Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Redox-neutral manganese-catalyzed synthesis of 1-pyrrolines†
Tingting
Feng
a,
Canxiang
Liu
a,
Zhen
Wu
a,
Xinxin
Wu
*a and
Chen
Zhu
 *ab
*ab
aKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu 215123, People's Republic of China. E-mail: chzhu@suda.edu.cn; xxwu99@suda.edu.cn
bFrontiers Science Center for Transformative Molecules, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, People's Republic of China
First published on 9th February 2022
Abstract
This report describes a manganese-catalyzed radical [3 + 2] cyclization of cyclopropanols and oxime ethers, leading to valuable multi-functional 1-pyrrolines. In this redox-neutral process, the oxime ethers function as internal oxidants and H-donors. The reaction involves sequential rupture of C–C, C–H and N–O bonds and proceeds under mild conditions. This intermolecular protocol provides an efficient approach for the synthesis of structurally diverse 1-pyrrolines.
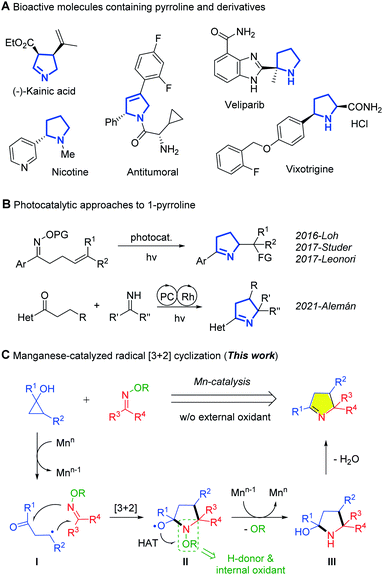
Pyrroline and its derivatives appear frequently as the core of the structure of natural products and biologically active molecules (Fig. 1A).1 Such compounds also serve as versatile feedstocks in various transformations, such as 1,3-dipolar cyclization, ring opening, reduction and oxidation, leading to diverse and valuable compounds.2–4 Over the past few decades, great effort has been devoted to the preparation of pyrrolines. This has resulted in several elegant approaches that rely on photoredox catalysis (Fig. 1B).5 The groups of Studer,5a,b Leonori,5c and Loh5d–f disclosed intramolecular addition of the intermediate iminyl radical to alkenes to construct pyrrolines. Generally, the synthetic value of a method can be further improved by using an intermolecular reaction pattern. For example, Alemán et al. recently reported a radical-polar cascade reaction involving the addition to ketimines of alkyl radicals formed in hydrogen atom transfer (HAT) reactions.5g That the existence of benzylic C–H bonds in the substrates is requisite for the HAT, compromises the substrate scope. Despite the appealing photochemical processes, development of new redox approaches to enrich the product diversity of pyrrolines, especially with inexpensive transition-metal catalysts, is still in demand.
Prompted by extensive applications of cyclopropanols in synthesis6 and our achievements in manganese-catalyzed ring-opening reactions,7 we conceived a radical [3 + 2] cyclization using cyclopropanol as a C3 synthon and oxime ethers as a nitrogen source (Fig. 1C). Hypothetically, single-electron oxidation of cyclopropanol by Mnn generates the β-keto radical (I), which undergoes a radical [3 + 2] cascade reaction with an oxime ether to give the alkoxy radical species (II). Conversion of II to the intermediate (III), the pyrroline precursor, requires an extra H-donor to support a HAT process and an oxidant for recovery of Mnn to perpetuate the catalytic cycle. In this scenario, the strategic inclusion of oxime ether is crucial to the overall transformation. The oxime ether is not only an internal oxidant and H-donor, but should also be subject to in situ deprotection by cleaving the N–O bond during the reaction. The choice of a proper Mnn/Mnn−1 pair with suitable redox potentials is also vital to the catalytic cycle.
Herein, we provide proof-of-principle studies for this hypothesis. The desired radical [3 + 2] cyclization of cyclopropanols and O-benzyl oxime ethers is accomplished with manganese catalysis. This redox-neutral process involves sequential rupture of C–C, C–H, and N–O bonds under mild conditions. The intermolecular protocol provides an ingenious approach to the synthesis of multi-functionalized 1-pyrrolines.
With these considerations in mind, phenylcyclopropanol (1a) and oxime ether (2a) were initially chosen as model substrates to evaluate reaction parameters in the presence of manganese salt (Table 1). With the use of 1.7 equiv. of manganic acetylacetonate (Mn(acac)3) and acetic acid, the pyrroline product (3a) was readily obtained at room temperature, albeit in low yield (entry 1). Acetic acid is crucial to the transformation in this case, presumably serves to activate the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of 2a (entry 2). The optimization of organic solvents was then conducted (entries 3–8), and it was found that the use of fluorinated alcohols, such as trifluoroethanol (TFE) and hexafluoroisopropanol (HFIP) as solvents provided excellent yields (entries 7 and 8). Decreasing the amount of Mn(acac)3 to 1.2 equiv. gave a comparable yield (entry 9), but further reducing the amount compromised the yield (entry 10). Replacing Mn(acac)3 with Mn(OAc)3 or MnCl2 significantly decreased the reaction yield (entries 11 and 12). However, the use of Mn(acac)2 gave a similar yield to Mn(acac)3 (entries 13 vs. 9). The above results prompted us to think over the counteranion effect that the acetylacetone (acac) anion may be requisite to the reaction. Indeed, the synergistic use of stoichiometric MnCl2 and acetylacetone led to a good yield of the desired product (entry 14). More importantly, a comparable yield was obtained with only 0.2 equiv. of MnCl2 and added acetylacetone, realizing this reaction under a catalytic amount of Mn salts (entry 15). Given that the low solubility of the Mn salt may lead to poor efficiency, a reaction with 0.067 M concentration was carried out and gave a 89% yield (entry 16). Further reducing the amount of acetylacetone to 1.0 equiv. had no influence on the outcome of the reaction (entry 17), but the reaction efficiency slightly decreased when 0.6 equiv. of acetylacetone was used as the additive (entry 18). Use of a decreased amount (1.0 equiv.) of acetic acid led to the best yield (91%, entry 19), whereas the reaction in the presence of 0.5 equiv. acetic acid (entry 20) or without acetic acid (entry 21) also gave high yields. It is noted that acetic acid is not crucial to the reaction using MnCl2 as catalyst, as the reaction could generate cat. HCl in situ. The reaction with substoichiometric amount (0.6 equiv.) of acac gave a decreased but also good yield (entry 22). Reducing the catalytic loading of MnCl2 to 10 mol% slightly compromised the yield (entry 23).
N bond of 2a (entry 2). The optimization of organic solvents was then conducted (entries 3–8), and it was found that the use of fluorinated alcohols, such as trifluoroethanol (TFE) and hexafluoroisopropanol (HFIP) as solvents provided excellent yields (entries 7 and 8). Decreasing the amount of Mn(acac)3 to 1.2 equiv. gave a comparable yield (entry 9), but further reducing the amount compromised the yield (entry 10). Replacing Mn(acac)3 with Mn(OAc)3 or MnCl2 significantly decreased the reaction yield (entries 11 and 12). However, the use of Mn(acac)2 gave a similar yield to Mn(acac)3 (entries 13 vs. 9). The above results prompted us to think over the counteranion effect that the acetylacetone (acac) anion may be requisite to the reaction. Indeed, the synergistic use of stoichiometric MnCl2 and acetylacetone led to a good yield of the desired product (entry 14). More importantly, a comparable yield was obtained with only 0.2 equiv. of MnCl2 and added acetylacetone, realizing this reaction under a catalytic amount of Mn salts (entry 15). Given that the low solubility of the Mn salt may lead to poor efficiency, a reaction with 0.067 M concentration was carried out and gave a 89% yield (entry 16). Further reducing the amount of acetylacetone to 1.0 equiv. had no influence on the outcome of the reaction (entry 17), but the reaction efficiency slightly decreased when 0.6 equiv. of acetylacetone was used as the additive (entry 18). Use of a decreased amount (1.0 equiv.) of acetic acid led to the best yield (91%, entry 19), whereas the reaction in the presence of 0.5 equiv. acetic acid (entry 20) or without acetic acid (entry 21) also gave high yields. It is noted that acetic acid is not crucial to the reaction using MnCl2 as catalyst, as the reaction could generate cat. HCl in situ. The reaction with substoichiometric amount (0.6 equiv.) of acac gave a decreased but also good yield (entry 22). Reducing the catalytic loading of MnCl2 to 10 mol% slightly compromised the yield (entry 23).
| Entrya | Mn salt (equiv.) | Additive (equiv.) | Solvent | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.45 mmol), 2a (0.3 mmol), AcOH (2.0 equiv.), and Mn salt (as shown) in solvent (2.0 mL), at room temperature (rt) under N2, for 16 h. b Without AcOH. c 0.067 M reaction. d 1.0 equiv. AcOH. e 0.5 equiv. AcOH. acac = acetylacetone. | ||||
| 1 | Mn(acac)3 (1.7) | None | CH3CN | 33 |
| 2b | Mn(acac)3 (1.7) | None | CH3CN | Trace |
| 3 | Mn(acac)3 (1.7) | None | DCM | 31 |
| 4 | Mn(acac)3 (1.7) | None | Acetone | 25 |
| 5 | Mn(acac)3 (1.7) | None | DMSO | Trace |
| 6 | Mn(acac)3 (1.7) | None | DMF | Trace |
| 7 | Mn(acac)3 (1.7) | None | TFE | 80 |
| 8 | Mn(acac)3 (1.7) | None | HFIP | 82 |
| 9 | Mn(acac)3 (1.2) | None | HFIP | 83 |
| 10 | Mn(acac)3 (0.9) | None | HFIP | 55 |
| 11 | Mn(OAc)3·2H2O (1.2) | None | HFIP | 36 |
| 12 | MnCl2 (1.2) | None | HFIP | Trace |
| 13 | Mn(acac)2 (1.2) | None | HFIP | 88 |
| 14 | MnCl2 (1.2) | acac (3.6) | HFIP | 80 |
| 15 | MnCl2 (0.2) | acac (3.6) | HFIP | 81 |
| 16c | MnCl2 (0.2) | acac (3.6) | HFIP | 89 |
| 17c | MnCl2 (0.2) | acac (1.0) | HFIP | 89 |
| 18c | MnCl2 (0.2) | acac (0.6) | HFIP | 83 |
| 19c,d | MnCl2 (0.2) | acac (1.0) | HFIP | 91 |
| 20c,e | MnCl2 (0.2) | acac (1.0) | HFIP | 83 |
| 21c,b | MnCl2 (0.2) | acac (1.0) | HFIP | 80 |
| 22c,d | MnCl2 (0.2) | acac (0.6) | HFIP | 82 |
| 23c,d | MnCl2 (0.1) | acac (1.0) | HFIP | 81 |
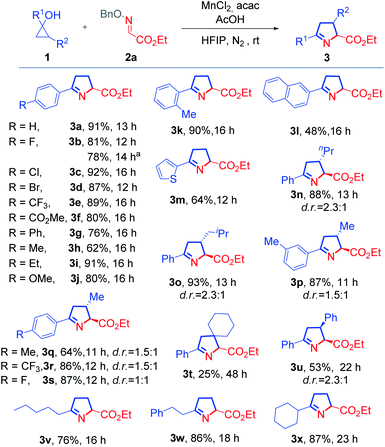
With the optimized conditions in hand for the synthesis of 1-pyrrolines, the compatibility of various cyclopropanols was inspected (Scheme 1). Common functional groups on the phenyl ring, including halides (3b–3d), ester (3f), ether (3j), were compatible under the reaction conditions. Regardless of the presence of electron-withdrawing or -donating substituents at the para-position of this phenyl ring, the reactions readily proceeded with generally high yields (3b–3j). The cyclopropanol (1k) with an ortho-methyl substituent underwent a cyclization reaction with excellent yield, demonstrating that steric effects had little effect on product of the reaction (3k). By replacing the phenyl group with a naphthyl or thienyl group, the corresponding products (3l and 3m) were produced with slightly lower yields. When 2-substituted cyclopropanols were utilized, these reactions gave rise to a portfolio of trisubstituted 1-pyrrolines (3n–3u).The relative configuration of 3u was determined by comparison with a reported structure.8 Remarkably, this protocol provided a convenient method for the construction of an N-containing spiro skeleton (3t). The reaction with alkyl cyclopropanols could also furnish the desired products (3v–3x) smoothly and with good yields.
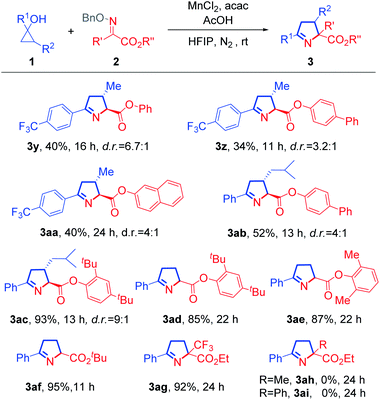
Next, we studied the scope of oxime ethers (Scheme 2). Steric hindrance from the ester moiety in the oxime ethers appeared not to influence the reaction outcome. Oxime ethers bearing various esters, such as phenyl (3y), biphenyl (3z and 3ab), 2-naphthyl (3aa), 2,4-di-tert-butylphenyl (3ac and 3ad), and 2,6-dimethylphenyl (3ae) esters all reacted smoothly. In addition, the substrate with tert-butyl ester also readily underwent cyclization to afford the desired product 3af with excellent yield. Remarkably, the trifluoromethyl-substituted pyrroline (3ag) was afforded almost quantitatively from the corresponding ketoxime ether. However, if the trifluoromethyl group was replaced by a methyl or phenyl group, the reaction failed to give rise to the desired product (3ah or 3ai), and this might be attributed to poorer electrophilic nature of the methyl or phenyl substituted substrate.
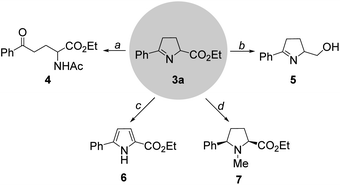
To illustrate the utility of this protocol, we carried out a set of synthetic applications using 1-pyrroline (3a) (Scheme 3). Upon treatment with acetyl chloride and pyridine at 42 °C, 1-pyrroline (3a) could be readily converted into the acyclic amino acid derivative (4). The reaction between 3a and LiAlH4 gave rise smoothly to the corresponding alcohol (5). In the presence of 2,3-dichloro-5,6-dicyano-1,4-benzoquin-4-one (DDQ) and triethylamine, the 2,5-disubstituted pyrrole (6) was obtained. Moreover, treatment of 3a with MeOTf and NaBH4 delivered the N-methyl proline derivative (7).9
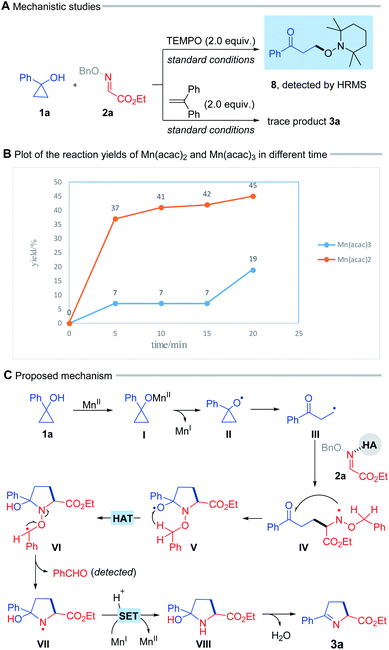
To probe the mechanistic pathways, we performed a radical trapping experiment in the presence of 2.0 equiv. of radical scavenger TEMPO. The radical trapping product (8) was detected by high-resolution mass spectrometry (HRMS) (Scheme 4A, top). In addition, the reaction was obviously suppressed when 1,1-diphenylethylene was added under standard condition (Scheme 4A, bottom). These results suggested that this process engaged in a radical pathway. Kinetic studies illustrated that the reaction immediately started with 20 mol% Mn(acac)2 but an approximate 15 min of induction period was appeared by using Mn(acac)3, which probably indicated that the reaction was initiated with Mn(II) rather than Mn(III), and the Mn(II)/Mn(I) cycle might be involved in the transformation (Scheme 4B, for details see ESI†).
On the basis of these results, a plausible mechanism for this radical process was proposed in Scheme 4C. Initially, the interaction between cyclopropanol (1a) and Mn(II) salt gives rise to the alkoxy manganese species (I), which undergoes a ligand-to-metal charge transfer (LMCT) process, leading to the alkoxy radical (II).5f Subsequent ring-opening of the alkoxyl radical (II) provides the alkyl radical (III). The addition of intermediate (III) to the oxime ether, possibly activated by HFIP or HOAc, furnishes the N-centered radical (IV), which intramolecularly attacks the ketone to afford a new alkoxy radical (V).10 The subsequent 1,5-hydrogen atom transfer (HAT) process delivers the alkyl radical (VI) at the α-position adjacent to the O atom, thus driving N–O bond cleavage to generate the N-centered radical (VII),5b,11 and benzaldehyde which was detected by TLC. This radical intermediate (VII) undergoes a single electron transfer (SET) mediated by the reduced-state Mn(I) species, and protonation to yield the cyclic pyrrolidine (VIII). Finally, dehydration of this intermediate produces 1-pyrroline (3a).
Conclusions
In conclusion, we have developed a novel Mn-catalyzed redox-neutral reaction, producing 1-pyrrolines under mild conditions. This method features a controlled radical cascade, good tolerance of functional groups, and a broad scope of cyclopropanols and oxime ethers. Mechanistic studies have implied the presence of alkyl radicals from ring-opening of cyclopropanols and a process involving HAT and N–O bond cleavage. The role of the oxime ether as an internal oxidant and H-donor is vital to the redox-neutral reaction. This protocol provides a versatile platform to further synthesis of N-containing heterocycles based on a strategy of combining manganese catalysis and ring-opening of cycloalkanols.Data availability
Data for this work, including experimental procedures and characterization data for all new compounds are provided in the ESI.†Author contributions
C. Z. and X. W. conceived of and directed the project, T. F., C. L., and Z. W. conducted the experiments and collected and analyzed the data, C. Z. and X. W. wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (Grant no. 22001185, 21971173, 22171201), the Natural Science Foundation of Jiangsu Province (BK20200852), the Natural Science Fund for Colleges and Universities in Jiangsu Province (20KJB150010), the Project of Scientific and Technological Infrastructure of Suzhou (SZS201905), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- (a) G. Giblin, A. Heseltine, W. Kiesman, D. MacPherson, J. Ramsden, R. Vadali, M. Williams and D. Witty, Org. Process Res. Dev., 2020, 24, 2802–2813 CrossRef CAS; (b) A. H. Hansen, E. Sergeev, D. Bolognini, R. R. Sprenger, J. H. Ekberg, C. S. Ejsing, C. J. McKenzie, E. R. Ulven, G. Milligan and T. Ulven, J. Med. Chem., 2018, 61, 9534–9550 CrossRef CAS PubMed; (c) A. Otero, M.-J. Chapela, M. Atanassova, J. M. Vieites and A. G. Cabado, Chem. Res. Toxicol., 2011, 24, 1817–1829 Search PubMed; (d) C. E. Stivala, E. Benoit, R. Aráoz, D. Servent, A. Novikov, J. Molgó and A. Zakarian, Nat. Prod. Rep., 2015, 32, 411–435 RSC; (e) M. Silva, V. K. Pratheepa, L. M. Botana and V. Vasconcelos, Toxins, 2015, 7, 859–885 CrossRef CAS PubMed; (f) J. Molgó, P. Marchot, R. Aráoz, E. Benoit, B. I. Iorga, A. Zakarian, P. Taylor, Y. Bourne and D. Servent, J. Neurochem., 2017, 142, 41–51 CrossRef PubMed.
- (a) X. Fang and C.-J. Wang, Org. Biomol. Chem., 2018, 16, 2591–2601 RSC; (b) Z.-Y. Xue, Z.-M. Song and C.-J. Wang, Org. Biomol. Chem., 2015, 13, 5460–5466 RSC; (c) Z.-Y. Xue, Y. Xiong and C.-J. Wang, Synlett, 2014, 25, 2733–2737 CrossRef CAS.
- (a) D.-S. Wang, Z.-S. Ye, Q.-A. Chen, Y.-G. Zhou, C.-B. Yu, H.-J. Fan and Y. Duan, J. Am. Chem. Soc., 2011, 133, 8866–8869 CrossRef CAS PubMed; (b) A. Abbaspour, S. S. Hecht and D. Hoffmann, J. Org. Chem., 1987, 52, 3474–3477 CrossRef CAS.
- (a) J. Zhang, X. Huo, J. Xiao, L. Zhao, S. Ma and W. Zhang, J. Am. Chem. Soc., 2021, 143, 12622–12632 CrossRef CAS PubMed; (b) Y. Peng, X. Huo, Y. Luo, L. Wu and W. Zhang, Angew. Chem., Int. Ed., 2021, 60, 24941–24949 CrossRef CAS PubMed.
- (a) H. Jiang and A. Studer, Angew. Chem., Int. Ed., 2017, 56, 12273–12276 CrossRef CAS PubMed; (b) H. Jiang and A. Studer, Angew. Chem., Int. Ed., 2018, 57, 1692–1696 CrossRef CAS PubMed; (c) J. Davies, S. G. Booth, S. Essafi, R. A. W. Dryfe and D. Leonori, Angew. Chem., Int. Ed., 2015, 54, 14017–14021 CrossRef CAS PubMed; (d) S.-H. Cai, J.-H. Xie, S. Song, L. Ye, C. Feng and T.-P. Loh, ACS Catal., 2016, 6, 5571–5574 CrossRef CAS; (e) S.-H. Cai, D.-X. Wang, L. Ye, Z.-Y. Liu, C. Feng and T.-P. Loh, Adv. Synth. Catal., 2018, 360, 1262–1266 CrossRef CAS; (f) Y.-H. Zhang, W.-W. Zhang, Z.-Y. Zhang, K. Zhao and T.-P. Loh, Org. Lett., 2019, 21, 5101–5105 CrossRef CAS PubMed; (g) R. I. Rodríguez, L. Mollari and J. Alemán, Angew. Chem., Int. Ed., 2021, 60, 4555–4560 CrossRef PubMed.
- For selected examples, see: (a) X. Cai, W. Liang and M. Dai, Tetrahedron, 2019, 75, 193–208 CrossRef CAS; (b) D. C. Davis, K. L. Walker, C. Hu, R. N. Zare, R. M. Waymouth and M. Dai, J. Am. Chem. Soc., 2016, 138, 10693–10699 CrossRef CAS PubMed; (c) X. Cai, W. Liang, M. Liu, X. Li and M. Dai, J. Am. Chem. Soc., 2020, 142, 13677–13682 CrossRef CAS PubMed.
- For selected reviews on radical-mediated ring-opening of cycloalkanols, see: (a) X. Wu and C. Zhu, Chem. Rec., 2018, 18, 587–598 CrossRef CAS PubMed; (b) X. Wu and C. Zhu, Chem. Commun., 2019, 55, 9747–9756 RSC . For selected examples, see:; (c) R. Ren, H. Zhao, L. Huan and C. Zhu, Angew. Chem., Int. Ed., 2015, 54, 12692–12696 CrossRef CAS PubMed; (d) R. Ren, Z. Wu, Y. Xu and C. Zhu, Angew. Chem., Int. Ed., 2016, 55, 2866–2869 CrossRef CAS PubMed; (e) L. Huan and C. Zhu, Org. Chem. Front., 2016, 3, 1467–1471 RSC; (f) D. Wang, R. Ren and C. Zhu, J. Org. Chem., 2016, 81, 8043–8049 CrossRef CAS PubMed; (g) R. Ren, Z. Wu and C. Zhu, Chem. Commun., 2016, 52, 8160–8163 RSC; (h) M. Wang, Z. Wu and C. Zhu, Org. Chem. Front., 2017, 4, 427–430 RSC.
- (a) W. Wen, L. Chen, M.-J. Luo, Y. Zhang, Y.-C. Chen, Q. Ouyang and Q.-X. Guo, J. Am. Chem. Soc., 2018, 140, 9774–9780 CrossRef CAS PubMed; (b) X.-F. Bai, Li. Li, Z. Xu, Z.-J. Zheng, C.-G. Xia, Y.-M. Cui and L.-W. Xu, Chem.–Eur. J., 2016, 22, 10399–10404 CrossRef CAS PubMed.
- (a) S. Fukuzawa and H. Oki, Org. Lett., 2008, 10, 1747–1750 CrossRef CAS PubMed; (b) F. Prause, J. Kaldun, B. Arensmeyer, B. Wennemann, B. Fröhlich, D. Scharnagel and M. Breuning, Synthesis, 2015, 47, 575–586 CAS.
- L. Benati, D. Nanni, C. Sangiorgi and P. Spagnolo, J. Org. Chem., 1999, 64, 7836–7841 CrossRef CAS.
- P.-Z. Wang, B.-Q. He, Y. Cheng, J.-R. Chen and W.-J. Xiao, Org. Lett., 2019, 21, 6924–6929 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2sc00015f |
| This journal is © The Royal Society of Chemistry 2022 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: