng, Sens. Actuators, B, 2020, 311, 127931 CrossRef CAS.
- S. K. Gurmessa, L. T. Tufa, J. Kim, K.-I. Lee, Y.-M. Kim, V. T. Tran, H.-Q. Nguyen, T. S. Shim, J. Kim, T. J. Park, J. Lee and H.-J. Kim, ACS Appl. Nano Mater., 2021, 4, 539–549 CrossRef CAS.
- S. Palanisamy, D. Senthil Raja, B. Subramani, T.-H. Wu and Y.-M. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 32468–32476 CrossRef CAS PubMed.
- Y. Si, Y. Bai, X. Qin, J. Li, W. Zhong, Z. Xiao, J. Li and Y. Yin, Anal. Chem., 2018, 90, 3898–3905 CrossRef CAS PubMed.
- G. Qiu, S. P. Ng and C.-M. L. Wu, Sens. Actuators, B, 2018, 265, 459–467 CrossRef CAS.
- Z. Huang, R. Zhang, H. Chen, W. Weng, Q. Lin, D. Deng, Z. Li and J. Kong, Biosens. Bioelectron., 2019, 142, 111542 CrossRef CAS PubMed.
- X. Xi, M. Wen, S. Song, J. Zhu, W. Wen, X. Zhang and S. Wang, Chem. Commun., 2020, 56, 6039–6042 RSC.
- H. Dong, L. Cao, H. Zhao, S. Liu, Q. Liu, P. Wang, Z. Xu, S. Wang, Y. Li, P. Zhao and Y. Li, Biosens. Bioelectron., 2020, 170, 112667 CrossRef CAS PubMed.
- X.-Y. Wang, Y.-G. Feng, A.-J. Wang, L.-P. Mei, X. Luo, Y. Xue and J.-J. Feng, Bioelectrochemistry, 2021, 140, 107802 CrossRef CAS PubMed.
- Z. Fu, W. Zeng, S. Cai, H. Li, J. Ding, C. Wang, Y. Chen, N. Han and R. Yang, J. Colloid Interface Sci., 2021, 604, 113–121 CrossRef CAS PubMed.
- D. Liu, C. Ju, C. Han, R. Shi, X. Chen, D. Duan, J. Yan and X. Yan, Biosens. Bioelectron., 2021, 173, 112817 CrossRef CAS PubMed.
- Y. Lin, S. Xu, J. Yang, Y. Huang, Z. Chen, B. Qiu, Z. Lin, G. Chen and L. Guo, Sens. Actuators, B, 2018, 267, 502–509 CrossRef CAS.
- J. Das, I. Ivanov, E. H. Sargent and S. O. Kelley, J. Am. Chem. Soc., 2016, 138, 11009–11016 CrossRef CAS PubMed.
- K. Wolska-Gawron, J. Bartosińska and D. Krasowska, Arch. Dermatol. Res., 2020, 312, 317–324 CrossRef CAS PubMed.
- M. M. H. Sohel, Life Sci., 2020, 248, 117473 CrossRef CAS PubMed.
- H. Park, M. K. Masud, J. Na, H. Lim, H.-P. Phan, Y. V. Kaneti, A. A. Alothman, C. Salomon, N.-T. Nguyen, M. S. A. Hossain and Y. Yamauchi, J. Mater. Chem. B, 2020, 8, 9512–9523 RSC.
- S. Mei, B. Liu, X. Xiong and X. Hun, J. Pharm. Biomed. Anal., 2020, 186, 113280 CrossRef CAS PubMed.
- F. Papaccio, F. Paino, T. Regad, G. Papaccio, V. Desiderio and V. Tirino, Stem Cells Transl. Med., 2017, 6, 2115–2125 CrossRef PubMed.
- K. Fujita, M. Kamiya and Y. Urano, Methods Mol. Biol., 2021, 2274, 193–206 CrossRef CAS PubMed.
- G.-W. Wu, Y.-M. Shen, X.-Q. Shi, H.-H. Deng, X.-Q. Zheng, H.-P. Peng, A.-L. Liu, X.-H. Xia and W. Chen, Anal. Chim. Acta, 2017, 971, 88–96 CrossRef CAS PubMed.
- J. Du, Z. Gu, L. Yan, Y. Yong, X. Yi, X. Zhang, J. Liu, R. Wu, C. Ge, C. Chen and Y. Zhao, Adv. Mater., 2017, 29, 1701268 CrossRef PubMed.
- H. Liu, W. Lin, L. He and T. Chen, Biomaterials, 2020, 226, 119545 CrossRef CAS PubMed.
- X. Ding, P. Yuan, N. Gao, H. Zhu, Y. Y. Yang and Q.-H. Xu, Nanomed.: Nanotechnol. Biol. Med., 2017, 13, 297–305 CrossRef CAS PubMed.
- B. Hu, F. Kong, X. Gao, L. Jiang, X. Li, W. Gao, K. Xu and B. Tang, Angew. Chem., Int. Ed., 2018, 57, 5306–5309 CrossRef CAS PubMed.
- P. Yuan, R. Ma, N. Gao, M. Garai and Q.-H. Xu, Nanoscale, 2015, 7, 10233–10239 RSC.
- P. Yuan, X. Ding, Z. Guan, N. Gao, R. Ma, X.-F. Jiang, Y. Y. Yang and Q.-H. Xu, Adv. Healthcare Mater., 2015, 4, 674–678 CrossRef CAS PubMed.
- F. Han, Z. Guan, T. S. Tan and Q.-H. Xu, ACS Appl. Mater. Interfaces, 2012, 4, 4746–4751 CrossRef CAS PubMed.
- K. Aleshire, I. M. Pavlovetc, R. Collette, X. T. Kong, P. D. Rack, S. B. Zhang, D. J. Masiello, J. P. Camden, G. V. Hartland and M. Kuno, Proc. Natl. Acad. Sci. U.S.A., 2020, 117, 2288–2293 CrossRef CAS PubMed.
- C. Kuppe, X. Z. Zheng, C. Williams, A. W. A. Murphy, J. T. Collins, S. N. Gordeev, G. A. E. Vandenbosch and V. K. Valev, Nanoscale Horiz., 2019, 4, 1056–1062 RSC.
- S. S. Li, Q. Y. Kong, M. Zhang, F. Yang, B. Kang, J. J. Xu and H. Y. Chen, Anal. Chem., 2018, 90, 3833–3841 CrossRef CAS PubMed.
- T. Tumkur, X. Yang, C. Zhang, J. Yang, Y. Zhang, G. V. Naik, P. Nordlander and N. J. Halas, Nano Lett., 2018, 18, 2040–2046 CrossRef CAS PubMed.
- M. M. Richter, Chem. Rev., 2004, 104, 3003–3036 CrossRef CAS PubMed.
- Y. Fang, H.-M. Wang, Y.-X. Gu, L. Yu, A.-J. Wang, P.-X. Yuan and J.-J. Feng, Anal. Chem., 2020, 92, 3206–3212 CrossRef CAS PubMed.
- N. Zhang, X.-T. Wang, Z. Xiong, L.-Y. Huang, Y. Jin, A.-J. Wang, P.-X. Yuan, Y.-B. He and J.-J. Feng, Anal. Chem., 2021, 93, 17110–17118 CrossRef CAS PubMed.
- J. Hu, S. Zhou, Y. Sun, X. Fang and L. Wu, Chem. Soc. Rev., 2012, 41, 4356–4378 RSC.
- B. Weigelt, J. L. Peterse and L. J. van't Veer, Nat. Rev. Cancer, 2005, 5, 591–602 CrossRef CAS PubMed.
- Y. Yang, J. Huang, X. Yang, K. Quan, H. Wang, L. Ying, N. Xie, M. Ou and K. Wang, J. Am. Chem. Soc., 2015, 137, 8340–8343 CrossRef CAS PubMed.
- M. Luan, M. Shi, W. Pan, N. Li and B. Tang, Chem. Commun., 2019, 55, 5817–5820 RSC.
- R. Zhan, X. Li, L. Zang and K. Xu, Analyst, 2020, 145, 1008–1013 RSC.
- Y. Song, Q. Shi, C. Zhu, Y. Luo, Q. Lu, H. Li, R. Ye, D. Du and Y. Lin, Nanoscale, 2017, 9, 15813–15824 RSC.
- Y. Wang, B. Yan and L. Chen, Chem. Rev., 2013, 113, 1391–1428 CrossRef CAS PubMed.
- W. Ma, P. Fu, M. Sun, L. Xu, H. Kuang and C. Xu, J. Am. Chem. Soc., 2017, 139, 11752–11759 CrossRef CAS PubMed.
- Z. Zhang, K. Bando, A. Taguchi, K. Mochizuki, K. Sato, H. Yasuda, K. Fujita and S. Kawata, ACS Appl. Mater. Interfaces, 2017, 9, 44027–44037 CrossRef CAS PubMed.
- P. Jiang, Y. Hu and G. Li, Talanta, 2019, 200, 212–217 CrossRef CAS PubMed.
- J. He, J. Dong, Y. Hu, G. Li and Y. Hu, Nanoscale, 2019, 11, 6089–6100 RSC.
- H. Zhu, Y. Wang, C. Chen, M. Ma, J. Zeng, S. Li, Y. Xia and M. Gao, ACS Nano, 2017, 11, 8273–8281 CrossRef CAS PubMed.
- J. Cui, R. Jiang, C. Guo, X. Bai, S. Xu and L. Wang, J. Am. Chem. Soc., 2018, 140, 5890–5894 CrossRef CAS PubMed.
- S. Cho, W. Park, H. Kim, J. R. Jokisaari, E. W. Roth, S. Lee, R. F. Klie, B. Lee and D.-H. Kim, ACS Appl. Nano Mater., 2018, 1, 4602–4611 CrossRef CAS PubMed.
- C. Wang, W. Fan, Z. Zhang, Y. Wen, L. Xiong and X. Chen, Adv. Mater., 2019, 31, 1904329 CrossRef CAS PubMed.
- R. Yang, S. Fu, R. Li, L. Zhang, Z. Xu, Y. Cao, H. Cui, Y. Kang and P. Xue, Theranostics, 2021, 11, 107–116 CrossRef CAS PubMed.
- S. Yu, Y. Zhou, Y. Sun, S. Wu, T. Xu, Y.-C. Chang, S. Bi, L.-P. Jiang and J.-J. Zhu, Angew. Chem., Int. Ed., 2021, 60, 5948–5958 CrossRef CAS PubMed.
- M. Wang, M. Chang, Q. Chen, D. Wang, C. Li, Z. Hou, J. Lin, D. Jin and B. Xing, Biomaterials, 2020, 252, 120093 CrossRef CAS PubMed.
- S. J. Chen, S. C. Wang and Y. C. Chen, Int. J. Mol. Sci., 2021, 22, 12836 CrossRef CAS PubMed.
- T. G. Davidson, Am. J. Health-Syst. Pharm., 2001, 58, S8–S15 CrossRef PubMed.
- K. Antunac, Acta Clin. Croat., 2019, 58, 46–59 Search PubMed.
- S. Li, W. Sun, Y. Luo, Y. Gao, X. Jiang, C. Yuan, L. Han, K. Cao, Y. Gong and C. Xie, J. Mater. Chem. B, 2021, 9, 4643–4653 RSC.
- R. Oun, Y. E. Moussa and N. J. Wheate, Dalton Trans., 2018, 47, 6645–6653 RSC.
- X. Yang, L. Li, D. He, L. Hai, J. Tang, H. Li, X. He and K. Wang, J. Mater. Chem. B, 2017, 5, 4648–4659 RSC.
- D. I. Abrams, G. Velasco, C. Twelves, R. K. Ganju and G. Bar-Sela, J. Natl. Cancer Inst. Monogr., 2021, 2021, 107–113 CrossRef PubMed.
- G. H. Nam, Y. Choi, G. B. Kim, S. Kim, S. A. Kim and I. S. Kim, Adv. Mater., 2020, 32, 2002440 CrossRef CAS PubMed.
- D. F. Zhi, T. Yang, J. O'Hagan, S. B. Zhang and R. F. Donnelly, J. Controlled Release, 2020, 325, 52–71 CrossRef CAS PubMed.
- H. Lv, D. Xu, L. Sun and B. Liu, J. Phys. Chem. Lett., 2020, 11, 5777–5784 CrossRef CAS PubMed.
- J. M. Chen, T. J. Fan, Z. J. Xie, Q. Q. Zeng, P. Xue, T. T. Zheng, Y. Chen, X. L. Luo and H. Zhang, Biomaterials, 2020, 237, 119827 CrossRef CAS PubMed.
- M. H. Lan, S. J. Zhao, W. M. Liu, C. S. Lee, W. J. Zhang and P. F. Wang, Adv. Healthcare Mater., 2019, 8, 1900132 CrossRef PubMed.
- Z. Zhou, J. Song, L. Nie and X. Chen, Chem. Soc. Rev., 2016, 45, 6597–6626 RSC.
- X. Wang, X. Zhong, Z. Liu and L. Cheng, Nano Today, 2020, 35, 100946 CrossRef CAS.
- Z. Tang, Y. Liu, M. He and W. Bu, Angew. Chem., Int. Ed., 2019, 58, 946–956 CrossRef CAS PubMed.
- G. Qi, Y. Zhang, J. Wang, D. Wang, B. Wang, H. Li and Y. Jin, Anal. Chem., 2019, 91, 12203–12211 CrossRef CAS PubMed.
- S. Bai, N. Yang, X. Wang, F. Gong, Z. Dong, Y. Gong, Z. Liu and L. Cheng, ACS Nano, 2020, 14, 15119–15130 CrossRef PubMed.
- Y. Yang, X. Wang, H. Qian and L. Cheng, Appl. Mater. Today, 2021, 25, 101215 CrossRef.
- W. Fan, B. Yung, P. Huang and X. Chen, Chem. Rev., 2017, 117, 13566–13638 CrossRef CAS PubMed.
- P. Huang, X. Qian, Y. Chen, L. Yu, H. Lin, L. Wang, Y. Zhu and J. Shi, J. Am. Chem. Soc., 2017, 139, 1275–1284 CrossRef CAS PubMed.
- J. Kim, S. Oh, Y. C. Shin, C. Wang, M. S. Kang, J. H. Lee, W. Yun, J. A. Cho, D. Y. Hwang, D. W. Han and J. Lee, Colloids Surf., B, 2020, 189, 110839 CrossRef CAS PubMed.
- E. B. Choi and J. H. Lee, Arch. Metall. Mater., 2017, 62, 1137–1142 CAS.
- S. Chen, X. C. Qiu, B. W. Zhang, J. B. Xu, F. G. Zhong, B. P. Zhu, Y. Zhang, J. Ou-Yang and X. F. Yang, J. Alloys Compd., 2021, 886, 161143 CrossRef CAS.
- M. Pietrzak and P. Ivanova, Sens. Actuators, B, 2021, 336, 129736 CrossRef CAS.
- Y. Huang, J. Ren and X. Qu, Chem. Rev., 2019, 119, 4357–4412 CrossRef CAS PubMed.
- X. Mu, J. Wang, Y. Li, F. Xu, W. Long, L. Ouyang, H. Liu, Y. Jing, J. Wang, H. Dai, Q. Liu, Y. Sun, C. Liu and X.-D. Zhang, ACS Nano, 2019, 13, 1870–1884 CAS.
- L. Rizzello and P. P. Pompa, Chem. Soc. Rev., 2014, 43, 1501–1518 RSC.
- H. Ji, K. Dong, Z. Yan, C. Ding, Z. Chen, J. Ren and X. Qu, Small, 2016, 12, 6200–6206 CrossRef CAS PubMed.
- S. Kumar, R. K. Majhi, A. Singh, M. Mishra, A. Tiwari, S. Chawla, P. Guha, B. Satpati, H. Mohapatra, L. Goswami and C. Goswami, ACS Appl. Mater. Interfaces, 2019, 11, 42998–43017 CrossRef CAS PubMed.
- M. Das, U. Goswami, R. Kandimalla, S. Kalita, S. S. Ghosh and A. Chattopadhyay, ACS Appl. Bio Mater., 2019, 2, 5434–5445 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |