Ontological orientations of educators’ sense of the atom and underlying source domains: a case study of Kotebe Metropolitan University, Ethiopia
Abayneh
Lemma
 *a and
Woldie
Belachew
*a and
Woldie
Belachew
 b
b
aDepartment of Chemistry, Fitche College of Teacher Education, Fitche, Ethiopia. E-mail: alsanabbay@gmail.com
bDepartment of Science and Mathematics Education, Addis Ababa University, Addis Ababa, Ethiopia
First published on 3rd June 2022
Abstract
This study aimed to uncover the ontological orientations of educators’ sense of the atom of Kotebe Metropolitan University (KMU), Ethiopia. Thus, an interpretative case study design was employed, with the analytic approach of grounded theory, due to the importance of atomic ontology as a case and the need for an in-depth analysis of their thinking and possible interpretations. The data were collected through a semi-structured interview of eight chemistry educators of the university and coded using Charmaz's approach to coding. The History and Philosophy of Science (HPS) was employed for identifying and sorting the data into the ontological categories, while the theory of experientialism (ToE) was used for locating source domains. Consequently, the interpretative ontological view was traced with two atomic notions: the functional and chemical atomic notions. This means that the educators do believe in the existence of the atom in the interpretative way in which their knowledge is well informed by interpretations and inferences of the experimental evidence and applications of quantum mechanics. However, their narrative lacks some essential priori philosophical arguments and scientific contexts and evidence. As a result, this ‘the latest is the best’ thinking pattern was traced as a major source domain to which the interpretative orientation is attributed.
Introduction
What needs to be taught as a scientific truth or reality has remained so controversial throughout the history of science education. As a result, the essence of some topics was found to be so questionable that their omission from the science curriculum has strongly been claimed (Bensaude-Vincent and Simon, 2008). The atom and its theories are among the debatable topics of science in the history of chemistry and its education (Taber, 2003; Erduran, 2014). The most controversial and problematic aspect is its ontology (Taber, 2003; Bensaude-Vincent and Simon, 2008; Matthews, 2011). Atomic ontology, in this sense, is concerned with the issue of whether an atom exists as a fundamental reality or not. Four principal categories of ontological views can be traced from the history of chemistry and its education: hypothetical, mechanical, interpretative, and operational ontology (Chalmers, 1999; Bensaude-Vincent and Simon, 2008).As can also be realized from Table 1, the hypothetical perspective rejects the existence of an atom with different positions and scopes of its essence in the science curriculum. Thus, anti-atomism and agnostic atomism are accordingly shared within this ontological position. Anti-atomists do not agree with the existence of the atom and the essence of issues such as atomic theories in science education. Agnostic atomists prefer to avoid considering or thinking about the atom. They were historically known for deliberately neglecting these controversial issues of the atom and trying to explain the nature of matter and its phenomenon in terms of other variables such as energy. Some claim that the chemists’ atom does not refer to any real particle of matter and, therefore, argue that atomic theories are not worth being learned, while others philosophically rejected the atom, but chemically support the inclusion of its topics as theoretical synthesis (Chalmers, 1999; Bensaude-Vincent and Simon, 2008; Matthews, 2011). August Kekulé's reflection (apud Bensaude-Vincent and Simon, 2008, 190), quoted below, is a typical example of the latter. He scientifically supported the essence of atomic theories, while philosophically rejecting the existence of the atom.
| Important aspects | Ontological positions | |||
|---|---|---|---|---|
| Hypothetical | Mechanical | Interpretative | Operational | |
| Philosophical basis | Anti-realism; idealism | Naïve or direct realism | Scientific realism | Operational realism; “chemists’ realism” |
| Claimed existence | An ideal entity beyond human experience; that is not worth learning | A physically or mechanically existing and perceptible object of different nature (size, color, odor, etc.) | An invisible region or area holding the already discovered and verifiable sub-atomic particles; a projected model of the area holding electrons, protons, and neutrons of different quantity | An unobservable, chemically indivisible, and fundamental constituents of matter that can be quantified; indirectly counted |
| Corresponding atomistic view | Anti-atomism; agnostic atomism | Corpuscularism or mechanical atomism; kinetic atomism | Kinetic atomism; electrical atomism | Functional atomism: Sole core of phenomenal relationships; chemical atomism: Co-core of phenomenal relationships |
| Common metaphors and/or analogies | Ball, billiard ball; block; brick; ball | A region; field, cell; house; building | Cell; sole actor; co-actor; chemical mediator | |
… from a philosophical point of view, I do not believe in the actual existence of atoms, taking the word in its literal signification of indivisible particles of matter. As a chemist, however, I regard the assumption of atoms to be not only advisable, but absolutely necessary in chemistry.
Though he has not been regarded as anti-atomist by historians and philosophers of science, especially those from France, Jean-Baptiste Dumas's once utterance, on the other hand, is a popular quote that portrays the earlier position against the existence of the atom and its essence in science education. During his lectures at the College de France in 1836, he uttered that:
If I had my way, I should erase the word ‘atom’ from science, in the firm belief that it goes beyond the realm of experiment; and never in chemistry must we go beyond the realm of experiment (Dumas, 1836 apud Bensaude-Vincent and Simon, 2008, 180).
Chemists and philosophers of chemistry such as Pierre Duhem and Wilhelm Ostwald have been advocating the agnostic notion. They tried to examine and explain the chemical phenomena mathematically. Accordingly, they were able to predict whether a given reaction could proceed or not, and the conditions in which it would take place. This intent has led to the foundation of the interpretative notion of the atom as a diffused and quantized electrical region (Jensen, 2010).
The mechanical view, on the other hand, acknowledges the atom as a physically existing and perceptible object of different nature. The atom, from this ontological perspective that once originated in the classical era of Greek philosophy and was revived in the 17th century, is treated as a mechanical entity that possesses properties such as color, taste, acidity, hotness, and even coldness (Jensen, 2010). The early studies of Isaac Newton and Robert Boyle correspond to such a mechanical entertainment of the atom. Newton introduced the role of force in this corpuscular treatment of the atom in which the world was investigated and described in terms of the shapes, sizes, and motions of particles (Jensen, 2010). This is when the kinetic atomism emerged within the realm of mechanical ontology and made its way to Dalton's first theory of mixed gases (Rocke, 1984). Such a mechanical and kinetic perspective created critical insight into experimentation. The resulting laws and theories were also found to work very well in explaining the properties of atoms and simple molecules. However, such an ontological realm of the atom could not enable us to understand and explain the existence of atoms and their role in the formation and properties of chemical substances. This entertainment of atoms as balls or objects of different shapes, sizes, and colors – if not accurately communicated within its scope – could also lead to one of those persistent misconceptions in which atoms are considered to have macroscopic properties such as color, appearance, hardness, and the likes (Jensen, 2010).
The interpretative ontology is rooted in scientific realism in which the atom is ontologically acknowledged as a mind-independent and unobservable reality of the world. Besides, scientific realism epistemologically asserts that science as one's process of knowing and knowledge of the atom does not represent as it is, from a God's Eye position (Evangelopoulos, 2013). By ‘God's Eye’ position, it means that one cannot conceive the atom as a truth independent of his/her human perspective including socio-cultural experiences and resulting conceptual scheme. This view supports the inclusion of the atom not as an observable object, but ontologically as an unobservable reality and epistemologically as a theoretical entity (Bensaude-Vincent and Simon, 2008). Thus, the conceptualization of the atom as reality would not be mind-independent and free from historical and socio-cultural contexts though (Vihalemm, 2012).
This ontological perspective is more associated with the kinetic and electrical notions of the atom. The kinetic notion, in such an interpretative sense, is concerned with the inside of the atom in which its internal structure was able to be explained in terms of the motion of electrons while the atom, in general, is explained as a quantized electrical region in the electrical atomism (Jensen, 2010). Alternatively, the operational ontology asserts that something whose quantity can be, directly or indirectly, determined does exist (Bensaude-Vincent and Simon, 2008). It is based on stoichiometric evidence from chemical analysis and employs scientific laws such as the Avogadro principle and the mole concept to compute and claim quantity and, thus, ontology of atoms in a given sample based on the resulting values. Thus, it is also known as stoichiometric ontology (Chalmers, 1999; Bensaude-Vincent and Simon, 2008; Matthews, 2011). The synthesis of such an ontology first emerged as a critic of the deliberate neglect of agnosticism, of which the following quote from Emile Meyerson could be a typical instance. Such arguments led to the synthesis of operational ontology in which the atom was even able to be used as both an actor of chemical phenomena and a mediating concept of phenomenal relationships.
In his heart, Kekulé believed firmly in the existence of atoms, molecules and bonds, manipulated them exactly as if they were objects that could be experienced with unaided senses (Meyerson, 1911 apud Bensaude-Vincent and Simon, 2008, 191).
This ontology constitutes two atomistic views: functional and chemical atomism. Functional atomism acknowledges the atom as the sole core of phenomenal relationships, while chemical atomism claims an equivalent ontology for ions and molecules, especially in terms of fundamentality and natural existence (Taber, 2000).
The empirical literature, on the other hand, shows that some naïve ideas and misconceptions have been persistently diagnosed within the conceptualization of the atom and related topics. A cross-age study conducted on Turkish students’ mental models of the atom, for instance, reported that much of the representation manifests the classical Democritus’ notion in an ontologically problematic style (Gökdere and Çalik, 2010). Taber (2003) also indicated that the ‘everything made up of atoms’ notion is still implied by the curricular, pedagogical, and learners’ senses of the atom. Other studies have found that curricular and pedagogical concepts of the atom are oversimplified and contradictory to the 21st-century's experimental evidence (Justi and Gilbert, 2000; Niaz and Coştu, 2009; Gökdere and Çalik, 2010; Erduran, 2014). Abayneh (2013) has also reported that eighth-grade students and their chemistry teachers have the kind of problematic conceptions that British middle school students had four decades ago. In this diagnostic study, he found that the students and their chemistry teachers believe that the atoms of gold are yellow and shiny.
Bachelard (1968 apud Taber, 2003) suggested that the source domains of these persistent pre-conceived ideas and alternative conceptions could also be associated with a range of epistemological profiles of the scientists and experienced professors themselves. According to him, they have different epistemological profiles for which they were found to usually use contradicting and multifaceted styles of thinking and interpretations of those dimensions of reality of the atom. Taber (2003) claimed that such styles could potentially be misleading to learners and readers. In an illustration of his case on nucleophilic substitution of halogenoalkanes, he argued that expert chemists’ ‘habit’ of referring to reacting species such as chloride ions and leaving groups such as bromide ions unintentionally as an atom is so misleading to novices and learners that it implicitly endorses the ‘everything made up of atoms’ notion. These expert chemists were also cited in his case for calling molecules, such as hydrogen (H2) and nitrogen (N2) molecules, as atoms in explaining molecular structure in organic chemistry.
This means that what has been said and meant, even by experienced professors, chemists, instructors, educators, and teachers, do significantly differ (Taber, 2003). Two ontologically and conceptually wrong messages are depicted in such narratives. These are ideas about atoms having macroscopic properties and everything being formed from atoms. The first is associated with the mechanical ontological view and atomic notion (Jensen, 2010), while the latter is associated with the functional atomic notion (Taber, 2003). Lemke (1990), in this regard, stressed that science disciplines such as chemistry have a typical nature and, therefore, the pattern in their writing and talking needs to be understood by educators, teachers, and students. Such cases do all indicate that those persistent pre-conceived ideas and alternative conceptions had become hindrances to the teaching and learning of the related fundamental and advanced concepts (Taber, 2003; Erduran, 2014). Bachelard (1968 apud Taber, 2003) referred to them as epistemological obstacles, while Taber (2003) discussed them as learning impediments. Others, such as Erduran (2014) and Kiray (2016), implicitly addressed these attributes as cardinal problems.
Ahead of thinking about the possible measures, it is therefore essential to analyze what, how, and why such experienced educators think about the atom. The core issues that need to be addressed within this analysis are the ontological orientations and potential source domains to which the ontological orientations are attributed. It was therefore aimed in this study to uncover the ontological orientations of educators’ sense of the atom of Kotebe Metropolitan University (KMU) and the source domains to which their orientations are attributed through the following research questions.
(a) How is the atom ontologically manifested within the educators’ sense?
(b) Why are the educators thinking about the atom the way they are thinking?
Methodology
Research design
This study is concerned with understanding and describing the educators’ sense of the atom and the underlying source domains. By ‘source domains’ we mean the basis to which the educators’ thinking and interpretations are attributed. Atomic ontology is the case to be investigated within this sense of the atom. A case study was employed due to the importance of the issue and the need for in-depth analysis and thick description. Such an understanding, however, demands analyzing informants’ senses or thinking with a critical consideration of all the possible interpretations at the participants’, researchers’, and readers’ levels. Furthermore, the case was chosen not because it was so specific, nor because of people, programs, or phenomena. It is rather because it remained so controversial in the literature. Besides, it has been widely found in the literature in association with potential learning complications and difficulties in secondary and higher chemistry education.Among the three approaches to the case study design, Merriam's qualitative case study is the one that can fit the requirements of both circumstances (Merriam, 2009). Typical sampling, data collection, and analytical features of the grounded theory design were also integrated to maintain transferability (Charmaz, 2006).
Research settings
Kotebe Metropolitan University is the second-oldest public university in Ethiopia. It began in 1959 as the Kotebe College of Teacher Education of Haile Selassie I University. In 2014, it became Kotebe University College. In 2016, it was re-established as a Metropolitan University in the capital of the country with the primary aim of addressing the human resources of the city.At the time we are processing this article, we have learned that the university is once again being re-established as Kotebe Education University. This makes it the only educational university in the country. Currently, there are 16 academic members of the Chemistry Department, seven of whom are PhD holders. Three of the remaining nine lecturers (MSc holders) have their bachelor's degree (BEd) in chemistry education, while the other BSc holders do not. Of the seven PhD holders, one had his PhD in chemistry education. Most of the general and specific pedagogical content knowledge, practicum and teaching practice courses for pre-service teachers, and training and workshops for in-service teachers are handled by three lecturers and one PhD holder with a teaching educational background (BEd and PhD in chemistry education). All of them took the one-year Higher Diploma Program (HDP) training and are certified as Professional Teacher Educators. In this research, we decided to refer to all the teaching members of the department as “educators”, since all of them have been giving courses for both pre-service teachers and non-teaching. This number is greater than the number of educators in the departments of those four universities (Hawassa, Bahir Dar, Jimma, and Mekelle University) in which the new “Doctor of Education” (DEd) was launched in 2020 for educators in the regional colleges of teacher education. It is even greater than the corresponding value for the oldest and the only university in the country that offers a Doctor of Philosophy (PhD) program in Science and Mathematics Education, Addis Ababa University.
As a result, it is a place where educators’ rich experiences are found. Teacher education was not being offered consistently in other higher education institutions in the country, including the oldest, Addis Ababa University. But in Kotebe, it has been run almost consistently without such interruptions, since it offered pre-primary. It was able to win this reputation and social trust by being seen as the originator of more competent and disciplined teachers throughout the country. Being a graduate of the university remains such a worthwhile quality for getting accepted into many governmental and private schools.
Participants
The university, KMU, was selected due to the aforementioned consistency and rich experience in teacher education in Ethiopia. It is the only university in the country that is currently offering all modalities (pre-primary, environmental science and mathematics, integrated science and chemistry) and levels (pre-primary, lower primary, upper primary, secondary, and preparatory teacher education). Hence, purposive sampling was employed to select the university in general and its chemistry educators in particular. Upon communicating with the department head, those available educators were asked for their permission to take part in the study and provided with a preliminary survey. In this survey, information related to educational and professional profiles, including qualifications, specializations, teaching experience, and courses they frequently taught, was acquired. The educators were also asked for their willingness to take part in the study and permission to request and access their official profiles from the department. Besides, issues such as qualifications, specialization, experience, and teaching exposure to the selected courses (Chem 1011, Chem 2031, and Chem 3101) were asked about in the survey. Of the 14 educators who were accessed and asked to fill out the survey, 11 were able to complete and return the survey, in which they agreed to take part. Necessary profiles of the educators were also obtained from the department and triangulated against the findings from the survey. All of them are male. But, they have different specializations, teaching experiences, and exposure to the selected courses. The resulting information was summarized without violating the code of confidentiality (Table 2).| No. | Assigned namea | Code of the audio file (s) | Qualification | Teaching experience (years) | Exposure to the courses |
|---|---|---|---|---|---|
| a Assigned names are pseudo names assigned by the authors to keep anonymity. | |||||
| 1 | Leta Kebede | E6209.m4a/R120103-001 | BEd, MSc | Between 6 and 10 | Higher |
| 2 | Addisu Tadesse | E6210.m4a/R120103-002/14 | BSc, MSc | Between 6 and 10 | Higher |
| 3 | Belay Demie | E6211.m4a/R120103-003 | BEd, MSc | More than 10 | Moderate |
| 4 | Akmel Seid | E6213.m4a/R120103-004 | BSc, MSc | Below 5 | Lower |
| 5 | Kinfe Desta | R120104-001/12 | BSc, MSc, and PhD | Between 6 and 10 | Moderate |
| 6 | Tasisa Gonfie | E6213.m4a/R120103-006 | BSc, MSc, and PhD | Between 6 and 10 | Moderate |
| 7 | Temesgen Getahun | E6215.m4a/R120103-007 | BSc, MSc | Below 5 | Lower |
| 8 | Tura Abdisa | E6215.m4a/R120103-008 | BEd, MSc, PhD | More than 10 | Moderate |
Then, three educators with typical specializations and exposure to those courses were purposely selected for the initial data collection and analysis. As a result, an initial coding scheme was developed. The interview protocol was accordingly revised to include more probing cases. Accordingly, the profiles of the remaining educators were re-examined with which they were identified and designated as targets of average/normal, unique/exceptional, and, lower/higher characteristics. In general, eight (educators) were interviewed.
The remaining educators were categorized as per their field of specialization, exposure to the selected courses, overall experience, and experience in teacher education. Hence, the next interviewee was the one with an opposite magnitude of teaching experience, exposure to the selected courses, and different areas of specialization, in cases of overlapping themes and sub-themes. An educator of a similar or average profile was, on the other hand, selected in cases of different and emerging themes and sub-themes. This method of simultaneous data collection and analysis was used until the eighth interview when the major themes and sub-themes were found to be saturated.
Instruments
A semi-structured interview is the major data-gathering instrument of the study. First, two central issues were identified from the literature and used as leading questions in the initial data analysis. These are the nature and constituents of the world. Eleven open-ended items were formulated and organized into five themes based on those findings from the literature on diagnostic studies, HPS, HPS based case studies, and interventions, as well as that of the initial analysis. These themes are “views on reality in chemistry”, “constituents of the world,” “sense of the atom,” “reflection on learning experiences (as a student and pre-service teacher)”, and “teaching experience (as a teacher and an educator).” In addition, two probing cases were prepared for the third and fourth categories each (Appendix A). These items are concerned with issues such as what and how things are made of; senses and mental models of atoms; teaching-learning experiences; grade level they were introduced to the issue of atoms; the context in which they constructed such mental models; their belief in the relevancy of the contents under study; the nature of the selected substances at the microscopic and macroscopic levels.This interview protocol was prepared by the first author and reviewed by the second author (dissertation supervisor). Next, it was reviewed by three more experts, two chemistry teacher educators, and one English language educator. In addition, a preliminary survey was prepared and administered to first obtain necessary information on the educators’ academic and professional profiles, and willingness to take part in the study.
Data collection and analysis
Interviewing, transcription, and coding were carried out almost simultaneously. Each educator was communicated with and briefed on the scope, purpose, and impact of the study; his privilege, responsibility, and risk in participating in the study; and the right to withdraw from the interview anytime he felt he had to. Before starting every interview, it was therefore made sure that he knew all that he had to do and agreed to proceed. All interviews are of the one-to-one interview type and were audio-recorded. Each recorded audio was first organized, transcribed, and documented using a default code given by the recorder. This was followed by an iterative reading and coding of segments that seemed significant in relation to the research questions. Addressing the first research question involved identifying, examining, and sorting any segment that seemed significant for the ontological themes: hypothetical, mechanical, interpretative, and operational ontology. Hence, we used HPS as a framework to identify, examine, and sort significant segments as codes. To address the second research question, we used experientialism as a framework as it offers a philosophy-driven approach for tracing source domains in terms of the educators’ experiences and resulting thinking (Yu, 2013; Amin and Jepsson, 2015).As suggested in Merriam's approach to the qualitative case study (Merriam, 2009), Charmaz's (2006) approach to coding was employed. Much of the analysis was carried out using NVivo, version 12. It involved three stages – initial, focused, and theoretical coding. At the initial stage, the transcripts were read, again and again, from which any significant segment was identified and organized separately for each research question. World views, figurations, styles, and argumentations of typical implications were specially considered. Basic assumptions about the existence and nature of the world were treated as world views. Choice of words and syntax were considered as style, while narrative and essayistic expressions were dealt with under figuration. Expressions, claims, interpretations, and justifications were accordingly identified and examined under argumentation (Ornatowski, 2007).
In the focused coding, the segments of each category were re-examined in which some irrelevant codes were omitted and some codes of greater concern were selected, labeled, and defined under which the remaining were sorted. In the theoretical coding, a given sub-theme was selected and interpreted in as many ways as possible. Each possible interpretation was then taken and checked in an abductive way with the codes of sub-themes of other categories. This was carried out to figure out the association between every educator's atomic notion, implied ontological position, and his thinking in the form of source domains. So, segments corresponding to the root sources of the interviewees’ overall thinking were identified and examined in relation to the second research question as “source domains.”
This was done by taking all the sorted codes of one interviewee at a time. Next, one of the ontological sub-themes was picked from which major codes were identified. Each one of these major codes was taken and compared against the major codes of the sub-themes of the second research question. This was done for the remaining ontological sub-themes too. The comparison and tracing of underlying associations continued for all interviewees. Then, major associations from the cases of all interviewees were compared with each other from which the most plausible ones were identified as the causes of the diagnosed atomic notions and the implied ontology.
The entire interviewing and analysis of the resulting transcripts was an iterative process. Accordingly, some educators were revisited more than twice, misunderstandings were being cleared, and the interview protocol was being modified. Some important probing cases and questions were, for example, incorporated into the former interview protocol (Appendix A (Part II-3 and II-4)). Some of the initial themes were omitted, and a few sub-themes were merged with others. The initial coding scheme (Fig. 1) is also modified from which the finalized codebook (Appendix B) was exported.
In accordance with the requirements of ethical and quality issues, five educators were contacted back with findings on major themes and associations. It was also attempted to obtain feedback and confirmation on those major findings and the resulting implications. Concerning the ethical requirements, the quotations were taken in a very generic way so that the interviewees could not be identified by the statements discussed in the findings. We also used pseudo names both in the table summarizing their profiles and citation of their quotes. In addition, fields of specialization were also shaded in Table 2 to reduce their vulnerability.
Results
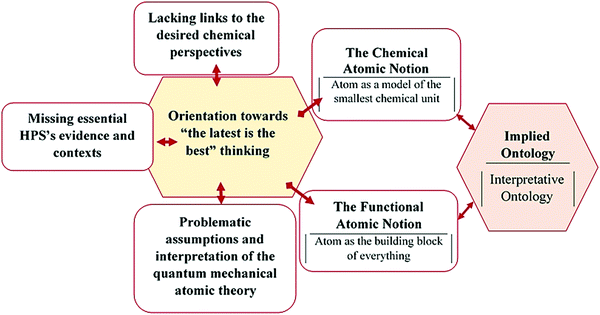
Three ontological themes were identified first as a result of the initial data collection and analysis. The themes were named “interpretative ontology”, “chemical ontology” and “assumed ontology”. Of the three, only the first belongs to the four ontological themes identified from the literature. Regarding the second, there has not been such an ontological view as “chemical ontology” in the literature. We just preferred to name and keep it so as not to lose those significant segments of the transcripts, which were identified in this initial analysis. Much of the data on this theme match the desired conception of chemical atomism. But, it lacks the very operational rationale for the claimed existence of the atom. The “assumed ontology” is also the theme that seemed to emerge in this analysis. Similar cases were also found within the category of the second research question. For example, it first seemed to us that few educators held the ontological positions, implied by their sense of it, because they think it should be part of their professional commitment or religious affiliation (Fig. 1).However, a single major theme was found for each research question as a result of the continued iterative interviewing, analysis, revisions, and modifications. Besides, a few more sub-themes were found in the cases of both research questions (Appendix B). The resulting findings were organized and discussed as follows in the order of the research questions. The first part corresponds to the atomic notions and implied ontology. The next part is dedicated to potential source domains to which the diagnosed atomic notions and implied ontology are attributed.
Implied ontology
It was generally found that all educators believed in the existence of the atom. No implication of anti-atomism or agnostic atomism was found at all. The implications of their sense of the atom were found to converge on a single ontological position, though it seemed to be of three types in the initial analysis. Thus, interpretative ontology is the only one implied by the educators’ sense of the atom.This means that they all acknowledge the atom as a hypothetical region that comprises protons and neutrons at its densest center and an electron cloud. According to them, this is the region in which the sub-atomic particles are held and interact accordingly. The atom, in this sense, is the smallest chemical unit that can take part in a chemical reaction. It is, however, not visible or detectable either with our bare eyes or through the aid of a microscope. Thus, the region to which they were referring is an imaginary region in which those “experimentally discovered and verifiable” sub-atomic particles are found. Temesgen Getahun (name changed) is one of the educators who claimed such invisibility and mode of existence.
But, there is a region, an abstract region. But, it is known. Like that, the magnet physically occupies only a certain limited space. But, the field goes beyond that until some imaginary limit. The field applies until that limit.
In much of the narrative, terms such as “abstract” and “magnetic field” were used to refer to the invisibility and imperceptibility of the atom. Accordingly, this imaginary region is figuratively addressed in terms of the boundary on which a magnetic field applies. Though they are invisible too, the sub-atomic particles were addressed by all the interviewees as something that can be indirectly tested, verified, and felt. Temesgen continued as follows to address this perceptibility.
Similarly, there is a region in which electrons, protons, and neutrons of a given atom operate. It has its limit. If an electron, for example, goes beyond that limit, it will be detached from that region. Hence, it is that imaginary region that is called the atom. We are not referring to a physical boundary or physical object. It is just like orbitals where the probability of finding an electron is high. That is the area allocated for a given atom. If there was no such region, the sub-atomic particles couldn’t be defined and located. Because the sub-atomic particles don’t exist discreetly.
According to this quote, the atom is just an imaginary region, a region projected either logically from the mutual existence of the “already discovered sub-atomic particles”, or mathematically from the plots of wave values such as Schrödinger's calculations. Either way, the atom is referred to as a “region” of limited boundary in which electrons, protons, and neutrons can co-exist. It was also made clear that such a boundary should not be considered as a “physical” or “objective”. Thus, the atom and its essence in science education are acknowledged within the sense of this category as a model of interpretations of values computed as wave functions of electrons. Belay Demie is an educator, with moderate exposure to the selected courses and more than ten years of experience in teacher education, whose explanation is found to portray this ontological orientation.
Being aware of the discovery and existence of the electron, and able to discover the nucleus, he [Rutherford] finally introduced a model of a two-region atom for the first time. Therefore, the wave nature of electrons was studied in detail through the Schrodinger equation. As a result, the location of electrons within each orbital was projected in the form of regions where the probability of finding an electron would be high.
Thus, from all these, we can assert the existence of the atom. Accordingly, I believe that atoms do exist and all the discoveries made so far and discussed are indicators of this existence.
In general, two atomistic views were found within such an interpretative ontological portrayal of the atom. These are the chemical and functional atomic notions. These are discussed as follows, along with typical evidence quoted from the analyzed transcripts.
As you know, many don’t exist in pure or elemental form. Only a few are found in free form. Most of them are found in the form of complex compounds on the earth's crust. When you dig somewhere you can get some of these in the form of rock. Most are found in the form of rock. When you go up to the atmosphere, all those gases are found in the form of a solution; air is a homogeneous mixture. You can’t find the atoms freely. It is therefore a solution.
In the probing discussion of NaCl salt, the majority of the interviewees made it clear that the salt would be the one that was created first and, therefore, found naturally in this world rather than its atomic (Na-atom and Cl-atom) or molecular (Cl-molecule or Cl2) counterparts. The following is just one example, quoted from Temesgen's view, among the many segments of such implications.
You can’t find sodium or chlorine atom in free form. Thus, the compound (NaCl) is the fundamental and the one that would be created first.
This is one of the key features targeted in the most recent, and the preferred atomic notion, chemical atomism. The epistemic justification and reflection located within the interviewees’ sense were, however, found to miss much of the philosophical account of operational ontology. That justification and those reflections from the interviewees are of the kind of interpretative ontology. Such senses of the atom cannot ontologically fit into the desired account of the philosophy of chemistry though this feature of acknowledging the status of ions and molecules and the chemical indivisibility of the atom is very chemical. This sense of imaginariness was found to also be strongly implied by the following excerpts from the transcripts of Kinfe Desta, another educator with moderate teaching experience and exposure to the selected courses.
Hence, it is that imaginary region that is called an atom. We are not referring to a physical boundary or physical object. It is just like a body of nucleus at the center and orbitals where the probability of finding an electron is high. That is the area allocated for a given atom. If there was no such region, the sub-atomic particles couldn’t be defined and located. Because the sub-atomic particles don’t exist discreetly.
It can also be realized from much of the data that this notion portray a model of a region of the nucleus and atomic orbitals because their descriptions constitute much of the attributes of Rutherford's nucleus and Schrödinger's orbitals. It denotes a region that is ideally shaped into a spherical denser core and corresponding structure of the orbitals. Many more segments of such implications were identified from the data of which the one from Leta is quoted as follows as one more example.
Individuals constructed different models; one of these is the quantum–mechanical model. Let us leave the details; but, it talks about a denser nucleus at the center and an electron cloud. It claims an electron doesn’t have an exact position; it has only a region where the probability of finding it is high.
Ok, an atom, for me is a building block of everything. Atom is the smallest particle from which matter is made.
Such segments of the data are linguistically clear and explicit in explaining how those substances, such as water and table salt, can be formed through the reaction of constituent elements. But, much of the argumentation is not found in the context and evidence of the scientific investigation, especially for reactivity and natural occurrence. Akmel Seid has the lowest experience in both the exposure to the selected courses and teacher education. Despite being able to address the reactivity attributes associated with atoms of those reactive elements such as chlorine and sodium, he concluded his reflection on the probing case of fundamentality and natural existence of NaCl salt as:
What I think is that the atoms were created or existed first. I mean atoms of sodium and chlorine were created first. The NaCl salt was then formed through the reaction of the two atoms. That is my hypothesis.
Such segments of the data imply a completely different sense of fundamentality and natural existence. Contrary to the previous atomic notion, it does not acknowledge the molecular and ionic occurrence of atoms of the majority of the elements. There are a few more pieces of data that do sound even more mechanical. The following excerpt from Akmel's elaboration of his view on reality in chemistry could be interpreted into such connotations.
This cloth [pointing to his jacket] is made of atoms of cloth. This chalk [pointing to a white chalk box on his table] is also made up of those tiny particles of chalk called atoms. So, we can say atoms exist.
Addisu Tadesse is the other participant with moderate teaching experience (6 to 10 years) as a teacher educator and higher exposure to the selected courses. Much of his narrative belongs to this sub-theme of the atomic notion. The following excerpt from his narrative not only explains the aforementioned features of the functional notion but also prescribes an atomic model that matches the hypothetical hybrid version of the historical models of the atom.
From Thomson, we have electrons. From Rutherford, we know about the nucleus that comprises protons and neutrons. Bohor enabled us to know about the orbits while we know about orbitals from Schrodinger. This means that we can understand and illustrate what the atom looks like by combining all these discoveries and features.
Source domains
Those segments associated with the interviewees’ thinking of the atom did at first seem to be linked to numerous attributes, including the curricula, adequacy of the instructional approaches and materials, educators’ awareness, and concern about the essential evidence and contexts of HPS and religious affiliation. However, the implicit rationale behind all these segments was found to be a single pattern of thinking. It is something similar to the old ‘the latest is always the best’ thinking.The precision of the latest theory in explaining the world, in general, is better than that of its earlier versions. This does not mean that the older versions are totally wrong. Some could be, some could not be. The practice of philosophy of chemistry and corresponding theories such as the chemical theory of the atom emerged before that of quantum mechanics. Underlying chemical approaches such as gravimetric analysis and principles such as Avogadro's law still work well with a variety of applications. The evidence from such analyses and computations forms the rationale and justification for such a practical philosophy of chemistry, called operational realism. It asserts that “something quantifiable does exist.” In addition, the identification of the nature of a given atom and its pre-planned manipulation in a chemical reaction is the other way of both observing reality in action and making it appear as a result of the reaction.
Quantum mechanics does not disprove such an existence and quantification of the atom; it rather elaborates the nature and structure of the atom. It was founded on and acknowledges essential context, arguments, and evidence of HPS. Conversely, the connotation in all those justifications sounds as if the quantum mechanical atomic theory is complete enough to solely enable us to address all those issues related to the atom. This is how the educator with moderate exposure to the selected courses and higher exposure to professional courses of teacher education, Tasisa Gonfie (name changed), started justifying his claimed existence as follows.
Currently, we are following the quantum theory, after the discovery of the electron, proton, nucleus, and neutron.
Such a maxim of “the newest is always the best” can also be realized from another narrative of the atom and atomic theories. It is quoted by Leta, the educator with a BEd background in chemistry education. According to him, the quantum mechanical model of the atom is the “new science that asserts the atom as an idea.” This addresses the quantum mechanical theory of the atom with some revolutionary sense as if previous discoveries and evidence would be disregarded.
So, this quantum mechanical model or the new science asserts the atom as an idea. The currently agreeable working science is this until it is disproved. What we, therefore, are using to explain this is the atom. If enough research will be conducted on it, and it will be disproved and decided to be changed, it will be changed. But, it is what we are using until then. This implies that things are made of atoms.
The problem with such narratives is that most of them sound as if the previous theories’ evidence, discoveries, and propositions are completely wrong and should be replaced. There was no remarkable reference to those desired historical and philosophical evidence and contexts. Besides, some parts of those “latest” or “modern” theories could be conceptually problematic. Here is an example quoted from Belay's sense of divisibility.
In modern atomic theory, what we are using is this divisibility concept. There are sub-atomic particles.
The problem with this excerpt is that an atom is not chemically divisible. The discovery and existence of sub-atomic particles by themselves do not imply the chemical divisibility of the atom. Atoms can be disintegrated into sub-atomic particles in the form of rays only by nuclear approaches. Such divisibility is, however, not chemical. This means that specific observations and evidence from a few experiments were interpreted according to the quantum mechanics and solely used to explain issues such as chemical divisibility, fundamentality, and existence. Explaining and justifying such issues of potential ontological implications cannot be explained in terms of the sizes, speed, and collisions of the atoms or molecules. It demands evidence from HPS and related experiments that are informed by theories of a chemical nature. Thus, the narrative, in general, appeared to lack an adequate link to or consideration of the very chemical perspective in general and some essential evidence and contexts of HPS in particular. The findings of this study, in general, can be summarized as follows in terms of the atomic notions, implied ontology, and underlying source domains (Fig. 2).
Discussion
Philosophers of science in general, and chemists in particular, have taken a variety of positions, ranging from anti-atomism to the very operational chemical atomism (Bensaude-Vincent and Simon, 2008). Jean-Baptiste Dumas (1800–1880) and August Kekulé (1829–1896) are among those who were against the existence of the atom and stood differently regarding its essence in science education. Other philosophers and chemists, such as Pierre Duhem (1861–1916) and Wilhelm Ostwald (1853–1922), avoided thinking about the atom and the essence of its education and tried to mathematically explain the nature and phenomena of matter. This corresponds to the agnostic ontological view (Bensaude-Vincent and Simon, 2008). On the other hand, John Dalton (1766–1844), Charles Adolphe Wurtz (1817–1884), and Jacob Berzelius (1779–1848) are among the prominent chemical atomists who were able to operationally demonstrate the existence of the atom (Rocke, 1984). Their scientific journey made its way to the emergence and advancement of the chemists’ version of reality, operational ontology, as a chemically produced, manipulated, and demonstrated reality (Rocke, 1984; Bensaude-Vincent and Simon, 2008).Historians of science and chemistry concluded, in this regard, that almost all the chemists and philosophers of chemistry believe in the existence of the atom, though their underlying lenses and justifications diverge (Rocke, 1984; Bensaude-Vincent, 1999; Bensaude-Vincent and Simon, 2008). Similarly, the literature on chemistry education indicates that chemistry educators, teachers, and even undergraduate science students believe in the existence of the atom and its essence in science education in one way or another (Taber, 2003; Gökdere and Çalik, 2010; Erduran, 2014; Perez et al., 2016; Wiener, 2020). Their narratives have been reported to be under-rooted in a variety of ontological and epistemic positions, most of which are conceptually and pedagogically problematic. Hence, the findings of this study are consistent with those findings from both the philosophical and educational literature. The findings in terms of the first sub-theme of atomic notion, chemical atomism, are also somewhat different and, in fact, better aligned with the key features of chemical atomism. Much of the educators’ sense belongs to the category of this sub-theme. It is good that this notion of the atom acknowledges arrays of ions and discrete molecules as naturally existing constituents of matter. Thus, it ascribes to the type of atom that Taber (2000) proposed as a fundamental co-constituent (with ion and molecule) of matter. It portrays a model that is desirably more oriented towards chemical atomism than the hypothetical hybrid (Justi and Gilbert, 2000; Taber, 2003; Perez et al., 2016) and mechanical (Taber, 2003; Gökdere and Çalik, 2010; Niaz and Maza, 2011; Perez et al., 2016) images of the atom found in the literature. The attributes identified within this notion portray a kind of orbital model that Kiray (2016, 158) and Wiener (2020, 70) identified from their participants’ drawings.
The finding in terms of the second sub-theme of the atomic notion, functional atomism, is consistent with the one prescribed by the expert chemists’ jargon discussed in Taber (2003). Everything is attempted to be explained in terms of the atom in such a notion. The portrayed image, however, is the usual hypothetical hybrid model that was reported in different diagnostic, curriculum, and related document analyses (Taber, 2000, 2003; Gökdere and Çalik, 2010; Niaz and Maza, 2011; Perez et al., 2016). The description in general was found to comprise attributes from two or more historical models. This type of model, for example, constitutes a denser core marked with small circles from Rutherford, the orbitals K, L, M, N… from Bohr, and dumbbell-shaped orbitals from the quantum mechanical model.
With respect to the second research question, the educators’ frequent references in their arguments to the chronological narration of atomic theories in existing textbooks, references, and syllabuses, as well as their teaching-learning experiences, almost coincide with the findings of those curricular and related document analyses (Justi and Gilbert, 2000; Taber, 2003; Niaz and Maza, 2011; Perez et al., 2016). Beyond this, the literature does not offer much in the case of the source domains. So, there is not much available to discuss where these findings as source domains lie. However, what we found can be better explained by the following quote from the Stanford Encyclopedia of Philosophy (2014, 1).
By contrast, the knowledge of atoms that is now taken for granted in modern science is not established by a priori philosophical argument but by appeal to quite specific experimental results interpreted and guided by a quite specific theory, quantum mechanics.
A similar pattern can be noted from Bachelard's (1968 apud Taber, 2003) discussion of the “pre-conceived ideas and assumptions” as source domains of “epistemological profiles” of the experienced educators, which in turn, become “learning impediments” to their novice audiences, students. According to experientialism, such ideas and assumptions about the world at such an unobservable scale are highly influenced by the bodily interactions and experiences one had with the physical and socio-cultural environments (Yu, 2013; Amin and Jepsson, 2015). That is why experientialism is also known as embodied realism (Amin and Jepsson, 2015). As already addressed in the Introduction section, such a cause-and-effect interaction is also well acknowledged both in scientific and operational realism. Because both assert that one cannot conceive the atom as a truth independent of his/her prior experiences, thinking patterns, and assumptions (Vihalemm, 2012; Evangelopoulos, 2013).
This means that one's process of knowing and knowledge of the atom are influenced by his/her prior experiences and resulting thinking, corresponding philosophical and theoretical orientations as a scientist's planning of an experiment and interpretation of the resulting data are informed by his/her epistemic profiles and corresponding philosophical and theoretical orientations (Taber, 2003; Banchetti-Robino, 2020). So, it is most likely that the educators’ ontological stance is attributed to their philosophical and theoretical assumptions. In that case, we can claim that the thinking pattern we found as a source domain is consistent with such philosophy-driven analyses, including Bachelard (1968 apud Taber, 2003), Stanford Encyclopedia of Philosophy (2014), and Taber (2003). However, it is not yet the time to make this conclusion as there is still much to be known and negotiated. Accordingly, prioritizing such philosophy-driven studies and dialogues would be very valuable.
Summary and implications
We aimed in this study to carry out an in-depth analysis of the educators’ sense of the atom and identify their ontological orientations. It was also aimed to identify potential source domains and corresponding associations with the diagnosed atomic notions and implied ontological view. Basically, we found that all the educators, like the chemists, philosophers of chemistry, chemistry professors, and educators from the literature, believe in the existence of the atom. We also found that all the educators believe in such an existence in almost the same interpretative way. They only differ in the atomic notions implied by their sense as two sub-themes of atomic notions were located within the interpretative realm of their thinking. These are the chemical atomic notion (chemical atomism) and functional atomic notion (functional atomism). The first notion is being highly advocated as per the operational philosophy of chemistry and its education, while the second notion was found in the literature to be substantially associated with the ontologically-invalid “everything made up of atoms” idea.In accordance with the second research question, we found that getting very much oriented towards “the latest is the best” thinking is the core source domain or reason behind such an ontological orientation of the educators. As a result, the educators are inspired by and take these successes in discoveries and the application of quantum mechanics for granted as a sole means of justifying the existence of the atom. The educators’ narrative, in general, is fairly aligned with the practice, context, and evidence of the experimental inquiries, especially in the case of chemical atomism. The accuracy in terms of style and figuration was also found to be substantially promising. Because much of it is free of the jargon found in the literature. But, the arguments, claims, and justifications lack the very chemical perspective of essential contexts and evidence of the philosophical and scientific history of the atom and atomism.
What, therefore, can be implied by these findings is that they believe in the existence of the atom and its essence in science education in the interpretative sense that did not entertain some key contexts and evidence from HPS. Being refrained from rushing to premature judgments and conclusions, we believe that, at least, there have to be ways of reminding or exposing the educators to these essential cases, contexts, and evidence of HPS, in which communicating these findings to them by itself is the basic suggestion. Arranging departmental seminars, in which these argumentative and contradicting cases from the HPS will be discussed, could be another alternative. Most importantly, we do feel that more inquiries into educators’, teachers’, pre-service teachers’, students’, and the curricular sense of the atom need to be conducted to have enough understanding and evidence needed to make informative decisions.
Conflicts of interest
This is one part of a PhD dissertation that the first author has been working on with the guidance and support of the second author as a supervisor. The entire PhD study is sponsored by the cooperation and agreement of Addis Ababa University and the Education Bureau of Oromia Regional State, Ethiopia. We, the aforementioned authors, therefore declare that neither the university nor the Bureau has any significant competing financial, professional, or institutional interests that might have influenced the publication of this article in this journal or somewhere else.Appendix A
Interview protocol(The final version)
Interviewee's code______________ Status/rank____________ Specialization__________
Professional experience as educator_______ Professional experience as instructor______
Date of the interview______________ Place_____________ Time_____________________
Part I: Introduction
Thank you for being willing and coming! As already mentioned in the written consent, the purpose of this interview is to collect information and figure out how the atom can be better conceptualized in a way that fits available experimental evidence of this century. I'm looking into your idea and illustration of the atom, its interpretation, corresponding rationale, and reflection on related experiences.
Upon your permission, I am going to use an audio recorder so that I can get all the details, make sure that I am catching you up, and have an attentive conversation with you.
Part II: Interview questions
1. Getting to know each other
I am hoping that you could start by telling me a bit about yourself: who you are, where you attended your undergraduate and postgraduate studies, how long have you been teaching, course (s) you enjoyed teaching the most, and your previous/future plan [reminding that his/her information is secure with me].
2. Reality
2.1. Let me start with the very idea of reality or ontology in its philosophical sense; what does reality mean to you?
2.2. How about reality in chemistry [consider for example molecules, air, dust, samples of metals, and isomers] in relation to reality in biology [such as cells, organs, organism, osmosis, diffusion, and virus]; or in physics [such as force, light, wave, particles, and power], or in social sciences [such as an idea, humanity, and honesty], or mathematics [such as point, numbers, and geometric objects]?
3. Constituents of the world
3.1. What do you think the world, and the things within it, are made of?
3.2. Let us, on the other hand, consider the [mechanical, quantum mechanical, and chemical] divisibility of things around us. Does it have a limit? What will be obtained at the end?
Probing: let us for example, consider table salt or sodium chloride; we all learned in chemistry that it is formed by reactions of reactants comprising sodium and chlorine as a result of the electrostatic attraction between sodium (Na+) and chlorine (Cl−) ions. But, consider the very first creation of things in this world:
(a) which one do you think was created first? Is that the salt (NaCl), the individual ions (Na+ and Cl−), or the individual atoms (Na and Cl), which appeared or existed first in this world?
(b) Which one is then the fundamental entity?
4. Sense of atom
4.1. What does ‘atom’ mean to you?
4.2. Are atoms real? Do atoms exist?
4.3. How do you think one can know this existence or reality?
Probing: Based on your views of the existence of the atom:
(a) If you think atoms exist, how do you think their existence can be better shown, justified, or conceptualized to students during the teaching–learning process?
(b) If you think atoms do not exist, what is then the essence of contents such as atomic theories, particulate nature of matter, and electronic structure of the atoms in Chemistry education?
5. Learning experience
5.1. Try to remember the first time you were introduced to the idea of ‘atom’ in your learning experience. When and in what grade and subject was it? What idea or view did you make of it? What mental image of the atom did this experience leave with you?
5.2. Which of your learning experiences did influence you the most in constructing your current senses of ‘atom’, ‘what and how the world is made of’, and ‘the limit to the divisibility of matter’?
6. Teaching experience: the literature as well as the data I collected so far substantially imply that secondary and undergraduate students still possess the classical mechanical notion of the atom, while emerging orientations towards agnostic and anti-atomism have also been traced. The implications in either way are not in alignment with the scientific evidence and the very philosophical account of chemical atomism.
6.1. Why do you, as an educator/instructor, think the students construct such senses of the atom?
6.2. How do you think the issue of the atom, atomic theories, and related contents should be conceptualized to address the desired ontological shift and optimize chemical atomism?
Part III: Closing
We are done with the questions. I appreciate your generous willingness, commitment, and contribution and, therefore, would like to express my heartfelt thanks! Finally, I would like to hear from you about your feeling about our discussion and/or some remarks you may want to add.
[Listening to the interviewee's final remarks; appreciating and cheering.]
Appendix B
| The Final coding manual of the analysis | |
|---|---|
| Name | Description |
| Sense of the atom and implied ontology | RQ 1: how is the atom ontologically manifested within the educators’ sense? |
| Interpretative ontology | This is a major ontological theme that acknowledges the atom as an interpretative model of all the evidence in quantum mechanics. The atom is an ideal or hypothetical region comprising protons and neutrons in the nucleus and electron clouds projected based on the wave properties of the quantum mechanical theory of the atom. |
| Atom as the smallest chemical unit | This is the sub-theme of atomic notion or atomism. The atom, in this sense, is an interpretative model of the smallest chemically-indivisible unit that can freely exist in nature in the cases of noble gases and inert metals, but cannot exist freely in nature in the case of the remaining elements. |
| Atom as the building block of everything | This is another sub-theme of atomic notion or atomism. Atom in this sense is the building block of everything. It implies that atoms of all elements were created first, ahead of their molecular and ionic forms of matter. All atoms are also supposed to exist discretely in the nature. |
| Ways of knowing and corresponding source domains | RQ 2: why are the educators thinking about the atom the way they are thinking? |
| Ways of knowing and knowledge | A sub-category of the second research question that is associated with all those claimed ways of knowing and knowledge of the atom. It comprises all the segments of the data and themes with significant epistemological implications. |
| Experiential inference | Refers to an epistemological sub-theme in which the existence of the atom is logically inferred from: (a) electron, proton and neutron were already discovered, (b) can be felt and used through different applications such as X-ray, NMR, ultrasound and the likes, and only co-exist in the unobservable region (c) so, the atom does exist as this unobservable region. |
| Quantum-mechanical projection | Refers to an epistemological sub-theme in which the existence of the atom is quantum-mechanically projected – comprises segments of the data implying any implicit or explicit claim and justification for the existence of the atom in terms of all those evidence, computations, projections, and applications of the quantum mechanical theory. |
| Traced source domains | Another sub-category of the second research question that refers to the roots, sources, origins, reasons, or root causes that were identified from the educators' senses of the atom and its ontology. |
| The chronological conception of the atomic theories | This theme is associated with pieces or segments of the data associated with the chronological presentation of the atomic theories. |
| Sole interpretation of evidence of few experiments, based on few theories | It corresponds to any segment of the data (initial codes) that implies the quantum mechanical atomic theory as the latest and complete without enough link to the other perspectives. |
| Missing philosophical perspectives | HPS's evidence and contexts. |
| Missing chemical perspectives | Stoichiometric and analytical perspectives of the atom and its application in other fundamental and advanced topics and courses of chemistry. |
Acknowledgements
We would like to express our deepest gratitude and appreciation to our former instructor and advisor, Temechegn Engida (PhD), for raising the issue and for the generous guidance he has been offering since the beginning of our postgraduate study. We also thank Addis Ababa University for creating this opportunity and providing us with the necessary support. Most importantly, we thank the educators of Kotebe Metropolitan University (KMU) for believing in the very purpose and contribution of this study and for being willing to take part in such a prolonged interview.References
- Abayneh L., (2013), A diagnostic assessment of eighth-grade students and their teachers’ misconceptions about basic chemical concepts, Afr. J. Chem. Educ., 3(1), 39–59.
- Amin T. and Jepsson F., (2015), Conceptual metaphor and embodied cognition in science learning: Introduction to special issue, Int. J. Sci. Educ., 37(5–6), 745–758.
- Banchetti-Robino M. P., (2020), The Changing Relation between Atomicity and Elementarity: From Lavoisier to Dalton. Florida Atlantic University, Oxford Scholarship Online, 87–108, Viewed 25 March 2021, https://oxford.universitypressscholarship.com/view/10.1093/oso/9780190933784.001.0001/oso-9780190933784-chapter-6.
- Bensaude-Vincent B., (1999), Atomism and positivism: A legend about French chemistry, Ann. Sci., 56(1999), 81–94.
- Bensaude-Vincent B. and Simon J., (2008), Chemistry: The impure science, London: Imperial College Press, Viewed 12 October 2019, https://www.researchgate.net/publication/50411935_Bernadette_Bensaude-Vincent_and_Jonathan_Simon_Chemistry_The_Impure_Science/link/593ff8c40f7e9bf167f1ce6d/download.
- Chalmers A. F., (1999), What is this thing called Science? 3rd edn, Hackett Publishing Company Inc., Viewed 21 October 2018, https://silo.pub/download/what-is-this-thing-called-science-an-assessment-of-the-nature-and-status-of-science-and-its-methods.
- Charmaz K., (2006), Constructing Grounded Theory: A Practical Guide through Qualitative Analysis, SAGE Publications, Viewed 20 March, 2020, http://www.sxf.uevora.pt/wp-content/uploads/2013/03/Charmaz_2006.pdf.
- Erduran S., (2014), A holistic approach to the atom in school chemistry, Educ. Quím. Educ., 19, 39–42.
- Evangelopoulos G., (2013), Scientific Realism in the Philosophy of Science and International Relations, A Thesis Submitted for the Degree of Doctor of Philosophy at the London School of Economics and Political Science Department of International Relations, Viewed 20 November, 2020, http://etheses.lse.ac.uk/691/1/Evangelopoulos_Scientific_realism_philosophy_science.pdf.
- Gökdere M. and Çalik M., (2010), A cross-age study of Turkish students’ mental models: An “atom” concept, Didactica Slovenica – Pedagoška Obzorja, 25(2), 185–199.
- Jensen W. B., (2010), Four centuries of atomic theory: An overview, in Giunta C. J. (ed.), Atoms in Chemistry: From Dalton's Predecessors to Complex Atoms and Beyond, ACS Books, Washington, DC, pp. 7–19.
- Justi R. and Gilbert J. (2000), History and philosophy of science through models: Some challenges in the case of 'the atom, Int. J. Sci. Educ., 22(9), 993–1009 DOI:10.1080/095006900416875.
- Kiray S. A. (2016), The pre-service science teachers’ mental models for the concept of atoms and learning difficulties, Int. J. Educ. Math., Sci. Technol., 4(2), 147–162.
- Lemke J. L., (1990), Talking Science: Language, Learning, and Values, Ablex Publishing Corporation, Viewed 05 March 2020, https://files.eric.ed.gov/fulltext/ED362379.pdf.
- Matthews M. R., (2011), Alan F. Chalmers: The scientist's atom and the philosopher's stone: How science succeeded and philosophy failed to gain knowledge of atoms, Sci. Educ., 20, 173–190.
- Merriam S. B., (2009), Qualitative research: a guide to design and implementation (Revised and expanded from Qualitative research and case study applications in education), San Francisco: The Jossey-Bass Higher and Adult Education Series, Viewed 25 September 2020, http://eds-courses.ucsd.edu/tep288a/shortbook.pdf.
- Niaz M. and Coştu B., (2009), Presentation of atomic structure in Turkish general chemistry textbooks, Chem. Educ. Res. Pract., 10, 233–240.
- Niaz M. and Maza A., (2011), Nature of Science in General Chemistry Textbooks, Springer, Viewed 23 May, 2020, http://www7.uc.cl/sw_educ/educacion/grecia/plano/html/pdfs/biblioteca/LIBROS/LibNiazMaza.pdf.
- Ornatowski C. M., (2007), Rhetoric of science: Oxymoron or tautology? The Writing Instructor, 1-15, September 2007, Viewed 20 June 2020, https://files.eric.ed.gov/fulltext/EJ824640.pdf.
- Perez E. A., Guidote A. M., Yu G. U. and Mariano M. E., (2016), Content analysis of the discussion of the atom in general chemistry textbooks using evaluation criteria based on the nature of science and the philosophy of chemistry, Kimika, 27(2), 50–62.
- Rocke A., (1984), Atomism in the Nineteenth Century: From Dalton to Cannizzaro, Ohio: Ohio State University Press, Viewed 12 May, 2021, https://digital.case.edu/islandora/object/ksl%3Ax633gj985.
- Stanford Encyclopedia of Philosophy, (2014), Atomism from the 17th to the 20th Century, Viewed 13 Sept, 2020, https://plato.stanford.edu/entries/atomism-modern/.
- Taber K. S., (2000), Teaching chemistry without (too much emphasis on) atoms? Royal Society of Chemistry Teacher Fellowship Discussion, Education-line, London, October 2000, http://www.leeds.ac.uk/educol/documents.
- Taber K. S., (2003), The atom in the chemistry curriculum: Fundamental concept, teaching model or epistemological obstacle, Found. Chem., 5, 43–84.
- Vihalemm R. (2012), Practical realism: Against standard scientific realism and anti-realism, Stud. Philos. Estonica, 5(2), 7 – 22.
- Wiener J., (2020), Science teachers' conceptions of atomic models, Eur. J. Math. Sci. Educ., 1(2), 67–80.
- Yu X., (2013), What are the metaphors we live by? Theory Pract. Lang. Stud., 3(8), 1467–1472.
| This journal is © The Royal Society of Chemistry 2022 |