Open Access Article
Open Access ArticleHighly regioselective and diastereodivergent aminomethylative annulation of dienyl alcohols enabled by a hydrogen-bonding assisting effect†
Yao
Huang‡
a,
Suchen
Zou‡
a,
Bangkui
Yu
a,
Xuyang
Yan
a,
Song
Liu
 *c and
Hanmin
Huang
*c and
Hanmin
Huang
 *ab
*ab
aDepartment of Chemistry, School of Chemistry and Material Science, Center for Excellence in Molecular Synthesis of CAS, University of Science and Technology of China, Hefei, 230026, P. R. China. E-mail: hanmin@ustc.edu.cn
bKey Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education, Huaibei Normal University, Huaibei, Anhui 235000, P. R. China
cChongqing Key Laboratory of Environmental Materials and Remediation Technologies, Chongqing University of Arts and Sciences, Chongqing, 402160, P. R. China. E-mail: sliu@cqwu.edu.cn
First published on 2nd February 2022
Abstract
A ligand-controlled palladium-catalyzed highly regioselective and diastereodivergent aminomethylative annulation of dienyl alcohols with aminals has been established, which allows for producing either cis- or trans-disubstituted isochromans in good yields with complete regioselectivity and good to excellent diastereoselectivity. Moreover, the chiral cis-products were also obtained in good yields with up to 94% ee by using a chiral phosphinamide as the ligand. Mechanistic studies revealed that the hydroxyl group plays a key role in facilitating the Pd-catalyzed Heck insertion regioselectively taking place across the internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of conjugated dienes.
C bond of conjugated dienes.
Introduction
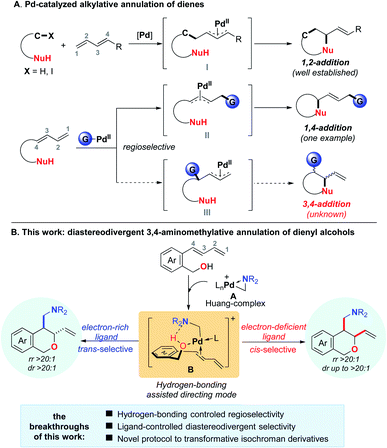
The ready availability and versatile reactivity as well as broad functionalization potential of conjugated dienes make them extremely promising and privileged starting materials for organic synthesis.1 In particular, the two-component Pd-catalyzed annulation of dienes involving the formation of π-allylpalladium species and intramolecularly intercepting them with a nucleophile allows for the rapid construction of cyclic molecules, and significant progress has been achieved.2,3 However, a serious limitation is that most of these reactions are performed via formation of a π-allylpalladium intermediate like I or II,2 which leads to the generation of 1,2 or 1,4-difunctionalization products (Scheme 1A). A different type of π-allylpalladium intermediate III would be expected that can deliver the 3,4-difunctionalized cyclic products via its subsequent intramolecular interception with a nucleophile. Such compounds are more synthetically useful as the terminal olefin functionality could act as a versatile handle group for further elaboration (Scheme 1A).4 However, in stark contrast to 1,2- and 1,4-annulations, the 3,4-annulation of 1,3-dienes has remained elusive.3 The underlying reason might be attributed to the lack of efficient strategy and appropriate alkylpalladium species to enable the Heck insertion to occur across the internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond for regioselective formation of intermediate III. Moreover, intramolecular interception of intermediate III may generate an inseparable mixture of trans- and cis-isomers, which makes such reactions more challenging.
C bond for regioselective formation of intermediate III. Moreover, intramolecular interception of intermediate III may generate an inseparable mixture of trans- and cis-isomers, which makes such reactions more challenging.
To address these limitations, we envisioned that once the nucleophile suspended in the diene backbone can complex with an appropriate alkylpalladium species, the chelation between the palladium species and the nucleophile will bring the palladium center close to the internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of the diene, thus facilitating the formation of the internal alkylated π-allylpalladium intermediate III. It is well known that the hydroxyl group can not only act as a nucleophile for palladium-catalyzed allylic C–O bond formation reactions,5 but also be frequently utilized as a directing group for various metal catalyzed reactions.6,7 However, due to the weak coordination ability of the oxygen atom to the palladium, the hydroxyl group may not act as an effective directing group to make the palladium interact with the internal C
C bond of the diene, thus facilitating the formation of the internal alkylated π-allylpalladium intermediate III. It is well known that the hydroxyl group can not only act as a nucleophile for palladium-catalyzed allylic C–O bond formation reactions,5 but also be frequently utilized as a directing group for various metal catalyzed reactions.6,7 However, due to the weak coordination ability of the oxygen atom to the palladium, the hydroxyl group may not act as an effective directing group to make the palladium interact with the internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of the diene. In this context, we surmised that the directing ability would be enhanced to facilitate the regioselective annulation once the alkylpalladium species interacts with the hydroxyl group like intermediate Bvia hydrogen-bonding. In line with our continuous interest in developing new transformations by using aminomethyl cyclopalladated complex A (Huang-complex) as a leading complex8 and inspired by our recent results on the hydrogen-bonding-assisted alcohol allylation reactions,5h we envisioned that complex A might be a suitable alkylpalladium species to coordinate with the hydroxyl group under the assistance of hydrogen-bonding. Once the diene-tethered benzyl alcohol reacted with complex A, intermediate B might be formed, in which the hydrogen-bonding between the hydroxyl group and the amine moiety would promote the palladium to coordinate with the O-atom of the hydroxyl and facilitate the internal C
C bond of the diene. In this context, we surmised that the directing ability would be enhanced to facilitate the regioselective annulation once the alkylpalladium species interacts with the hydroxyl group like intermediate Bvia hydrogen-bonding. In line with our continuous interest in developing new transformations by using aminomethyl cyclopalladated complex A (Huang-complex) as a leading complex8 and inspired by our recent results on the hydrogen-bonding-assisted alcohol allylation reactions,5h we envisioned that complex A might be a suitable alkylpalladium species to coordinate with the hydroxyl group under the assistance of hydrogen-bonding. Once the diene-tethered benzyl alcohol reacted with complex A, intermediate B might be formed, in which the hydrogen-bonding between the hydroxyl group and the amine moiety would promote the palladium to coordinate with the O-atom of the hydroxyl and facilitate the internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond binding to the palladium. Such interactions would guide the Heck insertion to take place selectively across the internal C
C bond binding to the palladium. Such interactions would guide the Heck insertion to take place selectively across the internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of the diene. Herein, we report for the first time a regioselective and diastereodivergent aminomethylative annulation of diene-tethered benzyl alcohols with aminals (Scheme 1B). This transformation offers an unprecedented efficient route for the selective synthesis of both trans- and cis-diastereoisomers of 1,2-disubstituted isochroman derivatives that are pervasive structural motifs omnipresent in myriad natural products and synthetic pharmaceuticals exhibiting an array of biological properties.9
C bond of the diene. Herein, we report for the first time a regioselective and diastereodivergent aminomethylative annulation of diene-tethered benzyl alcohols with aminals (Scheme 1B). This transformation offers an unprecedented efficient route for the selective synthesis of both trans- and cis-diastereoisomers of 1,2-disubstituted isochroman derivatives that are pervasive structural motifs omnipresent in myriad natural products and synthetic pharmaceuticals exhibiting an array of biological properties.9
Results and discussion
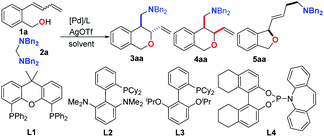
At first, we examined the reaction of (2-(buta-1,3-dien-1-yl)phenyl)methanol 1a with N,N,N′,N′-tetrabenzylmethanediamine 2a by using a series of palladium-catalysts bearing different phosphine ligands. On the basis of our previous work, the reaction was carried out in toluene at 80 °C with [Pd(allyl)Cl]2 as the palladium source. In the presence of AgOTf as the counter anion source, a Xantphos-ligated Pd-catalyst showed high activity, and an almost quantitative conversion was observed, which resulted from either internal (trans-3aa and cis-4aa) or terminal Heck-insertion (5aa) (Table 1, entry 1). To our delight, when PPh3 was used as the ligand, the selectivity dramatically improved to give trans-3aa as the major product, although concomitantly with a trace amount of 1,4-difunctionalized cyclization product 5aa. The high regioselectivity observed by using the monodentate ligands might be attributed to the fact that the ligated-palladium complex can provide a vacant coordination site to complex with the hydroxyl group to furnish the directing effect. Encouraged by this promising result, a series of mono-phosphine ligands were examined. Biaryl monophosphines were identified as privileged ligands for the present reaction, offering satisfactory conversions and selectivities of trans-products while effectively suppressing the formation of 5aa. When Cphos (L2) was used, 3aa could be generated exclusively in 59% yield. Further optimization of the reaction parameters by changing the solvent and the ratio of 1a to 2a improved the yield to 86% while maintaining high regio- and diastereoselectivities (Table 1, entry 9). Intriguingly however, the use of more electron-deficient phosphinamide L4 reversed the diastereoselectivity, giving an uncommon cis-enriched diastereoisomer mixture, albeit with poor selectivity (trans-3aa/cis-4aa = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (Table 1, entry 10). Switching the pre-catalyst to PdBr2 increased the cis-selectivity to trans-3aa/cis-4aa = 1
5) (Table 1, entry 10). Switching the pre-catalyst to PdBr2 increased the cis-selectivity to trans-3aa/cis-4aa = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 with a yield as high as 90%. Remarkably, the cis-selectivity could be further improved to trans-3aa/cis-4aa = 1
7 with a yield as high as 90%. Remarkably, the cis-selectivity could be further improved to trans-3aa/cis-4aa = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 at room temperature without losses in yields (Table 1, entry 15).
14 at room temperature without losses in yields (Table 1, entry 15).
| Entry | [Pd] | Ligand | T (°C) | Solvent | Yield (%) | 3aa/4aa | 5aa Yield (%) |
|---|---|---|---|---|---|---|---|
| 3aa + 4aa | |||||||
| a Reaction conditions: 1a (0.3 mmol), 2a (0.36 mmol), [Pd] (5 mol%), AgOTf (6 mol%), ligand (5 mol%), solvent (1.0 mL), 12 h, isolated yield, the dr value was determined by 1H NMR analysis of the crude reaction mixture. b Ligand (10 mol%). c AgOTf (12 mol%). d 1a (0.36 mmol), 2a (0.30 mmol). e 24 h. | |||||||
| 1 | [Pd(allyl)Cl]2 | L1 | 80 | Toluene | 40 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
45 |
| 2b | [Pd(allyl)Cl]2 | PPh3 | 80 | Toluene | 63 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 |
| 3b | [Pd(allyl)Cl]2 | L2 | 80 | Toluene | 59 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Trace |
| 4b | [Pd(allyl)Cl]2 | L3 | 80 | Toluene | 43 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Trace |
| 5b,c | PdCl2 | L2 | 80 | Toluene | 48 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Trace |
| 6b,c | PdBr2 | L2 | 80 | Toluene | 50 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Trace |
| 7b | [Pd(allyl)Cl]2 | L2 | 80 | CH2Cl2 | 65 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Trace |
| 8b | [Pd(ally)Cl]2 | L2 | 80 | CH2Cl2/toluene = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
67 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Trace |
| 9b,c | [Pd(allyl)Cl]2 | L2 | 80 | CH2Cl2/toluene = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
86 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Trace |
| 10b,c | [Pd(allyl)Cl]2 | L4 | 80 | CH2Cl2 | 79 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Trace |
| 11b,c,d | PdBr2 | L4 | 80 | CH2Cl2 | 90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
Trace |
| 12b,c,d | PdBr2 | L4 | 60 | CH2Cl2 | 84 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
Trace |
| 13b,c,d | PdBr2 | L4 | 40 | CH2Cl2 | 81 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Trace |
| 14b,c,d | PdBr2 | L4 | rt | CH2Cl2 | 62 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
Trace |
| 15b,c,d,e | PdBr2 | L4 | rt | CH2Cl2 | 89 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
Trace |
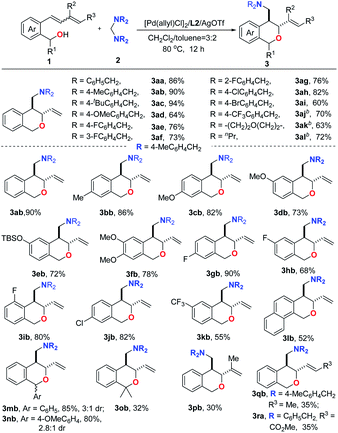
Having established the highly regioselective and diastereodivergent conditions for the annulation of 1a with 2a as shown above, we then examined the substrate scope with an L2/ or L4/PdBr2/AgOTf catalysis system. Table 2 summarizes the substrates compatible for trans-selective annulation. Aminals derived from benzylamines bearing electron-rich and -deficient substituents worked smoothly with 1a to exclusively give the corresponding trans-products (3aa–3aj) in 60–94% yields with complete regioselectivity. Functional groups such as halides (F, Cl, Br), CF3, and OMe were compatible. Gratifyingly, aminals derived from aliphatic amines, such as dipropylamine and morpholine, could react with 1a to give the corresponding products as well (3ak and 3al) in good yields in the presence of 10 mol% of catalyst. Next, the scope of the diene-tethered benzyl alcohols was also explored under optimized conditions. As shown in Table 2, a variety of substrates bearing electron-donating and -withdrawing functional groups on the phenyl-rings underwent regioselective and diastereoselective annulations to provide the trans-products (3ab–3kb) in good to excellent yields (55–90%). The naphthyl-containing 1l was also compatible, generating the corresponding annulation product 3lb in 52% yield. Meanwhile, the dienes containing a substituent at the benzyl site could also be converted into the desired products with three chiral centers (3mb and 3nb) in good yields. Importantly, substrates containing substituents at C2 or C1 positions also proved to be competent reaction partners to afford the corresponding products (3pb, 3qb and 3ra) in moderate yields with excellent diastereoselectivities and regioselectivities. It is noteworthy that the regio- and diastereoselectivities of this reaction were not affected by the E/Z ratio of the corresponding diene-tethered benzyl alcohols (see the ESI†).
a Reaction conditions: 1 (0.36 mmol), 2 (0.3 mmol), [Pd(allyl)Cl]2 (2.5 mol%), AgOTf (6 mol%), L2 (10 mol%), solvent (CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene = 3 toluene = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1.0 mL), 80 °C, 12 h, isolated yields, >20 2, 1.0 mL), 80 °C, 12 h, isolated yields, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rr, and >20 1 rr, and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr in all cases as shown by 1H NMR analysis of the crude reaction mixture, unless otherwise noted.
b [Pd(allyl)Cl]2 (5 mol%), AgOTf (12 mol%), L2 (20 mol%). 1 dr in all cases as shown by 1H NMR analysis of the crude reaction mixture, unless otherwise noted.
b [Pd(allyl)Cl]2 (5 mol%), AgOTf (12 mol%), L2 (20 mol%).
|
|---|
|
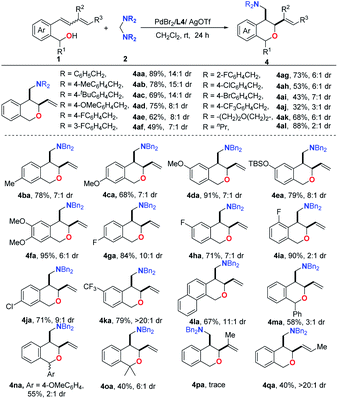
We next focused on exploring the scope of the cis-diastereoselective annulation reaction by using PdBr2/L4/AgOTf as the catalyst. As shown in Table 3, the diastereoselectivity was significantly influenced by the substrates, though the exclusive internal carbo-oxygenation products were observed in all cases. Evaluation of the scope was initiated with the investigation of various aminals. The para-methyl-, t-Bu-substituted benzylamine-derived aminals exhibited high cis-selectivity (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 14
1 and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively) to give the corresponding cis-annulation products 4ab and 4ac in 69–78% yields. Other benzyl aminals bearing electron-withdrawing substituents showed decreased selectivities (4ae–4aj), among which the lowest was given by aminal 2j containing CF3 (4aj). The substrates 2k and 2l derived from alkyl amines were also compatible, albeit with low to moderate selectivities (4ak and 4al). Next, with 2a as the standard coupling partner, a series of diene-tethered benzyl alcohols were tested. Obviously, the introduction of a substituent on the phenyl ring of the substrate resulted in lower diastereoselectivities, and the lowest cis-selectivity (2
1, respectively) to give the corresponding cis-annulation products 4ab and 4ac in 69–78% yields. Other benzyl aminals bearing electron-withdrawing substituents showed decreased selectivities (4ae–4aj), among which the lowest was given by aminal 2j containing CF3 (4aj). The substrates 2k and 2l derived from alkyl amines were also compatible, albeit with low to moderate selectivities (4ak and 4al). Next, with 2a as the standard coupling partner, a series of diene-tethered benzyl alcohols were tested. Obviously, the introduction of a substituent on the phenyl ring of the substrate resulted in lower diastereoselectivities, and the lowest cis-selectivity (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was observed when the substrate bore an ortho-fluoro substituent on the phenyl ring (4ia). However, when there was a CF3 at the 5-position, up to >20
1) was observed when the substrate bore an ortho-fluoro substituent on the phenyl ring (4ia). However, when there was a CF3 at the 5-position, up to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity was observed (4ka). In addition, substrate 1l containing naphthalene gave a moderate yield with 11
1 diastereoselectivity was observed (4ka). In addition, substrate 1l containing naphthalene gave a moderate yield with 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity (4la). Substrates with substituents at the benzyl carbon bearing the hydroxyl group could also give moderate yields with lower selectivity (4ma–4oa). Nevertheless, the introduction of a methyl group at the C2-position of the diene resulted in no reaction, which might be due to steric hindrance. However, when the methyl group was instead introduced at the C1 position, the desired product could be obtained in 40% yield with excellent selectivity (>20
1 diastereoselectivity (4la). Substrates with substituents at the benzyl carbon bearing the hydroxyl group could also give moderate yields with lower selectivity (4ma–4oa). Nevertheless, the introduction of a methyl group at the C2-position of the diene resulted in no reaction, which might be due to steric hindrance. However, when the methyl group was instead introduced at the C1 position, the desired product could be obtained in 40% yield with excellent selectivity (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) (4qa).
1 dr) (4qa).
a Reaction conditions: 1 (0.36 mmol), 2 (0.3 mmol), PdBr2 (5 mol%), AgOTf (12 mol%), L4 (10 mol%), CH2Cl2 (1.0 mL), rt, 24 h, isolated yield, the dr value was determined by 1H NMR analysis of the crude reaction mixture, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rr in all cases. 1 rr in all cases.
|
|---|
|
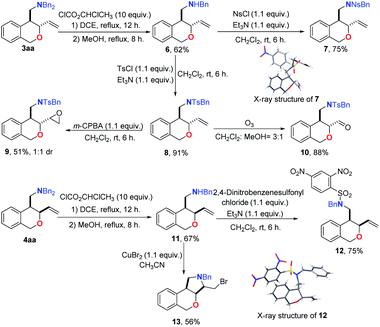
To explore the synthetic potential of the present reaction, several transformations of the obtained trans-3aa and cis-4aa were conducted (Scheme 2). One of the benzyl groups in 3aa could be selectively removed to give 6 by treatment with ClCO2CHClCH3.10a To further confirm the structure of the product, the easily crystallizable Ns-protected amide 7 was obtained, whose structure was unambiguously confirmed by an X-ray diffraction analysis. The nucleophilic substitution reaction of 6 with TsCl in the presence of Et3N afforded the sulfamide 8. Oxidation of the sulfamide 8 by using m-CPBA afforded the epoxide 9.10b Highly valuable amino aldehyde 10 was cleanly formed by exposure of 8 to O3 in CH2Cl2/MeOH at −78 °C.10c Similarly, the cis-4aa could also be transformed into sulfonamide 12 in excellent yield via de-benzylation and sulfonation. The structure of 12 was also verified by single-crystal X-ray diffraction.11 The obtained cis-product 11 could be further converted into the ring-fused [6, 6, 5] tricyclic isochroman 13 by a CuBr2-mediated cyclization reaction.10d
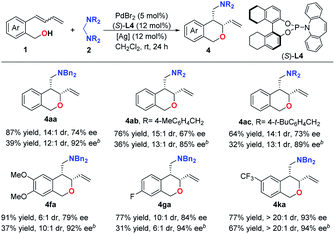
To achieve an enantioselective aminomethylative annulation reaction, (S)-L4 was utilized as the chiral ligand. As evident from the results compiled in Table 4, a series of chiral cis-diastereoselective products (4aa–4ac and 4fa–4ka) could be obtained in good yields with moderate to good enantioselectivities under the standard reaction conditions by using PdBr2/(S)-L4/AgOTf as the asymmetric catalysis system. Further optimization of the reaction revealed that the enantioselectivities could be increased when executing the reaction with TsO– as the counter anion of the palladium catalyst. However, although good to excellent enantioselectivities (85–94% ees) could be obtained, the yields of the desired products decreased. Following a two-step derivatization of chiral compound 4aa, the absolute configuration of 4aa was determined to be (3S, 4R) by X-ray diffraction analysis of chiral compound 12 (see the ESI†).
a Reaction conditions: 1 (0.36 mmol), 2 (0.3 mmol), PdBr2 (5 mol%), AgOTf (12 mol%), (S)-L4 (12 mol%), CH2Cl2 (1.0 mL), rt, 24 h, isolated yield, the dr value was determined by 1H NMR analysis of the crude reaction mixture, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rr in all cases, ee values were determined by chiral HPLC analysis.
b [Pd(allyl)Cl]2 (2.5 mol%), AgOTs (6 mol%). 1 rr in all cases, ee values were determined by chiral HPLC analysis.
b [Pd(allyl)Cl]2 (2.5 mol%), AgOTs (6 mol%).
|
|---|
|
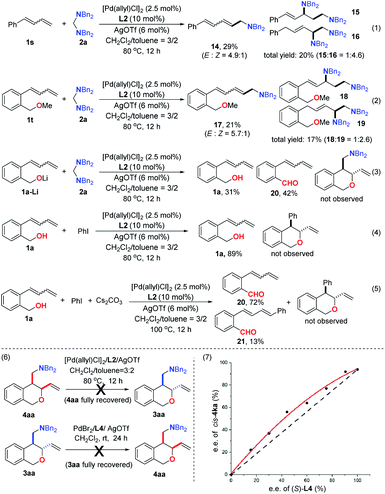
A series of experiments were conducted to shed light on the mechanism of this transformation. First, the simple diene 1s was subjected to the standard reaction conditions for the generation of trans-products. However, only three major products (14, 15 and 16)8a,b derived from terminal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond insertion were observed and none from internal C
C bond insertion were observed and none from internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond insertion was detected (Scheme 3-1), showing the importance of the directing-effect of the hydroxyl group. The diene-tethered benzyl methyl ether 1t was prepared and treated with 2a under identical conditions. Although the OMe should be able to coordinate with the palladium, there was no product originating from internal C
C bond insertion was detected (Scheme 3-1), showing the importance of the directing-effect of the hydroxyl group. The diene-tethered benzyl methyl ether 1t was prepared and treated with 2a under identical conditions. Although the OMe should be able to coordinate with the palladium, there was no product originating from internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond insertion detected again (Scheme 3-2). This result indicated that the coordination of O to Pd alone could not direct the desired Heck insertion. On the other hand, no desired product but the aldehyde 20 was detected in the reaction with the Li-salt of 1a, which excluded the possibility that the directing effect was furnished via the formation of the Pd–O sigma-bond (Scheme 3-3). In addition, replacing the aminal with PhI led to the recovery of the starting material 1a when the reaction was conducted in the absence of a base. In the presence of a base and at higher temperature, the aldehydes 20 and 21 were generated as major products, and yet gave no desired cyclization product. Besides, we observed an intriguing relationship between the regioselectivity of the reaction and the electronic nature of the substituted aminal. Higher regioselectivity was observed when the aminal contained an electron-rich para-substituent, and the selectivity diminished gradually as the substituent became more electron deficient. The aromatic electronic effect can be represented by a Hammett relationship. The Hammett plot of lg (ratio of regioisomers) against σp shows a linear free-energy relationship (ρ = −1.27, r = 0.969) between regioselectivity and the electronic character of the substituent (see the ESI†).12 These results further confirmed that the formation of hydrogen-bonding was key to furnish the 3,4-difunctionalized annulation. Subjecting the purified cis-4aa to the reaction conditions for producing trans-products resulted in fully recovered cis-4aa (Scheme 3-6). This result ruled out the possibility that C–O bond formation is reversible and the thermodynamically preferred trans-product is generated from the kinetically preferred cis-product in the case of Cphos. Furthermore, no cis-product was detected when a purified trans-product, 3aa, was subject to the reaction conditions for generation of cis-products. On the other hand, the non-linear relationship between the ee value of optically active ligand L4 and the ee value of the cis-4ka (Scheme 3-7) provided evidence that two phosphoramidite ligands and one palladium were involved in the enantioselectivity-determining step.
C bond insertion detected again (Scheme 3-2). This result indicated that the coordination of O to Pd alone could not direct the desired Heck insertion. On the other hand, no desired product but the aldehyde 20 was detected in the reaction with the Li-salt of 1a, which excluded the possibility that the directing effect was furnished via the formation of the Pd–O sigma-bond (Scheme 3-3). In addition, replacing the aminal with PhI led to the recovery of the starting material 1a when the reaction was conducted in the absence of a base. In the presence of a base and at higher temperature, the aldehydes 20 and 21 were generated as major products, and yet gave no desired cyclization product. Besides, we observed an intriguing relationship between the regioselectivity of the reaction and the electronic nature of the substituted aminal. Higher regioselectivity was observed when the aminal contained an electron-rich para-substituent, and the selectivity diminished gradually as the substituent became more electron deficient. The aromatic electronic effect can be represented by a Hammett relationship. The Hammett plot of lg (ratio of regioisomers) against σp shows a linear free-energy relationship (ρ = −1.27, r = 0.969) between regioselectivity and the electronic character of the substituent (see the ESI†).12 These results further confirmed that the formation of hydrogen-bonding was key to furnish the 3,4-difunctionalized annulation. Subjecting the purified cis-4aa to the reaction conditions for producing trans-products resulted in fully recovered cis-4aa (Scheme 3-6). This result ruled out the possibility that C–O bond formation is reversible and the thermodynamically preferred trans-product is generated from the kinetically preferred cis-product in the case of Cphos. Furthermore, no cis-product was detected when a purified trans-product, 3aa, was subject to the reaction conditions for generation of cis-products. On the other hand, the non-linear relationship between the ee value of optically active ligand L4 and the ee value of the cis-4ka (Scheme 3-7) provided evidence that two phosphoramidite ligands and one palladium were involved in the enantioselectivity-determining step.
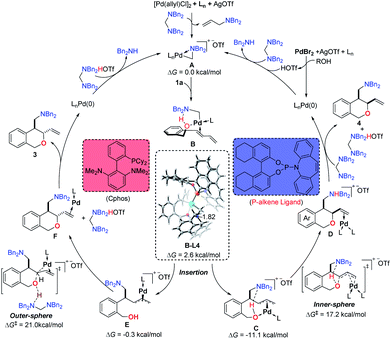
A tentative mechanism is thus proposed based on the above results and previous reports.8 As depicted in Fig. 1, the cyclopalladated complex A is generated via either oxidative addition of the Pd(0) to the protonated aminal or an SN2-type reductive elimination and oxidative addition process between the aminal and the π-allylpalladium catalyst. The hydroxyl group acts as a directing group to bring the palladium center close to the internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, in which the hydrogen-bonding between the aminal moiety of complex A and the hydroxyl group plays a key role in facilitating the formation of intermediate B. The relative free energy of intermediate B is only 2.6 kcal mol−1, when electron-deficient L4 is utilized as the ligand. Subsequently, the migratory insertion of the internal C
C double bond, in which the hydrogen-bonding between the aminal moiety of complex A and the hydroxyl group plays a key role in facilitating the formation of intermediate B. The relative free energy of intermediate B is only 2.6 kcal mol−1, when electron-deficient L4 is utilized as the ligand. Subsequently, the migratory insertion of the internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond into the C–Pd bond of complex A takes place to form the π-allylpalladium intermediate C which is exergonic by 11.1 kcal mol−1. The strong π-acceptor ability of the L4 would induce the palladium center to coordinate with the alcohol in intermediate C,13 which undergoes inner-sphere reductive elimination with a free energy barrier of 17.2 kcal mol−1 to afford the cis-diastereoselective product 4 and regenerates the Pd(0) to enter the next catalytic cycle. Notably, there are two phosphoramidite ligands that coordinate with the Pd(II) center in this inner-sphere reductive elimination transition state. The calculated results are consistent with the non-linear relationship. When the electron-rich L2 (Cphos) acts as the ligand,14 the alcohol does not coordinate with the Pd(II) center in π-allylpalladium intermediate E. Then the π-allylpalladium is intramolecularly trapped by the alcohol via the outer-sphere mode with an energy barrier of 21.0 kcal mol−1 to produce the trans-diastereoselective cyclization product 3, and the Pd(0) is regenerated with the assistance of aminal.
C bond into the C–Pd bond of complex A takes place to form the π-allylpalladium intermediate C which is exergonic by 11.1 kcal mol−1. The strong π-acceptor ability of the L4 would induce the palladium center to coordinate with the alcohol in intermediate C,13 which undergoes inner-sphere reductive elimination with a free energy barrier of 17.2 kcal mol−1 to afford the cis-diastereoselective product 4 and regenerates the Pd(0) to enter the next catalytic cycle. Notably, there are two phosphoramidite ligands that coordinate with the Pd(II) center in this inner-sphere reductive elimination transition state. The calculated results are consistent with the non-linear relationship. When the electron-rich L2 (Cphos) acts as the ligand,14 the alcohol does not coordinate with the Pd(II) center in π-allylpalladium intermediate E. Then the π-allylpalladium is intramolecularly trapped by the alcohol via the outer-sphere mode with an energy barrier of 21.0 kcal mol−1 to produce the trans-diastereoselective cyclization product 3, and the Pd(0) is regenerated with the assistance of aminal.
Conclusions
In summary, we have developed a new and efficient hydrogen-bonding assisted directing strategy to enable the Heck insertion to regioselectively take place across the internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of dienes. By using this strategy, a palladium-catalyzed regioselective and ligand-controlled diastereodivergent aminomethylative annulation of diene-tethered benzyl alcohols with aminals was established for the first time. The combination of the palladium catalyst with electron-rich ligands such as Cphos preferably affords the annulation products in a trans-selective fashion. In contrast, the homologous catalyst with electron-deficient phosphoramidite enables the selective formation of the cis-diastereoisomers. Moreover, the chiral cis-products were also obtained in good to excellent enantioselectivities when chiral phosphoramidite was utilized as the ligand. We envision that this work on the hydrogen-bonding-assisting effect will spur further investigations on exploring new and efficient directing strategies for metal-catalyzed reactions.
C bond of dienes. By using this strategy, a palladium-catalyzed regioselective and ligand-controlled diastereodivergent aminomethylative annulation of diene-tethered benzyl alcohols with aminals was established for the first time. The combination of the palladium catalyst with electron-rich ligands such as Cphos preferably affords the annulation products in a trans-selective fashion. In contrast, the homologous catalyst with electron-deficient phosphoramidite enables the selective formation of the cis-diastereoisomers. Moreover, the chiral cis-products were also obtained in good to excellent enantioselectivities when chiral phosphoramidite was utilized as the ligand. We envision that this work on the hydrogen-bonding-assisting effect will spur further investigations on exploring new and efficient directing strategies for metal-catalyzed reactions.
Data availability
The data that support the findings of this study are available in the ESI† or on request from the corresponding author.Author contributions
H. H. designed the project and wrote the manuscript. Y. H., S. Z., B. Y., and X. Y. conducted experimental studies. S. L. conducted the DFT calculations.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (21925111 and 21790333) and the Strategic Priority Research Program of the CAS (XDPB14) for financial support.Notes and references
- (a) M. Holmes, L. A. Schwartz and M. J. Krische, Chem. Rev., 2018, 118, 6026 CrossRef CAS PubMed; (b) X. Wu and L.-Z. Gong, Synthesis, 2019, 51, 122 CrossRef CAS; (c) G. Li, X. Huo, X. Jiang and W. Zhang, Chem. Soc. Rev., 2020, 49, 2060 RSC.
- For selected examples, see: (a) J. M. O'Connor, B. J. Stallman, W. G. Clark, A. Y. L. Shu, R. E. Spada, T. M. Stevenson and H. A. Dieck, J. Org. Chem., 1983, 48, 807 CrossRef; (b) R. C. Larock, N. Berrios-Peña and K. Narayanan, J. Org. Chem., 1990, 55, 3447 CrossRef CAS; (c) D. Flubacher and G. Helmchen, Tetrahedron Lett., 1999, 40, 3867 CrossRef CAS; (d) C. E. Houlden, C. D. Bailey, J. G. Ford, M. R. Gagne, G. C. Lloyd-Jones and K. I. Booker-Milburn, J. Am. Chem. Soc., 2008, 130, 10066 CrossRef CAS PubMed; (e) D. Xing and D. Yang, Org. Lett., 2013, 15, 4370 CrossRef CAS PubMed; (f) C. Um and S. R. Chemler, Org. Lett., 2016, 18, 2515 CrossRef CAS PubMed; (g) S.-S. Chen, J. Meng, Y.-H. Li and Z.-Y. Han, J. Org. Chem., 2016, 81, 9402 CrossRef CAS PubMed; (h) S.-S. Chen, M.-S. Wu and Z.-Y. Han, Angew. Chem., Int. Ed., 2017, 56, 6641 CrossRef CAS PubMed; (i) T. Zhang, H.-C. Shen, J.-C. Xu, T. Fan, Z.-Y. Han and L.-Z. Gong, Org. Lett., 2019, 21, 2048 CrossRef CAS PubMed.
- (a) Y. Zhu, R. G. Cornwall, H. Du, B. Zhao and Y. Shi, Acc. Chem. Res., 2014, 47, 3665 CrossRef CAS PubMed; (b) F. Burg and T. Rovis, J. Am. Chem. Soc., 2021, 143, 17964 CrossRef CAS PubMed.
- For selected reviews, see: (a) G. Liu and Y. Wu, Topics in Current Chemistry, Springer, Berlin, Heidelberg, 2009, vol 292 Search PubMed; (b) S. J. Connon and S. Blechert, Angew. Chem., Int. Ed., 2003, 42, 1900 CrossRef CAS PubMed; (c) Z. Dong, Z. Ren, S. J. Thompson, Y. Xu and G. Dong, Chem. Rev., 2017, 117, 9333 CrossRef CAS PubMed; (d) M. Nishiura, F. Guo and Z. Hou, Acc. Chem. Res., 2015, 48, 2209–2220 CrossRef CAS PubMed; (e) K. A. Margrey and D. A. Nicewicz, Acc. Chem. Res., 2016, 49, 1997–2006 CrossRef CAS PubMed.
- For selected reviews, see: (a) B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921 CrossRef CAS PubMed; (b) B. M. Trost, T. Zhang and J. D. Sieber, Chem. Sci., 2010, 1, 427–440 RSC . For selected examples, see:; (c) A. Iourtchenko and D. Sinou, J. Mol. Catal. A: Chem., 1997, 122, 91 CrossRef CAS; (d) B. M. Trost, E. J. McEachern and F. D. Toste, J. Am. Chem. Soc., 1998, 120, 12702 CrossRef CAS; (e) B. M. Trost, B. S. Brown, E. J. McEachern and O. Kuhn, Chem.–Eur. J., 2003, 9, 4442 CrossRef CAS PubMed; (f) A. Khan, S. Khan, I. Khan, C. Zhao, Y. Mao, Y. Chen and Y. Zhang, J. Am. Chem. Soc., 2017, 139, 10733 CrossRef CAS PubMed; (g) S. Khan, H. Li, C. Zhao, X. Wu and Y. J. Zhang, Org. Lett., 2019, 21, 9457 CrossRef CAS PubMed; (h) R. Chang, S. Cai, G. Yang, X. Yan and H. Huang, J. Am. Chem. Soc., 2021, 143, 12467 CrossRef CAS PubMed.
- For selected reviews, see: (a) A. H. Hoveyda, D. A. Evans and G. C. Fu, Chem. Rev., 1993, 93, 1307 CrossRef CAS; (b) G. Rousseau and B. Breit, Angew. Chem., Int. Ed., 2011, 50, 2450 CrossRef CAS PubMed; (c) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef CAS PubMed; (d) C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes and M. Schnürch, Chem. Soc. Rev., 2018, 47, 6603 RSC.
- For selected examples, see: (a) Y. Lu, D.-H. Wang, K. M. Engle and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 5916 CrossRef CAS PubMed; (b) S. Nakanowatari and L. Ackermann, Chem.–Eur. J., 2014, 20, 5409 CrossRef CAS PubMed; (c) W. Guo, L. Martínez-Rodríguez, R. Kuniyil, E. Martin, E. C. Escudero-Adań, F. Maseras and A. W. Kleij, J. Am. Chem. Soc., 2016, 138, 11970 CrossRef CAS PubMed; (d) K. Meng, T. Li, C. Yu, C. Shen, J. Zhang and G. Zhong, Nat. Commun., 2019, 10, 5109 CrossRef PubMed; (e) T. Kang, N. Kim, P. T. Cheng, H. Zhang, K. Foo and K. M. Engle, J. Am. Chem. Soc., 2021, 143, 13962 CrossRef CAS PubMed.
- (a) Y. Liu, Y. Xie, H. Wang and H. Huang, J. Am. Chem. Soc., 2016, 138, 4314 CrossRef CAS PubMed; (b) C. Qiao, A. Chen, B. Gao, Y. Liu and H. Huang, Chin. J. Chem., 2018, 36, 929 CrossRef CAS; (c) B. Yu, S. Zou, H. Liu and H. Huang, J. Am. Chem. Soc., 2020, 142, 18341 CrossRef CAS PubMed; (d) H. Zhang, T. Jiang, J. Zhang and H. Huang, Acc. Chem. Res., 2021, 54, 4305 CrossRef CAS PubMed.
- (a) K. C. Nicolaou, J. A. Pfefferkorn, S. Barluenga, H. J. Mitchell, A. J. Roecker and G.-Q. Cao, J. Am. Chem. Soc., 2000, 122, 9968 CrossRef CAS; (b) C. A. Maier and B. Wünsch, J. Med. Chem., 2002, 45, 4923 CrossRef CAS PubMed; (c) G. P. Ellis and I. M. Lockhart, The Chemistry of Heterocyclic Compounds, Chromenes, Chromanones, and Chromones, Wiley, New York, 2007 Search PubMed; (d) Y. B. Zhou, J.-H. Wang, X. M. Li, X. C. Fu, Z. Yan, Y. M. Zeng and X. Li, J. Asian Nat. Prod. Res., 2008, 10, 827 CrossRef CAS PubMed; (e) K. Trisuwan, V. Rukachaisirikul, Y. Sukpondma, S. Phongpaichit, S. Preedanon and J. Sakayaroj, Tetrahedron, 2010, 66, 4484 CrossRef CAS.
- (a) R. A. Olofson, J. T. Martz, J. P. Senet, M. Piteau and T. Malfroot, J. Org. Chem., 1984, 49, 2081 CrossRef CAS; (b) L. Petersen, E.-B. Pedersen and C. Nielsenb, Synthesis, 2001, 4, 559 CrossRef; (c) F. Yang, J.-J. Newsome and D.-P. Curran, J. Am. Chem. Soc., 2006, 128, 14200 CrossRef CAS PubMed; (d) G.-Q. Liu, Z.-Y. Ding, L. Zhang, T.-T. Li, L. Li, L.-L. Duan and Y.-M. Li, Adv. Synth. Catal., 2014, 356, 2303 CrossRef CAS.
- CCDC 2111898 (7), CCDC 2111899 (12) and CCDC 2111895 ((3S,4R)-12) contain the supplementary crystallographic data for this paper.†.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165 CrossRef CAS.
- (a) J.-P. Chen, Q. Peng, B.-L. Lei, X.-L. Hou and Y.-D. Wu, J. Am. Chem. Soc., 2011, 133, 14180 CrossRef CAS PubMed; (b) D. C. Bai, F.-L. Yu, W.-Y. Wang, D. H. Li, Q.-R. Liu, C.-H. Ding, B. Chen and X.-L. Hou, Nat. Commun., 2016, 7, 11806 CrossRef PubMed; (c) L. Hu, A. Cai, Z. Wu, A. W. Kleij and G. Huang, Angew. Chem., Int. Ed., 2019, 58, 14694 CrossRef CAS PubMed; (d) M.-H. Yang, D. L. Orsi and R. A. Altman, Angew. Chem., Int. Ed., 2015, 54, 2361 CrossRef CAS PubMed; (e) A. Cai, W. Guo, L. Martínez-Rodríguez and A. W. Kleij, J. Am. Chem. Soc., 2016, 138, 14194 CrossRef CAS PubMed; (f) T. Jiang, H. Zhang, Y. Ding, S. Zou, R. Chang and H. Huang, Chem. Soc. Rev., 2020, 49, 1487 RSC.
- (a) A. Pfaltz and M. Lautens, Comprehensive Asymmetric Catalysis, Springer, New York, NY, 1999, vol. 2, p. 833 Search PubMed; (b) G. Helmchen, J. Organomet. Chem., 1999, 576, 203 CrossRef CAS; (c) J. Keith, D.-C. Behenna, N. Sherden, J.-T. Mohr, S. Ma, S.-C. Marinescu, R.-J. Nielsen, J. Oxgaard, B.-M. Stoltz and W.-A. Goddard, J. Am. Chem. Soc., 2012, 134, 19050 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2111895, 2111898 and 2111899. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc06479g |
| ‡ Y. H. and S. Z. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |