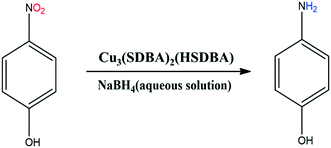Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper-based MOF, Cu3(SDBA)2(HSDBA), as a catalyst for efficient reduction of 4-nitrophenol in the presence of sodium borohydride†
Manel
Mansour
ab,
Hamza
Kahri
*c,
Mouhieddinne
Guergueb
d,
Houcine
Barhoumi
c,
Enrique
Gutierrez Puebla
 b,
Brahim
Ayed
a and
Umit B.
Demirci
b,
Brahim
Ayed
a and
Umit B.
Demirci
 *e
*e
aLaboratory Materials, Crystal Chemistry and Applied Thermodynamics, Faculty of Sciences of Monastir, University of Monastir, Monastir, Tunisia
bMaterials Science Factory, Materials Science Institute of Madrid (CSIC), C/Sor Juana Ines de La Cruz, 3, Madrid, Spain
cLaboratory of Interfaces and Advanced Materials, Faculty of Sciences, University of Monastir, Tunisia. E-mail: kahrihamza11@gmail.com
dUniversity of Monastir, Laboratoire de Physico-Chimie des Matériaux, Faculté des Sciences de Monastir, Avenue de l'Environnement, 5019 Monastir, Tunisia
eIEM (Institut Européen des Membranes), UMR5635 (CNRS, ENSCM, UM), Université de Montpellier, Place Eugene Bataillon, CC047, Montpellier, France. E-mail: umit.demirci@umontpellier.fr
First published on 5th January 2022
Abstract
Herein, we report the synthesis of the copper-based MOF, Cu3(SDBA)2(HSDBA), using a solvothermal method. The physicochemical properties of the as-prepared sample were examined by X-ray diffraction, scanning electron microscopy, energy-dispersive X-ray spectroscopy, Fourier-transform infrared and UV-vis spectroscopy techniques, and thermal analysis. Cu3(SDBA)2(HSDBA) was then investigated as a catalyst for the reduction of 4-nitrophenol (4-NP) in the presence of sodium borohydride (NaBH4) at 25 °C, and related kinetics and thermodynamics analyses were carried out. In a nutshell, it can be emphasized that, under our conditions, the Cu3(SDBA)2(HSDBA) catalyst is able to completely reduce 4-NP to 4-aminophenol (4-AP) in a very short time such as 20 s. Additionally, Cu3(SDBA)2(HSDBA) can be re-used for seven successive cycles without obvious change in its catalytic activity.
1. Introduction
Nitroaromatic compounds such as nitrophenols are important intermediates for industrial applications including pharmaceuticals, leather, plastics, fungicides, pesticides and insecticides.1–5 Nevertheless, some nitrophenols such as 4-nitrophenol (4-NP) are hazardous as they cause eye inflammation, skin allergies and respiratory problems.It is therefore important to remove 4-NP from aqueous solution. Different methods have been reported, and these methods cover adsorption, photocatalytic degradation, microwave-assisted catalytic oxidation and electro-Fenton.6–11 The catalytic reduction of 4-NP to 4-aminophenol (4-AP) has also been described. A variety of catalysts were reported for this reaction.12–18 These catalysts provide high efficiency and high recyclability, as is the case with N-doped reduced graphene metal oxide nanocomposites for example.19–21 Metal–organic frameworks (MOFs) are a class of hybrid porous materials, displaying high surface areas and tunable pore size/volume.22 MOFs, specifically catalytic nanoparticles supported on MOFs, can be used as catalysts for the reduction of 4-NP to 4-AP. Examples are as follows: nanoparticles of Ni@Pd supported on UiO-66-NH2,23 and Cu-based 2D MOFs.24 MOFs were also utilized as a template for the preparation of porous carbon. Examples are as follows: nanoparticles of Co supported on a porous carbon stemming from carbonization of benzene-1,4-dicarboxylic acid-based MOFs,25 and a comparable supported catalyst where the nanoparticles were made of Fe.26 Such MOF-based catalysts offer advantages such as high catalytic performance, as well as drawbacks such as lack of stability. Thus, it is important to further explore MOF-based catalysts showing excellent performance in terms of kinetics and very good stability for the reduction of 4-NP to 4-AP in aqueous solution and under mild conditions.
In this study, we report the synthesis of the copper-based MOF Cu3(SDBA)2(HSDBA) using a solvothermal method where 4,4′-sulfonyldibenzoic acid (H2-SDBA) is used as a precursor. The as-prepared catalyst was characterized employing various techniques such as SEM-EDX, FTIR, XRD, UV-vis and TGA-DSC. Our catalyst showed high efficiency as well as very good stability towards the reduction of 4-NP using sodium borohydride NaBH4 as a reducing agent.
2. Methodology
2.1. Synthesis method
Cu3(SDBA)2(HSDBA) was synthesized under hydrothermal conditions as follows (Fig. S1†). Copper nitrate trihydrate Cu(NO3)2·3H2O (0.5 mmol; 99%; Sigma-Aldrich) and 4,4′-sulfonyldibenzoic acid C14H10O6S (0.5 mmol; denoted H2-SDBA; 98%; Sigma-Aldrich) were dissolved in 5 mL of deionized water (resistivity >18.2 MΩ cm). The solution was transferred in a Teflon vessel (25 mL) that was sealed and placed in a stainless-steel autoclave. Then, the autoclave was placed in an oven preheated at 170 °C, and kept there for 3 days. It is worth mentioning that the time was optimized; the crystals with the best quality formed after 3 days. After cooling to room temperature, the as-obtained blue crystals of Cu3(SDBA)2(HSDBA) were filtered off and washed with water, ethanol and acetone.2.2. Characterization methods
Cu3(SDBA)2(HSDBA) was scrutinized by scanning electron microscopy (SEM) and its chemical composition was analyzed by electron dispersive X-ray spectroscopy (EDS). An S-3000N microscope equipped with an ESED and an INCAX-sight from Oxford Instruments was used. The samples to be analyzed were prepared as follows. Cu3(SDBA)2(HSDBA) was dispersed onto a double-sided adhesive conductive carbon tape that was attached to a flat aluminum sample holder, and it was metalized using a 12 nm gold layer with a Quorum Q150T-S sputter.Elemental analysis was performed using a PerkinElmer2400 CHN elemental analyzer.
Molecular analysis of Cu3(SDBA)2(HSDBA) was performed by Fourier-transform infrared spectroscopy (Perkin-Elmer spectrophotometer). A thin transparent pellet was made by compacting an intimate mixture of 2 mg of Cu3(SDBA)2(HSDBA) and 100 mg of potassium bromide KBr (99%; Sigma-Aldrich). The spectrum was recorded at 45 scans per minute at room temperature from 4000 to 400 cm−1.
Structural analyses of Cu3(SDBA)2(HSDBA) were carried out. In a first step, single crystal XRD experiment was performed upon selection of Cu3(SDBA)2(HSDBA) crystals using a polarized optical microscope. Single-crystal X-ray data were collected using a Bruker four-circle kappa diffractometer equipped with a Cu INCOATED micro-source, operated at 30 W (45 kV, 0.60 mA) to generate Cu Kα radiation (λ = 1.54178 Å), and with a Bruker VANTEC-500 detector (micro-gap technology). The diffraction data were collected, exploring over the reciprocal space in a combination of f and w scans to reach a resolution of 0.85 Å, with a completeness >95% and a redundancy >3. For this purpose, a generic hemisphere collection strategy has been developed using Bruker APEX3 and the software suite was used.27 The exposure time was adjusted on the basis of the size and diffraction quality of the specimens, each exposure covering 1° in ω or φ. Unit cell dimensions were determined for least-squares fit of reflections with I > 4 s. The structures were solved by direct methods implemented in the SHELX package.28 The hydrogen atoms were fixed at their calculated positions using distances and angle constraints. All calculations were performed using APEX3 software for data collection, and OLEX2-1.2 (ref. 29) and SHELXTL28 to resolve and refine the structure. All non-hydrogen atoms were anisotropically refined. In a first step, powder X-ray diffraction (PRXD) data were collected. The PRXD patterns were measured using a Bruker D8 diffractometer with a Cu source operated at 1600 W, with a step size of 0.02° and an exposure time of 0.5 s per step. The PXRD measurements were used to check the purity of the obtained microcrystalline product by comparison of the experimental results with the calculated pattern obtained from the single-crystal XRD data.
The solid-state UV-vis spectra of Cu3(SDBA)2(HSDBA) and of the ligand H2-SDPA were obtained at room temperature using a Perkin-Elmer Lambda 950 spectrometer within a 200–800 nm wavelength range. The spectra allowed the determination of the gap energy Egap (in eV) that is defined as the difference between the energy level of the highest occupied molecular orbital (EHOMO) and the energy level of the lowest unoccupied molecular orbital (ELUMO):
 | (1) |
 | (2) |
2.3. Catalytic reduction of 4-nitrophenol
A 5 mL aqueous solution containing 2.1 × 10−2 mol of sodium borohydride NaBH4 (96%; Sigma-Aldrich) and 7.1 × 10−4 mol of 4-nitrophenol (4-NP; 99%; Sigma-Aldrich) was prepared. To start the reduction reaction, 2 mg of Cu3(SDBA)2(HSDBA) was added to the solution. To follow the reduction of 4-NP, absorbance values at 400 nm were recorded using a UV-vis spectrometer (Varian Cary 5000 spectrophotometer; Agilent Technologies). The experiment was repeated at various temperatures (25, 35, 45 and 55 °C) to see the effect of the temperature and determine the apparent activation energy of the reaction under our conditions.3. Results and discussion
3.1. Morphological and molecular analyses of Cu3(SDBA)2(HSDBA)
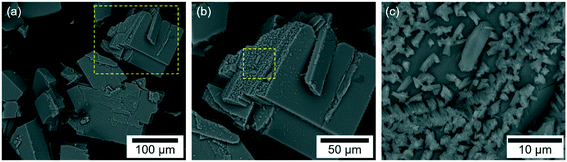
Cu3(SDBA)2(HSDBA) consists of blue crystals (Fig. S1†), in fact polyhedra of various sizes. There are polyhedra of dozens of micrometers (up to 130 μm; Fig. 1a and b and S2a†), as well as smaller particles located on the facets of the bigger ones (Fig. 1c, and S2b†). Upon SEM observation, the sample was analyzed by EDS and the presence of both Cu and S was detected (Fig. S3†). These two elements were quantified by elemental analysis, with 43 and 8.4 wt% in good agreement with the calculated contents (46.4 and 8.8 wt%). Two other elements, H and N, were found, with 2.9 and 0.02 wt% respectively (versus 2.2 and 0 wt% for the calculated values).The FT-IR spectra of Cu3(SDBA)2(HSDBA) and the ligand H2-SDBA are highly comparable (Fig. 2) in terms of the bands at 3470–3460 cm−1 (O–H stretching), 3100–2600 cm−1 (C–H aliphatic and aromatic stretching), 1700 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1588–1584 cm−1 (O–H deformation), 1570–1429 cm−1 (COO asymmetric and symmetric stretching), 1289–1166 cm−1 (C–SO2–C asymmetric and symmetric stretching), 1101–1019 cm−1 (C–C and C–O stretching), and 750–576 cm−1 (C–S deformation).30,31 The spectrum of H2-SDBA shows another band peaking at 937 cm−1 due to aromatic ring deformation and C–S stretching; this band cannot be seen in the spectrum of Cu3(SDBA)2(HSDBA), which may indicate bonding between the ligands and the metal nodes. The spectrum of Cu3(SDBA)2(HSDBA) shows an additional band, peaking at 519 cm−1 and corresponding to the vibrational signal of Cu–O,32 thereby confirming the formation of Cu3(SDBA)2(HSDBA) via Cu–O bonds between the metal nodes and the organic ligands.
O stretching), 1588–1584 cm−1 (O–H deformation), 1570–1429 cm−1 (COO asymmetric and symmetric stretching), 1289–1166 cm−1 (C–SO2–C asymmetric and symmetric stretching), 1101–1019 cm−1 (C–C and C–O stretching), and 750–576 cm−1 (C–S deformation).30,31 The spectrum of H2-SDBA shows another band peaking at 937 cm−1 due to aromatic ring deformation and C–S stretching; this band cannot be seen in the spectrum of Cu3(SDBA)2(HSDBA), which may indicate bonding between the ligands and the metal nodes. The spectrum of Cu3(SDBA)2(HSDBA) shows an additional band, peaking at 519 cm−1 and corresponding to the vibrational signal of Cu–O,32 thereby confirming the formation of Cu3(SDBA)2(HSDBA) via Cu–O bonds between the metal nodes and the organic ligands.
3.2. Crystallinity of Cu3(SDBA)2(HSDBA)
The single crystal XRD analysis reveals that Cu3(SDBA)2(HSDBA) is a 3D coordination polymer crystallizing in the triclinic crystal system, with the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Table S1†). The asymmetric unit of Cu3(SDBA)2(HSDBA) consists in 2 SDBA2− anions, 1 H-SDBA− anion and 3 Cu(II) cations (Fig. 3a). The structural arrangement of Cu3(SDBA)2(HSDBA) is shown in Fig. 3b along the [010] plane. The structure may be described as clusters (Cu3O14) made of three polyhedra with shared apices which are linked by SDBA2− anions. All of these dual strings run in parallel with each other. The carboxylate groups of SDBA2− are connected to Cu(II) in two ways: a bis-tetradentate form results in a diamond shape in the Cu2O10 cluster system, and the SDBA2− ligands take a bridging coordination mode μ6-COO−. Each 1D single chain string has microporous cavities with sizes of approximately 12.82(2) and 11.6(3) Å2 for Cu⋯Cu and S⋯S respectively, and one possesses a 1D related square plane geometry along the crystallographic a-axis (Fig. 3c).
space group (Table S1†). The asymmetric unit of Cu3(SDBA)2(HSDBA) consists in 2 SDBA2− anions, 1 H-SDBA− anion and 3 Cu(II) cations (Fig. 3a). The structural arrangement of Cu3(SDBA)2(HSDBA) is shown in Fig. 3b along the [010] plane. The structure may be described as clusters (Cu3O14) made of three polyhedra with shared apices which are linked by SDBA2− anions. All of these dual strings run in parallel with each other. The carboxylate groups of SDBA2− are connected to Cu(II) in two ways: a bis-tetradentate form results in a diamond shape in the Cu2O10 cluster system, and the SDBA2− ligands take a bridging coordination mode μ6-COO−. Each 1D single chain string has microporous cavities with sizes of approximately 12.82(2) and 11.6(3) Å2 for Cu⋯Cu and S⋯S respectively, and one possesses a 1D related square plane geometry along the crystallographic a-axis (Fig. 3c).
 | ||
| Fig. 3 (a) Asymmetric unit for Cu3(SDBA)2(HSDBA). (b and c) Polymer coordination geometry for the ligand SDBA2− with Cu(II) metal centers. | ||
The phase purity of bulk Cu3(SDBA)2(HSDBA) (blue precipitate) was examined by PXRD study. The PXRD pattern shows sharp peaks showing high crystallinity (Fig. S4†). In addition, the Bragg peak positions of the PXRD pattern match well with those of the pattern calculated from the single crystal XRD data, which indicates the successful synthesis of a pure phase of Cu3(SDBA)2(HSDBA). There is nevertheless the presence of a small amount of CuO (peak at ca. 35° due to the (111) plane; JCPDS: card no. 48-1548).
3.3. UV-VIS analysis of Cu3(SDBA)2(HSDBA)
The UV-vis absorption spectra of Cu3(SDBA)2(HSDBA) and the H2-SDBA ligand were recorded in the solid state from 200 to 800 nm. The spectrum of H2-SDBA (Fig. S5†) exhibits two strong absorption bands with the maximum being below 300 nm (i.e. λmax = 217 nm and λmax = 292 nm), which may be contributed by the n–π* transition as well as the π–π* transition of the aromatic rings, respectively.33 This value corresponds to λlim at 379 nm and the energy gap (Egap = 3.27 eV). This may classify the ligand as a semiconductor.34 The UV-vis absorption spectrum of Cu3(SDBA)2(HSDBA) (Fig. 4) shows a large band that corresponds to the charge transfer from O of the ligand to Cu.35 The energy gap, calculated on the basis of λlim of Cu3(SDBA)2(HSDBA), was found to be 2.13 eV, which allows classifying Cu3(SDBA)2(HSDBA) as a semiconductor.363.4. Thermal stability of Cu3(SDBA)2(HSDBA)
TG and DSC analysis were carried out to check the thermal stability of Cu3(SDBA)2(HSDBA) (Fig. 5). Cu3(SDBA)2(HSDBA) is stable up to 200 °C, and above this temperature, it overcomes five successive weight losses up to about 486 °C. The first weight loss of 5.9 wt% is indicated by an endothermic signal peaking at 223 °C. This is due to the desorption of water incorporated into the pores of Cu3(SDBA)2(HSDBA). Then, the next four weight losses (41 wt%) are associated with four exothermic signals peaking at 322, 391, 411 and 486 °C. These indicate a stepwise decomposition of the ligand.37 Between 493 and about 650 °C, the solid is stable, and it overcomes an additional weight loss of 8 wt% up to about 750 °C. One may thus conclude that Cu3(SDBA)2(HSDBA) is thermally stable up to 200 °C.3.5. Catalytic reduction of 4-nitrophenol
Cu3(SDBA)2(HSDBA) was used for the reduction of 4-NP in the presence of NaBH4 in aqueous solution (30-fold excess of NaBH4 over 4-NP). 4-NP has an adsorption maximum at 400 nm in the reaction mixture, and its reduction was followed by monitoring the decrease of the absorption band as a function of time by UV-vis spectrophotometry. Concomitantly, a new band appeared, at 300 nm, evidencing the presence of 4-AP as the reduction product of 4-NP (Fig. 6). Under our conditions, and at 25 °C (Fig. 7), the reduction of 4-NP started upon the addition of Cu3(SDBA)2(HSDBA) and it was totally reduced in 20 seconds. This catalytic performance may be compared to some state-of-the-art MOF-based catalysts (Table 1).24,38–41 Under roughly similar conditions, Cu3(SDBA)2(HSDBA) is as efficient as Pd@Co-MOF40 for reducing 4-NP in 20 seconds; however, our Cu3(SDBA)2(HSDBA) is free of a hydrogenation metal like Pd. It is difficult to further discuss the performance of the listed catalysts because of discrepancies in the reaction conditions, but it is reasonable to observe that Cu3(SDBA)2(HSDBA) is highly efficient in quickly reducing 4-NP at 25 °C.| Catalyst | Weight | [4-NP] | [NaBH4] | Temp. | Conv. | Time | Ref. |
|---|---|---|---|---|---|---|---|
| mg | mmol L−1 | mmol L−1 | °C | % | s | ||
| a FMOF for ferrocene based metal–organic framework. b CCFs for carboxymethylated cellulose fibers. c rGO for reduced graphene oxide. d BDC for benzene-1,4-dicarboxylic acid. e Used in the form of membrane. | |||||||
| Au@FMOFa | 1.5 | 7.2 | 500 | 50 | 100 | 200 | 38 |
| Ag@MOF-199/CCFsb | —e | 0.1 | 10 | 25 | 91 | 600 | 39 |
| Pd@Co-MOF | 5 | 2 | 48 | 25 | 90 | 20 | 40 |
| Cu2O/Cu-MOF/rGOc | 1 | 0.1 | 100 | 25 | 100 | 120 | 41 |
| Cu-BDCd | 5 | 0.1 | 100 | 25 | 100 | 120 | 24 |
| Cu3(SDBA)2(HSDBA) | 2 | 0.7 | 21 | 25 | 100 | 20 | This study |
Fig. 8a shows the decrease in the concentration of 4-NP (Ct/C0) at 25 °C, as well as three other temperatures such as 35, 45 and 55 °C, as a function of time. The curves were exploited to plot −ln(Ct/C0) as a function of time, as shown in Fig. 8b. There is a good linear correlation, thereby confirming that the reduction of 4-NP is consistent with first-order reaction kinetics.42 This allowed us to calculate the pseudo-first order rate constants k that were found to be 0.25, 0.31, 0.34 and 0.36 s−1 at 25, 35, 45 and 55 °C, respectively.
The activation and thermodynamic parameters for the reduction of 4-NP by Cu3(SDBA)2(HSDBA) were calculated using the Arrhenius and Eyring equations:
 | (3) |
 | (4) |
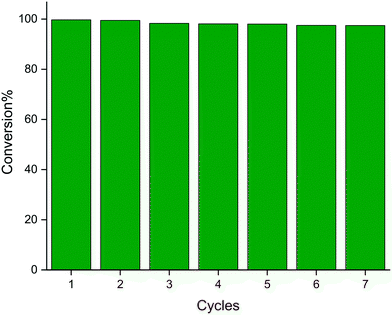
The recyclability of Cu3(SDBA)2(HSDBA) for the reduction of 4-NP was studied. To do this, after each cycle, the spent solution was filtered by centrifugation (3500 rpm, 10 min); most of the filtrate was removed and the remaining solid was washed with water and ethanol (with intermediate centrifugation); the washed solid was then dried at 80 for 1 h; finally, the as-recovered Cu3(SDBA)2(HSDBA) was re-used for another cycle by recharging with the reactants. Seven cycles were performed (Fig. 10 and S6†). Under our conditions, Cu3(SDBA)2(HSDBA) is relatively stable: it exhibits a conversion of 97% after the seventh cycle, and the time of reaction slightly increases from 20 s for the first cycle to 25 s for the sixth and seventh cycle. This is comparable to the stability performance of Ag-nanoparticle-embedded FeO3O4@MIL-100(Fe)48 and better than that of Ag nanoparticles immobilized onto MOF-199 supported by carboxymethylated cellulose fibers.39 One may however mention that Cu-BDC is fully stable over 5 cycles when used under more favorable conditions (see Table 1). In sum, Cu3(SDBA)2(HSDBA) is active for the reduction of 4-NP in 20 seconds at 25 °C (under our conditions) and it is able to maintain 97% of its initial performance in terms of conversion after 7 successive uses. Cu3(SDBA)2(HSDBA) thus positions itself as one of the most efficient catalysts for the catalytic reduction of 4-NP in the presence of NaBH4.
Cu3(SDBA)2(HSDBA) recovered after the aforementioned 7 cycles of use (it is now called ‘used Cu3(SDBA)2(HSDBA)’) was analyzed by FTIR and XRD. The FTIR spectrum (Fig. S7†) of used Cu3(SDBA)2(HSDBA) is comparable to that of Cu3(SDBA)2(HSDBA) (Fig. 2), except for one signal at about 1500 cm−1. This could be explained by the reduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the ligand to C–O–H by NaBH4.49 The PXRD pattern of used Cu3(SDBA)2(HSDBA) (Fig. S8†) is different from that of Cu3(SDBA)2(HSDBA) (Fig. S7†). This shows only the diffraction peak at about 43.5°, which is ascribed to the (111) plane of metallic Cu, formed by reduction of CuO ((111) plane) by NaBH4. Otherwise, used Cu3(SDBA)2(HSDBA) is amorphous, and the presence of one or more amorphous phases could be explained by the agglomeration of the Cu3(SDBA)2(HSDBA) particles.50 These changes are in line with the slight evolution of the catalytic activity of Cu3(SDBA)2(HSDBA) after 7 cycles.
O of the ligand to C–O–H by NaBH4.49 The PXRD pattern of used Cu3(SDBA)2(HSDBA) (Fig. S8†) is different from that of Cu3(SDBA)2(HSDBA) (Fig. S7†). This shows only the diffraction peak at about 43.5°, which is ascribed to the (111) plane of metallic Cu, formed by reduction of CuO ((111) plane) by NaBH4. Otherwise, used Cu3(SDBA)2(HSDBA) is amorphous, and the presence of one or more amorphous phases could be explained by the agglomeration of the Cu3(SDBA)2(HSDBA) particles.50 These changes are in line with the slight evolution of the catalytic activity of Cu3(SDBA)2(HSDBA) after 7 cycles.
4. Conclusion
Cu3(SDBA)2(HSDBA) has been successfully prepared using a solvothermal method. Its purity and morphology were examined by different techniques such as XRD, SEM-EDS, FTIR, UV-vis and TGA-DSC. Under our conditions, Cu3(SDBA)2(HSDBA) has a high catalytic performance for the reduction of 4-NP to 4-AP in the presence of NaBH4 at 25 °C: indeed, the reduction of 4-NP started upon the addition of Cu3(SDBA)2(HSDBA) and 4-NP was totally reduced in 20 seconds. The activation energy, activation enthalpy, and activation entropy, which were all determined through a systematic study, are of 9.7 kJ mol−1, 7.0 kJ mol−1, and 232.4 J mol−1 K−1, respectively. Cu3(SDBA)2(HSDBA) was also assessed for re-use over several successive cycles, and it has shown very good stability; it typically kept 97% of its initial performance in terms of conversion after seven cycles. Cu3(SDBA)2(HSDBA) is thus a suitable catalyst for the reduction of 4-NP to 4-AP in the presence of NaBH4 at 25 °C.Conflicts of interest
The authors have no conflicts of interest to disclose.Acknowledgements
This work was supported by the Tunisian National Ministry of Higher Education and Scientific Research, an Agreement of Collaboration between the University of Monastir and the Materials Science Factory, Materials Science Institute of Madrid (CSIC; C/ Sor Juana Ines de La Cruz, 3, Madrid, Spain).References
- T. He, C. Zhang, L. Zhang and A. Du, Single Pt atom decorated graphitic carbon nitride as an efficient photocatalyst for the hydrogenation of nitrobenzene into aniline, Nano Res., 2019, 12, 1817–1823 CrossRef CAS.
- M. M. Kgatitsoe, S. Ncube, H. Tutu, I. A. Nyambe and L. Chimuka, Synthesis and characterization of a magnetic nanosorbent modified with Moringa oleifera leaf extracts for removal of nitroaromatic explosive compounds in water samples, J. Environ. Chem. Eng., 2019, 7, 103128 CrossRef CAS.
- Y. Xu, X. Shi, R. Hua, R. Zhang, Y. Yao, B. Zhao, T. Liu, J. Zheng and G. Lu, Remarkably catalytic activity in reduction of 4-nitrophenol and methylene blue by Fe3O4@COF supported noble metal nanoparticles, Appl. Catal., B, 2020, 260, 118142 CrossRef CAS.
- A. H. Kianfar and M. A. Arayesh, Synthesis, characterization and investigation of photocatalytic and catalytic applications of Fe3O4/TiO2/CuO nanoparticles for degradation of MB and reduction of nitrophenols, J. Environ. Chem. Eng., 2020, 8, 103640 CrossRef CAS.
- F. Liu, X. Liu, D. Astruc and H. Gu, Dendronized triazolyl-containing ferrocenyl polymers as stabilizers of gold nanoparticles for recyclable two-phase reduction of 4-nitrophenol, J. Colloid Interface Sci., 2019, 5, 161–170 CrossRef PubMed.
- J. N. Jebaranjitham, C. Mageshwari, R. Saravanan and N. Mu, Fabrication of amine functionalized graphene oxide-Ag NPs nanocomposite with improved dispersibility for reduction of 4-nitrophenol, Composites, Part B, 2019, 171, 302–309 CrossRef.
- J. Singh, A. Mehta, M. Rawat and S. Basu, Green synthesis of silver nanoparticles using sun dried tulsi leaves and its catalytic application for 4-nitrophenol reduction, J. Environ. Chem. Eng., 2018, 6, 1468–1474 CrossRef CAS.
- M. T. Nakhjiri, G. B. Marandi and M. Kurdtabar, Preparation of magnetic double network nanocomposite hydrogel for adsorption of phenol and p-nitrophenol from aqueous solution, J. Environ. Chem. Eng., 2021, 9, 105039 CrossRef CAS.
- L. L. Bo, Y. B. Zhang, X. Quan and B. Zhao, Microwave assisted catalytic oxidation of p-nitrophenol in aqueous solution using carbon-supported copper catalyst, J. Hazard. Mater., 2008, 153, 1201–1206 CrossRef CAS PubMed.
- M. Heibati, J. Elham and V. Farzaneh, Photocatalytic Degradation of p-nitrophenol in an annular volumn photoreactor and the intermediates, Water Environ. Res., 2015, 87, 437–443 CrossRef CAS PubMed.
- P. Menon, T. S. A. Singh, N. Pani and P. V. Nidheesh, Electro-Fenton assisted sonication for removal of ammoniacal nitrogen and organic matter from dye intermediate industrial wastewater, Chemosphere, 2021, 269, 128739 CrossRef CAS PubMed.
- F. M. Valadi and M. R. Gholami, Synthesis of CuCo2O4/BiVO4 composites as promise and efficient catalysts for 4-nitrophenol reduction in water: Experimental and theoretical study, J. Environ. Chem. Eng., 2021, 9, 105408 CrossRef.
- D. Chen, L. Wu, S. Nie and P. Zhang, Solvent-free synthesis of N-doped carbon-based catalyst for high-efficient reduction of 4-nitrophenol, J. Environ. Chem. Eng., 2021, 9, 105649 CrossRef CAS.
- S. Ni, L. Yang, H. Qu, X. Zhu, Z. Xu, M. Yuan, H. Xing, L. Wang, J. Yu and H. Lu, Tailoring the structure and energy level over transition-metal doped MoS2 towards enhancing 4-nitrophenol reduction reaction, J. Environ. Chem. Eng., 2021, 9, 105101 CrossRef CAS.
- P. Xiao, S. Wang, X. Xu and J. Zhu, In-situ template formation method to synthesize hierarchically porous carbon for electrocatalytic reduction of 4-nitrophenol, Carbon, 2021, 184, 596–608 CrossRef CAS.
- F. S. Alamro, A. M. Mostafa, H. A. Ahmed and A. Toghan, Zinc oxide/carbon nanotubes nanocomposite: Synthesis, characterization and catalytic reduction of 4-nitrophenol via laser assistant method, Surf. Interfaces, 2021, 26, 101406 CrossRef.
- W. Shen, Y. Qu, X. Pei, S. Li, S. You, J. Wang, Z. Zhang and J. Zhou, Catalytic reduction of 4-nitrophenol using gold nanoparticles biosynthesized by cell-free extracts of Aspergillus sp. WL-Au, J. Hazard. Mater., 2017, 321, 299–306 CrossRef CAS PubMed.
- S. Jana, S. Konar, B. C. Mitra, A. Mondal and S. Mukhopadhyay, Fabrication of a new heterostructure Au/Pt/SnO2: An excellent catalyst for fast reduction of para-nitrophenol and visible light assisted photodegradation of dyes, Mater. Res. Bull., 2021, 141, 111351 CrossRef CAS.
- M. J. S. Mohamed, U. S. Shenoy and D. K. Bhat, Enhanced photocatalytic performance of N-doped RGO-FeWO4/Fe3O4 ternary nanocomposite in environmental applications, Mater. Today Chem., 2017, 4, 133–141 CrossRef.
- M. J. S. Mohamed, U. S. Shenoy and D. K. Bhat, Novel NRGO-CoWO4-Fe2O3 nanocomposite as an efficient catalyst for dye degradation and reduction of 4-nitrophenol, Mater. Chem. Phys., 2018, 208, 112–122 CrossRef CAS.
- M. J. S. Mohamed, U. S. Shenoy and D. K. Bhat, Synthesis of BaWO4/NRGO–g-C3N4 nanocomposites with excellent multifunctional catalytic performance via microwave approach, Front. Mater. Sci., 2018, 12, 247–263 CrossRef.
- D. Wu, P. F. Zhang, G. P. Yang, L. Hou, W. Y. Zhang, Y. F. Han and Y. Y. Wang, Supramolecular control of MOF pore properties for the tailored guest adsorption/separation applications, Coord. Chem. Rev., 2021, 434, 213709 CrossRef CAS.
- S. M. Sadeghzadeh, R. Zhiani and S. Emrani, The reduction of 4-nitrophenol and 2-nitroaniline by the incorporation of Ni@Pd MNPs into modified UiO-66-NH2 metal–organic frameworks (MOFs) with tetrathia-azacyclopentadecane, New J. Chem., 2018, 42, 988–994 RSC.
- H. N. Abdelhamid, High performance and ultrafast reduction of 4-nitrophenol using metal-organic frameworks, J. Environ. Chem. Eng., 2021, 9, 104404 CrossRef CAS.
- M. A. Ahsan, O. Fernandez-Delgado, E. Deemer, H. Wang, A. A. El-Gendy, M. L. Curry and J. C. Noveron, Carbonization of Co-BDC MOF results in magnetic C@Co nanoparticles that catalyze the reduction of methyl orange and 4-nitrophenol in water, J. Mol. Liq., 2019, 290, 111059 CrossRef CAS.
- M. A. Ahsan, O. Fernandez-Delgado, E. Deemer, H. Wang, A. A. El-Gendy, M. L. Curry and J. C. Noveron, Fe nanoparticles encapsulated in MOF-derived carbon for the reduction of 4-nitrophenol and methyl orange in water, Catal. Commun., 2019, 130, 105753 CrossRef CAS.
- M. Y. Pan, S. Q. Xia and X. T. Tao, Crystal structure of Ba5In4Sb6, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2015, 71, i4.933 Search PubMed.
- L. J. Farrugia, WinGX suite for small-molecule single-crystal crystallography, J. Appl. Crystallogr., 1999, 32, 837–838 CrossRef CAS.
- G. M. Sheldrick, A short history of SHELX, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- K. Y. A. Lin and Y. T. Hsieh, Copper-based metal organic framework (MOF), HKUST-1, as an efficient adsorbent to remove p-nitrophenol from water, J. Taiwan Inst. Chem. Eng., 2015, 50, 223–228 CrossRef.
- U. Jamil, A. H. Khoja, R. Liaquat, S. R. Naqvi, W. N. N. W. Omar and N. A. S. Amin, Copper and calcium-based metal organic framework (MOF) catalyst for biodiesel production from waste cooking oil: A process optimization study, Energy Convers. Manage., 2020, 215, 112934 CrossRef CAS.
- R. Rani, A. Deep, B. Mizaikoff and S. Singh, Enhanced hydrothermal stability of Cu MOF by post synthetic modification with amino acids, Vacuum, 2019, 164, 449–457 CrossRef CAS.
- S. Bhattacharya and S. Natarajan, Stabilization of Co3 –oxoclusters in a pcu net: Synthesis, structure, solvent exchange (single crystal to single crystal) and magnetic studies, Z. Anorg. Allg. Chem., 2014, 14, 2922–2930 CrossRef.
- J. Zhou, Y. Liu, J. Tang and W. Tang, Surface ligands engineering of semiconductor quantum dots for chemosensory and biological applications, Mater. Today, 2017, 20, 360–376 CrossRef CAS.
- S. Biswas, R. Saha and A. Ghosh, Copper (II)−Mercury (II) Heterometallic complexes derived from a salen-type ligand: A new coordination mode of the old Schiff base ligand, Organometallics, 2012, 31, 3844–3850 CrossRef CAS.
- Q. Q. Huang, Y. J. Lin, R. Zheng, W. H. Deng, C. Kashi, P. N. Kumar, G. E. Wang and G. Xu, Tunable electrical conductivity of a new 3D MOFs: Cu-TATAB, Inorg. Chem. Commun., 2019, 105, 119–124 CrossRef CAS.
- A. Pal, S. Chand, S. M. Elahi and M. C. Das, A microporous MOF with a polar pore surface exhibiting excellent selective adsorption of CO2 from CO2–N2 and CO2–CH4 gas mixtures with high CO2 loading, Dalton Trans., 2017, 46, 15280–15286 RSC.
- J. Liu, H. Yu and L. Wang, Effective reduction of 4-nitrophenol with Au NPs loaded ultrathin two dimensional metal-organic framework nanosheets, Appl. Catal., A, 2020, 599, 117605 CrossRef CAS.
- C. Duan, C. Liu, X. Meng, W. Lu and Y. Ni, Fabrication of carboxymethylated cellulose fibers supporting Ag NPs@MOF-199s nanocatalysts for catalytic reduction of 4 nitrophenol, Appl. Organomet. Chem., 2019, 33, e4865 CrossRef.
- Z. Liu, L. Ning, K. Wang, L. Feng, W. Gu and X. Liu, A new cobalt metal–organic framework as a substrate for Pd nanoparticles applied in high-efficiency nitro phenol degradation and cinnamaldehyde hydrogenation, Dalton Trans., 2020, 49, 1191–1199 RSC.
- E. Akbarzadeh, H. Z. Soheili and M. R. Gholami, Novel Cu2O/Cu-MOF/rGO is reported as highly efficient catalyst for reduction of 4-nitrophenol, Mater. Chem. Phys., 2019, 237, 121846 CrossRef CAS.
- S. P. Shet, S. Shanmuga Priya, K. Sudhakar and M. Tahir, A review on current trends in potential use of metal-organic framework for hydrogen storage, Int. J. Hydrogen Energy, 2021, 46, 11782–11803 CrossRef CAS.
- M. Guergueb, S. Nasri, J. Brahmi, F. Loiseau, F. Molton, T. Roisnel, V. Guerineau, I. Turowska-Tyrk, K. Aouadi and H. Nasri, Effect of the coordination of π-acceptor 4-cyanopyridine ligand on the structural and electronic properties of meso-tetra(para-methoxy) and meso-tetra(para-chlorophenyl) porphyrin cobalt(ii) coordination compounds, Application in the catalytic degradation of methylene blue dye, RSC Adv., 2020, 10, 6900–6918 RSC.
- S. R. Thawarkar, B. Thombare, B. S. Munde and N. D. Khupse, Kinetic investigation for the catalytic reduction of nitrophenol using ionic liquid stabilized gold nanoparticles, RSC Adv., 2018, 8, 38384–38390 RSC.
- M. A. Mahmoud, F. Saira and M. A. El-Sayed, Experimental evidence for the nanocage effect in catalysis with hollow nanoparticles, Nano Lett., 2010, 10, 3764–3769 CrossRef CAS PubMed.
- N. Sahiner, H. Ozay, O. Ozay and N. Aktas, A soft hydrogel reactor for cobalt nanoparticle preparation and use in the reduction of nitrophenols, Appl. Catal., A, 2010, 101, 137–143 CrossRef CAS.
- A. I. Ayad, D. Luart, A. O. Dris and E. Guénin, Kinetic analysis of 4-nitrophenol reduction by “water-soluble” palladium nanoparticles, Nanomaterials, 2020, 10, 1169 CrossRef CAS PubMed.
- S. Chang, C. Liu, Y. Sun, Z. Yan, X. Zhang, X. Hu and H. Zhang, Fe3O4 nanoparticles coated with Ag-nanoparticle-embedded metal-organic framework MIL-100(Fe) for the catalytic reduction of 4-nitrophenol, ACS Appl. Nano Mater., 2020, 3, 2302–2309 CrossRef CAS.
- D. E. Ward and C. K. Rhee, Chemsoselective reductions with sodium borohydride, Can. J. Chem., 1989, 67, 1206–1211 CrossRef CAS.
- A. Badri, S. Slimi, M. Guergueb, H. Kahri and X. Mateos, Green synthesis of copper oxide nanoparticles using Prickly Pear peel fruit extract: Characterization and catalytic activity, Inorg. Chem. Commun., 2021, 134, 109027 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1re00506e |
| This journal is © The Royal Society of Chemistry 2022 |