Pedagogical chemistry sensemaking: a novel conceptual framework to facilitate pedagogical sensemaking in model-based lesson planning
Meng-Yang M.
Wu
 and
Ellen J.
Yezierski
and
Ellen J.
Yezierski
 *
*
Department of Chemistry and Biochemistry, Miami University, Oxford, Ohio, USA. E-mail: yeziere@miamioh.edu
First published on 18th January 2022
Abstract
Researchers have typically identified and characterized teachers’ knowledge bases (e.g., pedagogical content knowledge and subject matter knowledge) in an effort to improve enacted instructional strategies. As shown by the Refined Consensus Model (RCM), understanding teacher learning, beliefs, and practices is predicated on the interconnections of such knowledge bases. However, lesson planning (defined as the transformation of subject matter knowledge to enacted pedagogical content knowledge) remains underexplored despite its central position in the RCM. We aim to address this gap by developing a conceptual framework known as Pedagogical Chemistry Sensemaking (PedChemSense). PedChemSense theoretically expands upon the RCM that generates actionable guidelines to support chemsistry teachers’ lesson planning. We incorporate the constructs of sensemaking, Johnstone's triangle, and the models for perspective to provide a lesson-planning mechanism that is specific, accessible, and practical, respectively. Lesson examples from our own professional development contexts, the VisChem Institute, demonstrate the efficacy of PedChemSense. By leveraging teachers’ sensemaking of the limitations and utility of models, PedChemSense facilitates teachers’ designing for opportunities to advance their students’ chemistry conceptual understanding. Implications and recommendations for chemistry instruction and research at secondary and undergraduate levels are discussed.
Introduction
A vignette of chemistry teaching and learning
Within a secondary chemistry classroom, students view a particulate-level animation of silver chloride precipitation. The teacher pauses the video iteratively, drawing students’ attention to and narrating key events of the reaction. After cycles of clarification and discussion, students then externalize their mental models through drawings and words. What emerges is a collection of precipitation representations in which non-spectator ions form a lattice and spectator ions are solvated by bulk water with appropriate dipole orientations. There are also lines trailing from chemical species, giving this momentary snapshot a dynamic flair of motion and interaction.Although students may be successfully attending to this system's description, there is something noticeably—if not alarmingly—absent. Mechanistic features, the how and why that explain precipitation, are less salient among students’ representations. While student reasoning may be present as evidenced by their descriptive labeling of precipitation features, the extent of student sensemaking via explaining precipitation remains unclear. This scenario raises compelling questions: How can we as chemistry education researchers and teachers better support students’ explanations to deepen their chemistry understanding? What is the distinction between reasoning and sensemaking? How should teachers plan for learning opportunities that advance students’ chemistry understanding?
The vignette illustrates a tension that resonates with our agenda and informs recommendations for advancing chemistry instruction at the secondary and introductory college levels. While others in chemistry education may investigate this context with a lens attuned to the learner, as researchers and professional developers, we focus our attention on the educator. Our ongoing collaboration and reflection with secondary chemistry teachers have enabled new insights to theoretically reframe how we should support their pedagogy and the development of their students’ conceptual chemistry learning.
The reality of using the pedagogical content knowledge model
A popular and robust framework to analyze teacher learning, beliefs, and practices is Shulman's (1986) pedagogical content knowledge (PCK) model. As a response to Rodriguez and Towns’ (2019) recommendation for more specific, accessible, and practical implications for enacted teaching practices, chemistry education researchers using the PCK model typically attend to the many professional knowledge bases that instructors possess. Marzabal et al. (2018) for instance list a criteria of content knowledge bases related to chemical kinetics to assist higher education instructors in responding to their students’ ideas. Hale et al. (2016) have developed an instrument to measure graduate teaching assistants’ knowledge bases about thin layer chromatography. Ekiz-Kiran et al. (2021) showcase a PCK-enriched course in which their pre-service chemistry teacher participants developed deeper knowledge of their learners and of instructional strategies.Scholars who characterize instructors’ knowledge bases often use specific types of data sources. Content Representations (CoRes), Pedagogical and Professional experience Repertoires (PaP-eRs), and/or results from an instrument have traditionally been used given their utility (Loughran et al., 2004; Mavhunga and Rollnick, 2011; Großschedl et al., 2019). Contextualized in teacher professional development (PD) and related initiatives, these knowledge bases-related teacher artifacts are also common for PCK analyses. Bertram and Loughran's (2012) two-year longitudinal ethnography with primary and secondary teacher participants found that CoRes and PaP-eRs are worthwhile tools for recognizing and understanding PCK. Gess-Newsome et al. (2017) studied the impact of their two-year professional development program for secondary biology teachers by measuring the growth of multiple knowledge bases with a validated instrument. Finally, Williams and Lockley's (2012) action research showed that combining CoRes with long-term secondary teacher/researcher collaboration can enable greater uptake of teaching and content knowledge bases.
Although CoRes, PaP-eRs, and instruments are beneficial for analysis with the PCK model, we recognize that PD work with secondary teacher populations require certain conditions. Studies that primarily use these data sources may have longer PD hours, a smaller sample size, and/or greater convenience of face-to-face participant interaction. Unfortunately, such prerequisites may be difficult to achieve for those who implement intensive PD for in-service secondary teachers sampled from across the US. One limitation is that conducting intensive PD during the academic year is unfeasible due to scheduling conflicts. Because PD facilitators and researchers must resort to the summer, obtaining artifacts related to teachers’ enacted practices becomes difficult. Remote implementation of PD, as recent circumstances have demanded, can further limit the availability of teacher data sources.
Alternatively, one potentially more accessible artifact given this context is a teacher's lesson plan. Lesson plans have greater precedence in other areas of teacher reform research beyond the PCK model. There exist instruments like SLPAIR which can evaluate lesson plans (Herrington et al., 2012) and rubrics like EQuIP to facilitate lesson plan development (NGSS, 2014; NGSS, 2016). Lesson plans can reveal large gaps between what is planned to be taught versus what is to expected to be taught at specific grade levels (Kellamis and Yezierski, 2019). However, teachers’ lesson plans are neither directly reflective of teachers’ knowledge bases nor their enacted teaching practices. Situated within the PCK literature, what teachers can do (i.e., their plans to teach) currently serve more for triangulating claims about what teachers know (i.e., their knowledge bases). We nevertheless assert that the absence of lesson plans as a primary data source given the PCK model is problematic. Those who occupy realities of facilitating remotely-delivered, intensive PD for in-service secondary teachers—just as we do—need to accordingly adapt.
The purpose of this paper is to offer a new conceptual framework known as Pedagogical Chemistry Sensemaking (PedChemSense) that enhances the analytical value of lesson plans within the PCK model. We establish our theory in chemistry education by delving into an underexplored PCK construct known as transformation, the mechanism that converts chemistry-specific subject matter knowledge to enacted pedagogical content knowledge. PedChemSense thus articulates guidelines for teachers to transform their chemistry understanding into teaching practices that advance their students’ chemistry sensemaking and explanations. By addressing the processes of how knowledge bases can be interconnected, we build upon the identification and classification that prior PCK-related studies have done. As a response to Rodriguez and Towns’ (2019) recommendation for greater specificity, accessibility, and practicality, PedChemSense draws from multiple theories as well as examples from our own PD program, the VisChem Institute, to yield recommendations for how secondary chemistry teachers can plan to advance their students’ chemistry understanding.
Background
Pedagogical content knowledge and subject matter knowledge
The PCK model was initially developed from Shulman's (1987) critique of the standards that policymakers and teacher educators had towards the “acceptable” quality of teaching, only requiring what Shulman lists as “basic skills, content knowledge, and general pedagogical skills” (p. 6). Shulman asserts that the “teacher has special responsibilities in relation to content knowledge, serving as the primary source of student understanding,” thereby establishing the expectation that an educator should be well-versed in both knowledge bases of discipline-specific content and pedagogy (p. 9). Teaching begins as a process of reasoning in which teachers must leverage “their knowledge base to provide the grounds for choices and actions” for the performance of teaching itself (p. 13).For this paper, we limit our focus on two of the broad knowledge bases: subject matter knowledge (SMK) and PCK. As operationalized in Shulman (1986), SMK is a teacher's capability of defining to students the accepted tenets of a domain, explaining why such ideas are warranted and relevant, and relating content to theory and practice both within and outside the discipline. SMK in our work would refer to the teacher's familiarity with chemistry concepts. Using the precipitation vignette, SMK would include the orientations of solvating water molecules and the rapid ion-pair formation of the lattice. Pedagogical content knowledge or PCK on the other hand is defined as “subject matter knowledge for teaching” (p. 9, emphasis in original). PCK is the teacher's capability to make the subject matter comprehensible for others by strategically selecting compelling forms of representations, analogies, demonstrations, and examples. Extending the vignette example, the teacher's PCK would lead to playing an animation, knowing key segments to highlight to visualize the particulate level, and eliciting students’ ideas and observations.
Although Shulman had originally classified SMK and PCK as being discrete, researchers who began using the PCK model realized its complexity and the blurriness between these two knowledge bases. Kind (2009) reports that the results from multiple studies have led to inconsistencies and disagreements about the PCK model such that, at the time this review was conducted, there was no overriding consensus. Some studies have concluded that the boundary between SMK and PCK should be entirely effaced. Teaching and scholarship were argued to be intertwined, that all SMK is fundamentally pedagogical in nature (McEwan and Bull, 1991; Segall, 2004). Other researchers follow Shulman's original conceptualizations and assert that SMK is distinct from PCK. Also known as the transformative model, this stance assumes that there is some mechanism which enables interaction and conversion between SMK and PCK (Grossman, 1990; Magnusson et al., 1999). Finally, there are those who have a blended interpretation of PCK, known as the integrative model. Gess-Newsome (1999) states that PCK subsumes SMK and other factors such as classroom context, suggesting that the former is fundamentally the knowledge that teachers possess and leverage within their classrooms.
The transformative model is typically considered for subject-specific contexts (Kind, 2009). Given our chemistry-specific focus—exemplified by the VCI's use of molecular-level visualizations—we follow this precedent established in the literature. In addition, the integrative model lacks a mechanism (and thus explanatory power) for how SMK can be converted to PCK, which the transformative model assumes (Abd El-Khalick, 2006). We thus respond to Kind's (2009) call for identifying a process of PCK development by narrowing our theoretical scope on the transformation of SMK into PCK.
Transformation situated in the refined consensus model
The notion of a mechanism which converts SMK to PCK was originally mentioned in Shulman's (1987) work as transformation. Shulman introduces transformation as a component of pedagogical reasoning, a process in which teachers identify the set of ideas to be taught and leverage their experiences to inform the choices and actions they take within the classroom. Transformation—a crucial step during said connection—is defined by Shulman as thinking “one's way from the subject matter as understood by the teacher into the minds and motivation of learners” (p. 16). Mavhunga (2016) similarly describes transformation as the pedagogical conversion and bridging of content knowledge for a particular topic, emergent from teachers interacting and understanding specific content knowledge components. For the purposes of this paper, we also interpret pedagogical reasoning as the broad connection between different knowledge bases (e.g., SMK to PCK).The transformative model of PCK interestingly underwent its own transformation throughout the years. Changes were eventually adopted after multiple findings have broadened, challenged, and refined the model's theoretical boundaries, culminating into what is now known as the Refined Consensus Model (RCM) (Hume et al., 2020). Shulman himself had offered insights on how PCK should be re-conceptualized. For example, the initial PCK model focused heavily on the cognitive attributes of the teachers to the extent that affective aspects of teacher understanding and action may have been overshadowed (Shulman, 2015). This over-reliance on the cognitive aspect is further stressed by Shulman's noting that the original PCK model did not “attend sufficiently to pedagogical action” (p. 10, emphasis added). Unsurprisingly, enacted PCK (ePCK) now exists as the RCM's hub of knowledge base connections.
Although the RCM is an updated and expanded version of the original PCK model, we observe that pedagogical reasoning—and embedded within it, the transformation—persists as a central component. In other words, a mechanism in which SMK is converted to ePCK is still theoretically relevant. However, transformation currently lacks information regarding practical implementation. To address this gap, we use PedChemSense as an additional theoretical lens to improve transformation's specificity, accessibility, and practicality. We first differentiate reasoning from sensemaking by comparing their definitions and describing why their distinction is important to increase transformation's specificity. Second, we use Johnstone's triangle, an accessible model of representation that has paradigmatically driven chemistry education research, to describe PedChemSense's mechanism. We finally offer a practical method for transforming teachers’ SMK to ePCK by applying the models for perspective to PedChemSense, highlighting how situating learning around the limitations of the triangle's vertices can foster deeper understanding.
Pedagogical chemistry sensemaking as a conceptual framework
On specificity: reasoning vs. sensemaking
PedChemSense requires simultaneously understanding reasoning and sensemaking from two perspectives: from the teacher's and from the student's. While reasoning/sensemaking of curricular design/pedagogy and of chemistry concepts are separate processes, we demonstrate that the two are intertwined, requiring precise definitions. For clarification purposes, pedagogical reasoning and pedagogical sensemaking refer to the teacher. Reasoning and sensemaking refer to the student. Finally, the term “PedChemSense” refers specifically to our conceptual framework as a lesson-planning tool for chemistry educators at secondary and early college levels.As previously described by the RCM, transformation is a component of pedagogical reasoning. Based on the PCK literature, we interpret pedagogical reasoning as the connection of teachers’ professional knowledge bases during lesson-planning. Student reasoning is also similarly defined as the connection of concepts throughout chemistry and science education literature. Taber (2019) for example conveys that learners draw upon a vast conceptual net of various linkages when engaging in reasoning processes. Another process known as productive reasoning has been operationalized as the understanding and application of structure–property relationships for explaining and predicting the behavior of chemical phenomena (Cooper et al., 2013; Maeyer and Talanquer, 2013). Even the National Research Council's (2012) Framework for K-12 Science Education and the Next Generation Science Standards (NGSS) call for the integration of content knowledge.
Sensemaking on the other hand originates from Dewey's (1997) work in which meaningful learning occurs by experiencing complex and confusing problems that initially raise doubt. Sensemaking is thus using scientific ideas and past experiences to figure out complex phenomena that may conflict with current mental schemes (Schwarz et al., 2016). Doing so requires identifying and working through the incongruities in one's understanding to develop more cohesive knowledge structures (Phillips et al., 2017). Pedagogical sensemaking consequently requires teachers’ creating moments of uncertainty within the classroom (Manz, 2015). A teacher who plans for sensemaking should strategically plan which ambiguities and decisions their students will encounter (Manz and Suárez, 2018). Unlike sensemaking that deals primarily with chemistry concepts, pedagogical sensemaking is teachers’ planned and enacted practice of responding and adapting to emergent student ideas—what Russ and Berland (2018) call a disciplined improvisation.
Transformation under the umbrella of pedagogical reasoning currently lacks detailed instructions for how and why chemistry teachers can and should transform their SMK to ePCK during their lesson-planning. We accordingly view pedagogical sensemaking as a more specific solution for the chemistry discipline. Manz and Suárez (2018) state that sensemaking as a pedagogical construct involves teachers figuring out how to warrant chemistry knowledge for learning. For PedChemSense, teachers use their own chemistry-specific SMK to create a need to know for their students, thereby informing ways to introduce uncertainties. Teachers simply asking students how or why questions related to chemistry phenomena may be insufficient as it may incentivize the latter's pattern recognition in lieu of sensemaking (Tang et al., 2020). Furthermore, by ignoring or discouraging moments of uncertainty in the classroom, students’ understanding may be treated as deficient and/or incomplete (Larkin, 2012).
Researchers in teacher reform have empirically demonstrated the efficacy of pedagogical sensemaking. Oliveira et al. (2012) find that teachers can influence their students’ authentic co-construction of knowledge by purposefully capitalizing on moments of ambiguity. Chen et al. (2019) add that the enacted practices of raising, maintaining, and reducing uncertainty are productive for students’ learning insofar that the problematizing of the phenomenon is authentic, meaningful, and ambiguous enough. Teachers’ incorporating uncertainties in their curriculum can also encourage greater student participation and understanding of a specific discipline's practices (Engle, 2011; Reiser, 2004). Based on prior work with sensemaking as a teacher construct, PedChemSense offers teachers specific ideas about coordinating how and why students learn chemistry. To address accessibility, we employ Johnstone's triangle as the conduit in which teachers can convert SMK to ePCK for their students’ chemistry-specific understanding.
On accessibility: Johnstone's triangle
We use Johnstone's triangle to frame how SMK should be transformed into ePCK for chemistry classrooms. Johnstone (1982) described that learning chemistry encompasses representations at three different levels: the macroscopic, the symbolic, and the submicroscopic or particulate. These three different levels not only attest to the complexities of chemistry learning itself but also imply that comprehensive chemistry understanding requires connection of one level to another (Taber, 2013). Specifically, Johnstone's triangle can enable learners to engage in modelling practices as they use the different levels to visualize the unobservable, describe complex relationships, and overcome spatial and temporal restrictions (Bussey and Orgill, 2015).The representation of chemistry knowledge as at least three distinct levels and its implications have indubitably become a paradigmatic driver of chemistry education research (Talanquer, 2011). For example, Seethaler et al. (2018) developed a rubric to assess the extent to which general chemistry textbooks can support students in navigating the representational levels of Johnstone's triangle. Edwards and Head (2016) designed a lesson plan around Johnstone's triangle, part of which encourages students to organize index card-sized pictorial representations along a triangular shaped continuum with the vertices labeled “macroscopic,” “particulate,” and “symbolic.” There have also been studies that have recommended an additional dimension such as the human element or a level more relevant to biochemistry for greater breadth of understanding (Towns et al., 2012; Sjöström and Talanquer, 2014). The work demonstrated from prior studies support Johnstone's triangle as an accessible model to organize how chemistry teachers can undergo PedChemSense during their lesson planning. Although the triangle has been expanded (Mahaffy, 2006), we limit our framework to the macroscopic, particulate, and symbolic levels to enhance secondary teacher usability.
When comparing the chemistry education work with Johnstone's triangle to the PCK literature, we detect a fascinating parallel. Transitioning between levels and/or promoting comprehension at multiple levels as evidence for chemistry expertise is the primary focus for most, if not all, studies inspired by Johnstone's triangle. We thus interpret Johnstone's triangle to be primarily used for promoting students’ reasoning, evidenced by their connection of different representational levels when explaining chemistry phenomenon. Because of the implicit weight on reasoning with Johnstone's triangle, we advocate for theoretical expansion using PedChemSense. We assert that while Johnstone's triangle possesses an almost ubiquitous presence throughout chemistry education literature, its utility for chemistry sensemaking and pedagogical sensemaking is largely underexplored at both secondary and early college levels.
Johnstone's triangle is essentially a composite of models at various representational levels. Models are the functional units of scientific thought and are integral to day-to-day scientific activities (Giere, 1988; Nersessian, 2002). Furthermore, explanations, data visualizations, and experimental measurements are inherently limited (Latour, 1987). Researchers constantly negotiate with uncertainty in their everyday practices (Allchin, 2012). However, a novice perception of a model oftentimes is dissimilar to an expert's, resulting in an ongoing issue of students’ assuming that models perfectly correspond to chemistry phenomena (Morrison, 2015). To emulate authentic scientific practices of working with uncertainty, we recommend that chemistry teachers frame their SMK-ePCK transformation around Johnstone's triangle. Considering the built-in limitations at each vertex, there is an opportunity for teachers to undergo PedChemSense for their students’ sensemaking at the macroscopic, symbolic, and particulate levels. We now shift to the models for perspective to practically demonstrate what PedChemSense with Johnstone's triangle entails.
On practicality: the models for perspective
Modelling is a process that constructs abstracted representations of the various features within a science concept or phenomenon (Krajcik and Merritt, 2012). Studies in chemistry education that focus on modelling can include sketching the dissolving of a sodium chloride lattice in water (Cooper et al., 2017), building three-dimensional molecular representations of chloroethanol or dodecaborane (Moreno et al., 2018), or creating graphs of reaction rates (Rodriguez et al., 2020). However, models can serve a much greater purpose beyond representation. This treatment of solely depiction would ignore the ways in which a model is sensitive to context and how a model should actually be used (Knuuttila, 2011). Gouvea and Passmore (2017) accordingly recommend shifting attention away from the model's structure and towards its function for explaining.As the names suggest, the models of perspective emphasizes description while the models for perspective hones in on the fidelity of how a model communicates casual mechanisms. The latter stance subsumes the former, implying that a learner would identify what a model is characterizing before discerning its limitations and utility for providing a specific explanation. The models for perspective would then position learners as epistemic agents who actively decide “in what respects and degrees the model represents features of some phenomenon” as well as “the knowledge the model is intended to generate” (Gouvea and Passmore, 2017, p. 53). Learners would thus be incentivized to enact generative and evaluative practices with respect to scientific knowledge (Giere, 1988). Chinn and Buckland (2012) argue that generation and evaluation of models are essential for learners’ conceptual learning.
Using Johnstone's triangle, leveraging a models of versus a models for perspective leads to starkly different teaching implications. A models of perspective would result in pedagogy that stimulates student reasoning employing all three levels in Johnstone's triangle (see Fig. 1). Essentially, students would identify how their models correspond with disciplinary canonical models at the macroscopic, particulate, and symbolic levels. Contextualized within the silver chloride precipitation vignette, using the models of perspective with reasoning principles would promote students’ conceptions of silver and chloride ions forming a lattice, writing the equation for the chemical reaction, and relating these ideas back to the white solid they had observed precipitation out of solution (see Table 1). More examples applying the models of perspective to the preparation of copper(II) sulfate solution are also provided in Table 1. The degree to which students can transition between representational levels would then be indicative of their understanding.
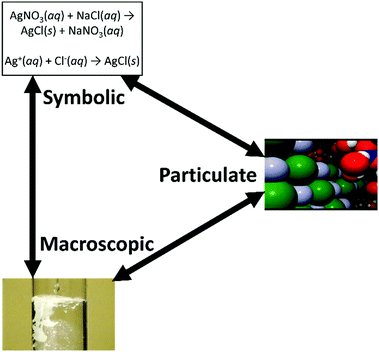 | ||
| Fig. 1 A models of perspective of Johnstone's triangle entails connecting the macroscopic, symbolic, and particulate levels in their understanding of silver chloride precipitation. | ||
| Silver chloride precipitation | Copper(II) sulfate solution | |||
|---|---|---|---|---|
| Models of | Models for | Models of | Models for | |
| Macroscopic | Students identify what is a precipitate and where they have seen this in their everyday lives | Students share ideas on how the precipitate is formed. Do the reactants just switch partners? Do Ag+ and Cl− ions form a molecular pair? | Students discuss their observations when a teacher prepares a solution of copper(II) sulfate | Students discuss how different chemical species account for the shift in colour (from white to blue) |
| Particulate | In groups, students draw which ions are attracted to each other and represent the appropriate ratios in a lattice | Students determine what words they could add to improve the explanations that their static representations do not convey | Students record what happens to the yellow ball (Cu2+ ion) in the VisChem animation (Tasker and Dalton, 2006) | Students reflect on how the atom and/or molecule speed and the crowdedness may have been exaggerated or simplified for better viewing purposes |
| Symbolic | Students write the molecular equation and then practice writing the net ionic equation | In a think-pair-share, students discuss what the net ionic equation does not convey (e.g., lattice formation, entropy, and enthalpy) | Students assign (aq) and (s) for reactants and products in various equations to practice describing states of matter | Students discuss what Cu2+(aq) really means with respect to solvating water molecules and the corresponding dipole interactions |
The models for perspective within Johnstone's triangle would encourage teachers to promote modelling practices differently. Instead of facilitating students to solely integrate the three representational levels, teachers would prioritize the generation and evaluation of learners’ models with respect to the utility of the contextualized explanation. Gouvea and Passmore (2017, p. 58) accordingly recommend the following questions to situate activities with a models for context:
• To what extent are there puzzling or unknown aspects worth investigating associated with the phenomenon?
• How clear is the question and does the question enable learners to make sense of what is puzzling or unknown?
• To what extent is there a clear epistemic aim and how well do the learners themselves understand their role in generating and evaluating the knowledge?
Combining Johnstone's triangle and the models for perspective during lesson planning leads the teacher to demonstrate how one representational level's utility complements another. By initially demarcating each of the representational levels in terms of its idiosyncratic limitations, one could create a need to know that can be later resolved by viewing Johnstone's triangle as a gestalt. Given the silver chloride precipitation vignette, a pedagogy inspired by the models for perspective would promote students’ initial experiencing of the macroscopic level and identifying incongruities with their mental schema, thereby warranting the use of a chemistry model for resolving uncertainties (see Table 1). Similarly, application of the models for perspective on copper(II) sulfate solution preparation is also listed in Table 1. Students could then make sense of the particulate animation, attending to its design and how it is functionally useful/limited for explaining precipitation. Finally, students can compare their ideas with the net ionic equation, discovering what essential precipitation processes are expressed or effaced by the conventions of chemistry symbolism.
Instead of purely a connection of macroscopic, symbolic, and particulate representations, Johnstone's triangle via PedChemSense becomes reappropriated as a toolkit for providing mechanistic explanations (see Fig. 2). PedChemSense therefore involves teachers planning for opportunities in which students confront uncertainties of each representational level and make sense of how each support another for a more complete explanation. The macroscopic, symbolic, and particulate levels become distinct lenses to understand phenomena with which students determine the degree of overlap/separation for greater and purposeful explanatory resolution (see Fig. 3). We argue that when a student explicitly discerns the context in which a model is both limited and useful, the discovered utility may enable stronger connections of chemistry concepts. In addition, PedChemSense will enable teachers to further develop their students’ ideas about the nature of models and, subsequently, the nature of science in general. To further reinforce the practicality of transformation with PedChemSense, we now transition into examples from a PD context.
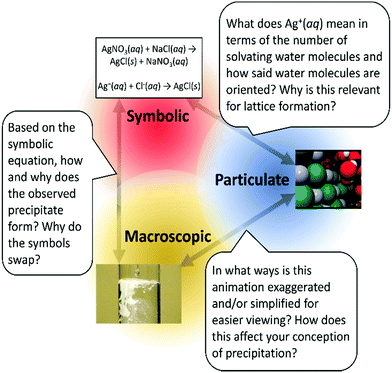 | ||
| Fig. 2 A models for perspective of Johnstone's triangle in which students make sense of the macroscopic, symbolic, and particulate levels for explaining silver chloride precipitation. | ||
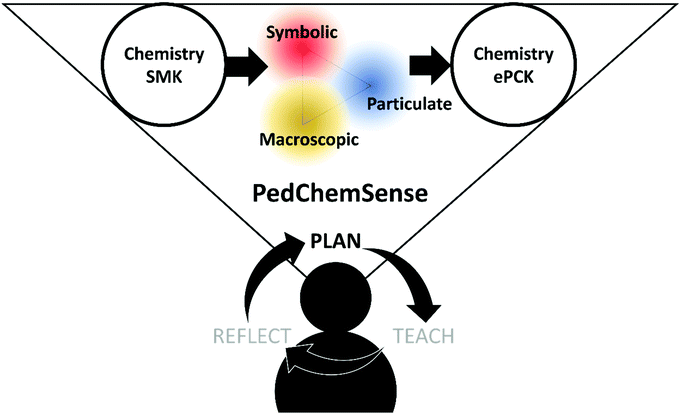 | ||
| Fig. 3 PedChemSense builds upon the RCM. It provides a mechanism for transforming SMK to ePCK through the use of Johnstone's levels during lesson planning. | ||
Contextualizing PedChemSense
| Macroscopic | How would you explain the presence of orange stains in your bathtub using molecular-level interactions? | How is waste water treated? What atomic/molecular processes do you imagine occurring? | You are a medical doctor. How would you explain to your patient the formation of kidney stones? |
| Particulate | In what ways has the animation been adjusted for more effective viewing and understanding of precipitation? | How can you modify your models to compensate for the animation's limitations on explaining precipitation? | In the animation, are water molecules simply carrying silver chloride ion pairs to the lattice? |
| Symbolic | What do the (aq) and (s) notations in our written equation fail to show in terms of electrostatic interactions? | What interactions that explain lattice formation does AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) fail to convey? | Given AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq), how does NaNO3(aq) interact in solution? |
In the case of copper(II) sulfate solution, our ePCK as PD facilitators involves initially presenting a macroscopic image of anhydrous copper(II) sulfate as a jar containing white solid. There is also a container of hydrated copper(II) sulfate containing blue solids within the same picture. Finally, there are additional images of the copper(II) sulfate solution shown as a clear, blue liquid and a test tube of clear, colorless liquid containing sodium and sulfate ions. We then ask participants, “Why is copper(II) sulfate solution blue and anhydrous copper(II) sulfate white? How do relevant chemical species interact that account for the colour change?” By drawing attention to the appearances of the solutions and solids with our ePCK, we attempt to introduce some cognitive dissonance for participants who must now make sense of the molecular rationale explaining the contrasting colours. By contrasting the presence of chemical species, one may initially interpret the presence of water molecules accounting for the blue appearance of copper(II) sulfate, which would raise some initial uncertainty.
We suggest initiating PedChemSense with the limitations of the macroscopic level because it can be readily experienced. Johnstone (2000) argues for a psychological approach of curricular order which starts with the tangible. Appropriate and relevant anchoring concepts should be introduced before learning new academic material to increase familiarity and meaningfulness (Ausubel, 1960). Similarly, we notice that VCI participants adhere to this recommendation. When designing a silver chloride precipitation learning design (i.e., student-centered lesson plan), some participants had shown images of iron(II) sulfate precipitate appearing in bathtubs or planned discussions on the removal of heavy metals in water filtration plants (see Table 2). Although these connections to everyday life may facilitate student understanding (Moje et al., 2001; Pinto and Garrido-Escudero, 2016), solely referencing these examples may not be enough for the sensemaking process. PedChemSense aims to expand upon Johnstone's ideas by using the macroscopic level's accessibility to promote student recognition of its uncertainties and, consequently, the utilities embedded in the particulate and symbolic levels.
While an animation can enable viewers to observe how solvating copper(II) ions results in the colour change at the macroscopic level, the animation may also be inappropriately treated as the canonical explanation. Circumventing this issue necessitated another form of ePCK in which we facilitated participants’ dialogue on features that the animation does not communicate well. For the copper(II) sulfate animation, we discussed how past students interpreted the water molecules deliberately carrying the ions to their corresponding places. Atom/molecule speed and the crowdedness of bulk water were also adjusted for easier viewing purposes (Tasker and Dalton, 2006). PedChemSense thus directs teachers to use their SMK to make sense of what the animation does not show with fidelity (see Table 2). For instance, are molecules and ions necessarily coloured as the animation depicts? In what ways may the speed of molecules and ions be slowed down to allow easy viewing? To what extent do students understand that atoms/molecules move randomly as opposed with intention? Finally, how difficult is it to see the chemical species of interest?
PedChemSense recognizes the importance of adopting the models for perspective because the models of perspective with particulate animations may raise major problems. For example, Kelly et al. (2017) examined how general chemistry students responded when shown two contrasting animations of a reduction–oxidation reaction: one that accurately conveyed electron exchange while another inaccurately showed a physical exchange between ions. Their results indicate that participants struggle with evaluating supporting and refuting evidence, perceiving both animations to be correct with almost half revising their drawings to fit the erroneous model. PedChemSense aims to avoid this scenario by framing students’ sensemaking of how a particulate-level model should not be treated as direct evidence. Teachers undergoing PedChemSense should incorporate the contextual function of particulate-level models and the gaps in their correspondence to phenomenon in their lesson plans. Doing so may help develop students’ recognition of the utility within other representational levels, the nature of models, and the nature of science at large.
During the VCI, our symbolic-level ePCK consisted of highlighting the information the symbolic level does not explicitly express in the copper(II) sulfate solution. The number of solvating water molecules that are present and the corresponding orientations to the copper(II) ion due to ion–dipole interactions remain unclear based on the written equation. We provide an opportunity for participants to determine that although the symbolic level is practical, it is by no means comprehensive for understanding the mechanistic explanation of dissolving. Based on participants’ silver chloride precipitation learning designs, our emphasis on the symbolic level's limitations did not appear to be as salient during participants’ planning. Instead, participants typically used the symbolic level to describe how a net ionic equation was written and as an introduction to a table of solubility rules which—given their curricular requirements—is to be expected. This may be detrimental for developing chemistry understanding, as learners may re-appropriate the swapping of symbols in a “double replacement” reaction as de facto explanation.
The repurposing of the symbolic level for explanation is likely due to its prioritization in chemistry teaching (Gabel, 1993). Students have been reported to mentally flip through formulas until they find one that fits the chemistry problem's conditions without ever pondering about the phenomenon itself (Bunce and Gabel, 1991). Although these findings are approximately 30 years old, we perceive a lingering emphasis on the symbolic level when teachers plan for symbol manipulation and solubility rules at the expense of particulate-level concepts. To highlight the limitations of the symbolic level, PedChemSense incentivizes teachers to ask the following questions when they plan for their ePCK: “What essential information related to particulate-interaction is not being conveyed when writing the equation for the reaction? To what extent might students be obligated to rely on symbol manipulation for explanatory purposes of the phenomenon? How aware are students with respect to the decisions to abbreviate chemical interactions as symbolic reactions?” Chemistry teachers who engage in PedChemSense should identify uncertainties with the symbolic level to support its recognition as shorthand for summarizing, and not for providing mechanistic explanations (see Table 2).
Limitations
Just as how uncertainty and limitations function are essential features of PedChemSense and the models for perspective, we must also evaluate our own conceptual framework in the same manner. According to the RCM, ePCK is currently more prioritized (Hume et al., 2020). PedChemSense does not delve into teachers’ PCK enacted in the classroom, but instead on the transformative process of SMK to ePCK prior to instruction. Our conceptual framework is currently incompatible for explaining how teachers should improvise and orchestrate sensemaking opportunities for their students during the class (Russ and Berland, 2018). We acknowledge that teaching and learning within-the-moment is exceedingly complex and requires additional theoretical constructs (e.g., affect, identity, multimodality, and discourse) for further clarification.Another limitation lies within our integration of sensemaking and the models for perspective. Our rationale was to align PedChemSense with the design of the VCI itself. Because the VCI highlights modelling practices and particulate animations for reforming secondary instructional strategies, our inspiration for developing PedChemSense was a response to our preliminary findings and reflections of PD implementation. PedChemSense may consequently be so tailored to the VCI that theoretical adjustments are likely necessary to enable better fit for other teacher learning contexts. However, we note that the use of particulate animations, modelling practices, and Johnstone's triangle are still popular endeavors for chemistry education research (e.g., Ovens et al., 2020; Long et al., 2021). PedChemSense also has useful applications for other PD programs that heavily feature molecular visualizations. As a result, PedChemSense will nevertheless remain salient in the chemistry education community, as its theoretical underpinnings can still inspire future avenues of teaching, learning, and research.
Implications for practitioners and researchers
We recommend that fellow professional developers provide more structured learning opportunities for teachers to undergo PedChemSense. From our VCI experiences, we observed that positioning secondary teacher participants as both learners of chemistry and of pedagogy is helpful for advancing their SMK and ePCK knowledge bases. On one hand, teachers can confront their own conceptual uncertainties, instigating a need to expand their chemistry knowledge. On the other, how a professional developer facilitates PedChemSense can also model effective strategies for teachers to adopt and author for their own instruction. Teacher educators should also consider the epistemic aim of their PD program. If the goal were to ease chemistry teachers into responding to uncertainty within their lesson planning and their classrooms, we recommend designing activities related to Johnstone's triangle and discussions on the limitations of models. Just as how teachers undergo PedChemSense to facilitate their students’ chemistry sensemaking, teacher educators should also undergo PedChemSense to facilitate teachers’ transformation of SMK into ePCK.PedChemSense similarly directs secondary chemistry teachers and undergraduate chemistry faculty to holistically evaluate pre-existing and future lesson plans in terms of the epistemic purpose. For example, is the epistemic aim of a laboratory to provide a visual demonstration of abstract chemistry concepts? To what extent are educators encouraging students to view particulate models as perfect representations of chemistry phenomena? Should educators problematize a chemical equation in terms of the information it does not convey? PedChemSense warns that solely emphasizing the transitioning between representational levels may not fully realize the potential of Johnstone's triangle. We advise that its purpose is not just connecting the three representational levels but viewing them as an ensemble of tools, each with limited function for specific circumstances. Especially for introductory chemistry, educators should increase awareness of how and why the symbolic level is rarely useful for explaining phenomena.
For future chemistry education research, the ways and the extent to which educators undergo PedChemSense for specific chemistry topics need to be broadened. The examples that we provide are the preparation of aqueous copper(II) sulfate solution and the precipitation of silver chloride. However, how the vertices of Johnstone's triangle for PedChemSense are incorporated is dependent on the phenomenon. We recommend additional research on how the limitations of Johnstone's triangle can be re-contextualized in both K-12 and undergraduate chemistry curricula. In addition, the process in which lesson plans can be analysed to understand transformation of SMK to ePCK is still inchoate. Future research should consider devising PedChemSense-related methods for more effective and comprehensive analysis of a lesson plan's contents. Analysing lesson plans in this manner may reveal new opportunities for the application of the RCM in chemistry education.
Finally, how PedChemSense can be adapted for enacted teaching contexts beyond lesson planning has yet to be determined. Similar to work conducted in elementary/middle school contexts, research should identify the ways secondary and undergraduate chemistry educators can raise, maintain, and reduce uncertainty in classroom spaces (Chen and Techawitthayachinda, 2021). Identifying best practices can assist educators in negotiating the difficulties of arriving at a scientifically-acceptable answer while still meaningfully integrating students’ accurate and inaccurate conceptions (Chen, 2021). There may also be opportunities to apply PedChemSense to various content and pedagogical knowledge bases conveyed in the RCM (Hume et al., 2020). Investigating how teachers confront uncertainties on the underlying components that culminate into ePCK may stimulate new understandings regarding their experiences and practices.
In addition, there are also inherent challenges with supporting pedagogical sensemaking within the moment due to classroom, curricular, and district obligations. The preponderance of the “five types of reactions” classification in secondary US chemistry, for example, is a risky perspective that overly simplifies chemistry concepts. Similar to Carlone et al.'s (2014) work, more research should investigate what teachers are obligated to do that may detract from what teachers want to do. This is useful especially in secondary chemistry contexts, which have been highly influenced by early college chemistry teaching. Implementing longitudinal observations and/or ethnographic methods may be an appropriate means to understand how planning with and the enacting of PedChemSense functions and stabilizes in classroom settings.
Conclusions
PedChemSense theoretically expands the RCM by providing a mechanism to transform SMK to ePCK. As shown with the constructs of sensemaking and models for, limitation is an imperative component both for promoting chemistry learning and mirroring the uncertain nature of science itself. PedChemSense itself is also limited, presently meant to assist teachers when planning their lessons and to reappropriate lesson plans as a useful data source for RCM-related analysis. However, we do not view the limitation itself as a weakness of the theory. Instead, we interpret limitation as utility. The two are fundamentally the same: where a tool is limited in one context means that it gains utility in another. Although PedChemSense requires further refinement, we assert its potential for productively maximizing students’ reasoning and sensemaking processes to advance chemistry conceptual understanding.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to Roy Tasker for serving as a consultant on the VisChem project as well as developing VisChem-related resources and the VisChem Approach. We also thank our participants for their time and commitment. This material is based upon work supported by the National Science Foundation under Grant No. DRL-1908121.References
- Allchin D., (2012), Teaching the nature of science through scientific errors, Sci. Educ., 96(5), 904–926 DOI:10.1002/sce.21019.
- Ausubel D. P., (1960), The use of advance organizers in the learning and retention of meaningful verbal material, J. Educ. Psychol., 51(5), 267–272 DOI:10.1037/h0046669.
- Bertram A. and Loughran J., (2012), Science teachers views on CoRes and PaP-eRs as a framework for articulating and developing pedagogical content knowledge, Res. Sci. Educ., 42, 1027–1047 DOI:10.1007/s11165-011-9227-4.
- Brandriet A. R. and Bretz S. L., (2014), The development of the redox concept inventory as a measure of students’ symbolic and particulate redox understandings and confidence, J. Chem. Educ., 91(8), 1132–1144 DOI:10.1021/ed500051n.
- Bunce D. M. and Gabel D. L., (1991), Enhancing chemistry problem-solving achievement using problem categorization, J. Res. Sci. Teach., 28(6), 505–521 DOI:10.1002/tea.3660280605.
- Bussey T. J. and Orgill M., (2015), What do biochemistry students pay attention to in external representations of protein translation? The case of the Shine-Dalgarno sequence, Chem. Educ. Res. Pract., 16(4), 714–730 10.1039/c5rp00001g.
- Carlone H. B., Scott C. M. and Lowder C., (2014), Becoming (less) scientific: A longitudinal study of students’ identity work from elementary to middle school science, J. Res. Sci. Teach., 51(7), 836–869 DOI:10.1002/tea.21150.
- Chen Y.-C., (2021), Epistemic uncertainty and the support of productive struggle during scientific modeling for knowledge co-development, J. Res. Sci. Teach., 1–40 DOI:10.1002/tea.21732.
- Chen Y.-C. and Techawitthayachinda R., (2021), Developing deep learning in science classrooms: Tactics to manage epistemic uncertainty during whole-class discussion, 58(8), 1083–1116 DOI:10.1002/tea.21693.
- Chen Y.-C., Benus M. J. and Hernandez J., (2019), Managing uncertainty in scientific argumentation, Sci. Educ., 103(5), 1235–1276 DOI:10.1002/sce.21527.
- Chinn C. A. and Buckland L. A., (2012), Model-based instruction: Fostering change in evolutionary conceptions and in epistemic practices, in Evolution challenges: integrating research and practice in teaching and learning about evolution, 211–232 DOI:10.1093/acprof:oso/9780199730421.003.0010.
- Cooper M. M., Corley L. M. and Underwood S. M., (2013), An investigation of college chemistry students’ understanding of structure–property relationships, J. Res. Sci. Teach., 50(6), 699–721 DOI:10.1002/tea.21093.
- Cooper M. M., Stieff M. and DeSutter D., (2017), Sketching the invisible to predict the visible: From drawing to modeling in chemistry, Top. Cogn. Sci., 9(4), 902–920 DOI:10.1111/tops.12285.
- Dewey J., (1997), How we think, Courier Corporation.
- Edwards A. D. and Head M., (2016), Introducing a culture of modeling to enhance conceptual understanding in high school chemistry courses, J. Chem. Educ., 93(8), 1377–1382 DOI:10.1021/acs.jchemed.6b00125.
- Ekiz-Kiran B., Boz Y. and Oztay E. S., (2021), Development of pre-service teachers’ pedagogical content knowledge through a PCK-based school experience course, Chem. Educ. Res. Pract., 22(2), 415–430 10.1039/d0rp00225a.
- El-Khalick A. F., (2006), Pre-service and experienced biology teachers’ global and specific subject matter structures: Implications for conceptions of pedagogical content knowledge, Eurasia J. Math., Sci. Technol. Educ., 2(1), 1–29.
- Engle R. A., (2011), The productive disciplinary engagement framework: Origins, key concepts, and developments, in Dai D. (ed.), Design research on learning and thinking in educational settings: Enhancing growth and functioning, New York, NY: Routledge, pp. 170–209.
- EQuIP Rubric for Science Released, (2014), Next Generation Science Standards, http://www.nextgenscience.org/news/equip-rubricscience-released (accessed September 2021).
- EQuIP Rubric for Lessons & Units Version 3, (2016), Next Generation Science Standards, http://www.nextgenscience.org/sites/default/files/EQuIP%20Rubric%20for%20Science%20v3.pdf (accessed September 2021).
- Gabel D. L., (1993), Use of the particle nature of matter in developing conceptual understanding, J. Chem. Educ., 70(3), 193–194 DOI:10.1021/ed070p193.
- Gess-Newsome J., (1999), Pedagogical content knowledge: An introduction and orientation, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge, Dordrecht: Springer, pp. 3–17.
- Gess-Newsome J., Taylor J. A., Carlson J., Gardner A. L., Wilson C. D. and Stuhlsatz M. A. M., (2017), Teacher pedagogical content knowledge, practice, and student achievement, Int. J. Sci. Educ., 41(7), 944–963 DOI:10.1080/09500693.2016.1265158.
- Giere R. N., (1988), Explaining Science: A Cognitive Approach, Chicago, IL: University of Chicago Press.
- Gouvea J. and Passmore C., (2017), ‘Models of’ versus ‘Models for’, Sci. Educ., 26, 49–63 DOI:10.1007/s11191-017-9884-4.
- Großschedl J., Welter V. and Harms U., (2019), A new instrument for measuring pre-service biology teachers’ pedagogical content knowledge: The PCK-IBI, J. Res. Sci. Teach., 56(4), 402–439 DOI:10.1002/tea.21482.
- Grossman P., (1990), The Making of a Teacher, New York, NY: Teachers College Press.
- Hale L. V. A., Lutter J. C. and Shultz G. V., (2016), The development of a tool for measuring graduate students’ topic specific pedagogical content knowledge of thin layer chromatography, Chem. Educ. Res. Pract., 17(4), 700–710 10.1039/c5rp00190k.
- Herrington D. G., Luxford K. and Yezierski E. J., (2012), Target inquiry: Helping teachers use a research experience to transform their teaching practices, J. Chem. Educ., 89(4), 442–448 DOI:10.1021/ed1006458.
- Hume A., Cooper R. and Borowski A. (ed.), (2020), Repositioning pedagogical content knowledge in teachers’ knowledge for teaching science, Singapore: Springer.
- Johnstone A. H., (1982), Macro- and microchemistry, Sch. Sci. Rev., 64, 377–379.
- Johnstone A. H., (2000), Teaching of chemistry – logical or psychological? Chem. Educ. Res. Pract., 1(1), 9–15 10.1039/A9RP90001B.
- Kellamis N. M. and Yezierski E. J., (2019), Applying the next generation science standards to current chemistry classrooms: How lessons measure up and how to respond, J. Chem. Educ., 96(7), 1308–1317 DOI:10.1021/acs.jchemed.8b00840.
- Kelly R. M., Barrera J. H. and Mohamed S. C., (2010), An analysis of undergraduate general chemistry students’ misconceptions of the submicroscopic level of precipitation reactions, J. Chem. Educ., 87(1), 113–118 DOI:10.1021/ed800011a.
- Kelly R. M., Akaygun S., Hansen S. J. R. and Villalta-Cerdas A., (2017), The effect that comparing molecular animations of varying accuracy has on students’ submicroscopic explanations, Chem. Educ. Res. Pract., 18(4), 582–600 10.1039/C6RP00240D.
- Kind V., (2009), Pedagogical content knowledge in science education: perspectives and potential for progress, Stud. Sci. Educ., 45(2), 169–204 DOI:10.1080/03057260903142285.
- Knuuttila T., (2011), Modelling and representing: An artefactual approach to model-based representation, Stud. Hist. Philos. Sci. Part A, 42(2), 262–271 DOI:10.1016/j.shpsa.2010.11.034.
- Krajcik J. and Merritt J., (2012), Engaging students in scientific practices: What does constructing and revising models look like in the science classroom, Sci. Teach., 79(3), 38–41.
- Larkin D., (2012), Misconceptions about “misconceptions”: Preservice secondary science teachers’ views on the value and role of student ideas, Sci. Educ., 96(5), 927–959 DOI:10.1002/sce.21022.
- Latour B., (1987), Science in action: How to follow scientists and engineers through society, Cambridge, MA: Harvard University Press.
- Lin C.-Y. and Wu H.-K., (2021), Effects of different ways of using visualizations on high school students’ electrochemistry conceptual understanding and motivation towards chemistry learning, Chem. Educ. Res. Pract., 22(3), 786–801 10.1039/D0RP00308E.
- Long S., Andreopoulos S., Patterson S., Jenkinson J. and Ng D. P. (2021), Metabolism in motion: Engaging biochemistry students with animation, J. Chem. Educ., 98(5), 1795–1800 DOI:10.1021/acs.jchemed.0c01498.
- Loughran J., Mulhall P. and Berry A., (2004), In search of pedagogical content knowledge in science: Developing ways of articulating and documenting professional practice, J. Res. Sci. Teach., 41(4), 370–391 DOI:10.1002/tea.20007.
- Maeyer J. and Talanquer V., (2013), Making predictions about chemical reactivity: Assumptions and heuristics, J. Res. Sci. Teach., 50(6), 748–767 DOI:10.1002/tea.21092.
- Magnusson S., Krajcik J. and Borko H., (1999), Secondary teachers’ knowledge and beliefs about subject matter and their impact on instruction, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge, Dordrecht: Springer, pp. 95–132.
- Mahaffy P., (2006), Moving chemistry education into 3D: A tetrahedral metaphor for understanding chemistry. Union carbide award for chemical education, J. Chem. Educ., 83(1), 49 DOI:10.1021/ed083p49.
- Manz E., (2015), Representing student argumentation as functionally emergent from scientific activity, Rev. Educ. Res., 85(4), 553–590 DOI:10.3102/0034654314558490.
- Manz E. and Suárez E., (2018), Supporting teachers to negotiate uncertainty for science, students, and teaching, Sci. Educ., 102(4), 771–795 DOI:10.1002/sce.21343.
- Marzabal A., Delgado V., Moreira P., Barrientos L. and Moreno J., (2018), Pedagogical content knowledge of chemical kinetics: Experiment selection criteria to address students’ intuitive conceptions, J. Chem. Educ., 95(8), 1245–1249 DOI:10.1021/acs.jchemed.8b00296.
- Mavhunga E., (2016), Transfer of the pedagogical transformation competence across chemistry topics, Chem. Educ. Res. Pract., 17(4), 1081–1097 10.1039/c6rp00095a.
- Mavhunga E. and Rollnick M., (2011), The development and validation of a tool for measuring topic specific PCK in chemical equilibrium, in Proc. ESERA Conf.
- Mayer R. E., Hegarty M., Mayer S. and Campbell J., (2005), When static media promote active learning: Annotated illustrations versus narrated animations in multimedia instruction, J. Exp. Psychol.-Appl., 11(4), 256–265 DOI:10.1037/1076-898X.11.4.256.
- McEwan H. and Bull B., (1991), The pedagogic nature of subject matter knowledge, Am. Educ. Res. J., 28(2), 316–334 DOI:10.3102/00028312028002316.
- Moje E. B., Collazo T., Carrillo R. and Marx R. W., (2001), “Maestro, what is ‘Quality’?”: Language, literacy, and discourse in project-based science, J. Res. Sci. Teach., 38(4), 469–498 DOI:10.1002/tea.1014.
- Moreno L. F., Alzate M. V., Meneses J. A. and Marín M. L., (2018), Build your model! Chemical language and building molecular models using plastic drinking straws, J. Chem. Educ., 95(5), 823–827 DOI:10.1021/acs.jchemed.7b00300.
- Morrison M., (2015), Reconstructing reality: models, mathematics, and simulations, England: Oxford University Press.
- Nersessian N., (2002), The cognitive basis of model-based reasoning in science, in Carruthers P., Stich S. and Siegal M. (ed.), The cognitive basis of science, England, Cambridge: Cambridge University Press, pp. 133–153.
- NGSS Lead States, (2013), Next Generation Science Standards: For States, By States, Washing, DC: The National Academies Press.
- Oliveira A. W., Akerson V. L., Colak H., Pongsanon K. and Genel A., (2012), The implicit communication of nature of science and epistemology during inquiry discussion, Sci. Educ., 96(4), 652–684 DOI:10.1002/sce.21005.
- Ovens M., Ellyard M., Hawkins J. and Spagnoli D., (2020), Developing an augmented reality application in an undergraduate DNA precipitation experiment to link macroscopic and submicroscopic levels of chemistry, J. Chem. Educ., 97(10), 3882–3886 DOI:10.1021/acs.jchemed.0c00481.
- Phillips A. M., Watkins J. and Hammer D., (2017), Problematizing as a scientific endeavor, Phys. Rev. Phys. Educ. Res., 13(2), 1–13 DOI:10.1103/PhysRevPhysEducRes.13.020107.
- Pinto G. and Garrido-Escudero A., (2016), Chemistry and explosives: An approach to the topic through an artistic and historical contribution made by a Spanish global explosives supplier, J. Chem. Educ., 93(1), 103–110 DOI:10.1021/acs.jchemed.5b00079.
- Reiser B. J., (2004), Scaffolding complex learning: The mechanisms of structuring and problematizing student work, J. Learn. Sci., 13(3), 273–304 DOI:10.1207/s15327809jls1303_2.
- Rodriguez J.-M. G. and Towns M. H., (2019), Alternative use for the refined consensus model of pedagogical content knowledge: Suggestions for contextualizing chemistry education research, J. Chem. Educ., 96(9), 1797–1803 DOI:10.1021/acs.jchemed.9b00415.
- Rodriguez J.-M. G., Harrison A. R. and Becker N. M., (2020), Analyzing students’ construction of graphical models: How does reaction rate change over time? J. Chem. Educ., 97(11), 3948–3956 DOI:10.1021/acs.jchemed.0c01036.
- Russ R. S. and Berland L. K., (2018), Invented science: A framework for discussing a persistent problem of practice, J. Learn. Sci., 28(3), 279–301 DOI:10.1080/10508406.2018.1517354.
- Schwarz C. V., Passmore C. and Reiser B. J., (2016), Moving beyond “knowing about” science to making sense of the world, in Schwarz C. V., Passmore C. and Reiser B. J. (ed.), Helping students make sense of the world using next generation science and engineering practices, Arlington, VA: National Science Teachers Association, pp. 3–21.
- Seethaler S., Czworkowski J. and Wynn L., (2018), Analyzing general chemistry texts’ treatment of rates of change concepts in reaction kinetics reveals missing conceptual links, J. Chem. Educ., 95(1), 28–36 DOI:10.1021/acs.jchemed.7b00238.
- Segall A., (2004), Revisiting pedagogical content knowledge: The pedagogy of content/the content of pedagogy, Teach. Teach. Educ., 20(5), 489–504 DOI:10.1016/j.tate.2004.04.006.
- Shulman L. S., (1986), Those who understand: Knowledge growth in teaching, Educ. Res., 15(2), 4–14 DOI:10.3102/0013189X015002004.
- Shulman L. S., (1987), Knowledge and teaching: Foundations of the new reform, Harvard Educ. Rev., 57(1) DOI:10.17763/haer.57.1.j463w79r56455411.
- Shulman L. S., (2015), PCK: Its genesis and exodus, in Berry A., Friedrichsen P. and Loughran J. (ed.), Re-examining pedagogical content knowledge in science education, New York, NY: Routledge, pp. 3–13.
- Sjöström J. and Talanquer V., (2014), Humanizing chemistry education: From simple contextualization to multifaceted problematization, J. Chem. Educ., 91(8), 1125–1131 DOI:10.1021/ed5000718.
- Taber K. S., (2013), Revisiting the chemistry triplet: Drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168 10.1039/C3RP00012E.
- Taber K. S., (2019), The Nature of the Chemical Concept: Re-constructing Chemical Knowledge in Teaching and Learning (Vol. 3), Royal Society of Chemistry.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33(2), 179–195 DOI:10.1080/09500690903386435.
- Tang X., Elby A. and Hammer D., (2020), The tension between pattern-seeking and mechanistic reasoning in explanation construction: A case from Chinese elementary science classroom, Sci. Educ., 104(6), 1071–1099 DOI:10.1002/sce.21594.
- Tasker R., Dalton R., (2006), Research into practice: Visualisation of the molecular world using animations. Chem. Educ. Res. Pract., 7(2), 141–159 10.1039/B5RP90020D.
- Towns M. H., Raker J. R., Becker N., Harle M. and Sutcliffe J., (2012), The biochemistry tetrahedron and the development of the taxonomy of biochemistry external representations (TOBER), Chem. Educ. Res. Pract., 13(3), 296–306 10.1039/C2RP00014H.
- Williams J. and Lockley J., (2012), Using CoRes to develop the pedagogical content knowledge (PCK) of early career science and technology teachers, J. Technol. Educ., 24(1), 34–53.
- Wu M.-Y. M., Magnone K., Tasker R. and Yezierski E. J., (2021), Remote chemistry teacher professional development delivery: Enduring lessons for programmatic redesign, J. Chem. Educ., 98(8), 2518–2526 DOI:10.1021/acs.jchemed.1c00181.
| This journal is © The Royal Society of Chemistry 2022 |
