Open Access Article
Open Access ArticleA short, versatile route towards benzothiadiazinyl radicals†
Andryj M.
Borys
 a,
Ewan R.
Clark
a,
Ewan R.
Clark
 *a,
Paul J.
Saines
*a,
Paul J.
Saines
 a,
Antonio
Alberola
a,
Antonio
Alberola
 b and
Jeremy M.
Rawson
b and
Jeremy M.
Rawson
 *c
*c
aSchool of Physical Sciences, University of Kent, Ingram Building, Canterbury, Kent CT2 7NH, UK
bPol. Industrial Cheste, Vial 6, 46380 Cheste, Valencia, Spain
cDepartment of Chemistry and Biochemistry, The University of Windsor, 401 Sunset Ave., Windsor ON N9B 3P4, Canada. E-mail: jmrawson@uwndsor.ca
First published on 23rd November 2021
Abstract
A family of substituted 1,2,4-benzothiadiazine 1-chlorides have been prepared by treatment of N-arylamidines in neat thionyl chloride at reflux. The S(IV) 1-chlorides are readily reduced under mild conditions to persistent 1,2,4-benzothiadiazinyl radicals which have been characterised by EPR spectroscopy and cyclic voltammetry. Crystallographic studies on isolated radicals indicate that the radicals dimerise via pancake bonding in the solid-state, resulting in spin-pairing and net diamagnetism.
Introduction
Stable molecular radical species have long been targeted as potential molecular magnets for future magnetic storage applications, and as test-beds for fundamental studies into magnetic communication.1 Several metal-free radical families2 have been explored which exhibit divergent properties from metal centred species. These are of particular interest because, in addition to their magnetic properties, many exhibit a unique form of bonding wherein they dimerise via π–π interactions (termed “pancake bonding”) rather than formation of simple 2c2e− bonds.3 These interactions are key to dictating both magnetic and electronic properties of organic systems, and represent a versatile and important motif in supramolecular recognition.4This pancake bonding mode is accessible because many of the “organic” radical families known utilise π-delocalisation, typically onto electronegative elements or extended systems, to achieve radical stability, as exemplified by the verdazyl5 (N centred radicals), nitroxyl6 (N–O centred radicals), and dithiazolyl, and dithiadiazolyl (S–N centred radicals) systems.7 The five-membered ring S–N radicals provide a particularly rich vein of potential radical systems due to their amenability to isoelectronic substitutions and modular synthesis. For example, the 1,2,3,5-dithiadiazolyl class incorporates a substituent at the 4-position which is not directly conjugated into the radical heterocycle and whose influence on properties is therefore limited to steric and inductive effects.8 However, comparatively few S–N radicals based on six-membered rings are known, and studies on the benzo-1,2,4-thiadiazinyl class are sparse, with only two examples crystallised to date;9 some mesophases containing these ring systems have also been characterised.10
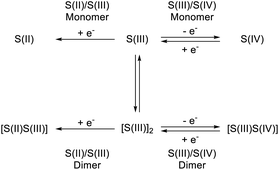
The benzo-1,2,4-thiadiazine heterocyclic system is accessible in a range of oxidation states from S(II) to S(VI), and incorporation of 2-pyridyl moieties at the pendant 3 position gives rise to chelating ligands for which first row transition metal11 and Ir(III)12 complexes are known. The S(III) radicals (see Scheme 1a) are accessible through one-electron oxidation of the S(II) systems,9 or more conveniently by one-electron reduction of S(IV) precursors.13 Work by Oakley has allowed the isolation of a range of mixed valent S(II)/S(III) thiadiazine based systems; in these cases, an extended π-system containing an additional S–N heterocycle is used to provide stability.14,15 Overall, these systems are comparatively unexplored and offer great potential as molecular radical building blocks as the substituents at the 3 position are not directly conjugated into the singly occupied molecular orbital (SOMO), analogous to the dithiadiazolyl radicals. The radical properties can therefore in principle be tuned as desired by chemical modification of both the benzo-fused ring, affecting the radical electronic structure, and the 3-substituent whose modification is expected to affect steric demand and packing interactions predominantly.
The expansion of this class of radicals is hampered by the lack of general synthetic methodologies available. The radicals themselves may be formed by either 1e− oxidation16 or reduction of appropriate closed shell precursors,14 but few synthetic routes towards these precursors are known and these are not particularly general (see Scheme 1), often involving long, multi-step syntheses. Indeed, for the five radicals reported by Kazsynski in 2004, five bespoke synthetic routes were required.9 The three points in common across these synthetic routes are the presence of an amidine intermediate to provide the NCN fragment of the heterocyclic ring, ring-closure effected by nucleophilic attack on an S(IV) species which is in turn generated by in situ oxidation of a S(II) precursor, and halogenation of the benzo-fused ring to stabilise the resultant radicals and enable their isolation. This last point introduces an additional synthetic consideration, necessitating pre-halogenated precursors, or exhaustive chlorination after ring-closure using Cl2. In Levchenko's original 1984 work,17 SCl2 acted simultaneously as the sulphur source, oxidising agent, and chlorinating agent to give benzothiadiazine 1-chlorides with varying degrees of chlorination about the benzo-fused ring, followed by direct chlorination with Cl2, whilst Oakley's mixed S(II)/(IV) parent species use S2Cl2 in the same role.14
Recognising this, we sought a simple, direct route to the required heterocyclic ring starting from the easily handled and cheap S(IV) source, thionyl chloride. We report here the development of a SOCl2-based route towards 1,2,4-benzothiadiazine 1-chlorides and studies on the derived S(III) radicals.
Results and discussion
Synthesis
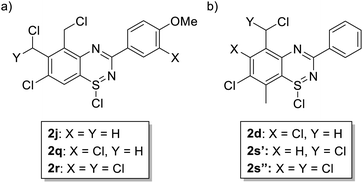
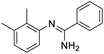
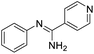
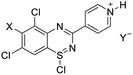
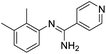
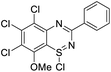
Initial studies on the reaction of the prototypical N-phenylbenzamidine, 1a, with stoichiometric SOCl2 were unsuccessful when performed in DCM, THF, and toluene, in each case producing a copious quantity of 1a·HCl as a colourless solid. Addition of stoichiometric base (pyridine, Et3N and iPr2EtN) did not improve matters and, for the trialkylamines, led to a violent side reaction which is likely due to oxidation of the trialkylamine by SOCl2.18 It was reasoned that a polar, high-boiling solvent would (i) retain the protonated amidine in solution and (ii) permit the HCl formed to be driven off at elevated temperatures, driving the desired cyclisation process. As thionyl chloride itself fulfils these criteria (μ = 1.44 D, bp = 74.6 °C), the reaction of 1a in neat SOCl2 was attempted. After reflux overnight, the reaction mixture was deep orange, characteristic of benzothiadiazine 1-chlorides. Layering the reaction mixture with nhexane, followed by slow diffusion, led to isolation of analytically pure, crystalline 2a in 71% yield. Reaction of the related amidines 1b–j in refluxing SOCl2 similarly led to the isolation of benzothiadiazine 1-chlorides 2b–j in moderate to good yields (31–81%) as yellow to red crystalline solids (Table 1). Notably, partial chlorination of both the benzo ring and any methyl substituents on the benzo ring occurs during all these reactions. However, the presence of excess SOCl2 led to a single product in nearly all cases. For 1d, the chloride salt 2d selectively precipitated from solution in 31% yield, but slow cooling of the mother liquor afforded orange needles which comprised a mixture of the isomeric compound 2s′ and the dichloromethyl derivative 2s′′ (Fig. 1b) in a ca. 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio based on crystallographic refinement, suggesting the potential for further chlorination when using extended reaction times. Attempts to prepare 2k yielded a yellow solid that was practically insoluble in most organic solvents and only poorly soluble in SOCl2. 1H NMR analysis was consistent with the formation of the expected heterocycle and partial chlorination at the 6 position (H
3 ratio based on crystallographic refinement, suggesting the potential for further chlorination when using extended reaction times. Attempts to prepare 2k yielded a yellow solid that was practically insoluble in most organic solvents and only poorly soluble in SOCl2. 1H NMR analysis was consistent with the formation of the expected heterocycle and partial chlorination at the 6 position (H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl = 3
Cl = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Prolonged reflux of 2k in the presence of excess SO2Cl2 and larger volumes of SOCl2 only slightly increased the degree of chlorination (H
2). Prolonged reflux of 2k in the presence of excess SO2Cl2 and larger volumes of SOCl2 only slightly increased the degree of chlorination (H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl = 2
Cl = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), possibly due to the poor solubility of the salt, whilst shorter reflux times afforded other unidentified species. Attempts to deprotonate compound 2k with basic amines (pyridine, Et3N, DABCO) resulted in the immediate formation of a dark blue, EPR active solution from which poor quality crystals of the neutral radical (H
3), possibly due to the poor solubility of the salt, whilst shorter reflux times afforded other unidentified species. Attempts to deprotonate compound 2k with basic amines (pyridine, Et3N, DABCO) resulted in the immediate formation of a dark blue, EPR active solution from which poor quality crystals of the neutral radical (H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl = 4
Cl = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) could be grown (see ESI† for details), indicating that these species are highly oxidising (vide infra).
1) could be grown (see ESI† for details), indicating that these species are highly oxidising (vide infra).
| Entry | N-Aryl-amidine | Yield (%) | Benzo-1,2,4-benzothiadiazine 1-chloride | Yield (%) | Entry | N-Aryl-amidine | Yield (%) | Benzo-1,2,4-benzothiadiazine 1-chloride | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|
| a Mixture of species. b Target product not obtained. | |||||||||
| a |

|
90 |

|
71 | i |

|
77 |
|
81 |
| b |

|
71 |

|
61 | j |

|
74 |

|
63 |
| c |
|
87 |

|
76 | k |
|
88 |
|
|
| d |

|
79 |

|
31 | l |
|
85 |

|
87 |
| e |

|
78 |

|
40 | m |

|
71 | 0b | |
| f |

|
66 |
|
46 | n |

|
72 | 0b | |
| g |

|
63 |

|
46 | o |

|
68 | 0b | |
| h |

|
66 |

|
53 | p |

|
41 | 0b | |
Selective chlorination of the benzo-fused ring is reminiscent of Levchenko's reaction of N-chloroamidines with SCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 in which the position para to the amidine nitrogen was always chlorinated. Similarly 1,2,3-dithiazolylium salts prepared by Herz from 2-mercaptoanilines and SOCl2 also undergo chlorination para to the amino group.19,20 For these benzo-fused 1,2,4-thiadiazolium salts, chlorination of the benzo ring occurred at all positions except the 8-position and chlorination of methyl groups on the benzo ring typically afforded selectively the chloromethyl derivatives unless the methyl group was at the 8 position where no chlorination was observed. The selectivity leading to retention of the C–H at C(8) is attributed to the combination of successive deactivation by chlorination elsewhere and conjugation into the larger heterocycle, lowering the HOMO energies, directed by the location of C(8) meta to the o,p-directing amidine group and ortho to the deactivating S(IV) functionality. This is consistent with the exhaustive chlorination reported by Levchenko and Kaszynski which required direct treatment with excess Cl2.9,17 In contrast to the benzo substituents, phenyl and tolyl groups at the 3-position generally appear resistant to chlorination under these conditions. However, activated aryl groups appear to promote chlorination of the 3-aryl group. For example, while 2i formed cleanly, reaction of 1j with SOCl2 formed 2j selectively under short reaction times but a mixture comprising 2j and up to ∼45% of 2q (Fig. 1a) was observed as a by-product under extended reaction times. Despite this, attempts to produce pure 2q under extended reaction times up to 72 hours was unsuccessful. Attempted purification of 2q by recrystallisation from thionyl chloride containing SO2Cl2 as stabiliser instead allowed the isolation of a few crystals of 2r with additional chlorination of the 6-chloromethyl group. Nevertheless, 2q was fully characterised by 1H and 13C{1H} NMR spectroscopy.
17 in which the position para to the amidine nitrogen was always chlorinated. Similarly 1,2,3-dithiazolylium salts prepared by Herz from 2-mercaptoanilines and SOCl2 also undergo chlorination para to the amino group.19,20 For these benzo-fused 1,2,4-thiadiazolium salts, chlorination of the benzo ring occurred at all positions except the 8-position and chlorination of methyl groups on the benzo ring typically afforded selectively the chloromethyl derivatives unless the methyl group was at the 8 position where no chlorination was observed. The selectivity leading to retention of the C–H at C(8) is attributed to the combination of successive deactivation by chlorination elsewhere and conjugation into the larger heterocycle, lowering the HOMO energies, directed by the location of C(8) meta to the o,p-directing amidine group and ortho to the deactivating S(IV) functionality. This is consistent with the exhaustive chlorination reported by Levchenko and Kaszynski which required direct treatment with excess Cl2.9,17 In contrast to the benzo substituents, phenyl and tolyl groups at the 3-position generally appear resistant to chlorination under these conditions. However, activated aryl groups appear to promote chlorination of the 3-aryl group. For example, while 2i formed cleanly, reaction of 1j with SOCl2 formed 2j selectively under short reaction times but a mixture comprising 2j and up to ∼45% of 2q (Fig. 1a) was observed as a by-product under extended reaction times. Despite this, attempts to produce pure 2q under extended reaction times up to 72 hours was unsuccessful. Attempted purification of 2q by recrystallisation from thionyl chloride containing SO2Cl2 as stabiliser instead allowed the isolation of a few crystals of 2r with additional chlorination of the 6-chloromethyl group. Nevertheless, 2q was fully characterised by 1H and 13C{1H} NMR spectroscopy.
In the case of 1l, the basic nature of the 3-(4′-pyridyl) group led to isolation of the hydrochloride salt [2l·H][HCl2]. No crystalline products could be isolated for the reactions of 1m–n with SOCl2. Since chlorination occurs para to the amidine functionality prior to, or at the same time as, ring-closure, it is believed that the presence of a methyl-group at this position inhibits the formation of the desired 1,2,4-benzothiadiazine 1-chloride. Attempts to prepare 2o were unsuccessful; yielding a dark brown insoluble solid assigned as a mixture of partially chlorinated species by 1H NMR spectroscopy. A low yield of pale peach solid was obtained during the attempted synthesis of 2p; a few, low quality colourless crystals, grown by recrystallisation of the crude solid from boiling SOCl2, were identified as doubly ortho-chlorinated 1p·HCl by SCXRD but were only a component of a complex mixture as seen by 1H NMR.
The patterns of partial chlorination observed imply that chlorination is rapid at the 5 and 7 positions, slow at the 6 position, and does not occur for the 8 position. The fact that no products could be isolated when substituents were present para to the amidine nitrogen (the pro-7 position) suggests that chlorination at this position is rapid, as seen for Herz reactions,19,20 producing highly reactive intermediates leading to uncontrolled further reactivity and decomposition. The isolation of partially chlorinated 1p·HCl suggests that chlorination of the aryl ring is more rapid than ring-closure and that chlorination at both positions ortho to the amidine nitrogen inhibits the formation of the fused-ring. This would indicate that the rates of chlorination and ring-closure are competitive for these systems. Independent synthesis of 1t, N-(4-chlorophenyl)-benzamidine, and reaction in SOCl2 led cleanly to 2a, supporting this species as an intermediate in ring closure. From these results, it is not possible to fully determine the mechanism, but it is plausible that it occurs by formation of an aminosulphonylchloride, 5 (Scheme 2), and subsequent electrophilic aromatic substitution, either directly or via an intermediate N-sulfinylamine, 6,21 followed by deoxygenation of an S(IV) 1-oxide, 7, by reaction with a further equivalent of SOCl2 to give the benzothiadiazine 1-chloride, 2, which may then undergo additional chlorination at carbon, as shown in Scheme 2.
Crystallographic discussion
Single crystals suitable for X-ray diffraction studies were obtained for all derivatives except 2e, either by layering the reaction mixture with dry nhexane or slow cooling of a saturated thionyl chloride solution. The benzothiadiazine 1-chlorides all adopt solid state structures distorted from planarity, exemplified by 2a (Fig. 2). The heterocyclic ring is not flat but instead bent along the 1,4 S⋯N axis, with angles between the two halves of the ring ranging from 7.11° to 15.10° (see Table 2). These nevertheless show on average less deviation from planarity than the related S(II) benzothiadiazines,22 likely due to the increased delocalisation in the formally 10π benzothiadiazine 1-chlorides vs. the 12π anti-aromatic S(II) benzothiadiazines. The pendant aryl rings are not coplanar with the heterocycle, showing torsion angles in the range of 0.8(6) to 30.09(3)°; the torsion angles are largest for 2g and 2h with ortho-methyl substituents on the pendant ring. Examination of the S–Cl bonds is informative - the bond lengths range from 2.221(2) Å to 2.3412(7) Å, considerably longer than the 2.072 Å expected for a formal S–Cl single bond,23 and comparable to the range seen for thiatriazines (2.283–2.357 Å).24,25 In addition to this, the transannular N–S–Cl angles are in a narrow range of 99.84(5)° to 106.80(5)°, consistent with a rigid and well defined bonding environment. These are therefore elongated and weakened covalent bonds, in contrast to the ionic character seen for dithiadiazolyl chlorides where the S–Cl contacts are much longer at 2.906–2.962 Å,26 albeit still within the sum of the van der Waals radii, 3.55 Å.27 This argues for a comparatively less stable cation following halide abstraction arising from poorer π-delocalisation about the heterocycle.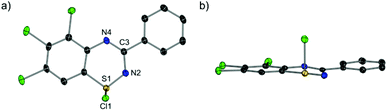 | ||
| Fig. 2 Molecular structure of 2a. Thermal ellipsoids shown at 50% probability. Hydrogen atoms omitted for clarity. (a) Top-down view; (b) side view. | ||
| Deviation from planarity/° | Torsion angles/° | S–CI bond length/Å | S–CI angle/° | ||
|---|---|---|---|---|---|
| N2–C3–CA–CB | N4–C3–CA–CF | N4–S1–CI | |||
| 2a | 11.74 | 4.6(6) | 0.8(6) | 2.221(2) | 100.79(8) |
| 8.17 | 13.7(6) | 9.9(6) | 2.306(1) | 106.24(7) | |
| 2b | 11.44 | 11.90(3) | 9.05(3) | 2.2811(8) | 102.58(4) |
| 2c | 7.32 | 6.65(3) | 4.18(3) | 2.3412(7) | 106.11(4) |
| 2d-α | 10.99 | 8.64(4) | 6.16(4) | 2.2974(9) | 102.32(5) |
| 2d-β | 7.11 | 6.1(8) | 1.7(8) | 2.261(2) | 107.0(1) |
| 2f | 9.61 | 3.83(2) | 0.89(2) | 2.2759(6) | 104.06(3) |
| 2g | 8.26 | 19.80(1) | 16.08(1) | 2.309(2) | 106.23(1) |
| 13.78 | 10.51(1) | 9.96(1) | 2.229(3) | 99.97(1) | |
| 2h | 15.10 | 30.90(3) | 28.91(3) | 2.2689(9) | 99.84(5) |
| 2i | 7.20 | 9.59(3) | 5.89(3) | 2.3205(8) | 106.80(5) |
| 2j | 8.25 | 8.25(5) | 5.10(5) | 2.279(1) | 105.33(7) |
| [21·H][HCl2] | 13.46 | 20.56(2) | 18.15(2) | 2.238(4) | 100.25(2) |
| 2r | 9.46 | 9.98(1) | 8.45(1) | 2.264(4) | 103.42(2) |
| 2s | 9.98 | 7.01(9) | 4.03(9) | 2.287(2) | 104.89(1) |
The benzothiadiazine 1-chlorides are chiral systems and, where partial chlorination of methyl groups occurs, the chloromethyl fragment may therefore lie either syn or anti to the S–Cl bond. In the case of 2c, 2h, 2j and 2r, the crystal structure shows both C–Cl bonds lying nearly co-parallel, anti to the S–Cl bond, whereas for [2l·H][HCl2], the two chloromethyl groups sit anti to one another. In contrast, 2d is polymorphic with the α-phase crystallising with the E–Cl bonds anti to one another whilst the β-phase adopts a syn configuration. Although not possessing a CH2Cl group, the OMe group of 2f is disordered equally above and below the plane of the ring relative to the S–Cl bond. In all cases, no splitting of the methylene protons is evident in the 1H NMR spectrum, consistent with rapid rotation and/or inversion about sulphur on the NMR timescale in solution.
Anion metathesis
Anion metathesis reactions are commonly employed to improve the solubility of S(IV) salts prior to reduction to form neutral radicals. With this in mind, some simple tests of the amenability of 2a towards halide abstraction were performed. Reaction of 2a with GaCl3 in DCM resulted in the immediate formation of [4a][GaCl4] as a dark purple solution whose identity was unambiguously confirmed by growth of single crystals by slow diffusion of nhexane into the reaction mixture (Fig. 3).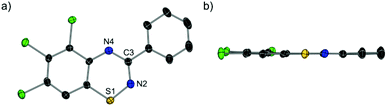 | ||
| Fig. 3 Molecular structure of [4a][GaCl4]. Thermal ellipsoids shown at 50% probability. Hydrogen atoms and counter-anion omitted for clarity. (a) Top-down view; (b) side view. | ||
The abstraction results in an almost completely planar system forming, with minimal trans-annular bend (0.51°) and a torsion angle of only 2.57(6)° between the pendant phenyl ring and heterocycle. The S–CAr bond length in [4a][GaCl4] is considerably shorter than that of 2a (1.683(4) Å vs. 1.733(2) Å), with minor alterations in other bond lengths, which coupled with the more planar structure argues for increased delocalization within the ring which is formally a 6π aromatic system (10π when considering the benzo-fused substituent). Two long cation–anion interactions of 3.341(1) Å and 3.463(1) Å (cf. 2.2572(7) Å for the S–Cl bond in 2a) are observed between sulphur and the chlorine atoms of two adjacent GaCl4− anions, confirming the structure as ionic.
Treatment of 2a with NaBArCl in DCM resulted in a deep blue, EPR active solution consistent with the formation of the neutral S(III) radical, 3a. Taken together, these results show that whilst benzothiadiazinyl cations are accessible, they are highly reactive and easily reduced to the radical such that judicious choice of weakly coordinating anions is required to allow their isolation.
1,2,4-Benzothiadiazinyl radicals
Benzothiadiazinyl radicals have been accessed via one electron oxidation of benzothiadiazines,9 but this requires considerable optimisation for each derivative. An alternative approach is one electron reduction of the benzothiadiazine 1-chloride salts. In our hands, we found that the 1,2,4-benzothiadiazine 1-chlorides (2a–j) were easily reduced to the corresponding radicals with both Ph3Sb and Fe(η5-C5H5)2 but attempts to remove the by-products and isolate the pure radicals from these reaction mixtures failed. Preliminary small-scale studies utilising Ph3P as a milder reducing agent in a variety of common organic solvents were promising, immediately yielding dark green, EPR active solutions, and clearly showing the formation of Ph3PCl2 (THF, toluene; δ −46 ppm) or [Ph3PCl]Cl (DCM, MeCN; δ +60 ppm) by 31P NMR spectroscopy. Solutions of the radicals generated in DCM, THF, and toluene swiftly discoloured to give yellow-brown suspensions, whilst the radicals were only poorly soluble in MeCN. The green solutions of these benzothiadiazinyl radicals were observed to become a rich blue colour on cooling in liquid nitrogen which is believed to originate from a monomer-dimer equilibrium (vide infra), with the formation of closed-shell dimers being favoured at low temperatures.EPR spectroscopy
Although concentrated solutions of these radicals decolourise in minutes, dilute solutions proved sufficiently robust in dry, degassed toluene to allow EPR studies to be performed. This indicates that the short half-life in solution is a result of kinetic instability and subsequent reactivity rather than inherent thermodynamic instability. This is consistent with the thermal stability of the more highly chlorinated analogues reported by Kaszynski which were stable to sublimation at elevated temperature but decomposed over hours in solution.9 They are therefore considerably more reactive than their valence isoelectronic analogues, the Blatter radicals.28 EPR spectra of radicals 3a–j, generated in situ by reduction with Ph3P, were recorded in toluene at ambient temperature on a continuous wave X-band EPR spectrometer. The EPR spectra for the 1,2,4-benzothiadiazinyl radicals are all similar, exhibiting slightly asymmetric 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
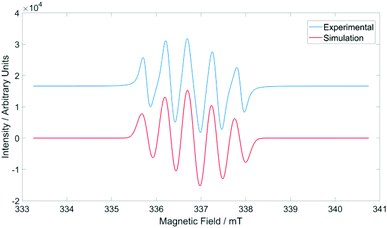
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 quintets arising from coupling to two similar but non-identical nitrogen atoms within the heterocyclic ring. Significant line-broadening was observed in all cases due to chlorination of the fused ring; since both 35Cl and 37Cl are quadrupolar nuclei with I = 3/2, the spectra consist of multiple superimposed spectra for each isotopalogue, resulting in net loss of resolution and increased line-broadening, meaning all features beyond nitrogen hyperfine coupling are lost. The EPR spectrum of 3a is shown in Fig. 4 and the g-values and hyperfine coupling constants for radicals 3a–j are summarized in Table 3.
1 quintets arising from coupling to two similar but non-identical nitrogen atoms within the heterocyclic ring. Significant line-broadening was observed in all cases due to chlorination of the fused ring; since both 35Cl and 37Cl are quadrupolar nuclei with I = 3/2, the spectra consist of multiple superimposed spectra for each isotopalogue, resulting in net loss of resolution and increased line-broadening, meaning all features beyond nitrogen hyperfine coupling are lost. The EPR spectrum of 3a is shown in Fig. 4 and the g-values and hyperfine coupling constants for radicals 3a–j are summarized in Table 3.
| g-Value | Line-width/MHZ | a N2/MHZ | a N4/MHZ | |
|---|---|---|---|---|
| 3a | 2.0037 | 0.27 | 15.66 | 13.13 |
| 3b | 2.0035 | 0.26 | 15.64 | 13.51 |
| 3c | 2.0034 | 0.31 | 15.80 | 13.60 |
| 3d | 2.0043 | 0.31 | 15.10 | 13.10 |
| 3e | 2.0046 | 0.37 | 14.69 | 13.30 |
| 3f | 2.0045 | 0.33 | 14.56 | 13.23 |
| 3g | 2.0046 | 0.27 | 15.88 | 13.35 |
| 3h | 2.0045 | 0.34 | 15.61 | 13.71 |
| 31 | 2.0041 | 0.29 | 15.82 | 12.89 |
| 3j | 2.0041 | 0.38 | 14.73 | 13.98 |
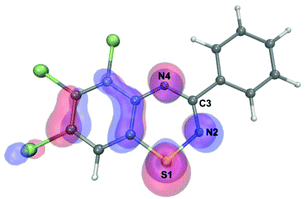
The SOMO geometry of 3a (Fig. 5), calculated at the UB3LYP/cc-pVDZ level using a UB3LYP/6-31G optimised geometry, reveals that it is largely independent of the substituents around the benzo-fused and pendant aryl rings. This is supported by the similarity of g-values and 14N hyperfine coupling constants observed across the range of 1,2,4-benzothiadiazinyl radicals indicating that the electronic structure is only slightly perturbed by substitution at these positions.
Electrochemical studies
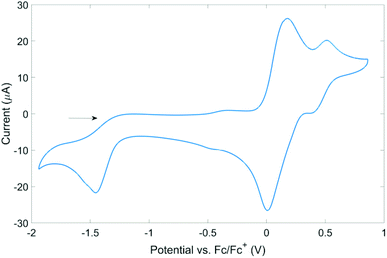
Although all radicals could be generated in situ, pure samples could only be isolated for a minority of the radicals (vide infra). As a result of this and their poor long-term stability in solution, cyclic voltammetry studies were performed on the chlorides 2a–j in dry, degassed DCM. The 1,2,4-benzothiadiazine 1-chlorides all displayed similar electrochemical behaviour, and the cyclic voltammogram of 2a is presented as typical of this series (Fig. 6). The two, often-overlapping, reversible processes at positive potentials (vs. Fc/Fc+) are assigned to S(III)/S(IV) couples, whilst the irreversible process at negative potential is attributed to the S(II)/S(III) couple. The two S(III)/S(IV) waves suggest the presence of radical monomers and dimers in solution, as shown in Scheme 3. Similar monomer-dimer equilibria have also been observed for 6-membered thiatriazines and independently confirmed by quantitative EPR measurements.29 Further support for a monomer-dimer equilibrium was observed via variable concentration studies – at low concentrations the monomer is favoured whilst at high concentrations the cyclic voltammogram is dominated by signals for the dimer. These studies permit initial assignment of the two S(III)/S(IV) redox couples and show that the one electron reduction potential of the monomer is positive relative to the dimer.29 In addition, the S(II)/S(III) reduction wave splits into two well resolved peaks at low concentrations (see ESI†).The irreversibility of the redox event at negative potentials is attributed to a rapid ErCi (a reversible electron transfer followed by an irreversible chemical reaction) comproportionation reaction between the electro-generated S(II) anion and the bulk S(IV) species 2a. This has been observed for several S–N systems.29 No evidence of quasi-reversibility was observed, even at scan rates up to 2 V s−1, indicating that the comproportionation reaction is extremely rapid. The variable scan-rate data nonetheless show that the S(III)/S(IV) processes for both radical monomer and dimer are reversible electron transfer processes involving a freely diffusing redox species according to the Randles–Sevcik equation,30 and confirm that the analyte is not adsorbed to the electrode surface (see ESI†).
The E1/2 potentials for the S(III)/S(IV) couple follow the expected trend (see Table 4), with 1,2,4-benzothiadiazine 1-chlorides bearing electron-withdrawing groups such as 2a, 2g and 2i being more easily reduced than those with electron-donating groups such as 2b–d. The same trends were also observed for the S(II)/S(III) couple. The substituents on the benzo-fused ring were found to have a greater influence on the electrochemical behaviour and redox potentials compared to substituents on the pendant aryl ring. This is in good agreement with DFT studies which indicate that there is negligible delocalisation of the unpaired electron onto the pendant aryl ring but significant π-delocalisation across the benzo-fused and heterocyclic rings. There are, however, only minor changes observed across the E1/2 potentials for substituted benzothiadiazine 1-chlorides.
| S(II)/S(III) | S(III)/S(IV) (dimer) | S(III)/S(IV) (monomer) | |||||
|---|---|---|---|---|---|---|---|
| E red/V | E red/V | E ox/V | E 1/2/V | E red/V | E ox/V | E 1/2/V | |
| 2a | −1.457 | 0.013 | 0.179 | 0.096 | 0.371 | 0.518 | 0.444 |
| 2b | −1.511 | −0.112 | 0.084 | −0.014 | — | 0.449 | — |
| 2c | −1.649 | −0.094 | 0.054 | −0.020 | — | 0.501 | — |
| 2d | −1.654 | −0.084 | 0.036 | −0.024 | — | 0.441 | — |
| 2e | −1.473 | −0.027 | 0.142 | 0.057 | 0.336 | 0.469 | 0.403 |
| 2f | −1.557 | −0.083 | 0.111 | 0.014 | 0.379 | 0.508 | 0.443 |
| 2g | −1.446 | −0.011 | 0.226 | 0.108 | 0.400 | 0.481 | 0.440 |
| 2h | −1.599 | −0.040 | 0.123 | 0.041 | — | 0.479 | — |
| 2i | −1.450 | 0.028 | 0.144 | 0.086 | 0.300 | 0.397 | 0.349 |
| 2j | −1.620 | −0.071 | 0.035 | −0.018 | — | 0.558 | — |
Solid state studies of 1,2,4-benzothiadiazinyl radicals
Isolated 1,2,4-benzothiadiazinyl radicals, 3a–j, were prepared by treatment of the S(IV) 1-chlorides with half a molar equivalent of Ph3P in thoroughly degassed and anhydrous MeCN (Scheme 4). Exploiting the poor solubility of radicals in MeCN, the radicals formed cleanly and rapidly, and precipitated as dark purple-green solids that were subsequently isolated by filtration and washed prior to drying under vacuum. The stability is significantly enhanced in the solid state, with samples giving clean elemental analysis and remaining spin active even after storage for several weeks at room temperature in an argon glove box. In contrast, the washings were found to swiftly discolour, even when stored at −20 °C. The growth of crystals suitable for SCXRD analysis was severely hampered by the poor stability of the 1,2,4-benzothiadiazinyl radicals in solution, with several approaches (slow cooling, vapour diffusion) and solvents (DCM, THF, toluene, pyridine, nhexane, Et2O) giving only rapid discolouration and formation of yellow-brown precipitates. Ultimately, single crystals suitable for SCXRD analysis of 3a and 3c were successfully grown by rapidly mixing the parent S(IV) 1-chloride and reducing agent in DCM and allowing the radical to slowly crystallise out of solution. This method, however, was found to be unreliable in forming X-ray quality crystals and often afforded just micro-crystalline material unsuitable for single crystal diffraction studies. Single crystals of 3e grew readily from saturated toluene solutions. A few, poor quality crystals of 3k could be isolated after attempting to dissolve 2k in pyridine.The discussion herein focuses on the thiadiazinyl ring (see ESI† for full structures). The structurally characterised 1,2,4-benzothiadiazinyl radicals are notably closer to planarity than the S(IV) 1-chlorides; the deviation from planarity for the heterocyclic ring ranges from 1.22° to 5.78° (cf. 7.11–15.10° for 2a–s), although the torsion angles for the pendant aryl ring are comparable 0.3(3)° to 9.6(5)° (cf. 0.89(2)–11.90(3)° for 2a–f). Crystallisation of 3a allows direct comparison of changes in heterocyclic ring structure between 2a, 3a, and [4a]GaCl4, as shown in Table 5. For 3c, the two CH2Cl groups adopt an anti configuration, in contrast to the syn configuration observed in 2c. Unlike Kaszynski's perfluoro- and perchloro-radicals which are monomeric in the solid state,9 radicals 3a, 3c, 3e, and 3k all crystallise as pancake dimers featuring short contacts between the heterocyclic rings.
Three different configurations of dimer (Fig. 7) were observed for the radicals. This is analogous to other sulphur–nitrogen radicals, especially those based on less delocalised five-membered ring systems such as dithiadiazolyls, for which multiple dimer motifs have been documented.8 Radical 3a dimerises in a twisted, suprafacial motif (Fig. 7a) with the two molecules related via a crystallographic 2-fold rotation axis. The two heterocyclic rings are not quite coplanar, exhibiting an interplanar angle of 7.82° and a pair of crystallographically equivalent S1···N2 contacts at 2.866(4) Å (cf. S⋯N contacts of 3.193(4) Å and 3.213(4) Å respectively for Kazsynski's monomeric perfluoro-analogue). Radicals 3c and 3k also adopt dimeric structures with molecules related via a crystallographic inversion centre. In this instance, the inversion centre generates a trans-antarafacial dimer motifs (Fig. 7b) featuring two short identical S1⋯N4 contacts at 3.087(2) Å and 3.010(3) Å for 3c and 3k respectively with parallel heterocyclic rings, whilst 3e dimerised in a trans-suprafacial motif (Fig. 7c) with a single short S1⋯S1′ contact of 2.846(1) Å and a non-parallel arrangement of the heterocyclic rings; the angle between the mean planes of 10.45°.
Computational investigation of these binding modes for the prototypical 3-phenyl benzothiadiazinyl radical found dimerization energies to be −14.3 and −14.8 kcal mol−1 for the trans-antarafacial and trans-suprafacial motifs respectively, approximately twice those seen for the related dithiadiazolyl system31 (see ESI† for details). No energy minimum corresponding to a pancake dimer could be located for the suprafacial binding mode; instead this geometry was found to correspond to a transition state between S–N bonded σ-dimers. This discrepancy between gas-phase calculated geometries and solid-state structure for this orientation of interaction suggests that this mode of dimerisation depends on additional, second–sphere interactions in the solid state; this is unsurprising as pancake bonding is known to be sensitive to subtle changes in structure.3
Magnetism
Of the structurally characterised radicals, only 3c and 3e could be isolated in sufficient purity for magnetic studies, and their magnetic susceptibilities were measured over the temperature range 2–300 K. These confirm that the radicals are diamagnetic in the solid-state, as expected for spin-paired dimers, with no evidence of any magnetic phase transition at high temperature (see ESI†). Broken symmetry calculations32,33 have been successfully applied to a variety of heterocyclic sulphur–nitrogen radicals,34–36 and were performed to explore the nature of the magnetic properties in 3c and 3e (see ESI† for details). For both systems, the calculated exchange interaction between the radical pair is strongly anti-ferromagnetic (J/k = −2629 and −6795 K respectively), consistent with observed spin pairing, net diamagnetism, and the large calculated dimerisation energies. The interaction between neighbouring radicals along the π-stack are also anti-ferromagnetic, albeit significantly weaker. This is in stark contrast to the behaviour seen for related benzothiadiazinyl radicals which retain their paramagnetism in the solid state9,16 and illustrates the sensitivity of these systems to substituents.Conclusions
A new, efficient route to 1,2,4-benzothiadiazine 1-chlorides is reported wherein the use of neat SOCl2 leads to reproducible patterns of chlorination on benzo and methyl substituents. This compact route proceeds directly from easily accessible precursors, with no need for pre-functionalisation of the amidines nor subsequent chlorination. Reduction of the benzothiadiazine 1-chlorides leads to the formation of persistent benzothiadiazinyl radicals which have been characterised in situ by EPR spectroscopy and cyclic voltammetry. The existence of solution-state monomer-dimer equilibria is supported by both thermochromism and voltametric measurements. Four of these radicals have been structurally characterized by X-ray diffraction and exhibit three differing modes of π*–π* pancake bonding. Two of these radicals were of sufficient purity for magnetic measurements, which confirm that they are diamagnetic in the solid state between 2–300 K, with pancake bonding quenching the inherent magnetism of the radicals. This is markedly different from the behaviour seen for related perhalogenated systems and shows that there remains much to be explored for this tunable radical scaffold.Data availability
Crystallographic data for all relevant compounds has been deposited at the CCDC; deposition numbers are listed in the ESI.† All computational parameters are provided in the ESI.†Author contributions
This work was initially undertaken by E. R. C., A. A., and J. M. R. at the University of Cambridge and completed by A. M. B, P. J. S., and E. R. C. at the University of Kent. Project design and oversight was led by J. M. R. and E. R. C. Synthetic work and physical measurements were completed by E. R. C, A. M. B., and P. J. S. with additional crystallographic measurements by A. A. Initial manuscript drafting was completed by A. M. B. with all authors contributing to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the EPSRC and University of Kent for funding, the University of Cambridge for a scholarship (E. R. C.), the University of Kent for a scholarship (A. M. B.), and Homerton College for a Research Fellowship (A. A.).Notes and references
- J. V. Yakhmi, in Handbook of Solid State Chemistry, Wiley, 2017 Search PubMed.
- R. G. Hicks, Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds, John Wiley & Sons, Wiltshire, 2010 Search PubMed.
- M. Kertesz, Chem. –Eur. J., 2019, 25, 400–416 CrossRef CAS.
- O. Anamimoghadam, L. O. Jones, J. A. Cooper, Y. Beldjoudi, M. T. Nguyen, W. Liu, M. D. Krzyaniak, C. Pezzato, C. L. Stern, H. A. Patel, M. R. Wasielewski, G. C. Schatz and J. F. Stoddart, J. Am. Chem. Soc., 2021, 143, 163–175 CrossRef CAS.
- B. D. Koivisto and R. G. Hicks, Coord. Chem. Rev., 2005, 249, 2612–2630 CrossRef CAS.
- J. F. W. Keana, Chem. Rev., 1978, 78, 37–64 CrossRef CAS.
- R. T. Oakley, Can. J. Chem., 1993, 71, 1775–1784 CrossRef CAS.
- M. A. Nascimento and J. M. Rawson, Encycl. Inorg. Bioinorg. Chem., 2019, 1–11 Search PubMed.
- J. Zienkiewicz, P. Kaszynski and V. G. Young, J. Org. Chem., 2004, 69, 7525–7536 CrossRef CAS.
- J. Zienkiewicz, A. Fryszkowska, K. Zienkiewicz, F. Guo, P. Kaszynski, A. Januszko and D. Jones, J. Org. Chem., 2007, 72, 3510–3520 CrossRef CAS.
- E. R. Clark, M. U. Anwar, B. J. Leontowicz, Y. Beldjoudi, J. J. Hayward, W. T. K. Chan, E. L. Gavey, M. Pilkington, E. Zysman-Colman and J. M. Rawson, Dalton Trans., 2014, 43, 12996 RSC.
- A. K. Pal, D. B. Cordes, K. Pringouri, M. U. Anwar, A. M. Z. Slawin, J. M. Rawson and E. Zysman-Colman, J. Coord. Chem., 2016, 69, 1924–1937 CrossRef CAS.
- L. N. Markovskii, V. S. Talanov, O. M. Polumbrik and Y. G. Shermolovich, Russ. J. Org. Chem., 1981, 17, 2338–2339 Search PubMed.
- L. Beer, R. C. Haddon, M. E. Itkis, A. A. Leitch, R. T. Oakley, R. W. Reed, J. F. Richardson, D. G. VanderVeer, L. Beer, J. L. Brusso, A. W. Cordes, R. C. Haddon, M. E. Itkis, K. Kirschbaum, D. S. MacGregor, R. T. Oakley, A. A. Pinkerton and R. W. Reed, Chem. Commun., 2005, 124, 1218–1220 RSC.
- A. a Leitch, R. T. Oakley, R. W. Reed and L. K. Thompson, Inorg. Chem., 2007, 46, 6261–6270 CrossRef PubMed.
- J. Zienkiewicz, P. Kaszynski and V. G. Young, J. Org. Chem., 2004, 69, 2551–2561 CrossRef CAS PubMed.
- E. S. Levchenko, G. S. Borovikova, E. I. Borovik and V. V. Kalinin, Russ. J. Org. Chem., 1984, 20, 176–181 Search PubMed.
- C. F. Marcos, C. Polo, O. A. Rakitin, C. W. Rees and T. Torroba, Angew. Chem., Int. Ed., 1997, 36, 281–283 CrossRef CAS.
- P. Hope and L. A. Wiles, J. Chem. Soc., 1967, 1965–1967 Search PubMed.
- Z. Wang, in Comprehensive Organic Name Reactions and Reagents, 2010, pp. 1395–1398 Search PubMed.
- G. Kresze, A. Maschke, R. Albrecht, K. Bederke, H. P. Patzschke, H. Smalla and A. Trede, Angew. Chem., Int. Ed., 1962, 1, 89–98 CrossRef.
- E. R. Clark, J. J. Hayward, B. J. Leontowicz, D. J. Eisler and J. M. Rawson, CrystEngComm, 2014, 16, 1755–1762 RSC.
- A. G. Orpen, L. Brammer, F. H. Allen, O. Kennard, D. G. Watson and R. Taylor, J. Chem. Soc. Dalt. Trans., 1987, S1–S83 Search PubMed.
- A. W. Cordes, P. J. Hayes, P. D. Josephy, H. Koenig, R. T. Oakley and W. T. Pennington, J. Chem. Soc. Chem. Comm., 1984, 1021–1022 RSC.
- N. Burford, T. Chivers, M. Hojo, W. G. Laidlaw, J. F. Richardson and M. Trsic, Inorg. Chem., 1985, 24, 709–715 CrossRef CAS.
- J. M. Rawson, A. J. Banister and I. Lavender, Adv. Heterocycl. Chem., 1995, 62, 137–247 CrossRef CAS.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- Y. Ji, L. Long and Y. Zheng, Mater. Chem. Front., 2020, 4, 3433–3443 RSC.
- R. T. Boere and T. L. Roemmele, Coord. Chem. Rev., 2000, 210, 369–445 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamental and Applications, John Wiley & Sons, NJ, 2nd edn, 2001 Search PubMed.
- M. A. Nascimento, E. Heyer, R. J. Less, C. M. Pask, A. Arauzo, J. Campo and J. M. Rawson, Cryst. Growth Des., 2020, 20, 4313–4324 CrossRef CAS.
- L. Noodleman, J. Chem. Phys., 1981, 74, 5737–5743 CrossRef CAS.
- L. Noodleman and E. R. Davidson, Chem. Phys., 1986, 109, 131–143 CrossRef.
- S. M. Winter, K. Cvrkalj, P. A. Dube, C. M. Robertson, M. R. Probert, J. A. K. Howard and R. T. Oakley, Chem. Commun., 2009, 7306 RSC.
- S. M. Winter, A. R. Balo, R. J. Roberts, K. Lekin, A. Assoud, P. A. Dube and R. T. Oakley, Chem. Commun., 2013, 49, 1603 RSC.
- D. Bates, C. M. Robertson, A. A. Leitch, P. A. Dube and R. T. Oakley, J. Am. Chem. Soc., 2018, 140, 3846–3849 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2101061–2101078. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc04248c |
| This journal is © The Royal Society of Chemistry 2022 |