Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A practical guide to automating fluorine-18 PET radiochemistry using commercially available cassette-based platforms†
Chris
Barnes
a,
Manoj
Nair
b,
Eric O.
Aboagye
a,
Stephen J.
Archibald
 cde and
Louis
Allott
cde and
Louis
Allott
 *cde
*cde
aComprehensive Cancer Imaging Centre, Faculty of Medicine, Department of Surgery and Cancer, Imperial College London, Hammersmith Hospital, Du Cane Road, London, W12 0NN, UK
bGE Healthcare, GEMS PET Systems, Uppsala, Sweden
cPositron Emission Tomography Research Centre, Faculty of Health Sciences, University of Hull, Cottingham Road, Kingston upon Hull, HU6 7RX, UK. E-mail: louis.allott@hull.ac.uk
dDepartment of Biomedical Sciences, Faculty of Health Sciences, University of Hull, Cottingham Road, Kingston upon Hull, HU6 7RX, UK
eHull University Teaching Hospital NHS Trust, Castle Hill Hospital, Castle Road, Cottingham, HU16 5JQ, UK
First published on 13th July 2022
Abstract
The automation of positron emission tomography (PET) radiochemistry using cassette-based automated radiosynthesis platforms is an essential component of clinical translation for the vast majority of 18F-based radiopharmaceuticals. The technology is widely adopted by good manufacturing practice (GMP) compliant radiopharmaceutical production facilities and research institutions developing novel tracers for clinical studies. Despite automation being fundamental to clinical translation, educational resources which introduce this branch of radiochemistry to the uninitiated are limited. Publications featuring automation assume previous experience of using these platforms and therefore, the detail they provide may not be sufficient for a novice user. In this Tutorial Account, we aim to bridge this knowledge gap and provide a resource for efficient automation for radiochemists across all levels of experience.
Introduction
Automating positron emission tomography (PET) radiochemistry is essential for the production of almost all fluorine-18 based radiopharmaceuticals intended for clinical use.1,2 With exception, the aluminium–[18F]fluoride ([18F]AlF) method permits a kit-based approach to labelling peptides mirroring gallium-68 radiopharmaceutical production methods, although automated [18F]AlF-labelling is proving popular for large scale syntheses.3–5 PET radiopharmaceuticals must be manufactured under controlled and regulated conditions which safeguard the quality of the product for use in humans; these are known as good manufacturing practices (GMP), which describe the required minimum standards in the production processes of medicines.6 The governance of GMP is overseen by regulatory agencies, for example the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK, the European Medicines Agency (EMA) in the EU, and the Food and Drug Administration (FDA) in the US.7 Automated radiochemistry offers batch reproducibility in PET radiopharmaceutical production which compliments the requirements for GMP compliant production.8 Reproducibility is confirmed by process validation (PV), which ensures the radiopharmaceutical is manufactured to its minimum quality specification.9 In addition to GMP considerations, automation provides radiation protection to the user, when the platform is isolated inside a hot cell, allowing for very large activities of [18F]fluoride (>100 GBq) to be manipulated safely.Increasing clinical demand for 18F-based radiopharmaceuticals has driven innovation in automated radiosynthesis platforms. There are numerous commercially available platforms on the market, and all have been exemplified for the GMP production of radiopharmaceuticals (Fig. 1).2,10,11 The plethora of available platforms and the necessity of automated radiosynthesis to support the clinical translation has led to their acquisition by academic institutions to progress proprietary tracers into first-in-human studies. As a result, automated radiosynthesis protocols are more frequently published as part of novel radiopharmaceutical research. Similarly, as new radiochemistry methodology emerges, automation usually follows with the aim of encouraging radiochemists to adopt new labelling techniques by demonstrating relevance to clinical tracer production.
 | ||
| Fig. 1 Examples of two commercially available automated radiosynthesis platforms: A) GE FASTlab 2; B) Trasis AllInOne. Images provided and approved for reproduction by GE Healthcare and Trasis. | ||
The challenge we face as a community, which this Tutorial Account aims to address, is that literature describing automated radiosynthesis assumes familiarity of using the platform. A publication may describe a particular operation, for example the mixing of two reagents, but an assumption is made that the reader will know exactly how to perform this operation efficiently using their system. Those skilled in the art are aware of several routes to achieve this seemingly simple step and have a grasp of how these routes influence robustness, reproducibility, accuracy and precision, as well as potential impact of cross-contamination on later synthetic steps.
In our experience, this insight is generally not published in the scientific literature, and there are no in-depth textbooks on the topic. Instead, users acquire knowledge from others with years of practical experience in the art. This is an excellent way to learn new skills, but the availability of such a resource varies between institutions. Manufacturers of automated platforms are often willing to help and provide newcomers with a comprehensive education on their system; however, for younger scientists in the field – particularly PhD students on short-term contracts – it isn't always feasible to engage with the manufacturer to provide this education.
This Tutorial Account is a focused guide designed to fill a knowledge gap in automating fluorine-18 radiochemistry on cassette-based platforms
We intend to provide novice users with a foundation of understanding to aid the implementation of published procedures, or to develop new automated methods for novel tracers. Although fluorine-18 is the focus of this Tutorial Account, there are concepts amendable to other isotopes. We focus on five fundamental areas of automated radiosynthesis: section 1) method planning and cassette design; section 2) liquid handling; section 3) reactors and reactions; section 4) purification and reformulation; section 5) optimisation and troubleshooting. The relationship between these is shown in Fig. 2. | ||
| Fig. 2 This Tutorial Account will discuss four fundamental aspects of automated radiosynthesis. RCY = radiochemical yield; Am = molar activity. | ||
This Tutorial Account is not a user manual for any specific cassette-based automated platform
All platforms share common characteristics in design and function, but some proprietary differences between manufacturers can occur. This Tutorial Account has been written so that key concepts are compatible with all platforms; therefore, general illustrations of cassettes have been used to demonstrate methods and operations. The authors have experience in using two popular systems: the GE FASTlab and Trasis AllInOne (AIO) and therefore, undoubtedly, this Tutorial Account will have been influenced by our experience in using these systems.Section 1: method planning & cassette design
The motivation behind automating a radiosynthesis is an important contributing factor towards planning the method. For example, automating a production for clinical translation requires careful consideration of GMP compatibility, especially of reagents. We recommend referring to the relevant regional pharmacopoeia for advice on “clinic friendly” reagents, as well as The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines.12–14 GMP compatibility does not need to be considered when automating a method for pre-clinical studies. Although it may seem like automation is solely to aid clinical translation, it can be incredibly useful in supporting pre-clinical work. For example, to allow higher activities of [18F]fluoride to be safely used in the radiosynthesis, boosting radioactive concentration and molar activity. Radiation safety is a vitally important consideration when developing new automated radiosynthetic methods. The guiding principle of radiation safety is “as low as reasonably achievable” (ALARA, US)15 or “as low as reasonably practicable” (ALARP, UK);16 in essence, consider using the lowest amount of radioactivity necessary to test the method during the development phase of your work, if you intend to enter the hot cell at the end of synthesis before significant radioactive decay has occurred (i.e. 24 h).15,16 An automated radiosynthesis protocol is comprised of three main components (Fig. 3): the sequence, the cassette, and the radiochemistry, which are interlinked and inform the design of one another. When approaching automation, it is likely that users already have radiochemistry established, either as a literature procedure or a radiosynthesis method performed in their laboratory by means of a manual process. | ||
| Fig. 3 The basics of automating fluorine-18 radiochemistry using cassette-based automated radiosynthesis platforms. Arrows show how each component interlinks and informs the design of one another. | ||
1.1 Basics of the cassette
The cassette is a consumable which contains all the necessary reagents, solid phase extraction (SPE) purification cartridges, dilution reservoirs (and more!) required to complete a radiosynthesis and subsequent purification/formulation of the radiopharmaceutical for biological or clinical use (Fig. 4). Cassette-based systems can increase the cost of a radiosynthesis because they are disposable, but their single-use nature is responsible in part for their popularity in clinical production owed to being highly compliant with GMP. The cassettes are manufactured, sterilised, and sealed under cleanroom conditions; once used, they can be disposed of, and a new cassette used for the next radiosynthesis. Cassettes are often pre-populated with pre-filled reagent vials, allowing for convenient set up and operation, reducing the potential for operator error and giving more control over the radiosynthesis. On fixed reactor systems, there is typically an extensive cleaning protocol is required between each radiosynthesis, which prolongs routine production time. The cleaning process must be validated prior to first use to ensure all chemical and biological contaminants are removed, which is also time-consuming.9 The cassette can also be linked to external equipment like a high-performance liquid chromatography (HPLC) purification system. | ||
| Fig. 4 Cassettes for the synthesis of [18F]FDG, proprietary to the A) Trasis AIO and B) GE FASTlab platform. Images provided and approved for reproduction by GE Healthcare and Trasis. | ||
A schematic representation of a cassette is shown in Fig. 5A. Cassettes are typically used in a left-to-right direction, indicated by the flow of inert gas for applying positive pressure at the start of the cassette; and a vacuum to create negative pressure at the end of the cassette. The vacuum typically sits over a waste bottle so that solvents/reagents removed from the cassette can be collected for disposal. Another key component to a cassette is it's valves, which are controlled by actuators on the automated platform. Changing the position of the valves, as indicated in Fig. 5B, will change the path of fluids in the cassette. They can also be used to seal a particular path to prevent fluid from flowing in one direction. Luer-lock fittings typically sit on top of the valves for attaching tubing, allowing fluids to be routed into reactors, moved around the cassette or off-board into larger dilution vessels. In addition to Luer-lock fittings, it is commonplace to find vial spikes for piercing septa of reagent vials. On some systems, these are fixed into the cassette and on others there is flexibility of their positioning. Common consumables used with cassette-based platforms are shown in Table 1, alongside a graphic of how these are represented throughout this Tutorial Account.
1.2 Basics of the sequence
The sequence is a program developed using proprietary software compatible with your automated platform. The sequence is developed and controlled via an intuitive graphical user interface (GUI), two examples are shown in Fig. 6. Its task is to control cassette in a logical, stepwise manner to move reagents, heat/cool reactors, load/wash/elute SPE cartridges. As well as sending instructions to the platform, the software also receives and displays real-time feedback (i.e. activity levels, reactor temperatures, actuator/syringe positions). Advanced logic operations like IF statements can be inserted into sequences, for example, to tell the system to progress onto the next step when certain conditions have been met (i.e. proceed once the reactor has reached a set temperature). A successful automated method requires both a well design cassette and sequence and therefore we will cover how to go about this throughout this Tutorial Account.There are almost endless possibilities for the type of reactions that can be automated; however, there are some limitations including challenging solvents, reaction conditions, number of reaction steps, physical properties of reagents and purification strategies, which will be discussed later. Usually, the vast majority of fluorine-18 radiolabelling follows one of three processes shown in Fig. 7, which can be conveniently accommodated on most cassette-based systems.
 | ||
| Fig. 7 Three common radiolabelling processes which can be conveniently automated by most cassette-based platforms. | ||
1.3 Planning to automate
Developing a new and efficient automated radiosynthesis method takes time and therefore, planning is key! The capacity of the cassette (i.e. the number of reagents and consumables it can hold) is an important consideration when deciding how to best automate a radiosynthesis. This is often a major limitation when automating a complex process, however innovative workarounds may be possible. It is good practice to sketch out the cassette and decide where components should be placed; even better, assemble a trial cassette in the laboratory and populate it with the necessary components. This gives a clearer view of the potential for automating a process, but also allows for the identification of potential snags early in the process. As many (if not all) cassette-based platforms are designed to be used in [18F]FDG production, the radiosynthesis of tracers by nucleophilic substitution followed by a simple hydrolysis and purification, is straightforward to automate; whereas the radiolabelling of more complex molecules like peptides and proteins, or small molecules which require multi-step post-radiolabelling assembly, can be a lot more challenging to automate, but not impossible!17,18Once satisfied that you can feasibly fit the entire radiosynthesis onto a single cassette, potential for cross-contamination and its impact on producing chemically and radiochemically pure tracers should be considered. Traces of reagents used in a radiosynthesis can stick to the inside of a cassette, and therefore it may be necessary to consider ways to minimise the impact of this. Washing steps are vitally important to include in a sequence; for example, after transferring a reagent to the reactor, consider washing the cassette with a water and/or solvent (i.e. ethanol, acetonitrile) mixture, drying under inert gas and vacuum. This can be an effective method for removing unwanted reagents from the cassette and minimising contamination on subsequent steps in the sequence. In addition to washing, it is also possible to design mitigations into a cassette which prevent or minimise unnecessary contamination. This includes reducing the distance between the reagent of concern and its destination on the cassette (i.e. the reactor). This will reduce the surface area of the contamination and potentiates more thorough removal of trace reagent via a washing step.
When designing a cassette, it is often useful to visualise the radiosynthesis process moving along the cassette from left to right, with a “dirty” zone on the left where the bulk of the synthesis of performed and a “clean” zone on the right for purification (Fig. 8); this helps remove potential cross-contamination of reagents with purified/reformulated product.
 | ||
| Fig. 8 A schematic highlighting the designation of “dirty” and “clean” zones in a cassette, and the general direction of workflow (white arrow). | ||
Reagents used in the radiosynthesis cannot be completely excluded from the “clean” zone, as the vacuum draws waste products to the right-hand side of the cassette; however, note that valves, syringes, and vials which handle concentrated reagents can be challenging to clean as they may harbour dead volumes. Isolating these components to zones on the cassette can significantly reduce the likelihood of contaminating a purified tracer.
Section 2: liquid handling
Different laboratories take different approaches to developing novel radiotracers for pre-clinical use. Some take a hands-on approach to radiochemistry, performing manual operations by pipette and syringe to label their precursors. This provides high precision and dexterity of manipulation, which can be challenging to automate – particularly when handling small volumes. Some laboratories use automated platforms like the GE TRACERLab, which doesn't require a cassette but allows flexibility in manipulating reagents on an automated platform. Either way, replicating accurate liquid handling using a cassette-based platform can be challenging. In this section, we will describe methods in which fluids can be moved around a cassette and how to overcome notorious challenges.2.1 Moving reagents from A to B by syringe
The most obvious way of moving liquids around a cassette is by utilising the on-board syringe drivers. Advantages of syringe drivers include control over volume and flowrate (Fig. 9). This tool is useful for automating careful and controlled operations such as flowing liquids through SPE cartridges and adding relatively consistent volumes of reagents to the reactor. If you are familiar with using syringes as part of a hands-on radiosynthesis, then these operations can be mirrored by the automated syringe. An important consideration is that any reagent vial which has had some liquid removed, either partially or completely, will reduce in pressure; therefore, reagent vials should be pressurised before the synthesis for more efficient transfer and to negate generating negative pressure inside the vial. This not only improves reagent transfer but reduces the accumulation of residual reagent in the cassette manifold. Similarly, to reduce the accumulation of residual reagents after each transfer, the cassette should be flushed with solvent and/or inert gas.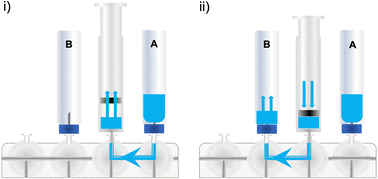 | ||
| Fig. 9 A schematic showing how liquids can be moved by syringe, allowing for control over volume and flowrate: i) syringe is loaded from A, and ii) expelled into B. | ||
2.2 Moving and mixing reagents by syringe
The syringe itself can also be useful as an additional vessel for mixing two different liquids. In Fig. 10, the syringe is used to take up reagent A, followed by reagent B which are mixed in the syringe. Unlike the manual operation of a syringe, whereby surface tension prevents liquids from flowing out until a positive pressure is exerted, the valve on the cassette provides additional functionality. If the valve is closed and the plunger is fully extended, negative pressure is created inside the barrel of the syringe (Fig. 11). Opening a pathway between a reagent vial and the syringe will result a high-pressure jet of liquid entering the syringe barrel, thus efficiently mixing one liquid into another. As liquids are non-compressible, the reverse operation whereby pressure is exerted in the syringe against a closed vial should be avoided and may result in damage to the cassette. | ||
| Fig. 11 Highly efficient mixing of two reagents in a syringe can be achieved by creating negative pressure in the barrel. | ||
2.3 Moving and mixing reagents by displacement with positive/negative pressure
Positive pressure (i.e. inert gas flow) and negative pressure (i.e. vacuum) can be used effectively to move liquids around a cassette. This is well suited to instances where complete transfer of liquid from one position to another is required; there is little control over the flowrate or volume of this transfer method. Typical use cases include removing the contents of the reactor to a dilution vial for further purification; this prevents contaminating the syringe, which can be particularly important for a GMP production (Fig. 12). The contents of reagent vials can also be transferred into a reactor by use of positive pressure; in this instance, the vial can be pressurised with inert gas and sealed by the cassette valve. Upon creating an open pathway to the reactor, the valve can be released, and the contents of the reagent vial will evacuate into the reactor (Fig. 12). This is a rapid and efficient way to add the entire contents of a reagent vial to a reactor, although it may need to be repeated twice for larger volumes. Again, this is useful for avoiding syringe contamination.2.4 Overcoming limitations of liquid transfer
A limitation of cassette-based platforms is their ability to effectively handle small reagent volumes (<300 μL) and one of the first considerations when automating a radiosynthesis is to ensure small volumes are appropriately scaled. Reagents are typically stored in inverted vials sealed with crimped septa and accessed through a hollow spike which pierces the septa; however, this design introduces a dead volume, sometimes up to 400 μL, where reagent becomes inaccessible (Fig. 13). This is of negligible concern where required reagent volumes are large but of significant impact for small volumes, or instances where a precise molar quantity of reagent is needed for the reaction. For example, if 10 nmol of reagent A is added to the reactor in 500 μL solvent, and the vial has a dead volume of 100 μL, then the vial should hold 12 nmol of reagent A in a total volume of 600 μL, to ensure the correct addition of 10 nmol in 500 μL. Systemic losses owed to moving reagents around a cassette can be challenging, and as a result, the quantity of reagent added to the reaction may be lower than anticipated. This is of particular concern when handling small reagent volumes or reagents with physical properties which result in their adhesion to the cassette or tubing. As previously mentioned, this may be addressed by using larger volumes and/or shortening the distance that the reagent travels across the cassette; in addition, washing the cassette into the reactor with solvent may remove some reagents, or in the case of proteins, washing the plastics with human serum albumin (HSA) can minimise surface interactions. On some cassettes, it is possible to use a 1 mL syringe filled with reagent and overcome the dead volume associated with septa-pierced vials.Although it is challenging to handle small volumes (<300 μL) on a cassette-based system, there are workarounds to achieve this. It is possible to use dead volumes in the cassette manifold to achieve the addition of smaller volumes of reagents. The GE FASTlab cassette has a dead volume of 25 μL between valves. This void (or multiples of) can be filled with reagent and trapped by closing both valves (Fig. 14). Excess reagent from either side of the valves is removed under vacuum, allowing the precise volume of liquids to be added to the reactor. This operation must be designed into the cassette layout and requires multiple positions, so it may be challenging to include in complicated multistep processes.
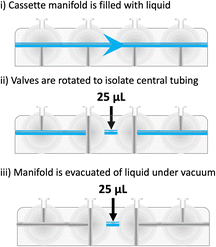 | ||
| Fig. 14 Using dead volumes between valves on a cassette manifold for more accurate measurement and addition of reagents. | ||
Section 3: reactors & reactions
The most frequently used chemistry for incorporating fluorine-18 into organic molecules is nucleophilic substitution (SN2) or nucleophilic aromatic substitution (SNAr); however, extensive research into new and more flexible approaches to forming carbon–[18F]fluorine bonds has highlighted some prominent additions to the repertoire of radiochemistry which are now utilised in the radiosynthesis of new tracers.19–21 In addition, radiochemistry has developed beyond carbon–[18F]fluorine bonds, and practical solutions including silicon–[18F]fluorine, boron–[18F]fluorine, sulfur–[18F]fluorine and aluminium–[18F]fluorine bonds have risen to prominence.22 These chemistries have been automated.43.1 The reactor
Radiolabelling reactions typically take place inside a reactor. Reactor vials are designed to allow for the addition and removal of reagents via a central tube which runs to the base of the reactor. It is also common for vials to be connected to inert gas and vacuum lines, particularly where azeotropic [18F]fluoride drying is required (Fig. 15).Reactors can be heated as a closed system and are sealed by closing valves on the cassette manifold. There are temperature limits to which reactors can be heated before they degrade. For example, the FASTLab platform can heat a reactor to 200 °C, but the material used in its reactor (cyclic olefin copolymer, COC) has a maximum tolerance of 140 °C. If high temperatures are required, then check compatibility with the manufacturer of your platform. There are reactors available which tolerate higher temperatures if required (e.g. glassy carbon). Reactors do not typically have stirring capabilities; however, this is rarely a limitation as excellent mixing and agitation can be achieved by applying a gentle flow of inert gas through the tube of the reactor which sits at the bottom of the reaction mixture, either continuously or periodically (Fig. 16). It is important to consider the flow rate when mixing a reaction, particularly when mixing a low reaction volume (<1 mL). If the flow rate is too high, then vigorous agitation of the reaction mixture may result in loss of reactor contents to the vessel walls above the solvent line and thus reduce overall yield. Increasing the reaction volume or reducing the vigour in which the reaction is mixed, can significantly improve radiochemical yield.
3.2 Automating [18F]fluoride drying
Nucleophilic [18F]fluoride is essential for the vast majority of labelling reactions, and can be achieved by azeotropic drying. An overview of the process is shown in Fig. 17. In brief, [18F]fluoride in oxygen-18 enriched water from the cyclotron target is brought onto the cassette under vacuum through a quaternary methyl ammonium (QMA) SPE cartridge; oxygen-18 enriched water is recovered into a separate vial. The [18F]fluoride is eluted from the QMA cartridge into the reactor using a mixture of acetonitrile/water (i.e. 80:20) containing an inorganic base (i.e. K2CO3) with a phase transfer catalyst (i.e. Kryptofix-222®); after which, the solvent is removed by evaporation under the flow of inert gas and vacuum. To the dry [18F]fluoride, a radiochemistry precursor (and any other necessary reagents) is added in solvent.While the process is straightforward, there are some important considerations when drying [18F]fluoride and using it for labelling. High gas flow rates can result in rapid drying, however they have a tendency to spray the [18F]fluoride solution around the reactor. This is a problem when a portion of the [18F]fluoride dries towards the top of the reactor, in a position inaccessible to the precursor and thus reduces the radiochemical yields (Fig. 19). A slow and steady approach to [18F]fluoride drying can improve the efficiency of subsequent chemistry.
3.3 Hydrolysis of protecting groups
The reactor is often used for [18F]fluoride incorporation but may be rinsed with water/solvent for use in post-labelling chemistry such as the hydrolysis of protecting groups. Typically, protecting groups used in PET radiochemistry are labile in acid and/or basic conditions and there are several ways to automate this chemistry.Firstly, protecting groups can be removed in the reactor without prior purification of the protected intermediate. Base-labile protecting groups (e.g. esters) can partially hydrolyse under basic 18F-fluorination conditions, and therefore the addition of more base at the end of the fluorination drives the reaction to completion. The hydrolysis of acid-labile protecting groups (e.g. tert-butyl) via this method requires some caution, as adding acid to unreacted [18F]fluoride will liberate volatile [18F]HF. To avoid this, trap the crude reaction mixture onto an SPE cartridge, rinse the reactor with water to remove the bulk of unreacted [18F]fluoride, and elute the crude material back into the reactor for the hydrolysis. If the reaction yield of the 18F-fluorination is high, and the starting activities are low (determined by permitted limits of volatile release from a given facility) then the liberation of [18F]HF may be of little concern.
Another approach may be to perform the hydrolysis in a separate reactor to the 18F-fluorination. Your automated platform may have two reactors available for use, or you may wish to use a reactor (i.e. Wheaton vial) external to the cassette. Either way, trapping the crude reaction material onto an SPE cartridge and eluting into another reactor for hydrolysis, or simply moving the bulk solvent containing the crude reaction mixture into another reactor, are feasible methods to perform a hydrolysis reaction in a separate vessel.
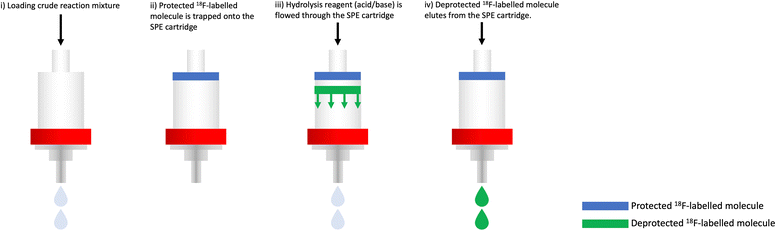
An alternative approach is to perform the hydrolysis on an SPE cartridge and remove the need for reaction vessel (Fig. 18). In this instance, the SPE cartridge can perform two functions. For example, if the protected 18F-fluorinated intermediate is lipophilic enough to be retained on a C18 SPE cartridge, then the SPE serves to purify the molecule from polar components (i.e. unreacted [18F]fluoride). With the protected 18F-fluorinated intermediate trapped on the SPE cartridge, acid/base can be flowed through the SPE cartridge to simultaneously hydrolyse the protecting groups and elute the more polar deprotected product. This is particularly useful if the hydrolysis is the final step in the radiosynthesis, as the eluent containing the desired hydrolysed radiotracer may be adjusted for pH and buffered for biological use. A very important consideration for developing GMP compliant methods, which is applicable but not exclusive to hydrolysis reactions, is the generation of “leachable” compounds. Harsh reagents can cause some degradation of consumables like tubing which can transfer into the final tracer dose and be identified by QC analysis. Avoiding the use of excessively strong acids and bases is an effective way to minimise this risk.
3.4 Automated radiochemistry on a solid support
One constraint of cassette-based platforms is the limited space on the cassette to fit the consumables and reagents necessary to perform a labelling, purification, and formulation. As a radiosynthesis becomes more complex and the number of steps required to label a compound increase, maximising the available cassette space is essential. Utilising solid supports as “reactors” or “reagents” is one way to increase the flexibility of the cassette.Using an SPE cartridge as a reactor was discussed in section 3.3, where an on-cartridge hydrolysis can serve as both a reaction vessel and a purification strategy. However, it is possible to perform different chemistries on SPE cartridges, as exemplified for copper-catalysed azide-alkyne cycloaddition (CuAAC) “click” chemistry.18 The benefits of this strategy are two-fold: firstly, the SPE cartridge is an alternative to a reaction vessel, allowing for greater flexibility in cassette design; secondly, the SPE cartridge allows for the concentration of reagents into small reaction volumes which are notoriously challenging to handle on cassette-based automated platforms. Concentrating reagents onto an SPE cartridge can improve reaction efficiency, and the handling of challenging radioactive intermediates (i.e. volatile radioactive molecules).
Solid-supported reagents can also be used in an automated radiosynthesis.23,24 There are many commercially available reagents fused onto solid supports, and these can be housed in cartridges assembled from commercially available components. This can be useful if a reagent exhibits challenging physical properties (i.e. poor solubility) and is difficult to handle with a cassette.
Section 4: purification & reformulation
Radiopharmaceuticals must exhibit high chemical and radiochemical purity (>95%), especially for clinical use. The most frequently adopted purification strategies include HPLC and SPE, both of which can be automated using cassette-based platforms.4.1 HPLC Purification
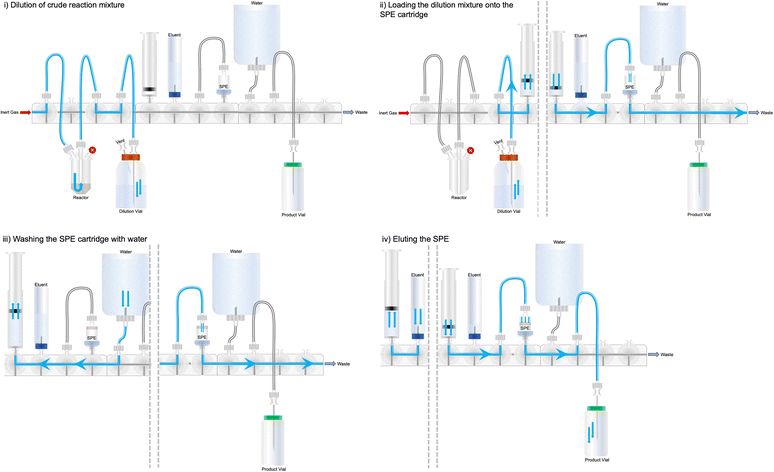
Developing HPLC purification methods to isolate radioactive compounds is beyond the scope of this tutorial account; however, these methods can be integrated into automated radiosynthesis, even if your platform does not natively support a HPLC system. It is often necessary to purify compounds by HPLC, and this can be done by using the automated platform to load the loop of a HPLC system. First, the crude mixture needs to be diluted in HPLC mobile phase, to the volume of the loop fitted on the HPLC system (i.e. 10 mL). This reduces the percentage organic solvent in the injection and facilitates better retention on the column. The dilution can be automated as shown in Fig. 20. The purification can be performed using the proprietary software/system associated with the HPLC system and the automated synthesis sequence is paused until given further notice to proceed working through the sequence. When a desired peak is “cut” on the HPLC system, it is collected in an external vial. By including a diluent in the collection vial, and a needle/tubing from the vial to the cassette, the purified compound can be returned to the cassette for reformulation, either to be used in subsequent reactions or for biological use.4.2 SPE purification & reformulation
Some radiopharmaceuticals can be purified using SPE cartridges alone (i.e. the synthesis of [18F]FDG) and this is highly desirable for future commercialisation of radiopharmaceutical cassettes. This method allows the full synthesis/purification to be performed on a single synthesis unit without the requirement for external HPLC equipment (which often varies between production sites), permitting a plug-and-play approach to radiopharmaceutical production. Automating SPE purification is straightforward and is exemplified in Fig. 21, for the purification of a crude reaction mixture. In brief, the reaction mixture which is often comprised of a highly organic solvent mixture, is diluted into an aqueous media. In Fig. 21, this dilution is shown as a transfer into an external vial; however, depending on the volume of organic solvent inside the reactor, and the trapping efficiency of the radioactive molecule on the chosen SPE cartridge, this dilution may be performed inside the reactor and/or syringe by the addition of water. This removes the need for an external dilution vial and therefore simplifies the protocol for setting up the synthesis. Secondly, the diluted mixture is pushed through the SPE cartridge, typically at a flow rate no greater than 2–3 mL min−1 using an on-board syringe, to avoid excessive backpressure. It should be noted that different types of SPE cartridge vary in their solid support packing density, so backpressure may vary. Depending on the volume of diluent, the SPE may require loading several times. An alternative method useful when large volumes of solvent are to be passed through an SPE cartridge is to pressurise the external vial and apply vacuum across the SPE cartridge. This removes the need for sequential filling and dispensing of the syringe and reduces process time; however, an additional cassette position will be required to pressurise the vial.The retention and elution of some compounds on SPE cartridges is highly temperature dependant, and variation of a few degrees can have a big impact on the purification. Once the diluent has been loaded onto the SPE, the cartridge can be washed with water to remove polar compounds and dried under a flow of inert gas. Finally, the radioactive compound can be eluted from the SPE using a pre-loaded eluent (i.e. ethanol). If the radioactive compound is intended for biological use, then a biocompatible solvent should be used. The size of the SPE cartridge dictates the minimum volume of eluent to remove the tracer. Larger SPE cartridges require more eluent, but if very small volumes are required, eluting the SPE cartridge in reverse may be a viable option (Fig. 22). This can also be useful to mitigate losses for compounds which elute poorly from an SPE cartridge.
This process concentrates the radioactivity, which may result in radiolysis depending on the susceptibility of the molecule in question and the quantity of radioactivity used in the synthesis. Free radical scavengers may be included in the diluent to limit or prevent this phenomenon (e.g. ascorbic acid). Although the example shown in Fig. 21 shows a simple water wash before elution, it may be that several eluents may be selected to remove components of the complex reaction mixture to produce a chemically and radiochemically pure dose; if this is desirable, then considerable evaluation and validation of the method is required prior to automation. If acidified water is required for an SPE purification, then consider modifying the pH of the water reservoir (i.e. water bag); this can free up space on the cassette and is an effective solution, so long as the acidification does not interfere with the rest of the labelling process.
Another essential application for SPE chromatography is reformulation or solvent exchange after HPLC purification. Quite often, desirable purification conditions for radiopharmaceuticals use solvents which are not biocompatible; this includes but is not limited to solvents such as methanol and acetonitrile, and pH modifiers such as trifluoroacetic acid (TFA). SPE cartridges can be used post-HPLC purification to exchange the radioactive compound into a biocompatible formulation. The automated process is the same as shown in Fig. 21, but instead of taking a crude mixture from the reactor vessel, the isolated radioactive product is cut from the HPLC system into a diluent which is subsequently loaded onto an SPE cartridge. Conscientious washing of the SPE cartridge can remove the mobile phase from the HPLC purification.
When developing purification methods for radiopharmaceuticals intended for clinical use, the early adoption of biocompatible solvents is advantageous and addresses potential problems which may arise later in the development progress. For example, developing HPLC methods with ethanol vs. methanol. Note that the use of ethanol in HPLC tends to result in higher back pressure and therefore modulation of flow rate and column type (i.e. stationary phase, dimensions) may be required to maintain efficient purification. In addition, exchanging TFA with an alternative acid like hydrochloric, acetic, or phosphoric acid can also be suitable for modifying pH.
Section 5: optimisation and troubleshooting
To avoid your automated platform becoming a “black box” where [18F]fluoride goes in, and tracer comes out, there are a few techniques to aid optimisation and troubleshooting. Firstly, most platforms contain radioactivity detectors around key components (i.e. reactor, SPE cartridges) which allow the radiochemist to quantify the levels of radioactivity. This can be useful for monitoring the transfer of radioactivity around the cassette, and as a method for identifying potential losses. This data is usually recorded and can be viewed post-synthesis.Identifying losses of radioactivity
When looking for ways to optimise performance, it can be advantageous to remove a cassette from the platform at the end of synthesis (or after a period of decay for safer handling) and strip it down into individual components to measure retained radioactivity using a dose calibrator. If the radiochemical yield is low, perhaps the product didn't elute fully from an SPE cartridge. Measuring the radioactivity retained on the SPE cartridge using a dose calibrator will help determine if this is the case. Some lipophilic compounds can be retained on tubing, so it can be useful to know when this occurs and design ways to mediate the loss (i.e. reduce length of tubing, change tubing material). Lipophilic compounds can also be retained on sterile filters, which can be mitigated by the addition of polysorbate-type surfactants or sodium dodecyl sulphate (SDS).If your radiosynthesis method produces little product
Consider taking a sample of the residual material in the reactor for HPLC analysis to rule out that the chemistry failed. It can also be useful to take a sample from the waste bottle for HPLC analysis to determine if the product was accidentally sent to waste by an inefficient purification strategy or programming error. Similarly, radio-TLC provides a rapid and effective method for determining if [18F]fluoride incorporation was problematic. If you're interested in monitoring reaction efficiency as part of your research, a pause can be programmed into the method to allow a sample of the reaction mixture to be obtained (if safe to do so); alternatively, if space allows, you could even programme the platform to remove a sample for analysis at the end of synthesis.Significant losses of radioactivity can occur during HPLC purification
Consider changing the column type, for example, losses of a lipophilic tracer may be mediated by switching from a C18 to C8 stationary phase, or by modulating the pH of the eluent.Case study – radiosynthesis of [18F]TTCO-IL2
We recently published the automated radiosynthesis of an 18F-labelled biologic, recombinant interleukin-2 (Proleukin™), via the facile inverse electron demand Diels–Alder (IEDDA) “click” chemistry using the GE FASTLab™ platform.17 In this Tutorial Account, we will use this radiochemistry as a case study to exemplify some of the methodology we have discussed herein; full experimental detail can be found in the previously published article but a summary of the radiochemistry is shown in Scheme 1. The automated radiosynthesis of [18F]TTCO-IL2 is complex but serves as a good example as it covers both small molecule fluorination (i.e. synthesis of 4-[18F]fluorobenzaldehyde, [18F]FBA), post-fluorination small molecule radiochemistry (i.e. the synthesis of the [18F]FBoxTz prosthetic group) as well as an approach to the radiolabelling more challenging biomolecules (i.e. Proleukin™). With this case study, we aim to consolidate the Tutorial Account and “bring to life” some of the concepts of automation covered in this article. To achieve this, we have animated the automated process in a video which can be found in the electronic ESI.† The process is described below against key timestamps. | ||
| Scheme 1 Radiosynthesis of [18F]TTCO-IL2. A) Radiochemistry precursors for IEDDA “click” chemistry; B) an overview of the synthesis of [18F]TTCO-IL2. Reaction conditions: a)[18F]F−, KHCO3, K222, 90 °C, 6.6 min; b) aniline hydrochloride, MeCN/H2O, 40 °C, 10 min; c) EtOH, 10 min, RT. Adapted from a published method.17 | ||
00:00–00:04 (mm:ss)
The animation starts with the assumption that [18F]fluoride has been dried in the reactor, following the process shown in Fig. 17. The cassette layout is shown, as well as the off-board components which include a semi-preparative HPLC system and external reaction vial.00:04–00:14 (mm:ss)
The radiochemistry precursor for the synthesis of 4-[18F]fluorobenzaldehyde ([18F]FBA) is transferred into the reactor by over-pressurising the vial and evacuating it into the reactor. This process allows for almost complete transfer of the contents of the vial into the reactor.00:16–00:28 (mm:ss)
The [18F]FBoxTz precursor is transferred into the reactor by the syringe, as described in Fig. 9.00:33–00:44 (mm:ss)
The entire contents of the reaction are transferred into an external dilution vial, as described in Fig. 12. This is performed by pressurising the reactor with inert gas and opening the flow through the central port of the reactor.00:42–01:06 (mm:ss)
The crude reaction material is purified by semi-preparative HPLC, as described in Fig. 20. The cut peak is collected in a second dilution vial ready for reformulation using a C18 SPE cartridge.01:06–01:41 (mm:ss)
The purified reaction mixture is trapped onto a C18 SPE cartridge and eluted with ethanol into an external reactor. This process is described in Fig. 21. The external reactor contains the IL2-PEG4-TCO precursor for the IEDDA “click” labelling with [18F]FBoxTz.01:41–01:15 (mm:ss)
The crude reaction mixture is diluted with the water bag mixture and brought back onto the cassette for purification by the C2 SPE cartridge.02:21–02:42 (mm:ss)
A 50% (v/v) mixture of ethanol and water bag solution is created inside the syringe. Thorough mixing was important in this step to ensure reproducible elution of unreacted [18F]FBoxTz. The technique is described in Fig. 11, and was used to provide turbulent mixing.02:42–03:08 (mm:ss)
Unreacted [18F]FBoxTz was eluted selectively from [18F]TTCO-IL2, followed by the elution of radiochemically pure [18F]TTCO-IL2 into the product vial containing formulation for biological evaluation.This automated procedure was able to produce [18F]TTCO-IL2 in >98% radiochemical purity within 110 min, with a decay-corrected radiochemical yield of 19.8 ± 2.6%, and a molar activity of 132.3 ± 14.6 GBq μmol−1.
Conclusions
We hope that this “Tutorial Account” has provided some insight into developing automated fluorine-18 radiochemistry methods on cassette-based platforms. Automation is a key component to GMP translation, yet there are few educational resources available for those who are new to the discipline. It was our intention to address this knowledge gap and provide a foundation from which others can build their skills in this fascinating and essential area of PET radiopharmaceutical development. Experienced individuals may have alternative approaches to the strategies outlined in this document, which is not intended to be an exhaustive account of all “tips and tricks” in automation, but instead share our experience in automating fluorine-18 PET radiochemistry. We hope that this manuscript encourages discussion and open sharing of ideas within the community, facilitating the transition of next generation of radiopharmaceuticals from the bench to the bedside.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank GE Healthcare and Trasis for providing high quality images for use in this manuscript. In addition, we are grateful to Dr Federica Pisaneschi for helpful discussion and proofreading of the manuscript.Notes and references
- L. Allott and E. O. Aboagye, Chemistry Considerations for the Clinical Translation of Oncology PET Radiopharmaceuticals, Mol. Pharmaceutics, 2020, 17, 2245–2259 CrossRef CAS PubMed.
- L. Bruton and P. J. H. Scott, in Handbook of Radiopharmaceuticals, ed. P. J. H. Scott and M. R. Kilbourn, John Wiley & Sons, Ltd, 2020, pp. 437–456 Search PubMed.
- S. J. Archibald and L. Allott, The aluminium-[18F]fluoride revolution: simple radiochemistry with a big impact for radiolabelled biomolecules, EJNMMI Radiopharm. Chem., 2021, 6, 30 CrossRef PubMed.
- L. Allott, C. Da Pieve, D. R. Turton and G. Smith, A general [ 18F]AlF radiochemistry procedure on two automated synthesis platforms, React. Chem. Eng., 2017, 2, 68–74 RSC.
- T. Tshibangu, C. Cawthorne, K. Serdons, E. Pauwels, W. Gsell, G. Bormans, C. M. Deroose and F. Cleeren, Automated GMP compliant production of [18F]AlF-NOTA-octreotide, EJNMMI Radiopharm. Chem., 2020, 5, 4 CrossRef PubMed.
- H. H. Boersma, M. G. G. Sturkenboom, M. N. Lub-De Hooge, P. H. Elsinga, G. Luurtsema, R. A. J. O. Dierckx and J. G. W. Kosterink, in Trends on the Role of PET in Drug Development, 2012 Search PubMed.
- S. Zhu, S. Mosessian, K. Kroeger, S. Sadeghi, R. Slavik, S. Kinloch, M. Moore, M. Allen-Auerbach, J. Czernin and M. Phelps, Transforming an Academic Radiochemistry Facility for Positron Emission Tomography Drug cGMP Compliance, Mol. Imaging Biol., 2020, 22, 256–264 CrossRef PubMed.
- F. Pisaneschi and N. T. Viola, Development and Validation of a PET/SPECT Radiopharmaceutical in Oncology, Mol. Imaging Biol., 2022, 24, 1–7 CrossRef CAS PubMed.
- S. Todde, P. K. Peitl, P. Elsinga, J. Koziorowski, V. Ferrari, E. M. Ocak, O. Hjelstuen, M. Patt, T. L. Mindt and M. Behe, Guidance on validation and qualification of processes and operations involving radiopharmaceuticals, EJNMMI Radiopharm. Chem., 2017, 2, 1–29 CrossRef PubMed.
- R. Krasikova, Synthesis Modules and Automation in F-18 Labeling BT - PET, Chemistry, 2007, 289–316 CAS.
- S. Boschi, F. Lodi, C. Malizia, G. Cicoria and M. Marengo, Automation synthesis modules review, Appl. Radiat. Isot., 2013, 76, 38–45 CrossRef CAS PubMed.
- S. W. Schwarz, D. Dick, H. F. VanBrocklin and J. M. Hoffman, Regulatory Requirements for PET Drug Production, J. Nucl. Med., 2014, 55, 1132–1137 CrossRef PubMed.
- S. Schwarz, J. Norenberg, M. Berridge, S. Dragotakes, J. Hung, J. Link, N. S. Mason, S. Mattmuller, R. A. Nickel, A. Packard, J. Paolino, N. Petry, J. Ponto, T. M. Quinton, K. L. Seifert, D. Swanson, R. E. Weiner and S. Zigler, The Future of USP Monographs for PET Drugs, J. Nucl. Med., 2013, 54, 472–475 CrossRef CAS PubMed.
- ICH, The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), https://www.ich.org, (accessed 7 July 2022) Search PubMed.
- CDC, Centers for Disease Control and Prevention (CDC) - ALARA - As Low As Reasonably Achievable, https://www.cdc.gov/nceh/radiation/alara.html, (accessed 7 July 2022) Search PubMed.
- HSE, Health and Safety Executive (HSE) - ALARP, https://www.hse.gov.uk/managing/theory/alarpglance.htm, (accessed 7 July 2022) Search PubMed.
- L. Allott, A. Amgheib, C. Barnes, M. Braga, D. Brickute, N. Wang, R. Fu, S. Ghaem-Maghami and E. O. Aboagye, Radiolabelling an 18F biologic via facile IEDDA “click” chemistry on the GE FASTLab™ platform, React. Chem. Eng., 2021, 6, 1070–1078 RSC.
- L. Allott, C. Barnes, D. Brickute and E. O. Aboagye, An improved automated radiosynthesis of [18F]FET-βAG-TOCA, React. Chem. Eng., 2019, 4, 569–574 RSC.
- A. V. Mossine, A. F. Brooks, V. Bernard-Gauthier, J. J. Bailey, N. Ichiishi, R. Schirrmacher, M. S. Sanford and P. J. H. Scott, Automated synthesis of PET radiotracers by copper-mediated 18F-fluorination of organoborons: Importance of the order of addition and competing protodeborylation, J. Labelled Compd. Radiopharm., 2018, 61, 228–236 CrossRef CAS PubMed.
- F. Guibbal, P. G. Isenegger, T. C. Wilson, A. Pacelli, D. Mahaut, J. B. I. Sap, N. J. Taylor, S. Verhoog, S. Preshlock, R. Hueting, B. Cornelissen and V. Gouverneur, Manual and automated Cu-mediated radiosynthesis of the PARP inhibitor [18F]olaparib, Nat. Protoc., 2020, 15, 1525–1541 CrossRef CAS PubMed.
- P. Xu, D. Zhao, F. Berger, A. Hamad, J. Rickmeier, R. Petzold, M. Kondratiuk, K. Bohdan and T. Ritter, Site-Selective Late-Stage Aromatic [18F]Fluorination via Aryl Sulfonium Salts, Angew. Chem., 2020, 132, 1972–1976 CrossRef.
- C. Fersing, A. Bouhlel, C. Cantelli, P. Garrigue, V. Lisowski and B. Guillet, A Comprehensive Review of Non-Covalent Radiofluorination Approaches Using Aluminum [18F]fluoride: Will [18F]AlF Replace 68Ga for Metal Chelate Labeling?, Molecules, 2019, 24, 2866 CrossRef CAS PubMed.
- L. Allott, C. Barnes, D. Brickute, S. F. J. Leung and E. O. Aboagye, Solid-supported cyanoborohydride cartridges for automation of reductive amination radiochemistry, React. Chem. Eng., 2019, 4, 1748–1751 RSC.
- F. Pisaneschi, L. E. Kelderhouse, A. Hardy, B. J. Engel, U. Mukhopadhyay, C. Gonzalez-Lepera, J. P. Gray, A. Ornelas, T. T. Takahashi, R. W. Roberts, S. V. Fiacco, D. Piwnica-Worms and S. W. Millward, Automated, Resin-Based Method to Enhance the Specific Activity of Fluorine-18 Clicked PET Radiotracers, Bioconjugate Chem., 2017, 28, 583–589 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2re00219a |
| This journal is © The Royal Society of Chemistry 2022 |