Open Access Article
Open Access ArticleManganese(I)-catalyzed access to 1,2-bisphosphine ligands†
Luo
Ge
 and
Syuzanna R.
Harutyunyan
and
Syuzanna R.
Harutyunyan
 *
*
Stratingh Institute for Chemistry, University of Groningen Institution, Nijenborgh 4, 9747 AG, Groningen, The Netherlands. E-mail: s.harutyunyan@rug.nl
First published on 13th January 2022
Abstract
Chiral bisphosphine ligands are of key importance in transition-metal-catalyzed asymmetric synthesis of optically active products. However, the transition metals typically used are scarce and expensive noble metals, while the synthetic routes to access chiral phosphine ligands are cumbersome and lengthy. To make homogeneous catalysis more sustainable, progress must be made on both fronts. Herein, we present the first catalytic asymmetric hydrophosphination of α,β-unsaturated phosphine oxides in the presence of a chiral complex of earth-abundant manganese(I). This catalytic system offers a short two-step, one-pot synthetic sequence to easily accessible and structurally tunable chiral 1,2-bisphosphines in high yields and enantiomeric excess. The resulting bidentate phosphine ligands were successfully used in asymmetric catalysis as part of earth-abundant metal based organometallic catalysts.
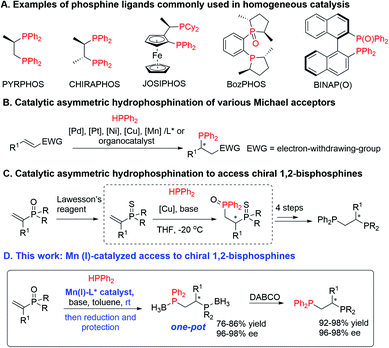
The vast majority of important catalytic transformations make use of very effective catalysts based on scarce, expensive and toxic noble transition metals and phosphine containing ligands that, especially when chiral, are often as expensive as the noble metals themselves due to their cumbersome synthetic accessibility.1 The past decade has witnessed significant progress towards the development of competitive catalysts that contain earth-abundant transition metals instead. These catalysts, however, still frequently rely on the use of chiral phosphine ligands. Bisphosphine ligands (Scheme 1A) for instance Pyrphos,2a Chiraphos,2b as well as Josiphos2c are among the most successful chiral ligands used in homogeneous catalysis. In recent years, bis(phosphine) monoxide compounds such as Bozphos,2d and Binap(o)2e have been shown to be powerful ligands in asymmetric catalysis as well. Unfortunately, the synthesis of these frequently and successfully used chiral phosphine-based ligands often requires stoichiometric amounts of chiral auxiliaries, enantiopure substrates, or separation by resolution to obtain them enantiomerically pure.1b–f
Catalytic asymmetric hydrophosphination is one of the most straightforward approaches for generating optically active P-chiral or C-chiral phosphines, from which chiral ligands can be derived.3 The potential of hydrophosphination reactions to access enantioenriched chiral phosphines catalytically was demonstrated for the first time by Glueck and coworkers in 2001 using a catalytic system based on Pt and the chiral bisphosphine ligand Me-DuPhos.4 Following the publication of this initial work, precious noble metal complexes such as chiral Pd or Pt catalysts have been widely used in the field of asymmetric hydrophosphination (Scheme 1B).5 Only few examples utilizing earth-abundant metals such as Ni,6 Cu7 and very recently Mn8 have been reported to date for catalytic asymmetric hydrophosphination. Apart from metal based catalytic systems, examples of asymmetric organocatalytic hydrophosphination reactions were also presented in the literature.9 So far, all successful methods that rely on the addition of phosphines to α,β-unsaturated conjugated systems provide chiral monophosphines.3 Interestingly, the only reported example of catalytic hydrophosphination that allows access to chiral 1,2-bisphosphine ligands utilizes a Michael acceptor with a P-containing electron-withdrawing group.7b
While α,β-unsaturated phosphine oxides are bench stable and readily available Michael acceptors, their application is less common when compared to conventional carbonyl based Michael acceptors, which is in part due to their lower reactivity.10 Yin and co-workers found an elegant solution to this problem by transforming α,β-unsaturated phosphine oxides into phosphine sulphides. This allows a ‘soft–soft’ interaction to be established between the Cu(I) atom of the chiral Cu(I)-catalyst and the S atom of the phosphine sulphide, enabling catalytic hydrophosphination towards the synthesis of chiral bisphosphines.7b While successful in applying this strategy for catalytic synthesis of variety of chiral bisphosphines, nevertheless it requires 6-steps synthetic sequence starting from α,β-unsaturated phosphine oxides (Scheme 1C).7b
Herein, we present a highly efficient, short and scalable catalytic protocol for the synthesis of chiral 1,2-bisphosphines from readily available, bench stable α,β-unsaturated phosphine oxides employing Mn(I)-catalyzed hydrophosphination as its core transformation (Scheme 1D).
The last five years witnessed remarkable success of Mn(I)-complexes as catalysts for reductive transformations of carbonyl compounds including asymmetric variants.11–13 Next to these reports, we have recently demonstrated that such complexes are capable of catalytic H–P bond activation of diarylphosphines.8 Based on these findings we hypothesised that Mn(I)-complexes should be able to bring the phosphine oxide and the phosphine reagents into closer proximity thus allowing the hydrophosphination reaction to take place directly with α,β-unsaturated phosphine oxides. This approach would avoid the additional synthetic steps and purifications procedures necessitated by the installation and removal of the sulphur atom that are intrinsic to the method utilising phosphine sulphides.
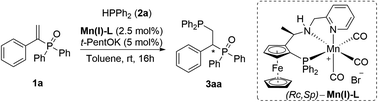
At the outset of this work, bench-stable α-substituted α,β-unsaturated phosphine oxide 1a was chosen as the model substrate in the reaction with HPPh2 (Table 1). Chiral Mn(I)-complex, Mn(I)-L, developed by Clark and co-workers13a,d for hydrogenation and transfer hydrogenation of carbonyl compounds, was selected as the chiral catalyst. After extensive optimization, the reaction with 5 mol% t-PentOK, 2.5 mol% Mn(I)-L, 1.05 equiv. of HPPh2 in toluene at room temperature for 16 hours was found to be optimal. Under these conditions, the product 3aa was obtained with 96% isolated yield and over 99% ee (entry 1).
| Entry | Deviation standard conditions | Conv.b (%) | Eec (%) |
|---|---|---|---|
| a General conditions: 1a (0.1 mol), Mn(I) (2.5 mol%), t-PentOK (5 mol%), 2a (0.105 mol) in toluene (1.0 ml) at rt for 16 h. b Determined by 1H NMR of reaction crude. c Determined by HPLC on a chiral stationary phase. d Isolated yield. | |||
| 1 | None | >99 (96)d | >99 |
| 2 | Without Mn(I)-L and t-PentOK | 0 | — |
| 3 | Without t-PentOK | 0 | — |
| 4 | Without Mn(I)-L | 99 | — |
| 5 | THF instead of toluene | 99 | 96 |
| 6 | 1,4-Dioxane instead of toluene | 98 | 97 |
| 7 | i-PrOH instead of toluene | 75 | 95 |
| 8 | MeOH instead of toluene | 90 | 52 |
| 9 | t-BuOK instead of t-PentOK | 99 | 97 |
| 10 | Barton's base instead of t-PentOK | 98 | 98 |
| 11 | t-PentOK (2.5 mmol%) | 56 | 99 |
| 12 | t-PentOK (7.5 mmol%) | 99 | 95 |
In the absence of both the base and the catalyst, as well as in the presence of only Mn(I)-L, no reaction occurs at room temperature (entries 2 and 3). In the presence of only the base (5 mol% of t-PentOK), however, 99% conversion towards the phosphine product 3aa was observed (entry 4).14
The screening of various solvents (entries 5–8) revealed excellent yields and enantiomeric ratios when using any of the following solvents: toluene, THF, and 1,4-dioxane. Given that the stereocenter in this reaction is generated upon formal stereospecific protonation, it was surprising that only a slight decrease in enantiomeric purity of the final product was observed in protic solvents, such as i-PrOH. On the other hand, running the reaction in MeOH led to a significant decrease in both substrate conversion and product ee.
As for the nature of the base we discovered that alkoxides and Barton's base provide the best results regarding the product yield and enantiopurity. The optimal performance of the base in the Mn(I)-catalyzed reaction is achieved with between 1.5 and 2 equivalents of the base with respect to the catalyst. A higher or lower amount of the base results in lower enantioselectivity or lower yield, respectively (compare entries 1, 11 and 12).
With the optimized conditions in hand, we moved to explore the scope of this methodology, first concentrating on the R2 substituent on the phosphine oxide. Various substitutions with aryl or alkyl groups led to excellent results in all cases (Scheme 2). Substrates with either an electron-donating group (3ba and 3ca) or an electron-withdrawing group (3da, 3ea, and 3fa) at the para-position of the phenyl ring led to the corresponding products with over 98% ee. The phenyl and ester functional groups at the para-position were also well tolerated, providing products 3ga and 3ha with high yields and enantiopurities. Similar results were obtained for substrates containing methyl- (3ia), chloro- (3ja) or methoxy- (3ka) substituents at the meta-position of the phenyl ring.
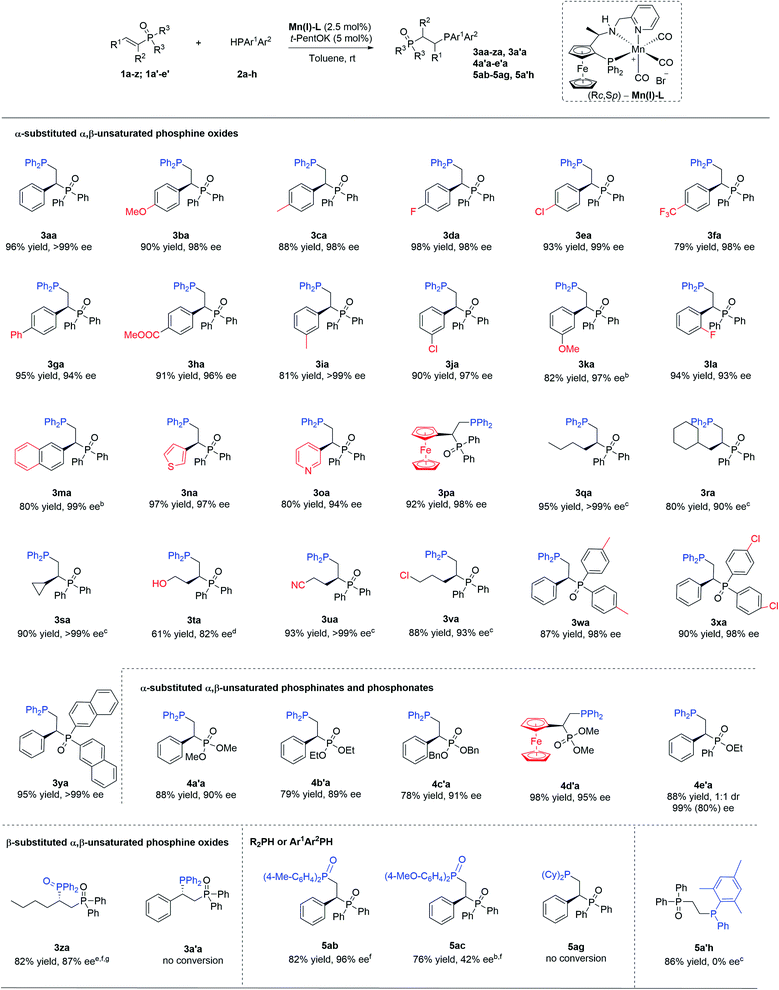 | ||
| Scheme 2 Product scope of Mn(I)-catalyzed asymmetric hydrophosphination of α,β-unsaturated phosphine oxidesa.aReaction conditions: 0.1 M of 1 in toluene, Mn(I)-L (2.5 mol%), t-PentOK (5 mol%), HP(Ar)2 (1.05 equiv) at rt. Isolated yields reported. For products 3aa and 3za the absolute configurations were determined by transforming them into the corresponding known compounds 6aa and 6da and for the remainder of the products by analogy (for details see ESI†); b5 mol% Barton's base used; c5 mol% Mn(I)-L,10 mol% t-PentOK used and reaction was carried out at rt for 72 h; d5 mol% Mn(I)-L,10 mol% t-PentOK used and reaction was carried out at rt for 5 days; e5 mol% Mn(I)-L,10 mol% t-PentOK used and reaction carried out at 60 °C; fthe reaction quenched with H2O2; gfor the absolute configuration of 3za, see the ESI.† | ||
α,β-Unsaturated phosphine oxides containing a heteroaryl moiety, such as 2-naphthyl (3ma), 3-thienyl (3na), and 3-pyridinyl (3oa), were well applicable in our catalytic system. We were pleased to see that substrate 3pa, bearing a ferrocenyl substituent – an essential structural component for many successful chiral ligands – can also be hydrophosphinated with excellent results. Next, α-alkyl substituted substrates were evaluated. The enantioselectivities observed for substrates with linear (3qa) and branched aliphatic substituents (3ra and 3sa) were in line with the results obtained for their aromatic counterparts. Substrates bearing functional groups amenable to further transformations, namely hydroxyl- (3ta), cyano- (3ua) or chloro-substituents provided the corresponding phosphine products with equally good results. We then move to study the effect of varying the substituents at the phosphorus atom. Various unsaturated diaryl phosphine oxides are compatible with this catalytic system and afford the corresponding products 3wa, 3xa, and 3ya with excellent enantiomeric excess and high isolated yield.
The relatively less reactive β-butyl-substituted α,β-unsaturated phosphine oxide is well tolerated as well, providing the corresponding enantioenriched oxide product 3za with 87% ee. On the other hand, no conversion to the product 3a′a was observed with β-phenyl-substituted α,β-unsaturated phosphine oxide. Interestingly, this catalytic system also supports α,β-unsaturated phosphonates, generating the corresponding final products (4a′a, 4b′a, 4c′a, and 4d′a) with enantiomeric excesses in the range of 89–95%. The catalytic protocol was also applied to a phosphinate substrate, allowing access to the product 4e′a with two chiral centers (dr 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with high ee. Finally, screening of various phosphine reagents revealed some limitations of the protocol. Hydrophopshination with (p-Me-C6H4)2PH and (p-MeO-C6H4)2PH led to the corresponding products 5ab and 5ac with good yields and good to excellent enantioselectivities. However, no conversion was obtained with the sterically more demanding (o-Me-C6H4)2PH, (3,5-CF3-C6H3)2PH, nor with Cy2PH and (p-CF3-C6H4)2PH. Attempts to access P-chiral phosphine product via addition of racemic diarylphosphine to α, β-unsaturated phosphine oxides led to the racemic P-chiral phosphine 5a′h.
1) with high ee. Finally, screening of various phosphine reagents revealed some limitations of the protocol. Hydrophopshination with (p-Me-C6H4)2PH and (p-MeO-C6H4)2PH led to the corresponding products 5ab and 5ac with good yields and good to excellent enantioselectivities. However, no conversion was obtained with the sterically more demanding (o-Me-C6H4)2PH, (3,5-CF3-C6H3)2PH, nor with Cy2PH and (p-CF3-C6H4)2PH. Attempts to access P-chiral phosphine product via addition of racemic diarylphosphine to α, β-unsaturated phosphine oxides led to the racemic P-chiral phosphine 5a′h.
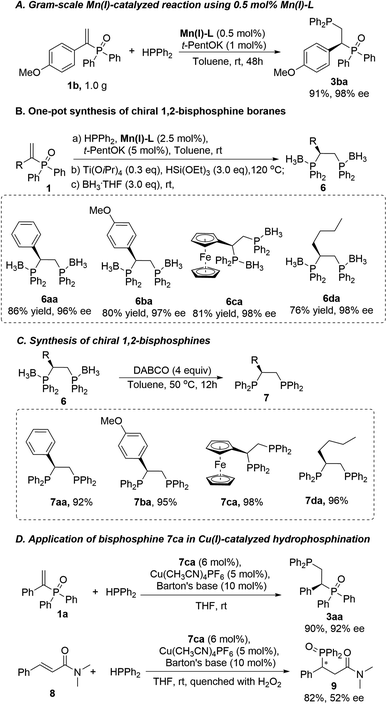
To demonstrate the potential application of our catalytic protocol in chiral phosphine ligand synthesis, we performed a gram-scale reaction between 1b and 2a (Scheme 3A). To our delight, the catalyst loading could be decreased to 0.5 mol%, leading to the product 3ba without deterioration of the yield (91%) or the enantioselectivity (98%).
Building on these results, we then developed a highly efficient one-pot method for the synthesis of four different chiral phosphine boranes (6aa–6da) (Scheme 3B) that yield the corresponding chiral 1,2-bisphosphine ligands (7aa–7da) in a single deprotection step (Scheme 3C). As is typical of any phosphines, the 1,2-bisphosphines 7 prepared in this study can easily oxidize during chromatographic purifications.7b
Therefore, to minimise chromatographic purification, as well as to facilitate product separation, degassed water was used to wash the reaction mixture, followed by the removal of volatiles under high vacuum. The free ligands 7 were obtained in good yields and high purity. Importantly, the 1,2-bisphosphine 7aa is a known, efficient chiral ligand for Rh-catalyzed asymmetric hydrogenation of α-amino-α,β-unsaturated esters.7b We also examined our bisphosphine ligand 7ca in the Cu-catalyzed hydrophosphination of α,β-unsaturated phosphine oxide 1a (Scheme 3D), obtaining the desired product 3aa in good yield (90%) and high enantioselectivity (92%). Similarly, α,β-unsaturated carboxamide 8 was investigated,7c providing the corresponding product 9 in good yield (82%) and moderate ee (52%).
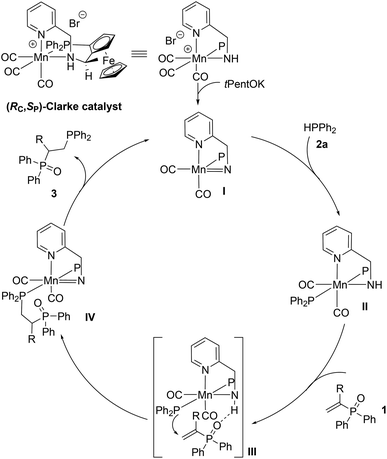
From a mechanistic point of view, we wondered whether our base activated Mn-catalyst I is involved in the activation of the phosphine reagent 2avia ligand–metal cooperation, as proposed in our previous work on α,β-unsaturated nitriles,8 or whether it also plays a role in the activation of the phosphine oxide substrate 1. Preliminary NMR spectroscopic studies did not reveal any interaction between I and 1 (see ESI†) leading us to hypothesise that the current transformation might follow a mechanistic path that primarily involves phosphine activation, as depicted in Scheme 4. Additional interaction between the NH and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moieties of the catalyst and phosphine oxide respectively is also possible and cannot be excluded at this stage. Detailed mechanistic studies are currently underway.
O moieties of the catalyst and phosphine oxide respectively is also possible and cannot be excluded at this stage. Detailed mechanistic studies are currently underway.
In summary, we have developed the first manganese(I) catalyzed enantioselective strategy for the hydrophosphination of α, β-unsaturated phosphine oxides. This methodology allows a high-yielding, catalytic, two-step sequence for the synthesis of enantiopure chiral 1,2-bisphosphine ligands, that were successfully applied in asymmetric catalysis. Since manganese is the third most abundant transition metal in the Earth's crust, a general catalytic method to access chiral bisphosphine ligands using this metal is further step towards more sustainable homogeneous catalysis. Further work is currently underway in order to unravel the mechanism of this transformation.
Data availability
Detailed synthetic procedures, complete characterization data for all new compounds can be found in the ESI.†Author contributions
L. G conducted all experiments and characterized the novel compounds. L. G and S. R. H designed the experiments and wrote the manuscript. S. R. H directed the research.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Financial support from the European Research Council (S. R. H. Grant No. 773264, LACOPAROM), The Netherlands Organization for Scientific Research (NWO-VICI to S. R. H.) and the China Scholarship Council (CSC, to L. G.) is acknowledged.Notes and references
- (a) P. W. N. M. van Leeuwen and J. C. Chadwick, Homogeneous Catalysts: Activity–Stability–Deactivation, Wiley-VCH, Weinheim, 2014 Search PubMed; (b) H. U. Blaser and H. J. Federsel, Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions, Wiley-VCH, Weinheim, 2010 CrossRef; (c) L. M. Pignolet, Homogeneous Catalysis with Metal Phosphine Complexes, Springer, Boston, 1983 CrossRef; (d) A. Börner, Phosphorus Ligands in Asymmetric Catalysis: Synthesis and Applications, Wiley-VCH, Weinheim, 2008 Search PubMed; (e) W. Tang and X. Zhang, Chem. Rev., 2003, 103, 3029–3070 CrossRef CAS PubMed; (f) A. Pfaltz and W. J. Drury III, Proc. Natl. Acad. Sci., 2004, 101, 5723–5726 CrossRef CAS.
- (a) M. D. Fryzuk and B. Bosnich, J. Am. Chem. Soc., 1978, 100, 5491–5494 CrossRef CAS; (b) M. D. Fryzuk and B. Bosnich, J. Am. Chem. Soc., 1977, 99, 6262–6267 CrossRef CAS PubMed; (c) A. Togni, C. Breutel, A. Schnyder, F. Spindler, H. Landert and A. Tijani, J. Am. Chem. Soc., 1994, 116, 4062–4066 CrossRef CAS; (d) A. A. Boezio, J. Pytkowicz, A. Côté and A. B. Charette, J. Am. Chem. Soc., 2003, 125, 14260–14261 CrossRef CAS PubMed; (e) S. Gladiali, S. Pulacchini, D. Fabbri, M. Manassero and M. Sansoni, Tetrahedron: Asymmetry, 1998, 9, 391–395 CrossRef CAS.
- (a) S. A. Pullarkat, Synthesis, 2016, 48, 493–503 CrossRef CAS; (b) L. Rosenberg, ACS Catal., 2013, 3, 2845–2855 CrossRef CAS; (c) D. Zhao and R. Wang, Chem. Soc. Rev., 2012, 41, 2095–2108 RSC; (d) C. A. Bange and R. Waterman, Chem.–Eur. J., 2016, 22, 12598–12605 CrossRef CAS PubMed; (e) R. J. Chew and P.-H. Leung, Chem. Rec., 2016, 16, 141–158 CrossRef CAS; (f) Z. Li and W. Duan, Chin. J. Org. Chem., 2016, 36, 1805–1813 CrossRef CAS; (g) K. Zheng, X. Liu and X. Feng, Chem. Rev., 2018, 118, 7586–7656 CrossRef CAS.
- (a) I. Kovacik, D. K. Wicht, N. S. Grewal, D. S. Glueck, C. D. Incarvito, I. A. Guzei and A. L. Rheingold, Organometallics, 2000, 19, 950–953 CrossRef CAS; (b) D. S. Glueck, Coord. Chem. Rev., 2008, 252, 2171–2179 CrossRef CAS.
- (a) Y. Huang, S. A. Pullarkat, Y. Li and P.-H. Leung, Chem. Commun., 2010, 46, 6950–6952 RSC; (b) J.-J. Feng, X.-F. Chen, M. Shi and W.-L. Duan, J. Am. Chem. Soc., 2010, 132, 5562–5563 CrossRef CAS PubMed; (c) Y. Huang, R. J. Chew, Y. Li, S. A. Pullarkat and P.-H. Leung, Org. Lett., 2011, 13, 5862–5865 CrossRef CAS; (d) Y.-R. Chen and W.-L. Duan, Org. Lett., 2011, 13, 5824–5826 CrossRef CAS; (e) B. Ding, Z. Zhang, Y. Xu, Y. Liu, M. Sugiya, T. Imamoto and W. Zhang, Org. Lett., 2013, 15, 5476–5479 CrossRef CAS PubMed; (f) J. Lu, J. Ye and W.-L. Duan, Org. Lett., 2013, 15, 5016–5019 CrossRef CAS; (g) R. J. Chew, K. Y. Teo, Y. Huang, B.-B. Li, Y. Li, S. A. Pullarkat and P.-H. Leung, Chem. Commun., 2014, 50, 8768–8770 RSC; (h) R. J. Chew, X.-R. Li, Y. Li, S. A. Pullarkat and P.-H. Leung, Chem.–Eur. J., 2015, 21, 4800–4804 CrossRef CAS PubMed; (i) X.-Y. Yang, W. S. Tay, Y. Li, S. A. Pullarkat and P.-H. Leung, Chem. Commun., 2016, 52, 4211–4214 RSC; (j) A. Sadeer, Y. J. Ong, T. Kojima, C. Q. Foo, Y. Li, S. A. Pullarkat and P.-H. Leung, Chem.–Asian J., 2018, 13, 2829–2833 CrossRef CAS PubMed; (k) Z. Lu, H. Zhang, Z. Yang, N. Ding, L. Meng and J. Wang, ACS Catal., 2019, 9, 1457–1463 CrossRef CAS; (l) Z.-H. Wu, A.-Q. Cheng, M. Yuan, Y.-X. Zhao, H.-L. Yang, L.-H. Wei, H.-Y. Wang, T. Wang, Z.-T. Zhang and W.-L. Duan, Angew. Chem., Int. Ed., 2021, 60, 27241–27246 CrossRef CAS PubMed.
- (a) A. D. Sadow, I. Haller, L. Fadini and A. Togni, J. Am. Chem. Soc., 2004, 126, 14704–14705 CrossRef CAS PubMed; (b) A. D. Sadow and A. Togni, J. Am. Chem. Soc., 2005, 127, 17012–17024 CrossRef CAS PubMed; (c) C. Wang, K. Huang, J. Ye and W.-L. Duan, J. Am. Chem. Soc., 2021, 143, 5685–5690 CrossRef CAS PubMed; (d) X.-T. Liu, X.-Y. Han, Y. Wu, Y.-Y. Sun, L. Gao, Z. Huang and Q.-W. Zhang, J. Am. Chem. Soc., 2021, 143, 11309–11316 CrossRef CAS PubMed.
- (a) Y.-R. Chen, J.-J. Feng and W.-L. Duan, Tetrahedron Lett., 2014, 55, 595–597 CrossRef CAS; (b) W.-J. Yue, J.-Z. Xiao, S. Zhang and L. Yin, Angew. Chem., Int. Ed., 2020, 59, 7057–7062 CrossRef CAS PubMed; (c) Y.-B. Li, H. Tian and L. Yin, J. Am. Chem. Soc., 2020, 142, 20098–20106 CrossRef CAS PubMed; (d) S. Zhang, J.-Z. Xiao, Y.-B. Li, C.-Y. Shi and L. Yin, J. Am. Chem. Soc., 2021, 143, 9912–9921 CrossRef CAS PubMed.
- J. M. Pérez, R. Postolache, M. Castiñeira Reis, E. G. Sinnema, D. Vargová, F. de Vries, E. Otten, L. Ge and S. R. Harutyunyan, J. Am. Chem. Soc., 2021, 143, 20071–20076 CrossRef PubMed.
- (a) A. Carlone, G. Bartoli, M. Bosco, L. Sambri and P. Melchiorre, Angew. Chem., Int. Ed., 2007, 46, 4504–4506 CrossRef CAS PubMed; (b) I. Ibrahem, R. Rios, J. Vesely, P. Hammar, L. Eriksson, F. Himo and A. Córdova, Angew. Chem., Int. Ed., 2007, 46, 4507–4510 CrossRef CAS PubMed; (c) I. Ibrahem, P. Hammar, J. Vesely, R. Rios, L. Eriksson and A. Córdova, Adv. Synth. Catal., 2008, 350, 1875–1884 CrossRef CAS; (d) M. Rakesh, J.-L. Yan, X. Yang, B. Mondal, J. Xu, H.-F. Chai, Z.-C. Jin and Y. R. Chi, Angew. Chem., Int. Ed., 2021, 60, 26616–26621 CrossRef.
- (a) V. Hornillos, C. Vila, E. Otten and B. L. Feringa, Angew. Chem., Int. Ed., 2015, 54, 7867–7871 CrossRef CAS; (b) K. M.-H. Lim and T. Hayashi, J. Am. Chem. Soc., 2017, 139, 8122–8125 CrossRef CAS PubMed; (c) Z. Wang and T. Hayashi, Angew. Chem., Int. Ed., 2018, 57, 1702–1706 CrossRef CAS PubMed; (d) Y. Zhang, F. Zhang, L. Chen, J. Xu, X. Liu and X. Feng, ACS Catal., 2019, 9, 4834–4840 CrossRef CAS.
- (a) A. Mukherjee, A. Nerush, G. Leitus, L. J. W. Shimon, Y. Ben David, N. A. Espinosa Jalapa and D. Milstein, J. Am. Chem. Soc., 2016, 138, 4298–4301 CrossRef CAS; (b) S. Elangovan, C. Topf, S. Fischer, H. Jiao, A. Spannenberg, W. Baumann, R. Ludwig, K. Junge and M. Beller, J. Am. Chem. Soc., 2016, 138, 8809–8814 CrossRef CAS PubMed.
- (a) Y. Wang, M. Wang, Y. Li and Q. Liu, Chem, 2021, 7, 1180–1223 CrossRef CAS; (b) F. Kallmeier and R. Kempe, Angew. Chem., Int. Ed., 2018, 57, 46–60 CrossRef CAS; (c) M. Garbe, K. Junge and M. Beller, Eur. J. Org. Chem., 2017, 2017, 4344–4362 CrossRef CAS; (d) G. A. Filonenko, R. Van Putten, E. J. M. Hensen and E. A. Pidko, Chem. Soc. Rev., 2018, 47, 1459–1483 RSC.
- (a) M. B. Widegren, G. J. Harkness, A. M. Z. Slawin, D. B. Cordes and M. L. Clarke, Angew. Chem., Int. Ed., 2017, 56, 5825–5828 CrossRef CAS; (b) M. Garbe, K. Junge, S. Walker, Z. Wei, H. Jiao, A. Spannenberg, S. Bachmann, M. Scalone and M. Beller, Angew. Chem., Int. Ed., 2017, 56, 11237–11241 CrossRef CAS PubMed; (c) A. Zirakzadeh, S. R. M. M. de Aguiar, B. Stöger, M. Widhalm and K. Kirchner, ChemCatChem, 2017, 9, 1744–1748 CrossRef CAS; (d) M. B. Widegren and M. L. Clarke, Org. Lett., 2018, 20, 2654–2658 CrossRef CAS PubMed; (e) V. Vasilenko, C. K. Blasius and L. H. Gade, J. Am. Chem. Soc., 2018, 140, 9244–9254 CrossRef CAS; (f) L. Zhang, Y. Tang, Z. Han and K. Ding, Angew. Chem., Int. Ed., 2019, 58, 4973–4977 CrossRef CAS PubMed; (g) L. Zhang, Z. Wang, Z. Han and K. Ding, Angew. Chem., Int. Ed., 2020, 59, 15565–15569 CrossRef CAS; (h) L. Zeng, H. Yang, M. Zhao, J. Wen, J. H. R. Tucker and X. Zhang, ACS Catal., 2020, 10, 13794–13799 CrossRef CAS; (i) H.-J. Pan and X. Hu, Angew. Chem., Int. Ed., 2020, 59, 4942–4946 CrossRef CAS PubMed; (j) C. Liu, M. Wang, S. Liu, Y. Wang, Y. Peng, Y. Lan and Q. Liu, Angew. Chem., Int. Ed., 2021, 60, 5108–5113 CrossRef CAS PubMed.
- (a) H. Brunner and S. Limmer, J. Organomet. Chem., 1991, 413, 55–63 CrossRef CAS; (b) C. A. Busacca, E. Farber, J. DeYoung, S. Campbell, S. Gonella, N. Grinberg, N. Haddad, H. Lee, S. Ma, D. Reeves, S. Shen and C. H. Senanayake, Org. Lett., 2009, 11, 5594–5597 CrossRef CAS PubMed; (c) A. M. Gonzalez-Nogal, P. Cuadrado and M. A. Sarmentero, Tetrahedron, 2010, 66, 9610–9619 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc06694c |
| This journal is © The Royal Society of Chemistry 2022 |