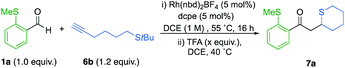Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Diverse saturated heterocycles from a hydroacylation/conjugate addition cascade†
Ndidi U. N.
Iwumene‡
 a,
Daniel. F.
Moseley‡
a,
Robert D. C.
Pullin
b and
Michael C.
Willis
a,
Daniel. F.
Moseley‡
a,
Robert D. C.
Pullin
b and
Michael C.
Willis
 *a
*a
aDepartment of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford OX1 3TA, UK. E-mail: michael.willis@chem.ox.ac.uk
bVertex Pharmaceuticals (Europe) Ltd, 86-88 Jubilee Avenue, Milton Park, Abingdon, OX14 4RW, UK
First published on 19th January 2022
Abstract
Rhodium-catalyzed hydroacylation using alkynes substituted with pendant nucleophiles, delivers linear α,β-unsaturated enone intermediates with excellent regioselectivity. These adducts are used to construct a broad range of diversely substituted, saturated O-, N- and S-heterocycles in a one-pot process. Judicious choice of cyclisation conditions enabled isolation of O-heterocycles with high levels of diastereoselectivity. A variety of derivatisation reactions are also performed, generating functionalised hydroacylation products. This sequence serves as a general approach for the synthesis of fully saturated heterocycles.
Introduction
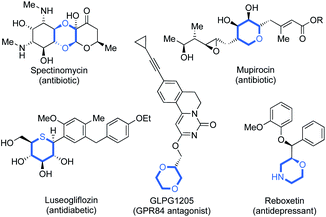
Since Lovering's seminal ‘flatland’ epiphany,1 more emphasis lies on increasing saturation within therapeutics.1,2 This increased three-dimensionality results in the improved aqueous solubility,1,2g,3 and target selectivity of drug candidates.4 Saturated N-, O- and S-heterocycles are ubiquitous in natural and pharmaceutical products which display an extensive range of biological activities (Fig. 1).5 Thus, the efficient synthesis of saturated heterocyclic fragments is an important application of new synthetic methodology.Recently, rhodium(I)-catalysed intermolecular hydroacylation,6 formally the atom-economic addition of a formyl C–H bond across a C–C π-bond,7 has been validated as a powerful C–C bond forming tool in heterocycle synthesis.7b,c,8 Of the variety of catalysts known to promote hydroacylation,9 rhodium(I)bisphosphines have proved to be highly effective.10 These catalysts have previously been exploited in intramolecular alkene hydroacylation approaches to the synthesis of 7- and 8-membered N-,11 S- and O-heterocycles,7a and in the preparation 5,5- and 5,6-polycyclic nitrogen heterocycles.12
Intermolecular alkyne hydroacylation methods have emerged as a modular alternative to intramolecular cyclisation. However, these reactions can be plagued by deleterious decarbonylation pathways.13 To date, the most successful remedy has been chelation control;14 where a directing group, most often positioned beta to the aldehyde, forms a stable 5-membered metallacycle.14c,15 Methodologies that employ low catalyst loadings and aldehydes containing P-, O-, N-, or S-coordinating groups, have been reported.16,10c,17,18,19 Elegant disconnections of certain heterocyclic scaffolds, typically those that incorporate partial or full unsaturation, have been realized through tandem hydroacylation cyclisation sequences.7b,c,8a–e The latter exploit highly electrophilic α,β-unsaturated enone intermediates (Scheme 1a), that contain aforementioned coordinating groups on the aldehyde (X) and/or nucleophilic groups on the alkyne components (Z).
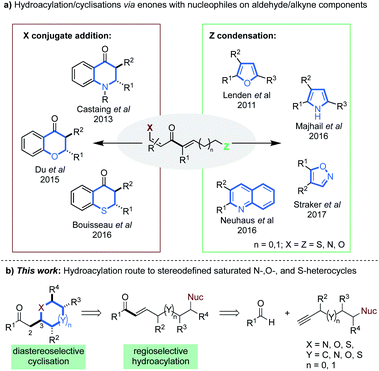 | ||
| Scheme 1 (a) Hydroacylation methods to various heterocycles via α,β-unsaturated enones; (b) our proposed alkyne hydroacylation route to stereodefined saturated heterocycles. | ||
Our laboratory has shown that β-heteroatoms on the aldehyde component can be exploited in conjugate–addition processes, yielding dihydroquinolones8b and thiochroman-4-ones.8a The Stanley group have also described the formation of 2,3-disubstituted chroman-4-ones via an analogous pathway, using O-coordinating aldehydes.20 The said enone intermediates, can also partake in condensation reactions with nucleophiles appended on the alkyne segment (Scheme 1a). This has allowed the synthesis of substituted furans,8c pyrroles,7c quinolines7b and isoxazoles.8e The above examples constitute encouraging precedent, however, most are either limited to specific substrate classes,7b,8a–c,20 rely on forcing conditions,8a–c,8e,20 or are not one–pot processes.8b,d,e Most importantly, no previous work achieves regio- and diastereo-defined syntheses of saturated heterocycles.
We envisioned that diversely substituted saturated heterocycles of varied ring-size could be disconnected to α,β-unsaturated enones bearing pendant N-, O- or S-nucleophiles (Scheme 1b). In turn, these intermediates may be prepared by the hydroacylative union of appropriately substituted aldehydes and terminal alkynes. Such a route has the potential to generate stereodefined structures, provided that stereo-control can be achieved at the C3 position of the products (Scheme 1b).
In this article we describe a one-pot hydroacylation/diastereoselective conjugate-addition sequence, that delivers an exceptional scope of saturated N-, O- and S-heterocycles from simple unactivated substrates. We also demonstrate the utility of this method through product derivatization; achieving the modular assembly of complex scaffolds.
Results and discussion
O- and N-heterocycles
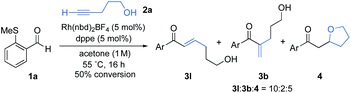
We began our investigation by determining the tolerance of alkynols and alkynamines in hydroacylation reactions. We have previously shown that Rh(I)-catalysts that include small-bite-angle diphosphine ligands produce efficient and selective hydroacylation reactions. When a similar catalyst system was applied to the targeted reaction, the conversion was initially low (Scheme 2). Nonetheless, selectivity for the linear hydroacylation adduct was high.21 Conversions were dramatically improved when employing 1,2-dichloroethane (DCE) solvent and 1,2 bis(dicyclohexylphosphino)ethane (dcpe) as a ligand (see the ESI† for further details). Under these optimal conditions, a significant proportion of the tetrahydrofuran product was detected, suggesting the intermediate enone undergoes intramolecular conjugate-addition spontaneously. This observation is consistent with a serendipitous finding of a previous study from our laboratory.22 The successful hydroacylation reactions with alkynol 2a confirmed the feasibility of a tandem hydroacylation/cyclisation protocol. Accordingly, we then set out to construct substituted alkynols, with the aim of achieving a diastereoselective cyclisation.A one-pot strategy was developed using alkynol 2b as a test substrate. Initially, hydroacylation of aryl sulfide 1a with 1.2 equiv. of alkynol, yielded dioxane 5a in a modest 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 86% total yield (Table 1, entry 1). Performing the cyclisation at room temperature was less efficient (entry 2). We then sought to improve the diastereoselectivity by inducing epimerisation of the forming stereocentre in product 5a. Stoichiometric quantities of the Brønsted acids, p-TsOH and MsOH increased selectivity to 11
1 dr and 86% total yield (Table 1, entry 1). Performing the cyclisation at room temperature was less efficient (entry 2). We then sought to improve the diastereoselectivity by inducing epimerisation of the forming stereocentre in product 5a. Stoichiometric quantities of the Brønsted acids, p-TsOH and MsOH increased selectivity to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and >20
1 and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, respectively (entries 3 and 4), but depreciated the overall yield. Dropping the loading of these acids to 20 mol% delivered a smaller increase in dr, without an overall reduction in yield (entries 5 and 6). When evaluating sub-stoichiometric quantities of Lewis acids at 55 °C (entries 7 to 10), the diastereoselectivity remained low. Conversely when BCl3 was used (entry 11), a dr of 7.3
1 dr, respectively (entries 3 and 4), but depreciated the overall yield. Dropping the loading of these acids to 20 mol% delivered a smaller increase in dr, without an overall reduction in yield (entries 5 and 6). When evaluating sub-stoichiometric quantities of Lewis acids at 55 °C (entries 7 to 10), the diastereoselectivity remained low. Conversely when BCl3 was used (entry 11), a dr of 7.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was obtained, with no yield degeneration. On addition of BCl3 to the reaction vial, fuming HCl was observed. Inspired by this, an organic solution of stoichiometric HCl (entry 12) was tested, providing the desired 1,4-dioxane ring in 13
1 was obtained, with no yield degeneration. On addition of BCl3 to the reaction vial, fuming HCl was observed. Inspired by this, an organic solution of stoichiometric HCl (entry 12) was tested, providing the desired 1,4-dioxane ring in 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 87% isolated yield. Attempts to extend the duration of epimerisation, or increase the equivalents of HCl, failed to produce a further increase in dr, without the accompaniment of product degradation (see the ESI† for further details).
1 dr and 87% isolated yield. Attempts to extend the duration of epimerisation, or increase the equivalents of HCl, failed to produce a further increase in dr, without the accompaniment of product degradation (see the ESI† for further details).
| Entry | Acid (equiv.) | Yielda (%) | drb |
|---|---|---|---|
a Determined using 1H NMR spectroscopy with methyl-3,5-dinitrobenzoate as an internal standard. Isolated yield in parentheses.
b Measured by 1NMR spectroscopy on the unpurified reaction mixture.
c Reaction at room temperature.
d >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 l 1 l![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b regioselectivity, and >20 b regioselectivity, and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 5a 1 5a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a determined by 1H NMR spectroscopy.
e 13.9 1a determined by 1H NMR spectroscopy.
e 13.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 l 1 l![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b regioselectivity, and 6.2 b regioselectivity, and 6.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 5a 1 5a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a determined by 1H NMR spectroscopy. 1a determined by 1H NMR spectroscopy.
|
|||
| 1 | — | 86d | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2c | — | 64e | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | p-TsOH (2.0) | 62 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | MsOH (2.0) | 33 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | p-TsOH (0.2) | 87 | 6.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | MsOH (0.2) | 98 | 4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | Sc(OTf)3 (0.2) | 78 | 4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | Ti(i-PrO)4 (0.2) | 96 | 4.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | AlCl3 (0.2) | 87 | 4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | BF3·OEt2 | 76 | 4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | BCl3 (0.2) | 83 | 7.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | HCl in dioxane (2.0) | 95(87) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
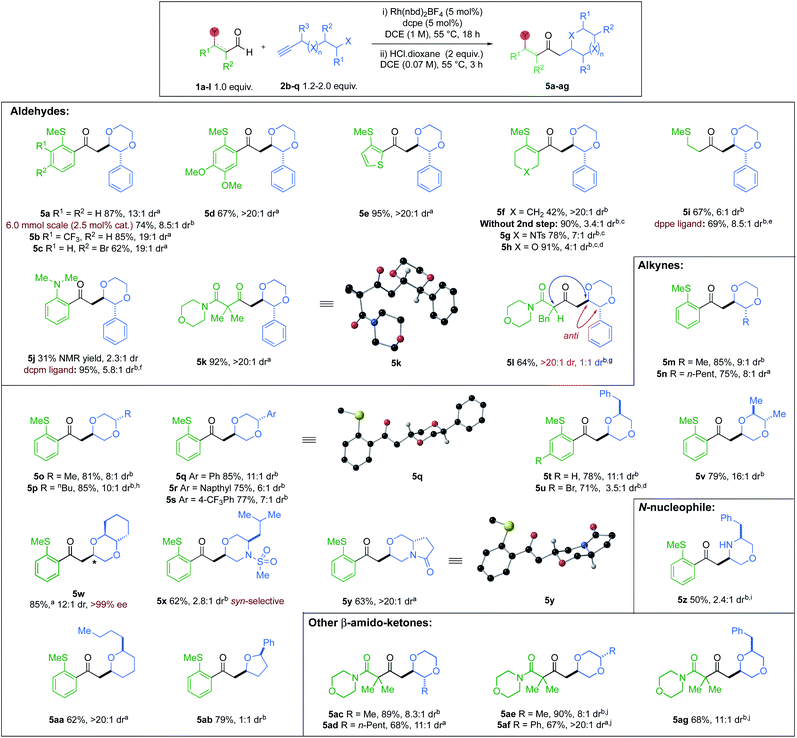
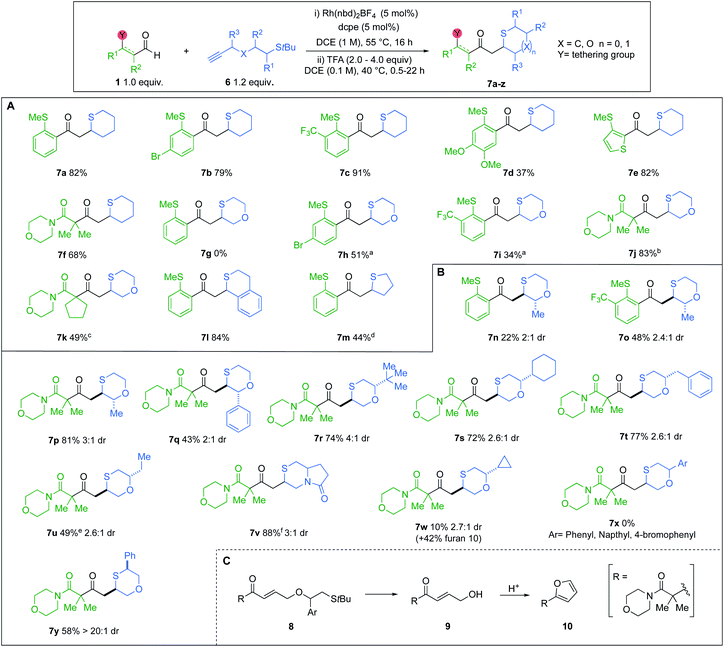
With the optimised conditions in hand, we explored the reaction substrate scope (Scheme 3). Electron-withdrawing CF3-substituent (5b) and donating OMe-substituents (5d) provided good yields and high selectivity. An aryl bromide (5c) was also compatible. Clean conversion of a thiophene-derived substrate, afforded the dioxane 5e in 95% yield and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Vinyl sulfide 5f decomposed under the acidic epimerisation conditions, resulting in a 42% yield with a >20
1 dr. Vinyl sulfide 5f decomposed under the acidic epimerisation conditions, resulting in a 42% yield with a >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. However, without the acid step, the yield increased to 90%, but the dr was reduced. Other heterocyclic vinyl sulfides (X = NTs, O) provided similar results in the absence of HCl (5g,h). Alkyl sulfide 5i and 2-aminobenzaldehyde-derived product 5j, were obtained with good to moderate dr, but required alternative Rh-catalysts.8b,23 Di- and Mono-substituted β-ketoamides 5k and 5l, both displayed excellent ring-closing diastereoselectivity, with yields of 92% and 64%, respectively. Exploration of the alkyne scope proved that good yields and selectivity could be obtained for all substitution patterns on the 1,4-dioxane ring (5m to 5y). An enantiopure alkynol was also compatible, delivering trans-6,6-bicyclic species 5w in 85% yield and 12
1 dr. However, without the acid step, the yield increased to 90%, but the dr was reduced. Other heterocyclic vinyl sulfides (X = NTs, O) provided similar results in the absence of HCl (5g,h). Alkyl sulfide 5i and 2-aminobenzaldehyde-derived product 5j, were obtained with good to moderate dr, but required alternative Rh-catalysts.8b,23 Di- and Mono-substituted β-ketoamides 5k and 5l, both displayed excellent ring-closing diastereoselectivity, with yields of 92% and 64%, respectively. Exploration of the alkyne scope proved that good yields and selectivity could be obtained for all substitution patterns on the 1,4-dioxane ring (5m to 5y). An enantiopure alkynol was also compatible, delivering trans-6,6-bicyclic species 5w in 85% yield and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, with retention of ee. A (rac)-leucine-derived, monocyclic morpholine 5x, was isolated in 62% yield, but with modest dr. A 6,5-pyrrolo-1,4-oxazine 5y could also be accessed in 63% yield with a >20
1 dr, with retention of ee. A (rac)-leucine-derived, monocyclic morpholine 5x, was isolated in 62% yield, but with modest dr. A 6,5-pyrrolo-1,4-oxazine 5y could also be accessed in 63% yield with a >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Tetrahydropyran 5aa and tetrahydrofuran 5ab, were also isolated in 62% and 79% yields, respectively. The diastereoselectivity of 5-exo-trig ring closure within tetrahydrofuran 5ab, could not be improved (see ESI, Section 4†).24 A (rac)-phenylalanine-derived free morpholine heterocycle (5z) could be accessed from an N-nucleophilic conjugate addition, in good yield and moderate dr, using p-TsOH for the cyclisation and N-Boc deprotection. The scope was extended to β-amido substrates; good yields and selectivities were obtained for the desired 1,4-dioxane products 5ac to 5ag. We were pleased to find that the reaction could be performed on a gram-scale (6 mmol) with half the catalyst loading (2.5 mol%), furnishing 5a in 74% isolated yield and 8.5
1 dr. Tetrahydropyran 5aa and tetrahydrofuran 5ab, were also isolated in 62% and 79% yields, respectively. The diastereoselectivity of 5-exo-trig ring closure within tetrahydrofuran 5ab, could not be improved (see ESI, Section 4†).24 A (rac)-phenylalanine-derived free morpholine heterocycle (5z) could be accessed from an N-nucleophilic conjugate addition, in good yield and moderate dr, using p-TsOH for the cyclisation and N-Boc deprotection. The scope was extended to β-amido substrates; good yields and selectivities were obtained for the desired 1,4-dioxane products 5ac to 5ag. We were pleased to find that the reaction could be performed on a gram-scale (6 mmol) with half the catalyst loading (2.5 mol%), furnishing 5a in 74% isolated yield and 8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr.
1 dr.
The relative configurations of the major diastereoisomers of products 5k, 5q and oxazine 5y were unambiguously determined by X-ray crystallography to be anti.25 For heterocycle 5x, the relative configuration was determined to be syn through NOESY analysis (see ESI, Section 6.3†), and is consistent with earlier studies.26
S-Heterocycles
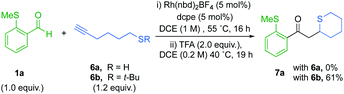
We next targeted the synthesis of sulfur containing heterocycles,27 and for our initial investigation aldehyde 1a was paired with alkanethiol 6a under our previously optimized hydroacylation conditions (Scheme 4). However, after 16 hours the enone product did not form. It is probable that the free thiol chelates to an intermediary rhodium species and prevents catalysis. The latter mandated the use of a protecting group in the hydroacylation step.We therefore selected t-Bu sulfide substituted-alkynes as suitable substrates. We were pleased to find that the coupling between aldehyde 1a and alkyne 6b proceeded to full conversion, with high selectivity for the linear regioisomer (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Subsequent treatment with 2.0 equivalents of TFA, afforded thiane 7a in 61% yield (Scheme 4). Encouraged by this result, the cyclisation step was further optimized (Table 2). It became apparent that higher equivalents of acid and longer reaction times depreciated the yield (Table 2, entries 1–3). Using a shorter reaction time had minimal effect on yield (entry 4). However, diluting the reaction favoured ring-formation (entries 5 and 6), ultimately delivering the thiane in 82% yield. Further dilution did not significantly affect the yield (entry 7).
1). Subsequent treatment with 2.0 equivalents of TFA, afforded thiane 7a in 61% yield (Scheme 4). Encouraged by this result, the cyclisation step was further optimized (Table 2). It became apparent that higher equivalents of acid and longer reaction times depreciated the yield (Table 2, entries 1–3). Using a shorter reaction time had minimal effect on yield (entry 4). However, diluting the reaction favoured ring-formation (entries 5 and 6), ultimately delivering the thiane in 82% yield. Further dilution did not significantly affect the yield (entry 7).
| Entry | Equiv. TFA | Time/h | Conc./M | Yieldb (%) |
|---|---|---|---|---|
| a 100% conversion for step (i) based on analysis by 1H NMR spectroscopy. b Yields determined using 1H NMR spectroscopy with 1,3,5-trimethoxybenzene as internal standard; isolated yields in parentheses. | ||||
| 1 | 2 | 19 | 0.2 | 61 |
| 2 | 5 | 19 | 0.2 | 54 |
| 3 | 10 | 19 | 0.2 | 55 |
| 4 | 2 | 6 | 0.2 | 57 |
| 5 | 2 | 4 | 0.5 | 64 |
| 6 | 2 | 4 | 0.1 | 74(82) |
| 7 | 2 | 4 | 0.025 | 70 |
Having established an efficient one-pot protocol, the scope of the reaction was explored (Scheme 5A). Aldehydes bearing electron-withdrawing groups were well tolerated (7b and 7c). However, when OMe substituents were present, a lower yield of cyclic sulfide 7d was obtained. Heteroaromatic and β-amido aldehydes were also compatible, yielding thianes 7e and 7f in 82% and 68%, respectively. The transformation was also successfully extended to t-Bu sulfide alkynes possessing an oxygen atom linker. However, when o-SMe-benzaldehydes were used with these alkynes, the ensuing α,β-unsaturated enones underwent a competitive 6-endo-trig cyclisation, delivering thiochroman-4-ones.8a Consequently, 1,4-oxathiane 7g could not be isolated. Pleasingly, incorporating electron-withdrawing groups on the o-SMe-benzaldehydes inhibited the competing pathway, providing improved yields of the desired oxathianes 7h and 7i. Employing β-amido chelating aldehydes eliminated the aforesaid competing cyclisation (7j and 7k). The reaction was also successfully applied to the synthesis of thiochromane derivative 7l, as well as tetrahydrothiophene 7m.
We next explored the scope of substituted t-Bu sulfide alkynes (Scheme 5B). Variation of groups at the R3 position was tolerated. Pairing methyl-substituted alkynes with o-SMe-aldehydes furnished lower yields of the desired cyclic sulfides (7n and 7o), due to the aforementioned competing 6-endo-trig ring closure. However, exploiting a dicarbonyl aldehyde enabled the synthesis of 1,4-oxathiane 7p in good yield. For the above cases, the crude diastereoselectivity did not exceed 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Attempts to increase the selectivity through treatment of the diastereoisomeric mixture with weak base, resulted in epimerization to a 1
1. Attempts to increase the selectivity through treatment of the diastereoisomeric mixture with weak base, resulted in epimerization to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture. Incorporating a phenyl R3 substituent did not enhance the dr, but instead impacted the yield of cyclised product 7q. Employing acyclic and aliphatic R2 groups gave rise to diversely substituted saturated S-heterocycles, in moderate to high yields (7r–w). Notable among these compounds is 6,5-pyrrolo-1,4-thiazinone 7v, which has the potential to serve as a versatile scaffold in drug discovery.28 Alkynes featuring R2 groups with sp2 character (8) displayed alternate reactivity to give furan 10, as opposed to oxathianes 7x, under the cyclisation conditions (Scheme 5C). Cyclopropyl-derivative 7w shows intermediate reactivity, with a low yield of the oxothiane being achieved, along with furan 10. Moderate drs were also obtained with the R2 substituted alkynes, however, we were pleased to achieve excellent selectivity, when using a phenyl R1 substituent, delivering cyclic sulfide 7y as a single diastereoisomer.
1 mixture. Incorporating a phenyl R3 substituent did not enhance the dr, but instead impacted the yield of cyclised product 7q. Employing acyclic and aliphatic R2 groups gave rise to diversely substituted saturated S-heterocycles, in moderate to high yields (7r–w). Notable among these compounds is 6,5-pyrrolo-1,4-thiazinone 7v, which has the potential to serve as a versatile scaffold in drug discovery.28 Alkynes featuring R2 groups with sp2 character (8) displayed alternate reactivity to give furan 10, as opposed to oxathianes 7x, under the cyclisation conditions (Scheme 5C). Cyclopropyl-derivative 7w shows intermediate reactivity, with a low yield of the oxothiane being achieved, along with furan 10. Moderate drs were also obtained with the R2 substituted alkynes, however, we were pleased to achieve excellent selectivity, when using a phenyl R1 substituent, delivering cyclic sulfide 7y as a single diastereoisomer.
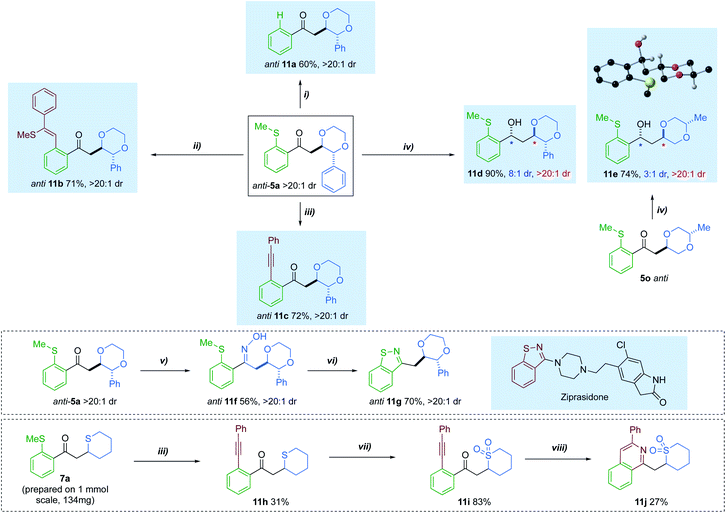
Having accessed a series of diversely substituted saturated heterocycles, we sought to extend the utility of the method by investigating a series of transformations to further functionalise the products (Scheme 6). For example, the directing SMe group could be removed using a silane-mediated desulfurization to give phenyl ketone 11a.14c Additionally, the thiomethyl tether was exploited as a ‘late-stage’ lynchpin in a rhodium-catalysed carbothiolation, giving product 11b in 70% yield.29 A Sonogashira-type coupling could also be performed on the anti-isomer of 5a, affording cross-coupled adduct 11c 72% yield.30 The ketone group of 5a could also be interconverted. A diastereoselective reduction with Eu(OTf)3 and LiBH4, afforded alcohol 11d in 90% yield and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr.31 When the same conditions were applied to dioxane 5o, with a distal Me-ring substituent, moderate diastereoselectivity could still be achieved. The anti-adduct was preferentially formed in these reductions.
1 dr.31 When the same conditions were applied to dioxane 5o, with a distal Me-ring substituent, moderate diastereoselectivity could still be achieved. The anti-adduct was preferentially formed in these reductions.
We additionally carried out two multi-step sequences to access bis-heterocyclic motifs with relevance to the pharmaceutical industry. Condensation of ketone 5a, with hydroxylamine hydrochloride, provided oxime 11f in 56% yield.32 This was further activated using acetic anhydride and base, resulting in the formation of benzoisothiazole 11g in 70% yield.33 Such a motif has relevance in a variety of FDA approved drug scaffolds,34 one of which being ziprasidone.35 Furthermore, cyclic sulfide 7a could also be coupled with phenylacetylene, delivering o-(1-alkynyl)phenylketone 11h. Treatment of 11h with m-CPBA resulted in clean conversion to the sulfone 11i. After modifying literature conditions,36 direct construction of isoquinoline 11j was achieved via annulation of 11i with ammonium acetate.
Conclusions
In summary, we have developed a one-pot tandem hydroacylation/conjugate-addition sequence that delivers a diverse array of fully saturated O, N, and S-heterocycles in good to excellent yields. Likewise, the robustness of a rhodium(I)/dcpe catalyst system is demonstrated through exemplary functional group tolerance and high regioselectivity. For oxygen ring-closure, significant enhancement of diastereoselectivity has been achieved, allowing access to single diastereomers (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) of O-heterocycles. The cyclised products have also been exploited in a series of derivatisation reactions, generating synthetically attractive and pharmaceutically intriguing molecules.
1 dr) of O-heterocycles. The cyclised products have also been exploited in a series of derivatisation reactions, generating synthetically attractive and pharmaceutically intriguing molecules.
Data availability
Full experimental and characterisation data are provided as part of the ESI.†Author contributions
N. U. N. I. and D. F. M. performed the experiments and analysed the results. All authors designed the project and wrote the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
N. U. N. I and D. F. M are grateful to the EPSRC Centre for Doctoral Training in Synthesis for Biology and Medicine (EP/L015838/1) for a studentship, generously supported by AstraZeneca, Diamond Light Source, Defence Science and Technology Laboratory, Evotec, GlaxoSmithKline, Janssen, Novartis, Pfizer, Syngenta, Takeda, UCB and Vertex. Antoine de Gombert and Richard Cooper (both University of Oxford), and Alasdair Mckay (University of Melbourne) are thanked for assistance with X-ray structure analysis.Notes and references
- F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS PubMed.
- (a) M. D. Delost, D. T. Smith, B. J. Anderson and J. T. Njardarson, J. Med. Chem., 2018, 61, 10996–11020 CrossRef CAS PubMed; (b) N. A. Meanwell, Chem. Res. Toxicol., 2011, 24, 1420–1456 Search PubMed; (c) T. J. Ritchie and S. J. F. Macdonald, Drug Discov. Today, 2009, 14, 1011–1020 CrossRef CAS PubMed; (d) T. J. Ritchie, S. J. F. MacDonald, R. J. Young and S. D. Pickett, Drug Discov. Today, 2011, 16, 164–171 CrossRef CAS PubMed; (e) R. D. Taylor, M. MacCoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845–5859 CrossRef CAS PubMed; (f) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed; (g) C.-V. T. Vo, M. U. Luescher and J. W. Bode, Nat. Chem., 2014, 6, 310–314 CrossRef CAS PubMed.
- (a) A. Nadin, C. Hattotuwagama and I. Churcher, Angew. Chem., Int. Ed., 2012, 51, 1114–1122 CrossRef CAS PubMed; (b) C. W. Murray and D. C. Rees, Angew. Chem., Int. Ed., 2016, 55, 488–492 CrossRef CAS PubMed.
- A. Gomtsyan, Chem. Heterocyc. Compd., 2012, 48, 7–10 CrossRef CAS.
- (a) J. Arp, S. Götze, R. Mukherji, D. J. Mattern, M. García-Altares, M. Klapper, D. A. Brock, A. A. Brakhage, J. E. Strassmann, D. C. Queller, B. Bardl, K. Willing, G. Peschel and P. Stallforth, Proc. Natl. Acad. Sci. U.S.A., 2018, 115, 3758–3763 CrossRef CAS PubMed; (b) L. M. Bang and L. J. Scott, Drugs, 2003, 63, 2413–2424 CrossRef CAS PubMed; (c) J. Cornil, L. Gonnard, C. Bensoussan, A. Serra-Muns, C. Gnamm, C. Commandeur, M. Commandeur, S. B. Reymond, A. G. R. Guérinot and J. Cossy, Acc. Chem. Res., 2015, 48, 761–773 CrossRef CAS PubMed; (d) W. H. El-Tantawy and A. Temraz, Arch. Physiol. Biochem., 2020, 126, 116–128 CrossRef CAS PubMed; (e) A. Markham and S. Elkinson, Drugs, 2014, 74, 945–950 CrossRef CAS PubMed; (f) C. Moennighoff, N. Thomas, F. Nienhaus, M. Hartmann, A. Menrath, J. Merkel, H. Detlefsen, L. Kreienbrock and I. Hennig-Pauka, BMC Vet. Res., 2020, 16, 1–14 CrossRef PubMed; (g) V. Terevnikov, G. Joffe and J. H. Stenberg, Int. J. Neuropsychop., 2015, 18, 1–14 CAS; (h) Z. Titarenko, N. Vasilevich, V. Zernov, M. Kirpichenok and D. Genis, J. Comp. Aid. Mol. Des., 2013, 27, 125–160 CrossRef CAS PubMed; (i) M. Valenza, A. Blasio, A. DiLeo, P. Cottone and V. Sabino, Pharmacol. Biochem. Behav., 2020, 192 Search PubMed; (j) C.-V. T. Vo and J. W. Bode, J. Org. Chem., 2014, 79, 2809–2815 CrossRef CAS PubMed.
- (a) C.-H. Jun, E.-A. Jo and J.-W. Park, Eur. J. Org. Chem., 2007, 2007, 1869–1881 CrossRef; (b) M. C. Willis, Chem. Rev., 2010, 110, 725–748 CrossRef CAS PubMed; (c) J. C. Leung and M. J. Krische, Chem. Sci., 2012, 3, 2202–2209 RSC; (d) A. Ghosh, K. F. Johnson, K. L. Vickerman, J. A. Walker and L. M. Stanley, Org. Chem. Front., 2016, 3, 639–644 RSC; (e) R. T. Davison, E. L. Kuker and V. M. Dong, Acc. Chem. Res., 2021, 54, 1236–1250 CrossRef CAS PubMed.
- (a) M. M. Coulter, P. K. Doman and V. M. Dong, J. Am. Chem. Soc., 2009, 131, 6932–6933 CrossRef CAS PubMed; (b) J. D. Neuhaus, S. M. Morrow, M. Brunavs and M. C. Willis, Org. Lett., 2016, 18, 1562–1565 CrossRef CAS PubMed; (c) M. K. Majhail, P. M. Ylioja and M. C. Willis, Chem. –Eur. J., 2016, 22, 7879–7884 CrossRef CAS PubMed.
- (a) A. Bouisseau, J. Glancy and M. C. Willis, Org. Lett., 2016, 18, 5676–5679 CrossRef CAS PubMed; (b) M. Castaing, S. L. Wason, B. Estepa, J. F. Hooper and M. C. Willis, Angew. Chem., Int. Ed., 2013, 52, 13280–13283 CrossRef CAS PubMed; (c) P. Lenden, D. A. Entwistle and M. C. Willis, Angew. Chem., Int. Ed., 2011, 50, 10657–10660 CrossRef CAS PubMed; (d) R. N. Straker, M. Formica, J. D. Lupton, J. Niu and M. C. Willis, Tetrahedron, 2018, 74, 5408–5414 CrossRef CAS; (e) R. N. Straker, M. K. Majhail and M. C. Willis, Chem. Sci., 2017, 8, 7963–7968 RSC.
- (a) R. Santhoshkumar, S. Mannathan and C. H. Cheng, J. Am. Chem. Soc., 2015, 137, 16116–16120 CrossRef CAS PubMed; (b) H. Miura, K. Wada, S. Hosokawa and M. Inoue, Chem. –Eur. J., 2013, 19, 861–864 CrossRef CAS PubMed; (c) Q. A. Chen, F. A. Cruz and V. M. Dong, J. Am. Chem. Soc., 2015, 137, 3157–3160 CrossRef CAS PubMed; (d) Y. Hoshimoto, Y. Hayashi, H. Suzuki, M. Ohashi and S. Ogoshi, Angew. Chem., Int. Ed., 2012, 51, 10812–10815 CrossRef CAS PubMed.
- (a) Y. Oonishi, A. Taniuchi, M. Mori and Y. Sato, Tetrahedron Lett., 2006, 47, 5617–5621 CrossRef CAS; (b) K. Tanaka and G. C. Fu, J. Amer. Chem. Soc., 2001, 123, 11492–11493 CrossRef CAS PubMed; (c) K. Kokubo, K. Matsumasa, M. Miura and M. Nomura, J. Org. Chem., 1997, 62, 4564–4565 CrossRef CAS.
- J. S. Arnold, E. T. Mwenda and H. M. Nguyen, Angew. Chem., Int. Ed., 2014, 53, 3688–3692 CrossRef CAS PubMed.
- (a) A. Ghosh and L. M. Stanley, Chem. Commun., 2014, 50, 2765–2768 RSC; (b) X. W. Du, A. Ghosh and L. M. Stanley, Org. Lett., 2014, 16, 4036–4039 CrossRef CAS PubMed.
- (a) R. J. Pawley, M. A. Huertos, G. C. Lloyd-Jones, A. S. Weller and M. C. Willis, Organometallics, 2012, 31, 5650–5659 CrossRef CAS; (b) A. B. Chaplin, J. F. Hooper, A. S. Weller and M. C. Willis, J. Am. Chem. Soc., 2012, 134, 4885–4897 CrossRef CAS PubMed; (c) J. Barwick-Silk, S. Hardy, M. C. Willis and A. S. Weller, J. Am. Chem. Soc., 2018, 140, 7347–7357 CrossRef CAS PubMed; (d) T. J. Coxon, M. Fernández, J. Barwick-Silk, A. I. McKay, L. E. Britton, A. S. Weller and M. C. Willis, J. Am. Chem. Soc., 2017, 139, 10142–10149 CrossRef CAS PubMed; (e) M. C. Willis, H. E. Randell-Sly, R. L. Woodward and G. S. Currie, Org. Lett., 2005, 7, 2249–2251 CrossRef CAS PubMed.
- (a) H. E. Randell-Sly, J. D. Osborne, R. L. Woodward, G. S. Currie and M. C. Willis, Tetrahedron, 2009, 65, 5110–5117 CrossRef CAS; (b) R. J. Pawley, G. L. Moxham, R. Dallanegra, A. B. Chaplin, S. K. Brayshaw, A. S. Weller and M. C. Willis, Organometallics, 2010, 29, 1717–1728 CrossRef CAS; (c) J. F. Hooper, R. D. Young, A. S. Weller and M. C. Willis, Chem. –Eur. J., 2013, 19, 3125–3130 CrossRef CAS PubMed.
- G. L. Moxham, H. E. Randell-Sly, S. K. Brayshaw, R. L. Woodward, A. S. Weller and M. C. Willis, Angew. Chem., Int. Ed., 2006, 45, 7618–7622 CrossRef CAS PubMed.
- P-coordinating group, H. Lee and C. H. Jun, Bull. Korean Chem. Soc., 1995, 16, 66–68 Search PubMed.
- O-coordinating groups: R. Pal, S. C. O'Brien and M. C. Willis, Chem. –Eur. J., 2020, 26, 11710–11714 CrossRef CAS PubMed.
- N-coordinating groups: (a) J. W. Suggs, J. Am. Chem. Soc., 1978, 100, 640–641 CrossRef CAS; (b) M. C. Willis and S. Sapmaz, Chem. Commun., 2001, 2558–2559 RSC; (c) C.-H. Jun, D.-Y. Lee, H. Lee and J.-B. Hong, Angew. Chem., Int. Ed., 2000, 39, 3070–3072 CrossRef CAS; (d) S. Seo, M. Gao, E. Paffenholz and M. C. Willis, ACS Catal., 2021, 11, 6091–6098 CrossRef CAS PubMed.
- S-coordinating groups: (a) M. C. Willis, S. J. McNally and P. J. Beswick, Angew. Chem., Int. Ed., 2004, 43, 340–343 CrossRef CAS PubMed; (b) G. L. Moxham, H. E. Randell-Sly, S. K. Brayshaw, R. L. Woodward, A. S. Weller and M. C. Willis, Angew. Chem., Int. Ed., 2006, 45, 7618–7622 CrossRef CAS PubMed.
- X. W. Du and L. M. Stanley, Org. Lett., 2015, 17, 3276–3279 CrossRef CAS PubMed.
- (a) C. Gonzalez-Rodriguez, R. J. Pawley, A. B. Chaplin, A. L. Thompson, A. S. Weller and M. C. Willis, Angew. Chem., Int. Ed., 2011, 50, 5134–5138 CrossRef CAS PubMed; (b) C.-H. Jun, H. Lee, J.-B. Hong and B.-I. Kwon, Angew. Chem., Int. Ed., 2002, 41, 2146–2147 CrossRef CAS.
- J. F. Hooper, S. Seo, F. R. Truscott, J. D. Neuhaus and M. C. Willis, J. Am. Chem. Soc., 2016, 138, 1630–1634 CrossRef CAS PubMed.
- (a) M. C. Willis, H. E. Randell-Sly, R. L. Woodward, S. J. McNally and G. S. Currie, J. Org. Chem., 2006, 71, 5291–5297 CrossRef CAS PubMed; (b) S. K. Murphy, A. Bruch and V. M. Dong, Angew. Chem., Int. Ed., 2014, 53, 2455–2459 CrossRef CAS PubMed.
- B. M. Trost, A. C. Gutierrez and R. C. Livingston, Org. Lett., 2009, 11, 2539–2542 CrossRef CAS PubMed.
- Depository numbers for X-ray structures with CCDC: 5k2091915; 5q2091914; 5y2091913; 10e2091916.†.
- S. J. Gharpure and J. V. K. Prasad, J. Org. Chem., 2011, 76, 10325–10331 CrossRef CAS PubMed.
- S. Pathania, R. K. Narang and R. K. Rawal, Eur. J. Med. Chem., 2019, 180, 486–508 CrossRef CAS PubMed.
- A. S. Newton, L. Deiana, D. E. Puleo, J. A. Cisneros, K. J. Cutrona, J. Schlessinger and W. L. Jorgensen, ACS Med. Chem. Lett., 2017, 8, 614–617 CrossRef CAS PubMed.
- M. Arambasic, J. F. Hooper and M. C. Willis, Org. Lett., 2013, 15, 5162–5165 CrossRef CAS PubMed.
- M. Arambasic, M. K. Majhail, R. N. Straker, J. D. Neuhaus and M. C. Willis, Chem. Commun., 2019, 55, 2757–2760 RSC.
- K. Asano, S. Matsubara and A. Matsumoto, Synlett, 2015, 26, 1872–1874 CrossRef.
- J.-L. Zhan, M.-W. Wu, D. Wei, B.-Y. Wei, Y. Jiang, W. Yu and B. Han, ACS Catal., 2019, 9, 4179–4188 CrossRef CAS.
- D. M. McKinnon and K. R. Lee, Can. J. Chem., 1988, 66, 1405–1409 CrossRef CAS.
- Y. Chen and M. C. Willis, Org. Lett., 2015, 17, 4786–4789 CrossRef CAS PubMed.
- A. W. Schmidt, L. A. Lebel, H. R. Howard and S. H. Zorn, Eur. J. Pharmacol., 2001, 425, 197–201 CrossRef CAS PubMed.
- V. Reddy, A. S. Jadhav and R. Vijaya Anand, Org. Biomol. Chem., 2015, 13, 3732–3741 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2091913–2091916. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc06900d |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |