Open Access Article
Open Access ArticleSite-specific doping of silver atoms into a Au25 nanocluster as directed by ligand binding preferences†
Wan-Qi
Shi
a,
Zong-Jie
Guan
a,
Jiao-Jiao
Li
a,
Xu-Shuang
Han
a and
Quan-Ming
Wang
 *ab
*ab
aDepartment of Chemistry, Tsinghua University, Beijing, 100084, PR China. E-mail: qmwang@tsinghua.edu.cn
bDepartment of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005, PR China
First published on 15th March 2022
Abstract
For the first time site-specific doping of silver into a spherical Au25 nanocluster has been achieved in [Au19Ag6(MeOPhS)17(PPh3)6] (BF4)2 (Au19Ag6) through a dual-ligand coordination strategy. Single crystal X-ray structural analysis shows that the cluster has a distorted centered icosahedral Au@Au6Ag6 core of D3 symmetry, in contrast to the Ih Au@Au12 kernel in the well-known [Au25(SR)18]− (R = CH2CH2Ph). An interesting feature is the coexistence of [Au2(SPhOMe)3] dimeric staples and [P–Au–SPhOMe] semi-staples in the title cluster, due to the incorporation of PPh3. The observation of only one double-charged peak in ESI-TOF-MS confirms the ordered doping of silver atoms. Au19Ag6 is a 6e system showing a distinct absorption spectrum from [Au25(SR)18]−, that is, the HOMO–LUMO transition of Au19Ag6 is optically forbidden due to the P character of the superatomic frontier orbitals.
1. Introduction
Atomically precise bimetallic nanoclusters have attracted intense attention owing to their structural diversities and potential applications.1–9 Gold–silver nanoclusters exhibit different catalytic, electrochemical and optical properties compared to their homometallic analogues, and the properties vary with the degree of doping.10–15 For example, Au57Ag53(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)40Br12 has a distinctly different optical absorption profile from [Au80Ag30(C
CPh)40Br12 has a distinctly different optical absorption profile from [Au80Ag30(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)42Cl9]Cl, owing to their different metal arrangements and Au/Ag ratios.16,17 [Au25−xAgx(SR)5(PPh3)10Cl2]2+ (x ≤ 13) has a 200-fold increase in luminescence quantum yield after doping the 13th Ag atom.18 These results indicate that the doping number and position of silver atoms should be considered in studying the structure–property relationships of alloy clusters.10,19,20
CPh)42Cl9]Cl, owing to their different metal arrangements and Au/Ag ratios.16,17 [Au25−xAgx(SR)5(PPh3)10Cl2]2+ (x ≤ 13) has a 200-fold increase in luminescence quantum yield after doping the 13th Ag atom.18 These results indicate that the doping number and position of silver atoms should be considered in studying the structure–property relationships of alloy clusters.10,19,20
The thiolated gold nanocluster [Au25(SR)18]− (R = CH2CH2Ph)21,22 has been extensively used as a parental cluster for heterometal doping, because of its easy preparation and high stability.23 In 2010, Au25−xAgx(SR)18 nanoclusters with x up to 11 were synthesized by Negishi et al.,24 and similar doping reactions were also reported by Murray et al.25 The structure of Au25−xAgx(SR)18 was determined by Dass et al.,26 which revealed a disordered arrangement of the icosahedral shell in Au1@Au5.3Ag6.7@6×Au2(SR)3. Later, different degrees of doping were achieved, including heavy-doped Au25−xAgx(SR)18 (x = 19.4, 20.45) by Jin et al.27,28 Although various degrees of doping can be roughly controlled by regulating the Au/Ag ratios in synthesis procedures, all these clusters are mixtures containing different numbers of heteroatoms as revealed by mass spectrometry.12,29–32 To establish a direct structure–property correlation, precise doping of heteroatoms in an ordered manner is highly demanded. Such a site-specific doping remains a big challenge, especially for Au–Ag alloy nanoclusters. The difficulty in obtaining non-disordered gold-silver alloy clusters lies mainly in the similarity in the atomic radius and electronic structures of Ag and Au.23 The substitution of silver atoms at equivalent sites of gold often occurs during heteroatom doping, leading to a distribution of Ag heteroatoms in a gold cluster. For example, the highly symmetric geometry of [Au25(SR)18]− makes the surface gold atoms hard to be distinguished, which increases the difficulty of site-specific doping.33
It is well known that surface organic ligands are critical in the formation of atomically precise metal nanoclusters.6,34–40 It has been found that the electronic nature, coordination preference, and bulkiness of ligands can have significant effects on the structures and properties of the clusters.41–45 In order to achieve site-specific doping of silver into a gold nanocluster, we develop a strategy to control the atomic arrangement through the combination of ligands with different ligating preferences. The use of thiolate and phosphine ligands resulted in the formation of a novel ordered Au19Ag6 core different from those with disordered Au25−xAgx cores. It is remarkable that the silver atoms are located at specific positions in the M13 kernel due to the different affinities of surface motifs.
Herein, we report the synthesis and structural determination of such an alloy (Au/Ag)25 cluster with ordered doping with the formula [Au19Ag6(MeOPhS)17(PPh3)6](BF4)2 (denoted as Au19Ag6). The composition and structure have been determined by electrospray ionization time-of-flight mass spectrometry and single-crystal X-ray structural analysis. An interesting feature is the coexistence of [Au2(SPhOMe)3] dimeric staples and [P–Au–SPhOMe] semi-staples in this cluster, due to the incorporation of PPh3, which dictates the specific sites for dopant silver atoms. In addition, we have also studied the optical properties and electronic structure of this 6e system, which are quite different from Au25−xAgx(SR)18 clusters.
2. Experimental
2.1 Materials
Triphenylphosphine (Ph3P, 99.5%), 2,2′-dipyridylamine (Hdpa), 4-methoxythiophenol and silver tetrafluoroborate (AgBF4, 99.0%) were purchased from J&K; sodium borohydride (NaBH4, 98%) and other reagents employed were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). All the reagents were used as received without further purification. Me2SAuCl and Ph3PAuCl were prepared according to literature methods.46,472.2 Synthesis of MeOPhSAu
To 60.0 ml acetone containing Me2SAuCl (589.2 mg, 2.0 mmol), MeOPhSH (336.4 mg, 2.4 mmol) and triethylamine (242.8 mg, 2.4 mmol) were added under vigorous stirring, and the mixture was stirred for 15 minutes in the dark. A large amount of white precipitate was formed. The resulting solution was rotary evaporated to dryness to give a white solid, which was washed with water, ethanol, and ether to give MeOPhSAu.2.3 Synthesis of MeOPhSAg
10 ml ethanol solution of MeOPhSH (350.5 mg, 2.5 mmol) was dropwise added into 100 ml aqueous solution of AgNO3 (425.0 mg, 2.5 mmol), followed by the addition of triethylamine (242.8 mg, 2.4 mmol), and the solution turned black. In an ice-water bath, the mixture was stirred for 15 minutes in the dark. After suction filtration, the obtained solid was washed with water, ethanol, and ether to give MeOPhSAg.2.4 Synthesis of Au19Ag6
To 4.0 ml CH2Cl2 containing Ph3PAuCl (24.7 mg, 0.05 mmol), 0.1 ml of methanol solution of AgBF4 (8.8 mg, 0.045 mmol) was added under vigorous stirring. After stirring for 15 minutes in the dark, the mixture was centrifuged and filtered to remove the AgCl precipitate. Hdpa (4.2 mg, 0.025 mmol) was added to the filtrate, the solution turned pale yellow, and the solution was stirred for 1 hour in the dark. Subsequently, MeOPhSAu (16.8 mg, 0.05 mmol) was added, and freshly prepared NaBH4 (0.7 mg in 1 ml ethanol) was added dropwise under stirring. The color of the solution changed from yellow to orange and then to dark brown. After stirring for 4 hours in the dark, MeOPhSAg (12.4 mg, 0.05 mmol) was added under stirring, and the color of the solution changed from dark brown to brown-black. The reaction solution was stirred for 12 hours at room temperature in the absence of light. After the reaction, the solvent was rotary evaporated to give a dark brown solid. The solid was dissolved in 2.0 ml of dichloromethane and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 minutes. The dark brown solution was collected, to which n-hexane was added for diffusion at 4 °C. After two weeks, black crystals were obtained (10.5 mg, yield 18% based on Au).
000 rpm for 3 minutes. The dark brown solution was collected, to which n-hexane was added for diffusion at 4 °C. After two weeks, black crystals were obtained (10.5 mg, yield 18% based on Au).
2.5 Characterization
UV-Vis-NIR absorption spectra were recorded on a Cary 5000. The mass spectrum was recorded on an ABI4800plus ESI-TOF-MS and a Waters Q-TOF mass spectrometer. Crystals of Au19Ag6 were dissolved in CH2Cl2 for ESI-MS measurement. X-ray photoelectron spectroscopy was performed using a Thermo ESCALAB Xi+ instrument. Intensity data of Au19Ag6 were collected on an Oxford Gemini S Ultra system (Cu Kα). Absorption corrections were applied by using the program CrysAlis (multi-scan). The structure was solved by direct methods, and non-hydrogen atoms except solvent molecules and counteranions were refined anisotropically by least-squares on F2 using the SHELXTL program. The diffuse electron densities resulting from the residual solvent molecules were removed from the data set using the Olex2 solvent mask.2.6 Computational details
Density functional theory (DFT) calculations were performed with the quantum chemistry program Gaussian 16.48 In the calculations, Au19Ag6 was mimicked by a model system of [Au19Ag6(PH3)6(SH)17]2+. The 6-31G(d) basis set was used for H, S, P, and LANL2DZ, for Au and Ag.49,50 Geometry optimizations were performed with the B3LYP functional, and time-dependent DFT calculations of the UV-Vis absorption spectrum were performed with the PBE0 functional.51 One hundred singlet states (nstates = 100, singlet) are chosen in the calculations of the UV-Vis absorption spectra. All the transitions together with their oscillator strengths were then convoluted with a Gaussian line shape of 0.15 eV broadening to make the whole optical-absorption spectrum. The molecular orbitals were visualized via the Multiwfn software and the VMD package.52,533. Results and discussion
The synthesis of Au19Ag6 involves the reduction of the gold precursor followed by the addition of silver thiolate. It is very important to remove chloride from Ph3PAuCl, otherwise the known [(p-Tol3P)10Au13Ag12Cl8] (PF6) with a biicosahedral core is obtained as reported previously.54 The halide-free strategy has been proved successful in synthesizing new phosphine/thiolate co-protected metal nanoclusters such as [Au55(p-MBT)24(Ph3P)6](SbF6)3 (p-MBT = 4-methylbenzenethiolate).55The composition of Au19Ag6 was determined by electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in positive-ion mode. As shown in Fig. 1, there is only one doubly charged peak observed at m/z 4164.83, which corresponds to the molecular ion [Au19Ag6(MeOPhS)17(PPh3)6]2+. The isotopic distribution pattern is in perfect agreement with the simulated one (Fig. 1, inset). No other doping products with different numbers of silver atoms were observed. The small doubly charged peak at m/z 4332.8 corresponds to [Au20Ag6(MeOPhS)18(PPh3)6]2+, due to the incorporation of MeOPhSAu into [Au19Ag6(MeOPhS)17(PPh3)6]2+.
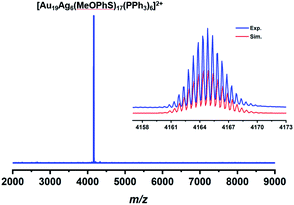 | ||
| Fig. 1 ESI-TOF-MS spectrum of Au19Ag6 in CH2Cl2. Inset: comparison of the experimental (blue) and simulated (red) isotope distribution patterns. | ||
The gold 4f7/2 binding energy of Au19Ag6 is 84.2 eV that is in between that of Au(0) (84.0 eV) and Au(1) (84.9 eV), confirming that gold atoms in Ag19Ag6 exist in mixed oxidation states of Au(0) and Au(I). The Ag 3d5/2 binding energy is 367.6 eV that is significantly lower than that of metallic silver (368.27 eV), indicating that Ag atoms in the cluster are positively charged.13
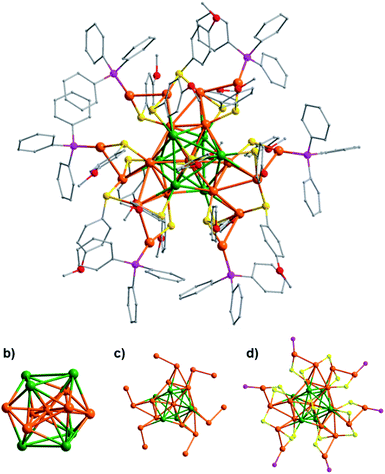
The structure of Au19Ag6 was determined by X-ray single-crystal diffraction.56 As shown in Fig. 2, the metal core of Au19Ag6 is a centered icosahedral Au@Au6Ag6 core, similar to the Au13 core in [Au25(SR)18]−. However, the icosahedron is slightly distorted due to the incorporation of Ag atoms. The Au–Au bond lengths from the central gold atom to the vertices of the Au6Ag6 icosahedron are between 2.7908(15) and 2.8189(14) Å (average 2.806 Å), and the Au–Ag bond lengths from the central gold atom to the silver vertices of the Au6Ag6 icosahedron are between 2.962(2) and 3.085(2) Å (average 3.022 Å). The Au–Au distances in the Au6Ag6 icosahedron can be classified into two groups: the shorter ones average 2.670 Å and the longer ones average 3.289 Å, which are distinguished by whether they interact with V-shaped [Au2(SR)3] staples or not. The Au–Ag bond lengths on the Au6Ag6 shell are in the range of 2.824(2)–3.056(2) Å (average 2.922 Å).
As shown in Fig. 2b, six dopant Ag atoms are located at two opposite triangles of the icosahedron, and the six gold atoms are in the arrangement of chair conformation. Each of the two Ag3 triangles is capped by a μ3 thiolate. In the Ag3 units, Ag atoms are bound by argentophilic interactions with Ag–Ag distances between 3.462(3) and 3.773(3) Å (average 3.623 Å), and the average bond length of the Ag–S bond is 2.469 Å.57,58 There are 12 peripheral gold atoms attached to this Au6Ag6 shell (Fig. 2c), but the gold-thiolate motifs are different from [Au25(SR)18]− due to the coordination of 6 PPh3 (Fig. 2d).
As shown in Fig. 3, Au19Ag6 has three dimeric staples ([Au2(SR)3], motif A) and six semi-staples ([P–Au–SR], motif B), which is quite different from [Au25(SR)18]− that has six identical [Au2(SR)3] dimeric staples. In the present case, each [Au2(SR)3] staple is connected to two Ag atoms in the Au6Ag6 shell, while each [P–Au–SR] semi-staple is linked to one Au atom of Au6Ag6.59 Therefore, the sites for silver and gold in the Au6Ag6 shell can be differentiated by the peripheral motifs through which they are connected. The doping of silver atoms lowers the symmetry from Ih of the Au13 kernel in [Au25(SR)18]− to D3 of the Au7Ag6 core in the title cluster.
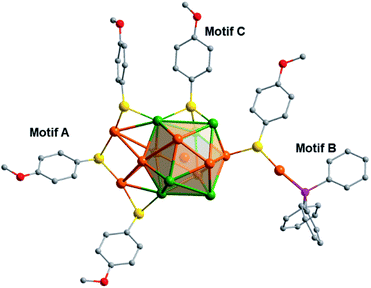 | ||
| Fig. 3 Gold-thiolate motifs attached to the Au6Ag6 shell (some of the surface binding motifs are removed for clarity). | ||
Studies have shown that the difficulty in achieving ordered doping in Au25−xAgx(SR)18 might be attributed to the highly symmetric geometry of [Au25(SR)18]−. Negishi et al. found that Ag substitution tends to occur at specific sites, where the substituted Au atoms are not equivalent to the other Au atoms.60 As for the title cluster, its synthesis involves two steps, that is, the reduction of the gold precursor and the subsequent addition of silver thiolate. We performed ESI-MS for the reaction mixture before adding silver thiolate, and no peak of [Au25(SR)18]− or [Au25(MeOPhS)17(PPh3)6]2+ was observed. Therefore, the formation mechanism is not a simple substitution of gold with silver in Au25, and it may involve the reaction of unknown intermediate clusters with silver thiolate.
We believe that the precise location of Ag atoms in Au19Ag6 is due to the introduction of the Ph3P ligand. Because of the stronger bonding of Au–P than Ag–P, the metal atoms that coordinate to the PPh3 ligands are all Au atoms. In comparison, Ag atoms preferentially take the positions that are bound to the S atoms of thiolates. 31P-NMR of Au19Ag6 in CD2Cl2 has only one peak at 37.48 ppm (Fig. S1†), which confirms that there is only one kind of P in Au19Ag6 consistent with its D3 symmetry.
In this dual-ligand system, Au and Ag atoms are fixed at special positions to form precisely substituted gold-silver nanoclusters. Similar situations have been observed in halogen/phosphine co-protected rod-like M25 nanoclusters as well as M38 alloy nanoclusters.61–65 For the M25 rod-like cluster, because the Ag–Cl bond is stronger than Au–Cl and Au–P is stronger than Ag–P, Ag atoms occupy all the positions bound to chlorides, while Au atoms preferentially take the positions linked to PPh3. It is worth mentioning that in a system where thiolates and phosphines coexist, it is easy to obtain thiolate/phosphine/chloride co-protected rod-like Au25−xAgx nanoclusters, but this problem can be avoided by removing the Cl in PPh3AuCl during the synthesis process.
The absorption spectrum of Au19Ag6 is shown in Fig. 4, which exhibits two prominent absorption peaks at 390 nm and 463 nm. In comparison with Au25−xAgx with disordered doping, it is found that the UV spectrum of Au19Ag6 is very different.24 Remarkably, the prominent peak around 700 nm in [Au25(SR)18]− disappears in Au19Ag6. Such a difference is generated from the alteration in electronic structures.66,67 The number of valence electrons of Au19Ag6 is 6 (n = 19 + 6 − 17 − 2), corresponding to the superatomic electronic configuration 1S21P4 in contrast to 1S21P6 of [Au25(SR)18]−.68,69 Thus, the HOMO–LUMO transition is an optically forbidden p–p transition as confirmed by the following TDDFT calculations, which explains the disappearance of the ∼700 nm peak. Notably, the geometry of Au19Ag6 is oblate as shown in Fig. 2. The Au atoms in the staples are distributed on the waist of the icosahedron. Such a core distortion in Au19Ag6 is in line with its 6e configuration.45 Unlike thiol-protected Au25 or Au25−xAgx clusters, the use of neutral phosphine ligands in Au19Ag6 makes the system more positively charged,45,70 leading to the formation of a 6e system instead of 8e. A similar case was observed in [Au40(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)20(dppm)4](SbF6)4 (dppm = bis(diphenylphosphino)methane), where it is a 16e system instead of 18e.59
C)20(dppm)4](SbF6)4 (dppm = bis(diphenylphosphino)methane), where it is a 16e system instead of 18e.59
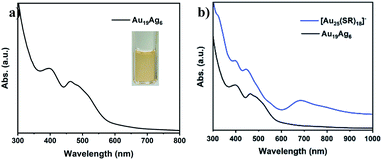 | ||
| Fig. 4 (a) The absorption spectrum of Au19Ag6 in CH2Cl2. Inset: photograph of the solution. (b) The comparison of Au19Ag6 and [Au25(SR)18]−. | ||
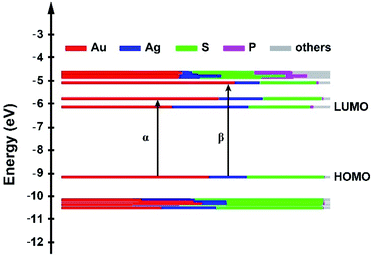
Time-dependent density functional theory (TDDFT) calculations were carried out to explore the relationship between the electronic structure and optical absorption properties. We adopted the structural data from X-ray single crystal diffraction as an input file to the quantum chemistry program Gaussian 16. As shown in Fig. 5a, there are characteristic absorption peaks at 2.78 eV (α) and 3.38 eV (β) in the simulated spectrum, which fit well with the experimental results except for a slight blue shift (Fig. 6b). The band at 2.78 eV was referred to as the transition from the HOMO to the LUMO+1, whereas the β peak at 3.38 eV was assigned to the HOMO to LUMO+2 transition. It is worth noting that Au19Ag6 exhibits highly symmetric superatomic orbital properties with the HOMO–LUMO showing P orbital characteristics, which accounts for the optically forbidden transition from the HOMO to the LUMO.
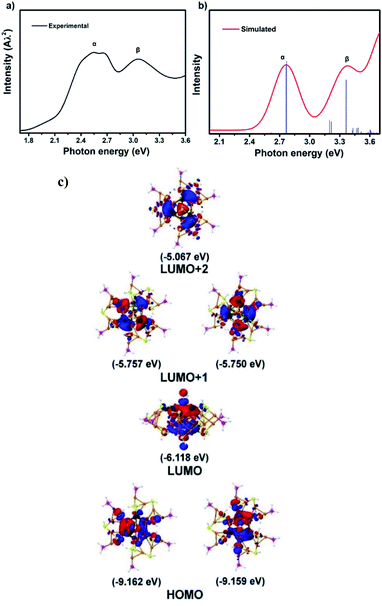 | ||
| Fig. 5 (a) Experimental absorption spectrum of Au19Ag6. (b) Simulated spectrum of Au19Ag6. (c) Frontier orbitals including the HOMO, LUMO, LUMO+1 and LUMO+2 of Au19Ag6. | ||
According to the Kohn–Sham molecular orbital (MO) energy level diagram of Au19Ag6 (Fig. 6), the HOMO and LUMO+1 orbitals are mainly constituted by the Au and Ag atomic orbitals in the Au7Ag6 icosahedral core. Therefore, the α absorption band at 2.78 eV and the β peak at 3.38 eV (Fig. 6b) are primarily attributed to the charge transfer within the metal kernel. Notably, the atomic orbitals of P contribute little to KS molecular orbitals.
4. Conclusion
In summary, we have obtained a bimetal nanocluster with ordered silver doping. This cluster has a precise composition and well-defined molecular structure, in which dopant silver atoms are located at the two opposite triangle sites in the distorted icosahedral Au@Au6Ag6 core. This cluster exhibits totally different optical properties from previously reported Au25−xAgx and Au25 clusters, due to its 1S21P4 electronic configuration. This is the first example of doping silver atoms in a spherical Au25 cluster at specific sites in an ordered way. This work underlies the effectiveness of using a ligand combination strategy to generate alloy nanoclusters with special geometric and electronic structures.Data availability
The X-ray crystallographic structure reported in this article has been deposited at the Cambridge Crystallographic Data Centre (CCDC) with deposition number (CCDC 2131886).Author contributions
Q.-M. W. conceived and directed the project. W.-Q. S. performed the experiments. Z.-J. G. conducted the calculations. Z.-J. G., J.-J. L and X.-S. H. analyzed the crystal data. All the authors discussed the results and co-wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Pei Li for her contribution at the beginning of this work. This work was supported by the National Natural Science Foundation of China (91961201, 21631007, 21971136 and 22001145).Notes and references
- Y. Du, H. Sheng, D. Astruc and M. Zhu, Chem. Rev., 2020, 120, 526–622 CrossRef CAS PubMed.
- Z. Lei, X.-K. Wan, S.-F. Yuan, Z.-J. Guan and Q.-M. Wang, Acc. Chem. Res., 2018, 51, 2465–2474 CrossRef CAS PubMed.
- Q. Yao, Z. Wu, Z. Liu, Y. Lin, X. Yuan and J. Xie, Chem. Sci., 2021, 12, 99–127 RSC.
- Z.-J. Guan, F. Hu, J.-J. Li, Z.-R. Liu and Q.-M. Wang, Nanoscale, 2020, 12, 13346–13350 RSC.
- J.-J. Li, Z.-J. Guan, Z. Lei, F. Hu and Q.-M. Wang, Angew. Chem., Int. Ed., 2019, 58, 1083–1087 CrossRef CAS PubMed.
- P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg, Science, 2007, 318, 430–433 CrossRef CAS PubMed.
- S.-F. Yuan, R.-L. He, X.-S. Han, J.-Q. Wang, Z.-J. Guan and Q.-M. Wang, Angew. Chem., Int. Ed., 2021, 60, 14345–14349 CrossRef CAS PubMed.
- X. Kang, H. Chong and M. Zhu, Nanoscale, 2018, 10, 10758–10834 RSC.
- Z. Gan, N. Xia and Z. Wu, Acc. Chem. Res., 2018, 51, 2774–2783 CrossRef CAS PubMed.
- W. Li, C. Liu, H. Abroshan, Q. Ge, X. Yang, H. Xu and G. Li, J. Phys. Chem. C, 2016, 120, 10261–10267 CrossRef CAS.
- C. Yao, J. Chen, M.-B. Li, L. Liu, J. Yang and Z. Wu, Nano Lett., 2015, 15, 1281–1287 CrossRef CAS PubMed.
- X. Dou, X. Yuan, Q. Yao, Z. Luo, K. Zheng and J. Xie, Chem. Commun., 2014, 50, 7459–7462 RSC.
- J. Xiang, P. Li, Y. Song, X. Liu, H. Chong, S. Jin, Y. Pei, X. Yuan and M. Zhu, Nanoscale, 2015, 7, 18278–18283 RSC.
- Y. Li, T.-Y. Luo, M. Zhou, Y. Song, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2018, 140, 14235–14243 CrossRef CAS PubMed.
- C. Kumara and A. Dass, Nanoscale, 2012, 4, 4084–4086 RSC.
- Z.-J. Guan, J.-L. Zeng, S.-F. Yuan, F. Hu, Y.-M. Lin and Q.-M. Wang, Angew. Chem., Int. Ed., 2018, 57, 5703–5707 CrossRef CAS PubMed.
- J.-L. Zeng, Z.-J. Guan, Y. Du, Z.-A. Nan, Y.-M. Lin and Q.-M. Wang, J. Am. Chem. Soc., 2016, 138, 7848–7851 CrossRef CAS PubMed.
- S. Wang, X. Meng, A. Das, T. Li, Y. Song, T. Cao, X. Zhu, M. Zhu and R. Jin, Angew. Chem., Int. Ed., 2014, 53, 2376–2380 CrossRef CAS PubMed.
- C. M. Aikens, J. Phys. Chem. C, 2008, 112, 19797–19800 CrossRef CAS.
- K. R. Krishnadas, A. Baksi, A. Ghosh, G. Natarajan and T. Pradeep, ACS Nano, 2017, 11, 6015–6023 CrossRef CAS PubMed.
- M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, J. Am. Chem. Soc., 2008, 130, 5883–5885 CrossRef CAS PubMed.
- M. W. Heaven, A. Dass, P. S. White, K. M. Holt and R. W. Murray, J. Am. Chem. Soc., 2008, 130, 3754–3755 CrossRef CAS PubMed.
- X. Kang, Y. Li, M. Zhu and R. Jin, Chem. Soc. Rev., 2020, 49, 6443–6514 RSC.
- Y. Negishi, T. Iwai and M. Ide, Chem. Commun., 2010, 46, 4713–4715 RSC.
- J.-P. Choi, C. A. Fields-Zinna, R. L. Stiles, R. Balasubramanian, A. D. Douglas, M. C. Crowe and R. W. Murray, J. Phys. Chem. C, 2010, 114, 15890–15896 CrossRef CAS.
- C. Kumara, C. M. Aikens and A. Dass, J. Phys. Chem. Lett., 2014, 5, 461–466 CrossRef CAS PubMed.
- R. Jin, S. Zhao, C. Liu, M. Zhou, G. Panapitiya, Y. Xing, N. L. Rosi, J. P. Lewis and R. Jin, Nanoscale, 2017, 9, 19183–19190 RSC.
- Q. Li, S. Wang, K. Kirschbaum, K. J. Lambright, A. Das and R. Jin, Chem. Commun., 2016, 52, 5194–5197 RSC.
- S. Wang, Y. Song, S. Jin, X. Liu, J. Zhang, Y. Pei, X. Meng, M. Chen, P. Li and M. Zhu, J. Am. Chem. Soc., 2015, 137, 4018–4021 CrossRef CAS PubMed.
- K. R. Krishnadas, A. Ghosh, A. Baksi, I. Chakraborty, G. Natarajan and T. Pradeep, J. Am. Chem. Soc., 2016, 138, 140–148 CrossRef CAS PubMed.
- K. R. Krishnadas, A. Baksi, A. Ghosh, G. Natarajan and T. Pradeep, Nat. Commun., 2016, 7, 13447 CrossRef CAS PubMed.
- S. Yamazoe, W. Kurashige, K. Nobusada, Y. Negishi and T. Tsukuda, J. Phys. Chem. C, 2014, 118, 25284–25290 CrossRef CAS.
- S. Hossain, T. Ono, M. Yoshioka, G. Hu, M. Hosoi, Z. Chen, L. V. Nair, Y. Niihori, W. Kurashige, D.-e. Jiang and Y. Negishi, J. Phys. Chem. Lett., 2018, 9, 2590–2594 CrossRef CAS PubMed.
- H. Qian, W. T. Eckenhoff, Y. Zhu, T. Pintauer and R. Jin, J. Am. Chem. Soc., 2010, 132, 8280–8281 CrossRef CAS PubMed.
- S.-F. Yuan, C.-Q. Xu, J. Li and Q.-M. Wang, Angew. Chem., Int. Ed., 2019, 58, 5906–5909 CrossRef CAS PubMed.
- Z.-J. Guan, J.-L. Zeng, Z.-A. Nan, X.-K. Wan, Y.-M. Lin and Q.-M. Wang, Sci. Adv., 2016, 2, e1600323 CrossRef PubMed.
- N. Kobayashi, Y. Kamei, Y. Shichibu and K. Konishi, J. Am. Chem. Soc., 2013, 135, 16078–16081 CrossRef CAS PubMed.
- X.-K. Wan, Z.-W. Lin and Q.-M. Wang, J. Am. Chem. Soc., 2012, 134, 14750–14752 CrossRef CAS PubMed.
- X.-S. Han, X. Luan, H.-F. Su, J.-J. Li, S.-F. Yuan, Z. Lei, Y. Pei and Q.-M. Wang, Angew. Chem., Int. Ed., 2020, 59, 2309–2312 CrossRef CAS PubMed.
- W.-D. Liu, J.-Q. Wang, S.-F. Yuan, X. Chen and Q.-M. Wang, Angew. Chem., Int. Ed., 2021, 60, 11430–11435 CrossRef CAS PubMed.
- J. Chen, Q.-F. Zhang, T. A. Bonaccorso, P. G. Williard and L.-S. Wang, J. Am. Chem. Soc., 2014, 136, 92–95 CrossRef CAS PubMed.
- F. Hu, J.-J. Li, Z.-J. Guan, S.-F. Yuan and Q.-M. Wang, Angew. Chem., Int. Ed., 2020, 59, 5312–5315 CrossRef CAS PubMed.
- Y. Chen, C. Zeng, C. Liu, K. Kirschbaum, C. Gayathri, R. R. Gil, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2015, 137, 10076–10079 CrossRef CAS PubMed.
- P. R. Nimmala and A. Dass, J. Am. Chem. Soc., 2014, 136, 17016–17023 CrossRef CAS PubMed.
- Y. Du, Z. J. Guan, Z. R. Wen, Y. M. Lin and Q. M. Wang, Chem.–Eur. J., 2018, 24, 16029–16035 CrossRef CAS PubMed.
- S. S. Zalesskiy, A. E. Sedykh, A. S. Kashin and V. P. Ananikov, J. Am. Chem. Soc., 2013, 135, 3550–3559 CrossRef CAS PubMed.
- I. Medina-Mercado, A. Colin-Molina, J. E. Barquera-Lozada, B. Rodríguez-Molina and S. Porcel, ACS Catal., 2021, 11, 8968–8977 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision B.01, Gaussian Inc., Wallingford CT, 2016 Search PubMed.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 299–310 CrossRef CAS.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS PubMed.
- D. Coskun, S. V. Jerome and R. A. Friesner, J. Chem. Theory Comput., 2016, 12, 1121–1128 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- B. K. Teo and H. Zhang, Angew. Chem., Int. Ed., 1992, 31, 445–447 CrossRef.
- X.-K. Wan, J.-Q. Wang and Q.-M. Wang, Angew. Chem., Int. Ed., 2021, 60, 20748–20753 CrossRef CAS PubMed.
- Crystal data for Au19Ag6: C227H209Ag6Au19B2F8O17P6S17, triclinic, P-1, a = 20.4536(6) Å, b= 20.7898(7) Å, c = 33.6586(9) Å, α = 72.945(3)°, β = 82.104(2)°, γ = 63.917(3)°, V = 12289.0(8) Å3, Z = 2, T = 173 K, 77948 reflections measured, 39715 unique (Rint= 0.0992), final R1=0.0853, and wR2= 0.2442 for 77948 observed reflections [I > 2σ(I)]. Deposition number 2131886 contains the supplementary crystallographic data for this paper.†.
- H. Schmidbaur and A. Schier, Angew. Chem., Int. Ed., 2015, 54, 746–784 CrossRef CAS PubMed.
- Q.-M. Wang and T. C. W. Mak, J. Am. Chem. Soc., 2001, 123, 7594–7600 CrossRef CAS PubMed.
- T. Wang, W.-H. Zhang, S.-F. Yuan, Z.-J. Guan and Q.-M. Wang, Chem. Commun., 2018, 54, 10367–10370 RSC.
- S. Hossain, Y. Niihori, L. V. Nair, B. Kumar, W. Kurashige and Y. Negishi, Acc. Chem. Res., 2018, 51, 3114–3124 CrossRef CAS PubMed.
- B. K. Teo and K. Keating, J. Am. Chem. Soc., 1984, 106, 2224–2226 CrossRef CAS.
- B. K. Teo, X. Shi and H. Zhang, J. Am. Chem. Soc., 1991, 113, 4329–4331 CrossRef CAS.
- B. K. Teo, X. Shi and H. Zhang, J. Chem. Soc., Chem. Commun., 1992, 1195–1196, 10.1039/C39920001195.
- B. K. Teo, K. Keating and Y. H. Kao, J. Am. Chem. Soc., 1987, 109, 3494–3495 CrossRef CAS.
- B. K. Teo, H. Zhang and X. Shi, J. Am. Chem. Soc., 1990, 112, 8552–8562 CrossRef CAS.
- K. Kwak, Q. Tang, M. Kim, D.-e. Jiang and D. Lee, J. Am. Chem. Soc., 2015, 137, 10833–10840 CrossRef CAS PubMed.
- T. Kawawaki, Y. Imai, D. Suzuki, S. Kato, I. Kobayashi, T. Suzuki, R. Kaneko, S. Hossain and Y. Negishi, Chem.–Eur. J., 2020, 26, 16150–16193 CrossRef CAS PubMed.
- D. M. P. Mingos, Dalton Trans., 2015, 44, 6680–6695 RSC.
- M. A. Tofanelli, K. Salorinne, T. W. Ni, S. Malola, B. Newell, B. Phillips, H. Häkkinen and C. J. Ackerson, Chem. Sci., 2016, 7, 1882–1890 RSC.
- A. Cirri, H. Morales Hernández, C. Kmiotek and C. J. Johnson, Angew. Chem., Int. Ed., 2019, 58, 13818–13822 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2131886. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d2sc00012a |
| This journal is © The Royal Society of Chemistry 2022 |