Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Catalytic asymmetric hydrometallation of cyclobutenes with salicylaldehydes
F. Wieland
Goetzke
a,
Mireia
Sidera
b and
Stephen P.
Fletcher
*a
aDepartment of Chemistry, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK. E-mail: stephen.fletcher@chem.ox.ac.uk
bVertex Pharmaceuticals (Europe) Ltd, 86–88 Jubilee Avenue, Milton Park, Abingdon, OX14 4RW, UK
First published on 12th January 2022
Abstract
Correction for ‘Catalytic asymmetric hydrometallation of cyclobutenes with salicylaldehydes’ by F. Wieland Goetzke et al., Chem. Sci., 2022, DOI: 10.1039/d1sc06035j.
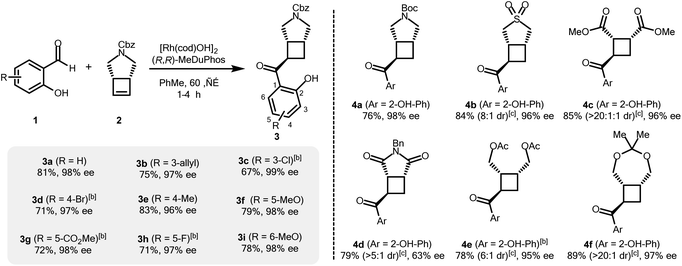
The authors regret that the absolute stereochemistry in Scheme 1 presented in the original manuscript was incorrect. The correct version of Scheme 1 is shown below.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2022 |