Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalyst choice impacts aromatic monomer yields and selectivity in hydrogen-free reductive catalytic fractionation†
Jacob K.
Kenny
 ab,
David G.
Brandner
ab,
David G.
Brandner
 b,
Sasha R.
Neefe
b,
William E.
Michener
b,
Yuriy
Román-Leshkov
b,
Sasha R.
Neefe
b,
William E.
Michener
b,
Yuriy
Román-Leshkov
 c,
Gregg T.
Beckham
c,
Gregg T.
Beckham
 *b and
J. Will
Medlin
*b and
J. Will
Medlin
 *a
*a
aDepartment of Chemical and Biological Engineering, University of Colorado Boulder, Boulder 80303, CO, USA. E-mail: will.medlin@colorado.edu
bRenewable Resources and Enabling Sciences Center, National Renewable Energy Laboratory, 15013 Denver W Pkwy, Golden, CO 80401, USA. E-mail: gregg.beckham@nrel.gov
cDepartment of Chemical Engineering, Massachusetts Institute of Technology, 25 Ames Street, Cambridge, MA 02139, USA
First published on 16th August 2022
Abstract
Hydrogen-free reductive catalytic fractionation (RCF) is a promising method to extract and depolymerize lignin from native biomass without the use of external hydrogen gas. Here, we show that Pt/C and Pd/C achieve comparable monomer yields regardless of hydrogen pressure, whereas Ru/C and Ni/C show lower yields under H2-free conditions. Ru/C and Ni/C primarily perform hydrodeoxygenation regardless of the hydrogen pressure, but Pt/C and Pd/C demonstrated the ability to form both ethyl products from dehydrogenation and propanol products through hydrogenation depending on the presence of external H2. Adding water to the solvent increased HDO selectivity to propyl products for both Pt/C and Pd/C. Monomer yields from poplar RCF showed similar trends in yield and selectivity to reactions with the model compound coniferyl alcohol, suggesting that H2-free RCF performance is dictated by the stabilization rate of reactive monomer intermediates.
Introduction
Lignin comprises 15–30% of lignocellulosic biomass and is the most abundant source of naturally available aromatic compounds.1 Techno-economic analyses and life cycle assessments have shown that conversion of lignin into fuels and chemicals is essential to the viability of biorefineries.2–5 During many conventional thermochemical pretreatments, which often target carbohydrate valorization, labile aryl-ether linkages in lignin are cleaved to form reactive intermediates that condense to form new C–C bonds, thus increasing its recalcitrance to downstream conversion.6 Conversely, reductive catalytic fractionation (RCF) is a lignin-first approach that achieves solvent-mediated extraction of lignin from biomass followed by reductive stabilization of reactive functionalities by a heterogeneous catalyst (e.g. Ni/C, Ru/C, or Pd/C), resulting in a lignin oil that is rich in aromatic monomers with saturated side chains.7–13In recent years, studies have revealed many of the crucial factors governing the extraction and stabilization phenomena. RCF reactions on whole biomass are governed both by the extent of lignin extraction and the rate of stabilization of reactive intermediates to monomers.10,14 Once in solution, lignin fragments are solvolytically depolymerized into smaller fragments that can be catalytically reduced to stable monomers.15 Polar-protic solvents such as methanol are usually used at high temperatures (up to 250 °C) to obtain high extents of extraction.7,8 The addition of water facilitates extraction at lower temperatures.9,12,16 Choice of substrate also has major implications for both monomer identity and overall yields, with hardwoods being more amenable to depolymerization and high monomer yields compared to softwoods and grasses.17,18
Investigations of the impact of catalyst identity have mainly focused on the selectivity to different product monomers, with comparably less emphasis on the rate of stabilization. Typically, Ru/C, Ni/C, and Rh/C are reported to form propyl substituted monomers through a combination of hydrodeoxygenation and hydrogenation,7,10,15,19 whereas Pd/C forms propanol substituted monomers.9,20,21 Catalyst dependent selectivity has been seen to change based on the other reaction conditions; Ru/C was shown to form mostly propanol products in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 butanol/water solvent.12 Similarly, embedding the catalytic Rh in porous hollow carbon spheres switched selectivity to favor propanol products.
1 butanol/water solvent.12 Similarly, embedding the catalytic Rh in porous hollow carbon spheres switched selectivity to favor propanol products.
Together, these advances have solidified RCF as an effective extraction and depolymerization method yielding a narrow slate of low molecular weight products. Nonetheless, recent techno-economic analysis from our group highlighted further developments that are needed to make RCF economically viable.22 One such recommendation was to employ a hydrogen-free (H2-free) RCF process (i.e. a process run without the addition of H2 gas), which enabled lower reactor operating pressure, in turn leading to an estimated 32% lower minimum RCF oil selling price, compared to a base case with methanol as a solvent and external H2 gas.22 Various H2-free RCF processes have been pursued to this end, and the pathway for utilizing alternative hydrogen donors appears to be dependent on the catalyst and solvent system. During extraction, multiple species can potentially serve as the source of hydrogen such as the alcohol solvent, hemicellulose, or even the lignin itself. Sels et al. reported high monomer yields for both Ru/C and Pd/C-catalyzed hydrogenolysis of birch in methanol at 250 °C regardless of whether the reaction was conducted in a N2 or H2 atmosphere.7 Solvent reforming was purported as the hydrogen source. Interestingly, ethyl substitued monomers formed from C–C bond hydrogenolysis predominated when Pd/C was used as a catalyst under H2-free conditions instead of propanol monomers as in reactions with H2. Hensen et al. achieved a monomer yield near the theoretical limit with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water/methanol solvent during Pt/Al2O3-catalyzed RCF of birch, similarly citing methanol reforming as the hydrogen source. Interestingly, only a 22% monomer yield was reported when Pd/C was used under the same conditions.16 Samec et al. demonstrated that Pd/C has a perhaps unique ability to utilize formic acid generated from hemicellulose degradation as a hydrogen donor during RCF of birch in an ethanol/water solvent mixture.23–25 Alternatively, Rinaldi et al. reported that RANEY®-Ni stabilizes reactive monomers through transfer hydrogenation using isopropanol as the hydrogen donor.26,27 Another interesting approach, termed atmospheric-RCF (ARCF), was described by He and co-workers in which ethylene glycol and sulfuric acid were used at comparatively lower temperatures (185–195 °C). Acid concentration was optimized to give 25.2% monomer yield after 6 hours using Ru/C as a catalyst. Lower monomer yields (4.8–6.9%) were obtained when using Pd/C regardless of the presence of H2SO4.28 This process reduced reactor pressure to atmospheric, but the separation of RCF products from ethylene glycol is expected to be difficult.22
1 water/methanol solvent during Pt/Al2O3-catalyzed RCF of birch, similarly citing methanol reforming as the hydrogen source. Interestingly, only a 22% monomer yield was reported when Pd/C was used under the same conditions.16 Samec et al. demonstrated that Pd/C has a perhaps unique ability to utilize formic acid generated from hemicellulose degradation as a hydrogen donor during RCF of birch in an ethanol/water solvent mixture.23–25 Alternatively, Rinaldi et al. reported that RANEY®-Ni stabilizes reactive monomers through transfer hydrogenation using isopropanol as the hydrogen donor.26,27 Another interesting approach, termed atmospheric-RCF (ARCF), was described by He and co-workers in which ethylene glycol and sulfuric acid were used at comparatively lower temperatures (185–195 °C). Acid concentration was optimized to give 25.2% monomer yield after 6 hours using Ru/C as a catalyst. Lower monomer yields (4.8–6.9%) were obtained when using Pd/C regardless of the presence of H2SO4.28 This process reduced reactor pressure to atmospheric, but the separation of RCF products from ethylene glycol is expected to be difficult.22
While these H2-free studies have demonstrated high monomer yields, there have been few direct comparisons between catalysts to benchmark the impact of catalyst choice on monomer yields in H2-free conditions. Excess catalyst loadings have often been used to achieve a high conversion of extracted lignin to monomers, making comparison between studies difficult. In situations where catalyst activity has been considered, the goal has be to find the required mass of catalyst for a given substrate loading.12 Catalyst choice therefore remains an open question for H2-free RCF processes. To that end, here we examine the impact of catalyst choice on monomer yields and selectivity in H2-free RCF.
Experimental
Batch RCF reactions
2 g of whole poplar sawdust biomass,29 100 mg of catalyst, 30 mL of methanol (Sigma-Aldrich) were added to a 75 mL Parr reactor with a magnetic stir bar. The reactor was sealed, purged three times, and pressure-tested with He up to reaction pressure (∼80 bar). For H2-free reactions, the pressure of He was reduced to ∼1 bar. For reactions with H2, H2 was loaded at a pressure of 30 bar. The stirring rate was set to 800 rpm, and reactor was heated to 225 °C for the desired reaction time (for simplicity “reaction time” is defined to start 30 minutes after heating was initiated). The reactors were quenched at the end of the reaction in an ice bath for 45 minutes. The headspace of H2-free reactions was sampled with a gas bag. Liquid contents were filtered first through a tared qualitative glass filter and then through a 0.2 μm PES syringe filter, and the methanol solvent was evaporated in a rotary evaporator. Ethyl acetate (Sigma-Aldrich) (20 mL) and DI-water (20 mL) were added to the crude RCF oil and separated in a separatory funnel. The aqueous layer was washed with an additional 20 mL ethyl acetate, and the organic layers were combined in a tared round bottom flask. The ethyl acetate was then removed via rotary evaporation, yielding an oil which was massed, and termed lignin oil. The lignin oil was dissolved in 15 mL acetone (Spectrum Chemical). Solid residue, including catalyst, was massed by massing the filter.Catalyst preparation of 5% Ni/C
A 5 wt% Ni/C catalyst was prepared similar to the preparation performed by Brandner et al., except at a 5 wt% loading.29 The other catalysts (Ru/C, Pd/C, Pt/C) were purchased from Sigma-Aldrich and used as received.Model compound reactions
60 mg of the selected model compound, coniferyl alcohol (Sigma-Aldrich), guaiacyl guaiacylglycerol-beta-guaiacyl ether (TCI America), or 30 mg of coniferyl aldehyde (Sigma-Aldrich) was added to a 75 mL Parr reactor along with 20 mg of catalyst and 30 mL of methanol. The reactor was then sealed, purged, and pressure-tested with He up to reaction pressure. For H2-free reactions, the pressure of He was reduced to ∼1 bar. For reactions with H2, H2 was loaded at a pressure of 30 bar. The stirring rate was set to 800 rpm, and reactor was heated to 225 °C for 1 hour before cooling in an ice bath for 45 minutes. The reaction mixture was filtered through a 0.2 μm filter. Reaction products were analyzed with an Agilent 1290 Infinity II LC equipped with a Phenomenex Luna C18(2)-HST column. Monomer yields for model compound reactions are reported on a molar basis (as opposed to mass basis like poplar RCF yields).Monomer analysis with GC
The lignin oil was dissolved in 15 mL of acetone. ¼ mL of this oil/acetone solution was added to a vial, along with ¼ mL of pure acetone, and ½ mL of 2 g L−1 tri-tertbutyl benzene (Sigma-Aldrich) in acetone as an internal standard. Samples were injected on an Agilent 8890 gas chromatograph equipped with an FID detector utilizing an HP-5 column. Quantification was performed using calibration curves with authentic standards for all compounds. All commercially available standards were purchased from Sigma Aldrich. 4-Propenylsyringol was purchased from AKos GmbH. Ethyl syringol was purchased from AAblocks. Several standards, 4-(3-methoxy)propylguaiacol, 4-propylsyringol, 4-(3-methoxy)propylsyringol, and 4-propanolsyringol, were synthesized in house.29 Monomer yield is calculated according to eqn (1)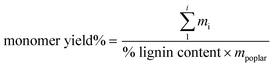 | (1) |
Headspace analysis
Gas from the headspace of H2-free reactions was captured from the reactor with a gas bag then withdrawn from the gas bag into a syringe. The sample was injected onto an Agilent 7890A gas chromatograph equipped FID and TCD, with two Wasson columns (part numbers 2428, 2378) to measure the mole fraction of each component. Moles of components were calculated using the ideal gas law assuming a headspace volume of 45 mL.Compositional analysis
Compositional analysis on the solids followed the NREL Laboratory Analytical Procedure (LAP).30,31Results and discussion
RCF of poplar
We first sought to investigate how the absence of hydrogen impacts extraction and monomer stabilization rates. Time course data allow for direct observation of the impact of H2-free conditions on the monomer formation rate, as well as whether low monomer yields can be overcome by increasing residence time. Thus, batch reactions were first performed for 1–6 hours (after reactor heat-up) using low catalyst loadings (50 mg 5 wt% Ru/C per g poplar) to prevent the reactions from reaching full conversion of lignin to monomers, allowing for monomer yields to reflect differences in the rate of hydrogenolysis between H2-free and conventional RCF conditions (see Fig. S1 for temperature and pressure profile, and Fig. S2† for RCF reaction at 200 mg Ru/C loading). As expected, nearly identical lignin oil yields are obtained regardless of the presence or absence of H2 (Fig. 1). p-Hydroxy benzoic acid (pHBA), methyl paraben, and phenol (likely produced via decarboxylation of pHBA) were produced during the RCF reaction as well, the amounts of these products were comparable in all conditions (see Fig. S3† for measurement of pHBA and methyl paraben). Notably, the selectivity to pHBA was higher in H2-free reactions (56 ± 8%) at 1 hour compared to reactions with H2 (27 ± 5%). By 3 hours, however, little pHBA was measured in either case. H2, CO, and CO2 were produced throughout the H2-free reaction, reaching a total of 7.1 ± 0.2, 3.86 ± 0.03, and 0.31 ± 0.02 mmol respectively, after 6 hours at reaction temperature (Fig. 1B, Table S3†); for reference, 30 bar H2 at room temperature is about 55 mmol. In the biorefinery, the reformed methanol will need to be replaced, incurring costs.22 Assuming all CO and CO2 are derived from methanol, the gaseous products represent a maximum loss of approximately 0.6% of the starting methanol solvent through decomposition.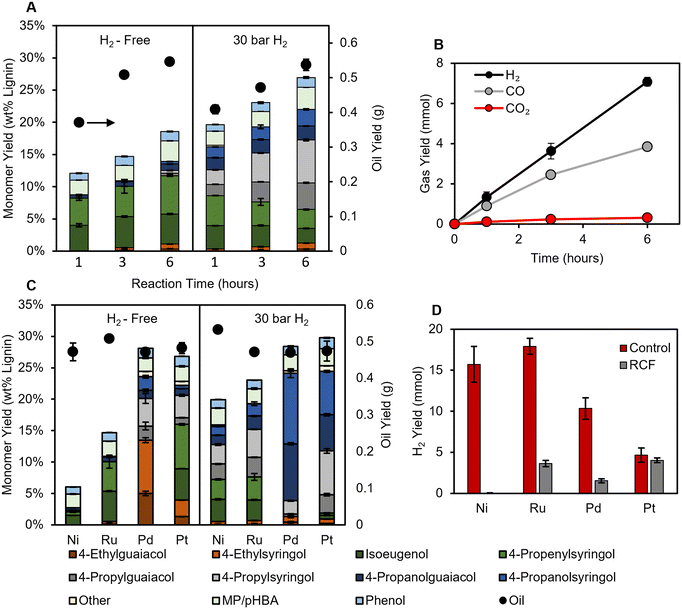 | ||
| Fig. 1 (A) Monomer yields (stacked bars, left axis) and oil yields (black circles, right axis) for time course RCF reactions without H2 (left) and with 30 bar H2 (right) using a Ru/C catalyst (Table S2†). (B) Gas yields during H2-free time course reactions with Ru/C (Table S3†). (C) Monomer yields and oil yields (black cirlces, right axis) for H2-free reactions (left) and with 30 bar H2 (right) for each catalyst (Table S4†). (D) H2 gas yields for control reactions (no poplar, red bars) and H2-free RCF (grey bars) (Table S5†). RCF conditions: 2 g poplar, 100 mg catalyst (5 wt% metal), 30 mL MeOH, 225 °C, 3 hours. Control reaction conditions: 30 mL methanol, 100 mg catalyst, 3 hours. MP/pHBA is the summed yield of methyl paraben and p-hydroxy benzoic acid. The error bars represent the standard deviation of triplicate measurements. | ||
Reactions with 30 bar H2 achieved higher monomer yields than H2-free reactions at each time point, reaching 27.0 ± 0.3% after 6 hours compared to 18.6 ± 0.3% for H2-free reactions (Fig. 1A). The lower monomer yield is a result of a lower rate of stabilization relative to condensation, indicating that adequate hydrogen is not available in the H2-free conditions with this catalyst and solvent system. By the first hour at reaction temperature, greater than 60% of the eventual delignification (as measured by oil yield) for both H2-free and 30 bar H2 reactions had occurred, yet only 1.3 ± 0.3 mmol of H2 was measured in the H2-free reactor headspace, showing that most of the extraction occurs in a low hydrogen environment. This is further evidenced by the lower yield of products with saturated propyl and propanol side chains under H2-free conditions.
Regardless of the hydrogen source, the disparity between reactions with hydrogen present and under H2-free conditions derives from the process of making hydrogen available on the surface of the catalyst. We hypothesized that other catalytic metals could be differentially active for H2-free RCF based on their hydrogen generation ability. Thus, batch reactions were performed with Ni/C, Pd/C, and Pt/C (all catalysts are 5 wt% metal loading) with and without 30 bar H2 for 3 hours (Fig. 1C).
With H2 loaded, Pt/C and Pd/C achieved the highest yields of 28 ± 2% and 30 ± 1%, respectively. Monomer yields for Ru/C and Ni/C were 23.0 ± 0.8 and 19.9 ± 0.5, and still produced unsaturated products at a selectivity of 30 ± 2% and 33.5 ± 0.5% respectively, presumably due to the low catalyst loadings. Ru/C and Ni/C formed primarily propenyl and propyl products, while Pd/C formed propanol products, as observed previously.7 Pt/C formed similar amounts of propyl and propanol products. pHBA, methyl paraben, and phenol were measured in similar amounts for all 3 hour reactions regardless of H2 pressure, suggesting that the pathways from pHBA are not substantially dependent on the presence of external hydrogen at high extents of conversion.
Without external H2 loaded, Pd/C and Pt/C retained high monomer yields, suggesting that monomer yields are limited by the rate of extraction rather than hydrogenolysis under these conditions. When Ni/C was used under H2-free conditions, monomer yields decreased to 6.0 ± 0.1%, indicating that Ni/C was unable to produce sufficient hydrogen for stabilization.18
It was expected that if the hydrogen donor was the methanol solvent, then the respective methanol reforming rates of the catalysts examined here would trend with monomer yield. However, in batch control experiments with methanol and catalyst (without poplar biomass), Ru/C and Ni/C produced the most hydrogen (Fig. 1D). Hydrogen yields during RCF were lower compared to control reactions, with only Pt/C achieving a similar H2 yield (Fig. 1D). This demonstrates the inhibitory role that the presence of the poplar has on the methanol reforming pathway. The higher monomer yields for Pd compared to Ru, despite the greater H2 production of Ru, indicates that H2-free monomer production depends on more than just reforming ability. The availability of routes involving ethyl products on Pd catalysts may be critical, as discussed in more detail below. Given that hemicellulose extraction is low in pure methanol,9 it is still likely that methanol is the predominant hydrogen donor. However, recent studies reported even the lignin itself to be the hydrogen donor,32,33 and the mechanism of H2-free activity remains unclear. Considering these results, it seems that complete reforming to H2 gas may be unnecessary, and it could be advantageous to limit the amount of excess reforming to minimize solvent loss.
Comparing monomer selectivity among the catalysts, Ru/C and Ni/C primarily performed hydrodeoxygenation (HDO) to form propenyl/propyl products, and the presence of external H2 only increased the rate of formation of these products. Conversely, the absence of external H2 gas changed the route of stabilization for Pd/C and Pt/C; namely, H2-free reactions formed ethyl products with selectivities of 48 ± 3% for Pd/C and 14.8 ± 0.2% for Pt/C (Fig. S4†). Running Pd/C reactions with higher catalyst loadings did not change the selectivity to ethyl products, and only slightly increased yields of propanol relative to propyl side chains potentially due to the increased H2 available from additional MeOH reforming (Fig. S5†). Previous authors proposed that without hydrogen coverage, Pd/C could prompt dehydrogenation followed by decarbonylation to form ethyl products.7 Interestingly, the use of Pd/C during H2-free reactions also exhibited higher selectivity to propyl products than propanol. The change in selectivity during H2-free reactions indicates that the pathway to form propanol side chains is enhanced by excess hydrogen. These results are in line with previous RCF reports in which higher H2 pressures (5–10 bar H2) were shown to change selectivity from propyl to propanol products.12 Overall, we conclude that the stabilization pathway over Pd/C and Pt/C, particularly that to form propanol products, exhibits a higher sensitivity to hydrogen pressure and potentially hydrogen coverage on the catalyst surface compared to Ru/C and Ni/C.
RCF selectivity is thought to be governed mainly by the catalytic metal,6 with additional effects from the support.34 However, a recent report demonstrated that other process conditions such as hydrogen pressure or solvent changed selectivity of Ru/C catalyzed RCF from expected propyl monomers to propanol monomers. To test the impact of water content on product selectivity, we ran RCF reactions with 25 and 50 volume percent water for the best performing catalysts, Pd/C and Pt/C (Fig. 2). When the water content of the reactions with Pt/C was increased to 25%, almost complete hydrogenation of propenyl side chains was measured (<0.5% propenyl products). A similar effect was reported by Hensen et al. in the H2-free RCF of birch with Pt/Al2O3 catalyst, except an even higher water content (approximately 50 vol%) was needed to fully saturate the side chains, which may be due to the different support as Al2O3 was found to be unstable under RCF conditions. When reactions were run at 50 vol% water in our studies, the monomer yield decreased, accompanied by the reappearance of unsaturated products such as propenyl syringol, contrasting with the near theoretical yields reported by Hensen, This is perhaps indicative of the impact of the different feedstock (birch versus poplar). Interestingly, when Pd/C was used in H2-free reactions with water, selectivity to ethyl products decreased with increasing water content, and propyl products were formed instead. However, the addition of water led to a monotonic decrease in monomer yield, reaching a yield of lignin derived products (omitting phenol and p-HBA) of 17.1 ± 0.3% for reactions with 50 vol% H2O, compared to 24.4 ± 0.3% in pure methanol. Water has been observed in previous studies to significantly affect the rate or selectivity of hydrogenation reactions; for example, water can decrease the magnitude of the enthalpy of adsorption of organic reactants35 or provide new pathways for hydrogen/proton transfer.36,37
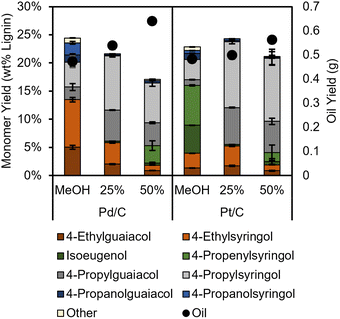 | ||
| Fig. 2 Lignin product monomer yields and oil yields (black circles, right axis) from H2-free RCF reactions with water/methanol solvent mixtures. Table S11.† Conditions: 2 g poplar, 100 mg catalyst, 3 hours, 225 °C. Percentages are volume percentages of water in the solvent mixture. The error bars represent the standard deviation of triplicate measurements. | ||
Model compound reactions
Previous work has shown that aryl-ether linkages in lignin can be cleaved in solution, followed by stabilization of smaller reactive intermediates.15 Furthermore, reports have postulated that the role of the catalyst is to stabilize coniferyl alcohol-like intermediates derived from solvolytic β-O-4 cleavage, rather than on dimers or oligomers with the β-O-4 linkage directly.38,39 Thus, to investigate the role of the metal catalyst further in H2-free RCF, batch reactions were performed on two common lignin model compounds, coniferyl alcohol (Fig. 3A) and guaiacylglycerol-beta-guaiacyl ether (GGE) (Fig. 3B). Both of these model compounds readily undergo degradation reactions at high temperatures,20,38 allowing for monomer yields to represent the relative rates of stabilization and condensation, similar to RCF reactions on poplar.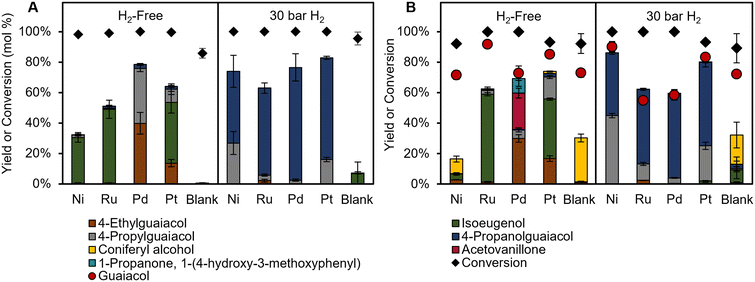 | ||
| Fig. 3 Monomer product yields for model compound reactions with (A) coniferyl alcohol and (B) guaiacylglycerol-beta-guaiacyl ether (GGE) under H2-free conditions (left) and with 30 bar H2 (right). Note: for clarity, the bar for coniferyl alcohol is not shown in A. Conditions: 60 mg substrate, 20 mg catalyst, 30 mL methanol, 1 hour at 225 °C. Error bars are the standard deviation of triplicate measurements. Numerical data are provided in Tables S6–S9.† | ||
Blank reactions of GGE without catalyst achieved 92 ± 6% conversion and a 29 ± 3% yield of coniferyl alcohol, confirming that the cleavage of the ether bond occurs readily in solution. Guaiacol yields were consistently lower than GGE conversion, suggesting that guaiacol may either be consumed in condensation pathways, or that GGE can condense before C–O bond cleavage to liberate guaiacol. Monomer yield trends for reactions with GGE differed from results obtained with poplar. When Pd/C was used under H2-free conditions, previously unreported products acetovanillone and 1-propanone, 1-(4-hydroxy-3-methoxyphenyl) were formed at 24 ± 3 and 10 ± 5% yield respectively. Reactions starting from these ketone products under identical conditions showed low conversion (<10%) to other conventional RCF products, ruling them out as intermediates. While GGE is not completely representative of lignin during RCF, the lack of ketone products during poplar RCF supports the mechanism proposed above, where dimers or oligomers with β-O-4 linkages are cleaved in solution to yield reactive intermediates that then undergo reductive stabilization or condensation.
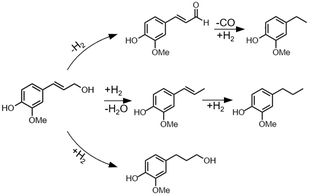
Coniferyl alcohol reactions under H2-free conditions showed good agreement compared to poplar RCF, with Pd/C and Pt/C achieving higher product yields than Ru/C and Ni/C. RCF typically forms propyl or propanol substituted products through hydrodeoxygenation and/or double bond hydrogenation. However, Pd/C, and to a lesser extent Pt/C, can form ethyl products from coniferyl alcohol under H2-free conditions, in line with this work and earlier reports.7 Through studies of simpler alcohols, Barteau and co-workers concluded that metals such as Pd, Pt, and Ni induce C–C scission through dehydrogenation to an acyl intermediate.40,41 To test this proposed mechanistic pathway, we used coniferyl aldehyde as a starting material in model compound experiments that mirror the RCF experimental conditions (Fig. S6†). The use of Pd/C and Pt/C in an H2-free context resulted in the formation of ethyl guaiacol in 79 ± 2% and 50 ± 4% yield, respectively, indicating that dehydrogenation followed by decarbonylation is a possible reaction step to form ethyl products. Conversely, the use of Ru/C and Ni/C in the same conditions exhibited ethyl guaiacol yields of 12 ± 5% and 4 ± 2%, respectively, which is higher than yields for reactions starting from coniferyl alcohol (<1%). This confirms that Ru/C and Ni/C do not dehydrogenate coniferyl alcohol to a large extent, and instead mainly perform HDO (Scheme 1).
Conclusions
Four common RCF catalysts were compared for their RCF monomer yields with and without external H2 gas added to the reaction. Clear differences were observed in monomer yield and selectivity under H2-free conditions. Pd/C and Pt/C retained high monomer yields, while the lack of H2 decreased monomer yields for Ru/C and Ni/C. Neither the H2 yields from poplar RCF nor from methanol reforming control reactions correlated with monomer yield. Coniferyl alcohol model compound reactions demonstrated good agreement with poplar RCF monomer yields relative to a β-O-4 model compound, GGE, which supports the previously proposed mechanism where the species reacting on the catalyst is a monomer rather than a dimer or larger. Ultimately, these results show that catalyst choice can impact monomer yields in H2-free reactions, and that more investigation into the stabilization mechanisms is needed to elucidate the origin of activity differences between catalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was authored by the National Renewable Energy Laboratory, operated by the Alliance for Sustainable Energy, LLC, for the U.S. Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. JKK, YRL, GTB, and JWM acknowledge funding from The Center for Bioenergy Innovation, a U.S. DOE Bioenergy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science. JKK acknowledges partial support from a Dept. of Education GAANN graduate fellowship. DGB, WEM, and GTB acknowledge funding from the U.S. DOE Office of Energy Efficiency and Renewable Energy Bioenergy Technologies Office. The views expressed in the article do not necessarily represent the views of the DOE or the U.S. Government. The U.S. Government retains and the publisher, by accepting the article for publication, acknowledges that the U.S. Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for U.S. Government purposes.References
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS.
- R. Davis, L. Tao, E. Tan, M. Biddy, G. Beckham, C. Scarlata, J. Jacobson, K. Cafferty, J. Ross and J. Lukas, Process design and economics for the conversion of lignocellulosic biomass to hydrocarbons: dilute-acid and enzymatic deconstruction of biomass to sugars and biological conversion of sugars to hydrocarbons, Report NREL/TP-5100-60223, National Renewable Energy Laboratory (NREL), Goldon, CO (US), 2013 Search PubMed.
- A. Corona, M. J. Biddy, D. R. Vardon, M. Birkved, M. Z. Hauschild and G. T. Beckham, Green Chem., 2018, 20, 3857–3866 RSC.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 1246843 CrossRef PubMed.
- K. Huang, P. Fasahati and C. T. Maravelias, iScience, 2020, 23, 100751 CrossRef CAS.
- W. Schutyser, T. Renders, S. Van Den Bosch, S. F. Koelewijn, G. T. Beckham and B. F. Sels, Chem. Soc. Rev., 2018, 47, 852–908 RSC.
- S. Van Den Bosch, W. Schutyser, S. F. Koelewijn, T. Renders, C. M. Courtin and B. F. Sels, Chem. Commun., 2015, 51, 13158–13161 RSC.
- W. Schutyser, S. Van Den Bosch, T. Renders, T. De Boe, S. F. Koelewijn, A. Dewaele, T. Ennaert, O. Verkinderen, B. Goderis, C. M. Courtin and B. F. Sels, Green Chem., 2015, 17, 5035–5045 RSC.
- T. Renders, S. Van Den Bosch, T. Vangeel, T. Ennaert, S. F. Koelewijn, G. Van Den Bossche, C. M. Courtin, W. Schutyser and B. F. Sels, ACS Sustainable Chem. Eng., 2016, 4, 6894–6904 CrossRef CAS.
- E. M. Anderson, M. L. Stone, M. J. Hu, G. T. Beckham and Y. Román-Leshkov, ACS Sustainable Chem. Eng., 2018, 6, 7951–7959 CrossRef CAS.
- Y. Li, L. Shuai, H. Kim, A. H. Motagamwala, J. K. Mobley, F. Yue, Y. Tobimatsu, D. Havkin-Frenkel, F. Chen, R. A. Dixon, J. S. Luterbacher, J. A. Dumesic and J. Ralph, Sci. Adv., 2018, 4 DOI:10.1126/sciadv.aau2968.
- T. Renders, E. Cooreman, S. Van Den Bosch, W. Schutyser, S. F. Koelewijn, T. Vangeel, A. Deneyer, G. Van Den Bossche, C. M. Courtin and B. F. Sels, Green Chem., 2018, 20, 4607–4619 RSC.
- N. Yan, C. Zhao, P. J. Dyson, C. Wang, L. T. Liu and Y. Kou, ChemSusChem, 2008, 1, 626–629 CrossRef CAS PubMed.
- E. M. Anderson, L. Michael, R. Katahira, G. T. Beckham, E. M. Anderson, M. L. Stone, R. Katahira, M. Reed, G. T. Beckham and Y. Román-Leshkov, Joule, 2017, 1, 613–622 CrossRef CAS.
- L. Chen, A. P. Van Muyden, X. Cui, Z. Fei, N. Yan, G. Laurenczy and P. J. Dyson, JACS Au, 2021, 1, 729–733 CrossRef CAS PubMed.
- X. Ouyang, X. Huang, J. Zhu, M. D. Boot and E. J. M. Hensen, ACS Sustainable Chem. Eng., 2019, 7, 13764–13773 CrossRef CAS.
- S. Su, L.-P. Xiao, X. Chen, S. Wang, X.-H. Chen, Y. Guo and S.-R. Zhai, ChemSusChem, 2022, 15, 1–10 CrossRef.
- I. Klein, B. Saha and M. M. Abu-Omar, Catal. Sci. Technol., 2015, 5, 3242–3245 RSC.
- X. Liu, S. Feng, Q. Fang, Z. Jiang and C. Hu, Mol. Catal., 2020, 495 CAS.
- Y. Li, B. Demir, L. M. Vázquez Ramos, M. Chen, J. A. Dumesic and J. Ralph, Green Chem., 2019, 21, 3561–3572 RSC.
- I. Klein, C. Marcum, H. Kenttämaa and M. M. Abu-Omar, Green Chem., 2016, 18, 2399–2405 RSC.
- A. W. Bartling, M. L. Stone, R. J. Hanes, A. Bhatt, Y. Zhang, M. J. Biddy, R. Davis, J. S. Kruger, N. E. Thornburg, J. S. Luterbacher, R. Rinaldi, J. S. M. Samec, B. F. Sels, Y. Román-Leshkov and G. T. Beckham, Energy Environ. Sci., 2021, 14, 4147–4168 RSC.
- M. V. Galkin, S. Sawadjoon, V. Rohde, M. Dawange and J. S. M. Samec, ChemCatChem, 2014, 6, 179–184 CrossRef CAS.
- M. V. Galkin, A. T. Smit, E. Subbotina, K. A. Artemenko, J. Bergquist, W. J. J. Huijgen and J. S. M. Samec, ChemSusChem, 2016, 9, 3280–3287 CrossRef CAS PubMed.
- M. V. Galkin and J. S. M. Samec, ChemSusChem, 2014, 7, 2154–2158 CrossRef CAS PubMed.
- P. Ferrini and R. Rinaldi, Angew. Chem., 2014, 126, 8778–8783 CrossRef.
- I. Graça, R. T. Woodward, M. Kennema and R. Rinaldi, ACS Sustainable Chem. Eng., 2018, 6, 13408–13419 CrossRef.
- T. Ren, S. You, Z. Zhang, Y. Wang, W. Qi, R. Su and Z. He, Green Chem., 2021, 23, 1648–1657 RSC.
- D. G. Brandner, J. S. Kruger, N. E. Thornburg, G. G. Facas, J. K. Kenny, R. J. Dreiling, A. R. C. Morais, T. Renders, N. S. Cleveland, R. M. Happs, R. Katahira, T. B. Vinzant, D. G. Wilcox, Y. Román-Leshkov and G. T. Beckham, Green Chem., 2021, 23, 5437–5441 RSC.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Determination of Structural Carbohydrates and Lignin in Biomass, NREL/TP-510-42618, US National Renewable Energy Laboratory, Golden, Colorado, 2008 Search PubMed.
- M. M. Abu-Omar, K. Barta, G. T. Beckham, J. S. Luterbacher, J. Ralph, R. Rinaldi, Y. Román-Leshkov, J. S. M. Samec, B. F. Sels and F. Wang, Energy Environ. Sci., 2021, 14, 262–292 RSC.
- Z. Dou, Z. Zhang, M. Wang, Z. Zhang and M. Wang, Appl. Catal., B, 2022, 301, 120767 CrossRef CAS.
- L. Li, L. Dong, D. Li, Y. Guo, X. Liu and Y. Wang, ACS Catal., 2020, 10, 15197–15206 CrossRef CAS.
- F. Héroguel, X. T. Nguyen and J. S. Luterbacher, ACS Sustainable Chem. Eng., 2019, 7, 16952–16958 CrossRef.
- N. Singh and C. T. Campbell, ACS Catal., 2019, 9, 8116–8127 CrossRef CAS.
- J. Shangguan, A. J. R. Hensley, M. V. Gradiski, N. Pfriem, J. S. McEwen, R. H. Morris and Y. H. C. Chin, ACS Catal., 2020, 10, 12310–12332 CrossRef CAS.
- B. Demir, T. Kropp, E. B. Gilcher, M. Mavrikakis and J. A. Dumesic, J. Catal., 2021, 403, 215–227 CrossRef CAS.
- S. Van Den Bosch, T. Renders, S. Kennis, S. F. Koelewijn, G. Van Den Bossche, T. Vangeel, A. Deneyer, D. Depuydt, C. M. Courtin, J. M. Thevelein, W. Schutyser and B. F. Sels, Green Chem., 2017, 19, 3313–3326 RSC.
- H. Li and G. Song, ACS Catal., 2019, 9, 4054–4064 CrossRef CAS.
- J. L. Davis and M. A. Barteau, Surf. Sci., 1987, 187, 387–406 CrossRef CAS.
- M. Mavrikakis and M. A. Barteau, J. Mol. Catal. A: Chem., 1998, 131, 135–147 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2re00275b |
| This journal is © The Royal Society of Chemistry 2022 |