Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Gram-scale synthesis of a covalent nanocage that preserves the redox properties of encapsulated fullerenes†
Daniel A.
Rothschild
 ,
William P.
Kopcha
,
Aaron
Tran
,
Jianyuan
Zhang
,
William P.
Kopcha
,
Aaron
Tran
,
Jianyuan
Zhang
 and
Mark C.
Lipke
and
Mark C.
Lipke
 *
*
Department of Chemistry and Chemical Biology, Rutgers, The State University of New Jersey, 123 Bevier Rd, Piscataway, NJ 08854, USA. E-mail: ml1353@chem.rutgers.edu
First published on 13th April 2022
Abstract
Discrete nanocages provide a way to solubilize, separate, and tune the properties of fullerenes, but these 3D receptors cannot usually be synthesized easily from inexpensive starting materials, limiting their utility. Herein, we describe the first fullerene-binding nanocage (Cage4+) that can be made efficiently on a gram scale. Cage4+ was prepared in up to 57% yield by the formation of pyridinium linkages between complemantary porphyrin components that are themselves readily accessible. Cage4+ binds C60 and C70 with large association constants (>108 M−1), thereby solubilizing these fullerenes in polar solvents. Fullerene association and redox-properties were subsequently investigated across multiple charge states of the host-guest complexes. Remarkably, neutral and singly reduced fullerenes bind with similar strengths, leaving their 0/1− redox couples minimally perturbed and fully reversible, whereas other hosts substantially alter the redox properties of fullerenes. Thus, C60@Cage4+ and C70@Cage4+ may be useful as solubilized fullerene derivatives that preserve the inherent electron-accepting and electron-transfer capabilities of the fullerenes. Fulleride dianions were also found to bind strongly in Cage4+, while further reduction is centered on the host, leading to lowered association of the fulleride guest in the case of C602−.
Introduction
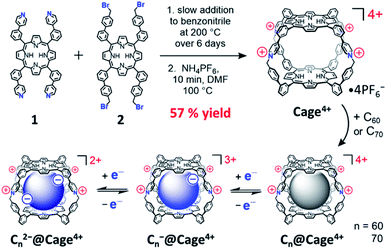
Macrocycles and nanocages with aromatic walls are popular synthetic targets for their interesting structures and ability to host aromatic guests.1 Many such molecular receptors have been examined for use in separating,2 sensing,3 or tuning the electronics4 and/or reactivity5 of various aromatic species. Fullerenes are one notable class of aromatic guests owing to their useful electron-accepting properties6 and the challenges that exist in purifying,2a,7 solubilizing,8 and derivatizing9 these aromatic carbon allotropes—challenges which might be overcome using the host-guest chemistry of suitable receptors. Nanocages with large aromatic components (e.g., porphyrins,2d,f,4a,10 naphthalene or perylene diimides,8a,11 pyrenes,12 anthracenes,13etc.14) often show particularly high affinities for binding fullerenes due to the large π–π overlap provided upon complexation. However, the synthesis of such hosts is typically challenging or costly, either relying on the stoichiometric use of precious metals to link the aromatic walls‡ or utilizing covalent linkages§ that result in low yields and challenging purifications. Furthermore, the individual components of these hosts often require numerous steps to synthesize,¶ compounding the inefficiency of cage formation.To overcome these limitations, we sought to develop an easily synthesized porphyrin nanocage of the appropriate shape and rigidity to bind fullerenes with high affinities and selectivity. Herein, we report the synthesis, characterization, and fullerene-binding properties of a tetracationic bis-porphyrin nanocage, Cage4+, which was prepared in good yield on a gram scale by the formation of pyridinium linkages between complementary pyridyl- and benzylbromide-substituted porphyrins (Scheme 1). This cage uptakes C60 and C70 quantitatively in MeCN, with essentially complete selectivity for extracting C70 from mixtures containing both fullerenes. Notably, the redox properties of the fullerenes are minimally perturbed by encapsulation in Cage4+ (Scheme 1) whereas previously reported hosts significantly alter the potentials and/or reversibility of fullerene redox processes.4a,c,d,8a The easy, scalable synthesis of Cage4+, its strong binding of fullerenes, and the preserved electronics of fullerene guests suggest that C60@Cage4+ and C70@Cage4+ might be directly useful as solubilized fullerene derivatives.
Results and discussion
Synthesis of Cage4+
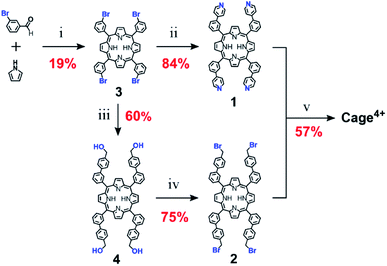
As shown in Scheme 1, Cage4+ was formed via simple SN2 reactions between complementary 4-fold-symmetric pyridyl- and benzylbromide-substituted porphyrins 1 and 2. Efficient synthesis of the cage was achieved using pseudo-high-dilution conditions in which the two components were added slowly to a moderate volume of PhCN at 200 °C over 6 days. The initial product Cage·4Br is insoluble in PhCN and DMF, allowing oligomeric byproducts to be removed by washing with DMF. Heating the remaining solid in a DMF solution of NH4PF6 provided the salt Cage·4PF6 as the only soluble product in a yield of up to 57%. The identity of Cage4+ was confirmed by a variety of NMR techniques (1H, 13C{1H}, and DOSY) in CD3CN and DMSO-d6 (see Fig. S13–S16 and S25†), and also by ESI(+)-HRMS characterization (Fig. S33–36†).The precursors of Cage4+ were also prepared easily in only a few steps from inexpensive starting materials (Scheme 2), suggesting the synthesis of Cage4+ could be economically scaled. Confirming this possibility, we prepared > 1 g of Cage·4PF6 in a single batch, with the percent yield (40%) suffering only a little from scale up. For comparison, we surveyed over 35 reported 3D fullerene receptors,|| finding that most were prepared on scales of <100 mg, with the largest synthesis providing only about 200 mg of the cage.12c To our knowledge, Cage4+ is the only fullerene-binding nanocage for which a gram scale synthesis has been demonstrated.
Association of fullerenes in Cage4+
Host-guest complexes of C60 and C70 in Cage4+ were formed after 3 h of sonicating suspensions of each fullerene in CD3CN solutions of the host. Fullerene encapsulation was evident from changes to all the 1H NMR resonances of Cage4+ (Fig. 1), with the upfield porphyrin NH signals of the host providing particularly useful NMR handles for tracking complexation. The 13C{1H} NMR spectra of the host-guest complexes confirm the encapsulation of the fullerenes. Most notably, a large signal at 140.40 ppm was observed for the C60 guest in C60@Cage4+ (Fig. S20†), and five resonances arising from encapsulated C70 were observed for C70@Cage4+ (Fig. S24†), whereas 13C NMR signals of C60 and C70 cannot otherwise be observed in CD3CN due to the negligible solubility of these fullerenes in this solvent.15 ESI(+)-HRMS characterization further confirmed the identity of the host-guest complexes, showing a series of peaks with the expected isotope patterns for C60@Cage4+ and C70@Cage4+ with 0 to 2 PF6− anions associated (Fig. S37–S44†).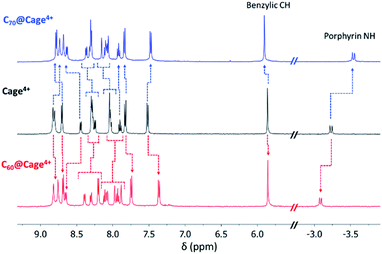 | ||
| Fig. 1 Partial 1H NMR spectra of Cage4+ (black), C60@Cage4+ (red), and C70@Cage4+ (blue) in CD3CN (500 MHz, 298 K). Changes to resonances of Cage4+ upon guest uptake are labelled with dotted arrows. | ||
The strength of association between C60 and Cage4+ was estimated based on the observation that the empty host could not be detected by 1H NMR spectroscopy after sonicating an excess of the fullerene in a saturated (0.65 mM) solution of Cage4+ in CD3CN (Fig. 1). Using the reported solubility of C60 in MeCN (0.56 μM)15 and the lower limit of 1H NMR detection of Cage4+ (1.94 μM), it can be concluded that C60@Cage4+ has a very large association constant of ≥ 6.0 × 108 M−1. It was not possible to measure the binding strength more precisely in MeCN, so the association of C60 in Cage4+ was also quantified in benzonitrile. Fluorescence measurements revealed that C60@Cage4+ forms with a Ka of 1.14 ± 0.30 × 107 M−1 in PhCN (Fig. S74†). Since PhCN is orders of magnitude better than MeCN at solvating C60,15 the fullerene should bind much more strongly in Cage4+ in MeCN, supporting the estimate of a Ka > 108 M−1 for the formation of C60@Cage4+ in MeCN.
Strong binding of C70 was also evident in CD3CN from complete disappearance of the 1H NMR signals of empty Cage4+ after sonication in the presence of this fullerene (Fig. 1), but like for C60, the association constant could not be quantified precisely in this solvent. Instead, the association constant for C70@Cage4+ was measured in PhCN (Fig. S75†), revealing a Ka (1.10 ± 0.17 × 108 M−1) that is an order of magnitude higher than that for binding C60 in PhCN. Competition experiments confirmed that this preference for binding C70 extends to MeCN solvent conditions. Sonication of a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of C60
1 mixture of C60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C70 in a solution of Cage4+ in CD3CN led to complete disappearance of the signals of the free host from the 1H NMR spectrum after 3 h, initially providing C60@Cage4+ and C70@Cage4+ in similar amounts (see Fig. S30†). However, the ratio of C70@Cage4+ to C60@Cage4+ steadily increased upon further sonication, until only the C70 complex could be observed by 1H NMR spectroscopy after 26 h (Fig. S30†). These observations show that the kinetics of fullerene uptake are similar for both guests, but C70@Cage4+ is the thermodynamically more favourable complex. Thus, Cage4+ is highly effective for separating C70 from mixtures of C60 and C70, showing perfect selectivity within the limits of 1H NMR detection (≥30-fold selectivity for C70).
C70 in a solution of Cage4+ in CD3CN led to complete disappearance of the signals of the free host from the 1H NMR spectrum after 3 h, initially providing C60@Cage4+ and C70@Cage4+ in similar amounts (see Fig. S30†). However, the ratio of C70@Cage4+ to C60@Cage4+ steadily increased upon further sonication, until only the C70 complex could be observed by 1H NMR spectroscopy after 26 h (Fig. S30†). These observations show that the kinetics of fullerene uptake are similar for both guests, but C70@Cage4+ is the thermodynamically more favourable complex. Thus, Cage4+ is highly effective for separating C70 from mixtures of C60 and C70, showing perfect selectivity within the limits of 1H NMR detection (≥30-fold selectivity for C70).
Structural analysis of host-guest complexes
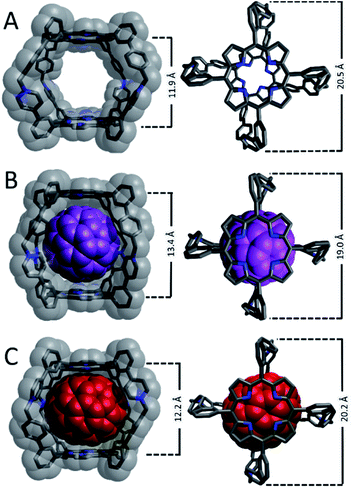
Crystals suitable for single-crystal XRD analysis could not be obtained for Cage4+ or its host-guest complexes after several attempts using a variety of solvents (MeCN and DMF) and antisolvents (Et2O, iPr2O, tBuOMe, DCM, hexanes), so DFT structural optimizations were performed. The optimized structure of Cage4+ (Fig. 2A) has a coplanar arrangement of its porphyrin faces, with a centroid-to-centroid separation of 11.9 Å. A distance of 20.5 Å was found between the benzylic carbon atoms at opposite ends of the cage. The dimensions of the host were altered only slightly in the optimized structure of C70@Cage4+ (Fig. 2C); spacing between the porphyrin faces is expanded to 12.2 Å and the distance between the benzylic carbon atoms is contracted to 20.2 Å. The spacing of the porphyrin faces is expanded much more (to 13.4 Å) in the optimized structure of C60@Cage4+ (Fig. 2B), and the benzylic carbon atom spacing is contracted considerably (to 19.0 Å). Thus, the geometry of Cage4+ is well suited to hosting C70, while considerable distortion is needed to host C60. These results help to explain the experimentally observed preference for binding C70vs. C60, especially since Cage4+ consists primarily of sp2 to sp2 linkages that are not expected to provide much conformational flexibility.The dimensions of Cage4+ and its fullerene complexes were evaluated experimentally using DOSY NMR spectroscopy (Fig. S25–S27†). A diffusion coefficient (D) of 8.76 × 10−11 m2 s−1 was determined for Cage4+ in dmso-d6, corresponding to an effective hydrodynamic radius16 (r) of 1.25 nm, which matches well with the van der Waals distance (ca. 2.5 nm) across the widest dimension of the computationally optimized structure (Fig. 2A). A similar diffusion constant was measured for C60@Cage4+ (D = 8.34 × 10−11 m2 s; r = 1.31 nm, Fig. S26†), while C70@Cage4+ diffuses more slowly (D = 7.01 × 10−11 m2 s; r = 1.56 nm, Fig. S27†) even though the optimized structures of the host-guest complexes suggest that the latter exhibits less structural distortion relative to the empty host. The disparity of the diffusion coefficient of C70@Cage4+ relative to the empty host may reflect altered interactions with other solutes, such as the PF6− counteranions. The influence of anions on the diffusion of the nanocage was assessed by comparing the diffusion of Cage4+ as its PF6−, BF4−, and BPh4− salts. The BF4− counteranions increase the rate of diffusion of Cage4+ substantially (D = 1.14 × 10−10 m2 s−1, Fig. S29†), while BPh4− anions result in Cage4+ diffusing only slightly faster (D = 9.46 × 10−11 m2 s−1, Fig. S28†) than observed for its PF6− salt. Since counteranions influence the diffusion of Cage4+, we speculate that the different diffusion rates for the host-guest complexes arise from the C60vs. C70 guests having differing influences on the association of the cage with its PF6− counteranions.
Travelling-wave ion-mobility spectrometry (TWIMS) was employed as an additional technique to compare the effective sizes—specifically the collisional cross sections—of Cage4+ and its complexes with fullerene guests.17 Conveniently, this gas-phase technique allows for selective measurement of the 4+ ions of the cage and host-guest complexes to eliminate any complicating influences of counteranions. Under optimized conditions, Cage4+ had a drift time of 23.99 ms, while the same experimental parameters provided slightly longer drift times of 25.62 ms and 26.01 ms for C60@Cage4+ and C70@Cage4+, respectively (Fig. S45†). Thus, the host-guest complexes have very similar effective cross sections, while the empty host appears slightly smaller, which is consistent with TWIMS measurements reported for other cationic hosts that encapsulate fullerenes.17a
Electrochemical characterization
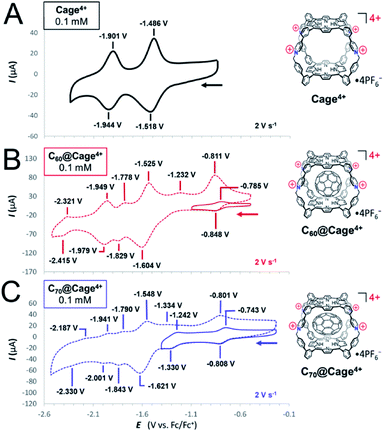
The electron-accepting properties of fullerenes are important to many of their possible applications,6 so cyclic voltammetry was used to examine how encapsulation by Cage4+ affects the redox properties of C60 and C70 (Fig. 3 and Table 1). First, Cage4+ was examined in the absence of fullerenes, revealing reversible reductions at −1.51 V and −1.92 V vs. Fc+/0 in DMF (Fig. 3A). By comparison with monomeric porphyrins representing each half of Cage4+ (Fig. S46 and S47†), the first reduction wave of the cage was assigned to the reductions of both porphyrin faces and the four pyridinium groups, while the smaller, more negative reduction feature corresponds to the second reduction of each porphyrin. However, the measured currents are closer to a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio rather than the expected 3
1 ratio rather than the expected 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.** Deviation from ideal behavior is unsurprising considering that anions affect the diffusion rate of Cage4+ (see above), and interactions between the cage and anions will be diminished as the cage is reduced. Assignments of the redox features of Cage4+ were confirmed by UV-vis-NIR monitoring of redox titrations (see below).
1.** Deviation from ideal behavior is unsurprising considering that anions affect the diffusion rate of Cage4+ (see above), and interactions between the cage and anions will be diminished as the cage is reduced. Assignments of the redox features of Cage4+ were confirmed by UV-vis-NIR monitoring of redox titrations (see below).
| 1st Cn reduction | 2nd Cn reduction | 1st Cage reduction | 3rd Cn reduction | 2nd Cage reduction | 4th Cn reduction | |
|---|---|---|---|---|---|---|
| a Unless otherwise noted, potentials refer to E1/2 values relative to Fc+/0 for reversible redox couples measured in DMF containing 0.1 mM analyte and 0.1 M [Bu4N][PF6] supporting electrolyte. b Reduction potentials of C60 are taken from ref. 18. c E pc value for a reduction of the C60 guest that overlaps with a reduction of the host. d This redox couple is poorly defined. | ||||||
| C60b | −0.77 V | −1.23 V | — | −1.82 V | — | −2.36 V |
| C70 | −0.77 V | −1.19 V | — | −1.71 V | — | −2.21 V |
| C60@Cage4+ | −0.82 V | [–1.33 V]c | −1.56 V | −1.80 V | −1.96 V | −2.37 V |
| C70@Cage4+ | −0.78 V | −1.29 V | −1.58 V | −1.82 V | −1.97 V | [–2.26 V]d |
| Cage4+ | — | — | −1.50 V | — | −1.92 V | — |
The host-guest complexes C60@Cage4+ and C70@Cage4+ both show additional redox couples arising from the fullerene guests (Fig. 3B and C). The first fullerene-centred reductions are reversible for both complexes and occur at potentials (E1/2 = −0.82 V, C60@Cage4+; −0.78 V, C70@Cage4+) that are slightly negative of those of free C60 (−0.77 V)18 and C70 (−0.77 V)†† in DMF (Table 1). Measurements in PhCN (Fig. S56–S59†) also showed that the first two reduction potentials of C60 and C70 are not altered much by encapsulation in Cage4+ (Table S1†). These observations are surprising since other cationic hosts shift the reduction potentials of fullerenes by as much as +500 mV.4c We reasoned that anions associated with C60@Cage4+ and C70@Cage4+ might mitigate the electrostatic influence of the host, especially given the high electrolyte concentration used in CV measurements. However, treatment of C60@Cage4+ with 1 equiv. of a diaryl viologen radical cation as a mild chemical reductant (E1/2 = −0.71 V, Fig. S53†) resulted in nearly the same equilibrium ratio of C60@Cage4+ to C60−@Cage4+ in samples containing from 0.1 mM to 100 mM concentrations of PF6− (Fig. S73†). Thus, the reduction potential of the guest does not appear to be affected by PF6− concentration. Likewise, addition of up to 100 mM concentrations of TBAOTf or TBABPh4 did not substantially alter the reduction of the guest by the chemical reductant. Since neither the anions nor solvent appear essential for preserving the reduction potentials of the fullerenes upon encapsulation, we speculate that the electrostatic influence of Cage4+ is balanced well by the electron-donating influence of its porphyrin walls towards the fullerene guests.
Regardless of the specific cause, it is notable that Cage4+ minimally affects the first reduction potentials of C60 and C70. Likewise, it is noteworthy that, even at scan rates as high as 50 V s−1, the first reductions of C60@Cage4+ and C70@Cage4+ show nearly ideal reversibility (ΔEpc ≤ 72 mV, Fig. S60†), whereas most hosts severely diminish the reversibility of fullerene redox processes,4a,d,8a indicating slowed electron-transfer rates or low stability of the reduced complexes. Thus, Cage4+ is unique as a host that solubilizes fullerenes while preserving their reduction potentials and electron transfer capabilities. These features are likely to be useful since the inherent electron-accepting properties of fullerenes are well studied for a variety of applications,19 and other solubilization strategies (e.g., covalent functionalization) also tend to alter the redox properties of fullerenes.20
Cage4+ and the fullerenes show greater mutual influence on each other's redox behaviour when more negative reductions of the host-guest complexes are examined, though even these processes are only modestly perturbed. The second reduction of C70@Cage4+ (E1/2 = −1.29 V) is observed clearly at a potential that is 100 mV negative of the free C70−/C702− redox couple (E1/2 = −1.19 V), while the second fullerene reduction of C60@Cage4+ overlaps partially with reduction of the host, also representing about a 100 mV cathodic shift relative to free C60−/C602− (Table 1). The first reductions of the host are also shifted cathodically by 60 to 80 mV for each complex (Table 1), suggesting the bound fullerides exert a small electrostatic influence on the host. However, the next cage-centered reductions are altered by a smaller amount (40 to 50 mV, Table 1). We speculate that electrostatic repulsion between the host and guests in their more reduced states (i.e., when both are in anionic states) triggers expulsion of the fulleride anions, such that reduction processes corresponding to the empty host and free guest are observed at more negative potentials. Consistent with this possibility, the most negative observable reduction of C60@Cage4+ appears at nearly the same potentials as the free C603−/C604− redox couple (note that the most negative reduction of C70@Cage4+ was not clear enough for comparisons).
1H NMR binding studies of C60n− (n = 1, 2)
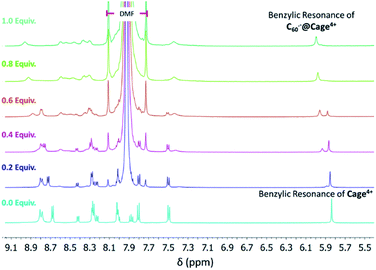
We were unable to acquire high quality CV data in MeCN, so we sought to directly observe the binding of fulleride anions by Cage4+ in CD3CN to complement the electrochemical measurements performed in DMF and PhCN. The association of C60− and C602− in Cage4+ was evident via titration experiments monitored by 1H NMR spectroscopy. A solution of [Cp*2Co][C60] in DMF was titrated in 0.1 equiv. increments into a solution of Cage4+ in CD3CN, resulting in the appearance of a new benzylic CH signal at ca. 5.95 ppm for the host-guest complex C60−@Cage4+. Despite the radical character of the guest, this new benzylic CH signal was resolved clearly enough to be observed as distinct from that of the empty host, which decreased steadily as C60− was added (Fig. 4 and S31†). Similar results were obtained upon titration of Cage4+ with a solution of C602− (Fig. S32†).Integration of the benzylic CH resonances of empty Cage4+vs. those of C60−@Cage4+ and C602−@Cage4+ provided association constants of approximately 107 M−1 and 105 M−1, respectively, which are lower than that (≥6.0 × 108 M−1) estimated for C60@Cage4+ in MeCN. Each order of magnitude decrease in the strength of association should correspond to a −59 mV shift of the reduction potential of the guest, so the binding constants determined in these experiments imply greater changes to the redox properties of C60 than were measured electrochemically in DMF and PhCN. However, the 1H NMR measurements likely underestimate the association constants because broadening of the resonances of the host-guest complexes leads to systematic lowering of the integrals of these signals, making it difficult to accurately quantify the concentrations of C60−@Cage4+ and C602−@Cage4+. Thus, while accurate quantitative comparisons cannot be made, the NMR studies confirm that C60− and C602− associate strongly with Cage4+ in MeCN, which provides qualitative agreement with CV measurements performed in DMF and PhCN. The effects of further reduction could not be examined by NMR methods but were examined by UV-vis-NIR spectroscopy as described in the next section.
UV-vis-NIR spectroscopy of Cage4+ and its host-guest complexes with fullerenes and fullerides
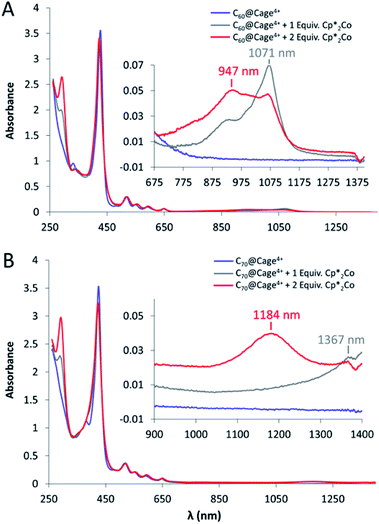
The UV-vis spectrum of Cage4+ in DMF displays one Soret band and one set of Q-peaks, suggesting the two distinct porphyrin faces of the cage are electronically similar, as is consistent with their overlapping redox features observed by cyclic voltammetry. Reduction of the cage with decamethylcobaltocene (Cp*2Co) causes the appearance of low-energy (>650 nm) absorbances that are characteristic of porphyrin radical anions (Fig. S62†).21 The intensity of these bands increases steadily with the addition of up to 4 equiv. Cp*2Co, and then less dramatically up to 6 equiv., which is consistent with nearly equal distribution of reduction over the two porphyrin faces and four pyridinium groups until all six sites are fully reduced. Addition of two more equivalents of Cp*2Co causes the low-energy bands to increase again, indicating further reduction of the porphyrins to their dianionic states (Fig. S62†). These results support the interpretation of the CV data described above for Cage4+, which is also consistent with DFT results indicating that the eight lowest unoccupied orbitals of the cage are centered on either the porphyrin faces or the pyridinium groups (Fig. S76†).Since the first two fullerene reductions occur at least slightly positive of the first reductions of Cage4+, it was possible to spectroscopically observe host-guest complexes of the singly and double reduced fullerenes in the 4+ charged host. The complex C60@Cage4+ was reduced via two sequential 1 equiv. additions of Cp*2Co, resulting in the appearance of characteristic absorbances for the C60 mono- and di-anions between 800–1200 nm (Fig. 5A).6 Similarly, sequential 1 e− reductions of C70@Cage4+ with Cp*2Co produces spectra that display absorbances consistent with those of C70− and C702− (Fig. 5B).22 The Soret band and Q-peaks of Cage4+ remain unchanged in these spectra, confirming that the host is unreduced.
Further reduction of the host-guest complexes was examined to determine if ejection of the fulleride guests occurs as was suggested by CV data. The complex C60@Cage4+ was treated with 8 equiv. of Cp*2Co in MeCN, reducing the host by 6 e− and the C60 guest by 2 e−. The reduced host has negligible solubility in MeCN, resulting in complete disappearance of any porphyrin spectral features, but a significant concentration of the C602− anion remained detectable by its NIR absorption band, representing 22% of the C60 that was initially present. This concentration represents a binding constant of 3.2 × 104 M−1 for C602-@Cage2- if it is assumed that full equilibration between bound and unbound C602− occurs prior to precipitation of the reduced host. This result confirms that reduction of the host weakens the binding of C602− by at least a small amount. In contrast, performing this experiment using C70@Cage4+ did not yield the free C702− anion in solution. These observations are consistent with CV results showing that the C702−/C703− redox couple differs by 100 mV for encapsulated vs. free C70 (Table 1), indicating the fulleride remains bound in the reduced host, at least on the short timescales of these experiments.
Conclusion
In summary, a new covalently linked nanocage Cage4+ has been synthesized on a gram scale using simple synthetic methods and inexpensive starting materials. This cage binds the fullerenes C60 and C70 with high affinities (Ka > 108 M−1 in MeCN) and displays excellent selectivity for extracting C70 from mixtures containing an excess of C60. Cage4+ also strongly binds the 1- and 2-states of the fullerenes. The different oxidation states of the host-guest complexes were characterized by several methods, including cyclic voltammetry and spectroscopic techniques, revealing that the electronic properties of the fullerenes are surprisingly unperturbed by encapsulation in Cage4+, in contrast to recent reports of fullerene guests in other cationic cages.4a,c,dThese findings suggest that Cage4+ may be a particularly useful host for separating and solubilizing fullerenes. Its performance is comparable to or better than that of many notable fullerene-binding hosts in terms of overall affinity and the ability to separate C70 from C60, while the low-cost scalable synthesis makes Cage4+ much more promising for applications. Additionally, since the encapsulation of fullerenes by this host does not appear to substantially alter their redox behaviour, C60@Cage4+ and C70@Cage4+ may be directly useful as solubilized fullerene derivatives that preserve this important functional property of the fullerene guests.
Author contributions
D. A. Rothschild and M. C. Lipke designed Cage4+. D. A. Rothschild and W. P. Kopcha carried out syntheses and performed experiments. D. A. Rothschild and A. Tran carried out DFT calculations. All authors contributed to analyzing data and preparing the results for publication.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. C. L. would like to thank the ACS Petroleum Research Fund (grant #61015-DNI3) and Rutgers, The State University of New Jersey for support. J. Z. would like to thank the support from the Department of Energy (grant no. DE-SC0020260).Notes and references
- (a) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810–6918 CrossRef CAS PubMed; (b) F. J. Rizzuto, L. K. S. von Krbek and J. R. Nitschke, Nat. Rev. Chem., 2019, 3, 204–222 CrossRef; (c) E. G. Percástegui and V. Jancik, Coord. Chem. Rev., 2020, 407, 213165 CrossRef; (d) D. Canevet, E. M. Pérez and N. Martín, Angew. Chem., Int. Ed. Engl., 2011, 50, 9248–9259 CrossRef CAS; (e) T. Y. Kim, R. A. S. Vasdev, D. Preston and J. D. Crowley, Chem. –Eur. J., 2018, 24, 14878–14890 CrossRef CAS PubMed.
- (a) C. García-Simón, M. Garcia-Borràs, L. Gómez, T. Parella, S. Osuna, J. Juanhuix, I. Imaz, D. Maspoch, M. Costas and X. Ribas, Nat. Commun., 2014, 5, 5557 CrossRef PubMed; (b) D. Zhang, T. K. Ronson, R. Lavendomme and J. R. Nitschke, J. Am. Chem. Soc., 2019, 141, 18949–18953 CrossRef CAS PubMed; (c) A. B. Grommet, J. B. Hoffman, E. G. Percástegui, J. Mosquera, D. J. Howe, J. L. Bolliger and J. R. Nitschke, J. Am. Chem. Soc., 2018, 140, 14770–14776 CrossRef CAS PubMed; (d) W. Brenner, T. K. Ronson and J. R. Nitschke, J. Am. Chem. Soc., 2017, 139, 75–78 CrossRef CAS PubMed; (e) E. Huerta, G. A. Metselaar, A. Fragoso, E. Santos, C. Bo and J. de Mendoza, Angew. Chem., Int. Ed. Engl., 2007, 46, 202–205 CrossRef CAS PubMed; (f) C. García-Simón, A. Monferrer, M. Garcia-Borràs, I. Imaz, D. Maspoch, M. Costas and X. Ribas, Chem. Commun., 2019, 55, 798–801 RSC.
- (a) M. Zhang, M. L. Saha, M. Wang, Z. Zhou, B. Song, C. Lu, X. Yan, X. Li, F. Huang, S. Yin and P. J. Stang, J. Am. Chem. Soc., 2017, 139, 5067–5074 CrossRef CAS PubMed; (b) T. Kawase, K. Tanaka, Y. Seirai, N. Shiono and M. Oda, Angew. Chem., Int. Ed. Engl., 2003, 42, 5597–5600 CrossRef CAS PubMed.
- (a) F. J. Rizzuto, D. M. Wood, T. K. Ronson and J. R. Nitschke, J. Am. Chem. Soc., 2017, 139, 11008–11011 CrossRef CAS PubMed; (b) R. L. Spicer, A. D. Stergiou, T. A. Young, F. Duarte, M. D. Symes and P. J. Lusby, J. Am. Chem. Soc., 2020, 142, 2134–2139 CrossRef CAS PubMed; (c) S. Hasegawa, S. L. Meichsner, J. J. Holstein, A. Baksi, M. Kasanmascheff and G. H. Clever, J. Am. Chem. Soc., 2021, 143, 9718–9723 CrossRef CAS PubMed; (d) K. Matsumoto, S. Kusaba, Y. Tanaka, Y. Sei, M. Akita, K. Aritani, M. A. Haga and M. Yoshizawa, Angew. Chem., Int. Ed. Engl., 2019, 58, 8463–8467 CrossRef CAS PubMed.
- (a) M. Morimoto, S. M. Bierschenk, K. T. Xia, R. G. Bergman, K. N. Raymond and F. D. Toste, Nat. Catal., 2020, 3, 969–984 CrossRef CAS; (b) W. X. Gao, H. N. Zhang and G. X. Jin, Coord. Chem. Rev., 2019, 386, 69–84 CrossRef CAS.
- C. A. Reed and R. D. Bolskar, Chem. Rev., 2000, 100(3), 1075–1120 CrossRef CAS PubMed.
- (a) W. Sun, Y. Wang, L. Ma, L. Zheng, W. Fang, X. Chen and H. Jiang, J. Org. Chem., 2018, 83, 14667–14675 CrossRef CAS PubMed; (b) M. J. Li, C. H. Huang, C. C. Lai and S. H. Chiu, Org. Lett., 2012, 14, 6146–6149 CrossRef CAS PubMed.
- (a) X. Chang, S. Lin, G. Wang, C. Shang, Z. Wang, K. Liu, Y. Fang and P. J. Stang, J. Am. Chem. Soc., 2020, 142, 15950–15960 CrossRef CAS PubMed; (b) K. Suzuki, K. Takao, S. Sato and M. Fujita, J. Am. Chem. Soc., 2010, 132, 2544–2545 CrossRef CAS PubMed.
- (a) E. Ubasart, O. Borodin, C. Fuertes-Espinosa, Y. Xu, C. García-Simón, L. Gómez, J. Juanhuix, F. Gándara, I. Imaz, D. Maspoch, M. von Delius and X. Ribas, Nat. Chem., 2021, 13, 420–427 CrossRef CAS PubMed; (b) V. Leonhardt, S. Fimmel, A. M. Krause and F. Beuerle, Chem. Sci., 2020, 11, 8409–8415 RSC.
- (a) H. Nobukuni, Y. Shimazaki, H. Uno, Y. Naruta, K. Ohkubo, T. Kojima, S. Fukuzumi, S. Seki, H. Sakai, T. Hasobe and F. Tani, Chem. –Eur. J., 2010, 16, 11611–11623 CrossRef CAS; (b) Y. Shi, K. Cai, H. Xiao, Z. Liu, J. Zhou, D. Shen, Y. Qiu, Q. H. Guo, C. Stern, M. R. Wasielewski, F. Diederich, W. A. Goddard and J. F. Stoddart, J. Am. Chem. Soc., 2018, 140, 13835–13842 CrossRef CAS PubMed; (c) J. Song, N. Aratani, H. Shinokubo and A. Osuka, J. Am. Chem. Soc., 2010, 132, 16356–16357 CrossRef CAS PubMed; (d) C. Zhang, Q. Wang, H. Long and W. Zhang, J. Am. Chem. Soc., 2011, 133, 20995–21001 CrossRef CAS PubMed; (e) F. Hajjaj, K. Tashiro, H. Nikawa, N. Mizorogi, T. Akasaka, S. Nagase, K. Furukawa, T. Kato and T. Aida, J. Am. Chem. Soc., 2011, 133, 9290–9292 CrossRef CAS PubMed; (f) M. Schmittel, B. He and P. Mal, Org. Lett., 2008, 10, 2513–2516 CrossRef CAS PubMed; (g) T. Nakamura, H. Ube, R. Miyake and M. Shionoya, J. Am. Chem. Soc., 2013, 135, 18790–18793 CrossRef CAS; (h) W. Meng, B. Breiner, K. Rissanen, J. D. Thoburn, J. K. Clegg and J. R. Nitschke, Angew. Chem., Int. Ed. Engl., 2011, 50, 3479–3483 CrossRef CAS; (i) Y. Xu, S. Gsänger, M. B. Minameyer, I. Imaz, D. Maspoch, O. Shyshov, F. Schwer, X. Ribas, T. Drewello, B. Meyer and M. von Delius, J. Am. Chem. Soc., 2019, 141, 18500–18507 CrossRef CAS PubMed; (j) A. R. Mulholland, C. P. Woodward and S. J. Langford, Chem. Commun., 2011, 47, 1494–1496 RSC; (k) A. L. Kieran, S. I. Pascu, T. Jarrosson and J. K. M. Sanders, Chem. Commun., 2005, 10, 1276–1278 RSC; (l) D. M. Wood, W. Meng, T. K. Ronson, A. Stefankiewicz, J. K. M. Sanders and J. R. Nitschke, Angew. Chem., Int. Ed. Engl., 2015, 54, 3988–3992 CrossRef CAS PubMed.
- (a) T. A. Barendt, W. K. Myers, S. P. Cornes, M. A. Lebedeva, K. Porfyrakis, I. Marques, V. Félix and P. De Beer, J. Am. Chem. Soc., 2020, 142, 349–364 CrossRef CAS PubMed; (b) K. Mahata, P. D. Frischmann and F. Würthner, J. Am. Chem. Soc., 2013, 135, 15656–15661 CrossRef CAS PubMed; (c) Z. Lu, T. K. Ronson and J. R. Nitschke, Chem. Sci., 2020, 11, 1097–1101 RSC.
- (a) V. Martínez-Agramunt, T. Eder, H. Darmandeh, G. Guisado-Barrios and E. Peris, Angew. Chem., Int. Ed. Engl., 2019, 58, 5682–5686 CrossRef PubMed; (b) V. Martínez-Agramunt, D. G. Gusev and E. Peris, Chem. –Eur. J., 2018, 24, 14802–14807 CrossRef PubMed; (c) T. K. Ronson, A. B. League, L. Gagliardi, C. J. Cramer and J. R. Nitschke, J. Am. Chem. Soc., 2014, 136, 15615–15624 CrossRef CAS PubMed.
- (a) N. Kishi, M. Akita, M. Kamiya, S. Hayashi, H. F. Hsu and M. Yoshizawa, J. Am. Chem. Soc., 2013, 135, 12976–12979 CrossRef CAS PubMed; (b) N. Kishi, Z. Li, K. Yoza, M. Akita and M. Yoshizawa, J. Am. Chem. Soc., 2011, 133, 11438–11441 CrossRef CAS PubMed; (c) T. K. Ronson, B. S. Pilgrim and J. R. Nitschke, J. Am. Chem. Soc., 2016, 138, 10417–10420 CrossRef CAS PubMed; (d) M. Yamashina, T. Yuki, Y. Sei, Y. Akita and M. Yoshizawa, Chem. –Eur. J., 2015, 21, 4200–4204 CrossRef CAS PubMed; (e) D. Canevet, M. Gallego, H. Isla, A. de Juan, E. M. Pérez and N. Martín, J. Am. Chem. Soc., 2011, 133, 3184–3190 CrossRef CAS PubMed; (f) G. Bastien, P. I. Dron, M. Vincent, D. Canevet, M. Allain, S. Goeb and M. Sallé, Org. Lett., 2016, 18, 5856–5859 CrossRef CAS PubMed.
- (a) J. Q. Wang, Y. Han and C. F. Chen, Chem. Commun., 2021, 57, 3987–3990 RSC; (b) M. Samanta, A. Rananaware, D. N. Nadimetla, S. A. Rahaman, M. Saha, R. W. Jadhav, S. V. Bhosale and S. Bandyopadhyay, Sci. Rep., 2019, 9, 9670 CrossRef PubMed; (c) C. Coluccini, D. Dondi, M. Caricato, A. Taglietti, M. Boiocchi and D. Pasini, Org. Biomol. Chem., 2010, 8, 1640–1649 RSC; (d) K. A. Nielsen, W. S. Cho, G. H. Sarova, B. M. Petersen, A. D. Bond, J. Becher, F. Jensen, D. M. Guldi, J. L. Sessler and J. O. Jeppesen, Angew. Chem., Int. Ed. Engl., 2006, 45, 6848–6853 CrossRef CAS PubMed; (e) T. Matsuno, S. Sato, R. Iizuka and H. Isobe, Chem. Sci., 2015, 6, 909–916 RSC; (f) A. Sygula, F. R. Fronczek, R. Sygula, P. W. Rabideau and M. M. Olmstead, J. Am. Chem. Soc., 2007, 129, 3842–3843 CrossRef CAS PubMed; (g) B. Chen, J. J. Holstein, S. Horiuchi, W. G. Hiller and G. H. Clever, J. Am. Chem. Soc., 2019, 141, 8907–8913 CrossRef CAS PubMed; (h) A. Ikeda, H. Udzu, M. Yoshimura and S. Shinkai, Tetrahedron, 2000, 56, 1825–1832 CrossRef CAS; (i) M. Zhang, H. Xu, M. Wang, M. L. Saha, Z. Zhou, X. Yan, H. Wang, X. Li, F. Huang, N. She and P. J. Stang, Inorg. Chem., 2017, 56, 12498–12504 CrossRef CAS PubMed; (j) S. I. Kawano, T. Fukushima and K. Tanaka, Angew. Chem., Int. Ed. Engl., 2018, 57, 14827–14831 CrossRef CAS PubMed; (k) J. Wang, X. Zhang, H. Jia, S. Wang and P. Du, Acc. Chem. Res., 2021, 54, 4178–4190 CrossRef CAS PubMed; (l) Y. Ni, F. Gordillo-Gamez, M. P. Alvarez, Z. Nan, Z. Li, S. Wu, Y. Han, J. Casado and J. Wu, J. Am. Chem. Soc., 2020, 142, 12730–12742 CrossRef CAS PubMed; (m) P. C. Purba, M. Maity, S. Bhattacharyya and P. S. Mukherjee, Angew. Chem., Int. Ed. Engl., 2021, 60, 14109–14116 CrossRef CAS PubMed; (n) G. Zango, M. Krug, S. Krishna, V. Marinas, T. Clark, M. V. Martinez-Diaz, D. M. Guildi and T. Torres, Chem. Sci., 2020, 11, 3448–3459 RSC; (o) H. Chen, Z. Xia and Q. Miao, Chem. Sci., 2022, 13, 2280–2285 RSC.
- K. N. Semenov, N. A. Charykov, V. A. Keskinov, A. K. Piartman, A. A. Blokhin and A. A. Kopyrin, J. Chem. Eng. Data, 2010, 55, 13–36 CrossRef CAS.
- L. Avram and Y. Cohen, Chem. Soc. Rev., 2015, 44, 586–602 RSC.
- (a) C. Vicent, V. Martinez-Agramunt, V. Ghandi, C. Larriba-Andaluz, D. G. Gusev and E. Peris, Angew. Chem., Int. Ed. Engl., 2021, 60, 15412–15417 CrossRef CAS PubMed; (b) E. Kalenius, M. Groessl and K. Rissanen, Nat. Rev. Chem., 2019, 3, 4–14 CrossRef CAS; (c) L. Polewski, A. Springer, K. Pagel and C. A. Schalley, Acc. Chem. Res., 2021, 54, 2445–2456 CrossRef CAS PubMed.
- D. Dubois, G. Moninot, W. Kutner, M. T. Jones and K. M. Kadish, J. Phys. Chem., 1992, 96, 7137–7145 CrossRef CAS.
- (a) A. V. Baskar, M. R. Benzigar, S. N. Talapaneni, G. Singh, A. S. Karakot, J. Yi, A. H. Al-Muhsaseb, K. Ariga, P. M. Ajayan and A. Vinu, Adv. Funct. Mater., 2021, 2106924 Search PubMed; (b) J. Liu, L. Qiu and S. Shao, J. Mater. Chem. C, 2021, 9, 16143–16163 RSC; (c) Z. Jiang, Y. Zhao, X. Lu and J. Xie, J. Energy Chem., 2021, 55, 70–79 CrossRef; (d) J. Coro, M. Suárez, L. S. R. Silvia, K. I. B. Eguiluz and G. R. Salazar-Banda, Int. J. Hydrogen Energy, 2016, 41, 17944–17959 CrossRef CAS; (e) Y. Fang, C. Bi, D. Wang and J. Huang, ACS Energy Lett., 2017, 2, 782–794 CrossRef CAS.
- L. Echegoyen and L. E. Echegoyen, Acc. Chem. Res., 1998, 31, 593–601 CrossRef CAS.
- K. G. Dutton, D. A. Rothschild, D. B. Pastore, T. J. Emge and M. C. Lipke, Inorg. Chem., 2020, 59, 12616–12624 CrossRef CAS PubMed.
- D. R. Lawson, D. L. Feldhiem, C. A. Foss, P. K. Dorhout, C. M. Elliott, C. R. Martin and B. Parkinson, J. Electrochem. Soc., 1992, 139, L68–L71 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic, experimental, and computational details; 1H, 13C{1H}, and DOSY NMR spectra; UV-vis-NIR spectra; ESI-HRMS data; Cyclic voltammograms. See https://doi.org/10.1039/d2sc00445c |
| ‡ For fullerene receptors utilizing precious metals as linking agents for aromatic walls, see ref. 2a, 3a, 7a, 8a, 8b, 12a, 12b, 13a, 13b, 13d, 13f, 14h, 14i, and 14m. |
| § For fullerene receptors formed using covalent linkages, see ref. 10c, 10b, 10d, 10i, 10j, 10k, 11a, 14a, 14b, 14e, 14f, 14k, 14l, 14n and 14o. |
| ¶ For fullerene receptors requiring numerous synthetic steps, see ref. 4d, 7a, 10a, 10d, 10e, 10f, 10i, 10j, 14f, 14j, and 14k. |
| || We define a 3D receptor as having a well-defined three dimensional pore (see ref. 2a, 2f, 4a, 7a, 8a, 8b, 9b, 10a, 10b, 10c, 10d, 10e, 10f, 10g, 10h, 10i, 10j, 10k, 10l, 11a, 11b, 11c, 12a, 12b, 13a, 13b, 13c, 13d, 14c, 14h, 14i, 14j, 14l, 14m, and 14n). This definition includes all nanocage structures as well as macrocycles with large aromatic surfaces that define a 3D volume between them, but excludes most molecular tweezers, macrocycles, and linear “wrap-around” hosts that typically show lower association constants for binding fullerenes. Even among these latter receptors, only one has been prepared on a scale of >1 g (see ref. 14a). |
| ** We also attempted to characterize Cage4+ and its host-guest complexes by differential pulse voltammetry, but reliable DPV measurements could not be obtained, possibly due to adhesion of Cage4+ to the electrode. |
| †† Reduction potentials of C70 were measured in DMF (0.1 M TBAPF6) following the technique described in ref. 18. |
| This journal is © The Royal Society of Chemistry 2022 |