Open Access Article
Open Access ArticlePyridine ionic liquid functionalized bimetallic MOF solid-phase extraction coupled with high performance liquid chromatography for separation/analysis sunset yellow
Jun Wu,
Shuyu Wan,
Ouwen Xu,
Hanyang Song,
Jing Yang and
Xiashi Zhu *
*
College of Chemistry and Chemical Engineering, College of Guangling, Yangzhou University, Yangzhou, 225002, China. E-mail: xszhu@yzu.edu.cn; Fax: +86-514-87975244; Tel: +86-514-87975244
First published on 28th October 2022
Abstract
An effective method based on the pyridine ionic liquid functionalized bimetallic MOF solid-phase extractant (Cu/Co-MOF@[PrPy][Br]) coupled with high performance liquid chromatography (HPLC) for the separation/analysis sunset yellow was established. Cu/Co-MOF@[PrPy][Br] was characterized by FTIR, XRD, SEM and TEM. Several important factors, such as pH, amount of extractant, extract time, and types of eluents were investigated in detail. Under the optimal conditions, linear range of the method was 0.05–40.00 μg mL−1, the detection limit was 0.02 μg mL−1, and the linear correlation was good (R2 = 0.9992). The analysis of sunset yellow in soda, effervescent tablet and jelly proved that the method was simple and effective.
1 Introduction
Dyes (natural dyes, synthetic dyes) are used in food industry due to their advantages of stability, uniform color and low cost in food processing.1 Sunset yellow as a food additive2 provided the desired color and appearance, and is also commonly used in cosmetics and pharmaceuticals.3 The overuse of this dye and its release into the environment and water through associated industry effluents cause environmental pollution and the spread of various diseases, including allergic and asthmatic reactions, liver cell damage, DNA damage, and potential immunotoxicity and reproductive toxics.4 Therefore, it is necessary to establish a sensitive, accurate and effective method for the detection of sunset yellow in real samples.The determination methods of sunset yellow include UV-visible spectrophotometry,5 high performance liquid chromatography,6 electrochemical analysis7 and fluorescence spectrometry.8 Due to the complex composition of the real sample and the low content of sunset yellow, liquid phase extraction and solid phase extraction are often used to pretreat the sample.9 Solid phase extraction (SPE) concentrates, separates and purifies samples by means of selective adsorption and selective elution. Compared with liquid phase extraction, SPE has the advantages of strong selectivity, high recovery, less solvent and simple operation.10
The selection of extractant is crucial in SPE. Initially-at first, the materials include activated carbon,11 SiO2,12 graphene,13 metal organic framework material,14 covalent organic framework material.15 With the deepening of the research, the existing functionalization of SPE extractant, such as magnesium hydroxide/carbon nanotube composite,16 ionic liquid functionalized silica,17 magnetic cyclodextrin modified graphene oxide,18 ionic liquid modified metal organic framework material19 could further improve the performance of SPE, expand the application range of the solid phase extraction technology.
Metal organic framework (MOF) is porous structures constructed from the coordinative bonding between organic linkers and metal ions.20 It has unique pore space, large specific surface area. Functionalization of MOF means to change the properties of materials by replacing metal ions,21 changing the organic ligands22 and introducing other components23 with MOF as the main body, so that the types of MOF are greatly enriched. In recent years, some metal organic framework functionalized with graphene oxide,24 magnetic nanoparticles,25 multi-walled carbon nanotubes,26 chitosan27 and ionic liquids28 have been widely used in solid-phase extraction. Ionic liquids (ILs) are liquid molten salts at temperature below 100 °C which generally consist of organic cations and inorganic or organic anions. Due to their outstanding characteristics, its have achieved lots of successful applications.19 Studies have shown that ILs functionalized MOF composites (MOF@ILs) integrate the advantages of MOF and ILs, it can be used as solid-phase extraction agents, enabling the further development of solid-phase extraction technology. There have been related reports on the use of MOF@ILs composites for solid-phase extraction,29,30 but there is no report on the use of pyridinium ionic liquid-functionalized bimetallic MOF composites for the separation and enrichment of sunset yellow.
In this experiment, Cu/Co-MOF@[PrPy][Br] composites were prepared and was as solid-phase extractant coupled with high-performance liquid chromatography for the separation and analysis sunset yellow. The Cu/Co-MOF@[PrPy][Br] composites combine the advantages of ILs and MOF, resulting in greatly improved extraction performance. The method is simple and effective for the determination of sunset yellow in real sample.
2 Experimental
2.1 Instruments and reagents
SH-C constant temperature oscillator, vacuum drying oven (Shanghai Yiheng Scientific Instrument Co., Ltd), Shimadzu high performance liquid chromatograph (Shimadzu Technology Co., Ltd), Mettler Toledo pH meter (Shanghai Jingke Lei Magnetic), Fourier transform infrared spectrometer (Bruker, Germany), Centrifuge TDL80-2B (Shanghai Anting Scientific Instrument Factory).Cupric nitrate, N,N-dimethylformamide (DMF), ethanol, methanol, acetone, acetonitrile, cobalt nitrate hexahydrate, trimesic acid, pyridine, n-butane bromide and sunset yellow are analytical grade, and acetonitrile is HPLC grade. The above reagents were purchased from Sinopharm Chemical Reagent Co., Ltd.
Sunset yellow stock solution: 10.0 μg mL−1.
Buffer solution: 0.1 mol/L HAc/NaAc, 0.1 mol/L NH3·H2O/NH4Cl.
2.2 Preparation of [PrPy][Br]
0.08 mol of pyridine and equimolar of bromoethane were added to a 100 mL three-necked flask with a reflux condenser, and stirred at 80 °C for 6 hours. After the reaction, it was washed three times with ethyl acetate and finally the obtained product was vacuum-dried at 70 °C for 12 hours to obtain [PrPy][Br].312.3 Preparation of Cu/Co-MOF
Weight 1.42 g copper nitrate trihydrate, 0.73 g cobalt nitrate hexahydrate and 1.00 g trimesic acid were added to the mixed solution containing 17.00 mL H2O, 17.00 mL DMF and 17.00 mL ethanol, and poured into the reactor after ultrasonication for 30 minutes. The hydrothermal reaction was carried out at 85 °C for 20 hours. After the reaction, it was washed with ethanol, and finally the obtained product was vacuum-dried at 60 °C for 12 hours to obtain Cu/Co-MOF.322.4 Preparation of Cu/Co-MOF@[PrPy][Br]
Added 0.50 g [PrPy][Br] to a small sealed beaker with 40.00 mL of acetone and stirred for an hour, then added 1.50 g Cu/Co-MOF and stirred in air to completely evaporate the acetone. After the acetone was completely removed, the product was washed with absolute ethanol, and finally placed in a vacuum drying oven at 60 °C for 12 hours to obtain Cu/Co-MOF@[PrPy][Br].332.5 Extraction and elution process
Added appropriate amount of sunset yellow (1.0 μg mL−1 standard solution or real sample solution) and 2.00 mL of pH 5.0 buffer solution to the centrifuge tube, dilute to 10.00 mL, accurately weigh 5.00 mg of the composite material, and shake at 25 °C for 15 minutes. Centrifuge, decant the supernatant, add 3.00 mL of methanol, and shake at 25 °C for 10 minutes. After centrifugation, the supernatant was decanted and measured by Shimadzu high performance liquid chromatograph.2.6 Sample handling
Soda: take 20.00 mL of carbonated beverages in a beaker, degassed by ultrasonic, then filter with a 0.22 μm filter to remove particulate matter in the sample, and store it in a 50 mL centrifuge tube for determination.Effervescent tablet: take one effervescent tablet (3.70 g) in a small beaker, add 10.00 mL of distilled water to dissolve, filter it with a 0.22 μm filter head, and store it in a 50 mL centrifuge tube for determination.
Jelly: take 2.00 g of jelly in a beaker, add 10.00 mL of distilled water, heat it in a water bath at 80 °C until it is completely dissolved, then filter it with a 0.22 μm filter and store it in a 50 mL centrifuge tube for measurement.34
2.7 Chromatographic conditions
Column was VP-ODS C18 Column (250 mm × 4.6 mm); mobile phase was acetonitrile -to-water volume ratio of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25; column temperature was 25 °C; injection volume was 25.0 μL; flow rate was 1.0 mL min−1; UV detector detection wavelength was 484 nm.
25; column temperature was 25 °C; injection volume was 25.0 μL; flow rate was 1.0 mL min−1; UV detector detection wavelength was 484 nm.
3 Results and discussion
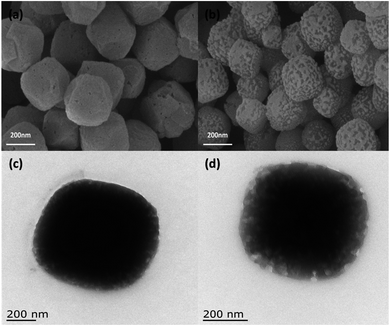
3.1 Characterization of Cu/Co-MOF@[PrPy][Br]
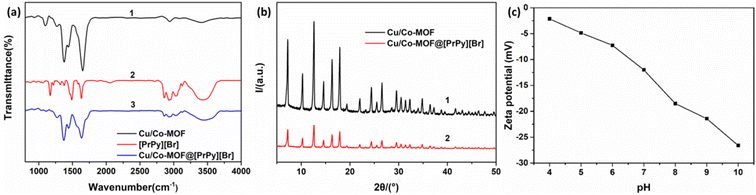
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration on the pyridine ring, while the peaks at 2680 cm−1, 2962 cm−1 and 3040 cm−1 were –CH3, –CH2 and C–H stretching vibration on the pyridine ring respectively. The characteristic peaks of Cu/Co-MOF@[PrPy][Br] correspond to Cu/Co-MOF and [PrPy][Br], and part of the characteristic peaks were offset, which could be attributed to the extension or shortening of the chemical bond between the MOF metal cluster and the connecting molecule caused by the addition of ionic liquid. These data indicated that Cu/Co-MOF@[PrPy][Br] composites were successfully synthesized.
N stretching vibration on the pyridine ring, while the peaks at 2680 cm−1, 2962 cm−1 and 3040 cm−1 were –CH3, –CH2 and C–H stretching vibration on the pyridine ring respectively. The characteristic peaks of Cu/Co-MOF@[PrPy][Br] correspond to Cu/Co-MOF and [PrPy][Br], and part of the characteristic peaks were offset, which could be attributed to the extension or shortening of the chemical bond between the MOF metal cluster and the connecting molecule caused by the addition of ionic liquid. These data indicated that Cu/Co-MOF@[PrPy][Br] composites were successfully synthesized.
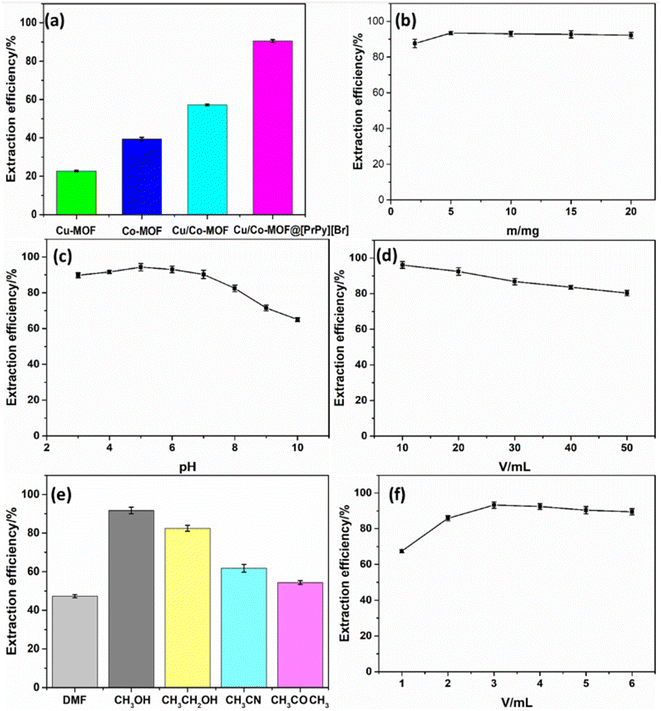
3.2 Optimization of extraction conditions
3.3 Optimization of elution conditions
3.4 Reuse times
Considering the maximization of material utilization, Cu/Co-MOF@[PrPy][Br] was eluted under the optimal elution conditions after extraction. The result showed that after 5 times of extraction-elution, the extraction efficiency remained above 85%. The results showed that Cu/Co-MOF@[PrPy][Br] had good stability and reusability.3.5 Interference experiment
The influence of interfering substances on the extraction efficiency of sunset yellow was studied. The allowable error range was ≤±5%. The results showed that when the concentration ratios of Rhodamine 6G, Sudan, lemon yellow, Allura Red, and methyl blue to sunset yellow were 100, 100, 5, 25, and 25 respectively, these interfering substances had no effect on the determination of sunset yellow.3.6 Analysis application
Under the optimal conditions, the linear range of sunset yellow was 0.05–40.0 μg mL−1. The linear equation was A (peak area) = 2.41 × 106c + 5.81 × 104 (μg mL−1). The linear correlation coefficient (R2) was 0.9992, detection limit was 0.02 μg mL−1.3.7 Sample analysis
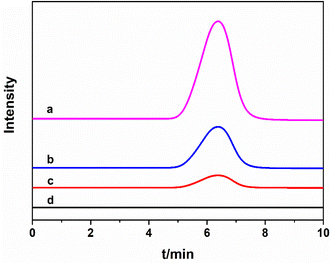
The chromatograms of samples measured under optimal conditions was obtained. As shown in Fig. 4, curve a represented the chromatogram of sunset yellow standard solution, and curves b, c and d represented extraction of real sample spiked with sunset yellow standard solution, the real sample with and without extraction respectively. It could be seen from Fig. 4 that: (1) the retention time of sunset yellow was about 6.38 min; (2) the sunset yellow signal in the real sample was significantly enhanced after the extraction (curves b, c and d).The real samples were determined by standard addition method. Table 1 showed that no sunset yellow was detected in soda, and the contents of sunset yellow in effervescent tablet and jelly were 0.051 and 0.062 μg g−1 respectively, which all met the Chinese national standard (GB 2760-2014). The recovery of sunset yellow in real samples ranged from 92.5% to 108.0%, which could be used for the determination of sunset yellow in real samples.
| Sample | Added value (mg L−1) | Measured value (mg L−1) | Recovery (%) |
|---|---|---|---|
| Soda | — | — | — |
| 0.500 | 0.514 | 102.8 | |
| 1.000 | 0.970 | 97.0 | |
| 2.000 | 1.850 | 92.5 | |
| Effervescent tablet | — | 0.051 | — |
| 0.500 | 0.487 | 97.4 | |
| 1.000 | 1.080 | 108.0 | |
| 2.000 | 1.910 | 95.5 | |
| Jelly | — | 0.062 | — |
| 0.500 | 0.508 | 101.6 | |
| 1.000 | 0.974 | 97.4 | |
| 2.000 | 1.980 | 99.0 |
3.8 Comparison of the proposed method with the reported methods
To further evaluate the performance of the present method, four previously reported methods for determining sunset yellow38–41 in different matrices were compared with the present method and the detailed results are summarized in Table 2. The linear range and LOD were comparable to those in the previously reported methods, demonstrating the reliability of the proposed method. It should be pointed out that the parameters, such as enhancement factor, sample volume, LOD of this method have a superior performance, and the extractant can be reused.| Methods | Samples | Enhancement factor | Linger range (μg L−1) | Extraction time (min) | Sample volume (mL) | LOD (μg L−1) | Ref. |
|---|---|---|---|---|---|---|---|
| SPE-HPLC | Sports drinks | 1.5 | — | 20 | 2 | 50.0 | 38 |
| SPE-UV | Beverage samples | 4 | 100–10000 | 9 | 4 | 37.0 | 39 |
| SPE-CE | Preserved fruit | 5 | 100–50000 | 10 | 15 | 35.0 | 40 |
| SPE-UV | Orange beverages, ice products, snacks, orange jelly powders | 10 | 1000–120000 | 15 | 20 | 410 | 41 |
| SPE-HPLC | Soda, effervescent tablet, jelly | 10 | 50–40000 | 15 | 30 | 20.0 | This work |
3.9 Adsorption (extraction) mechanism studies
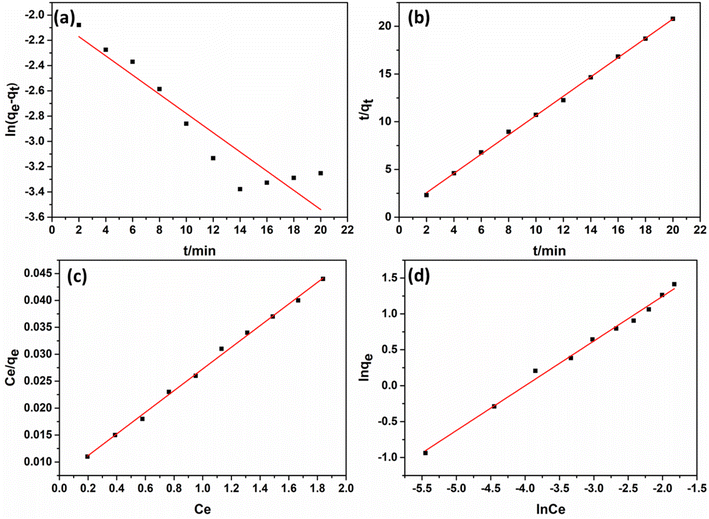
Pseudo-first-order: ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t |
| Pseudo-second-order: t/qt = 1/k2qe + t/qe |
The fitting curve were shown in Fig. 5a and b. It could be seen that the pseudo-second-order kinetic curve was better than the pseudo-first-order kinetic curve, and the linear correlation coefficient was 0.9987. Therefore, the pseudo-second-order kinetic model could better explain the adsorption process of sunset yellow of Cu/Co-MOF@[PrPy][Br], indicating that the process was chemical adsorption (extraction).
| Langmuir equation: Ce/qe = Ce/qmax + 1/qmaxKL |
Freundlich equation: ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce/n + ln Ce/n + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF KF |
The results were shown in Fig. 5c and d. The linear correlation coefficients of the Langmuir and Freundlich models are 0.9971 and 0.9927 respectively, so the Langmuir adsorption isotherm model was more suitable for the adsorption of sunset yellow on Cu/Co-MOF@[PrPy][Br]. The monolayer adsorption capacity calculated from the Langmuir isotherm was 50.00 mg g−1.
4 Conclusion
In this work, bisimidazole ionic liquid-functionalized metal organic framework composite (Cu/Co-MOF@[PrPy][Br]) was prepared and used as solid-phase extractant coupled with high performance liquid chromatography for the separation and analysis of sunset yellow in food. Compared with monometallic MOF, the bimetallic in Cu/Co-MOF@[PrPy][Br] endow the composites with more tunable active sites, due to the synergistic effect between the bimetals and has better recycling performance. The method is applied to the analysis of real samples with feasible, economical and practical, and the analytical results are satisfactory.Conflicts of interest
All authors declare there is no conflicts of interest.Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (21375117) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- T. Gan, J. Sun, W. Meng, L. Song and Y. X. Zhang, Electrochemical sensor based on graphene and mesoporous TiO2 for the simultaneous determination of trace colourants in food, Food Chem., 2013, 141(4), 3731–3737 CrossRef CAS PubMed.
- S. A. R. Hosseininia, M. H. Kamani and S. Rani, Quantitative determination of sunset yellow concentration in soft drinks via digital image processing, J. Food Meas. Char., 2017, 11(3), 1065–1070 CrossRef.
- W. Zhang, T. Liu, X. Zheng, W. S. Huang and C. D. Wan, Surface-enhanced oxidation and detection of Sunset Yellow and Tartrazine using multi-walled carbon nanotubes film-modified electrode, Colloids Surf., B, 2009, 74(1), 28–31 CrossRef CAS PubMed.
- L. Ji, Q. Cheng, K. Wu and X. F. Yang, Cu-BTC frameworks-based electrochemical sensing platform for rapid and simple determination of Sunset yellow and Tartrazine, Sens. Actuators, B, 2016, 231, 12–17 CrossRef CAS.
- Y. E. Unsal, M. Soylak and M. Tuzen, Column solid-phase extraction of sunset yellow and spectrophotometric determination of its use in powdered beverage and confectionery products, Int. J. Food Sci. Technol., 2012, 47(6), 1253–1258 CrossRef CAS.
- D. Ş. Zor, B. Aşçı and A. Ö. Dönmez, Simultaneous Determination of Potassium Sorbate, Sodium Benzoate, Quinoline Yellow and Sunset Yellow in Lemonades and Lemon Sauces by HPLC Using Experimental Design, J. Chromatogr. Sci., 2016, 54(6), 952–957 Search PubMed.
- K. Zhang, P. Luo, J. Wu, W. Wang and B. Ye, Highly sensitive determination of Sunset Yellow in drink using a poly (l-cysteine) modified glassy carbon electrode, Anal. Methods, 2013, 5(19), 5044–5050 RSC.
- Y. Yuan, X. Zhao, M. Qiao, J. H. Zhu, S. P. Liu, J. D. Yang and X. L. Hu, Determination of sunset yellow in soft drinks based on fluorescence quenching of carbon dots, Spectrochim. Acta, Part A, 2016, 167, 106–110 CrossRef CAS PubMed.
- W. A. Khan, M. B. Arain and M. Soylak, Nanomaterials-based solid phase extraction and solid phase microextraction for heavy metals food toxicity, Food Chem. Toxicol., 2020, 145, 111704 CrossRef CAS PubMed.
- J. S. Pyo, H. S. Lee and J.-H. Kwak, Determination of Gamma-Hydroxybutyric Acid in Urine by Solid Phase Extraction and Gas Chromatography-Mass Spectrometry, Anal. Lett., 2016, 49(2), 217–225 CrossRef CAS.
- H. Braus, F. Middleton and G. Walton, Orangic Chemical Compounds in Raw and Filtered Surface Waters, Anal. Chem., 1951, 23(8), 1160–1164 CrossRef CAS.
- K. Pyrzyñska and Z. Joñca, Multielement Preconcentration and Removal of Trace Metals by Solid-Phase Extraction, Anal. Lett., 2000, 33(7), 1441–1450 CrossRef.
- A. L. de Toffoli, E. V. S. Maciel and B. H. Fumes, The role of graphene-based sorbents in modern sample preparation techniques, J. Sep. Sci., 2018, 41(1), 288–302 CrossRef CAS PubMed.
- N. Manousi, D. A. Giannakoudakis and E. Rosenberg, Extraction of Metal Ions with Metal-Organic Frameworks, Molecules, 2019, 24(24), 4605 CrossRef CAS PubMed.
- Y. Ma, Y. Ruan and X. Gao, Preparation of a Novel Resin Based Covalent Framework Material and Its Application in the Determination of Phenolic Endocrine Disruptors in Beverages by SPE-HPLC, Polymers, 2021, 13(17), 2935 CrossRef CAS PubMed.
- Z.-L. Wu, Q. Liu, X.-Q. Chen and J. G. Yu, Preconcentration and analysis of Rhodamine B in water and red wine samples by using magnesium hydroxide/carbon nanotube composites as a solid-phase extractant, J. Sep. Sci., 2015, 38(19), 3404–3411 CrossRef CAS PubMed.
- J. P. Chen and X. S. Zhu, Ionic liquid coated magnetic core/shell Fe3O4@SiO2 nanoparticles for the separation/analysis of linuron in food samples, Spectrochim. Acta, Part A, 2015, 137, 456–462 CrossRef CAS PubMed.
- J.-Y. Chen, S.-R. Cao, C.-X. Xi and Y. Chen, ZQ Chen A novel magnetic β-cyclodextrin modified graphene oxide adsorbent with high recognition capability for 5 plant growth regulators, Food Chem., 2018, 239, 911–919 CrossRef CAS PubMed.
- X. Wei, Y. Wang, J. Chen, P. Xu and Y. G. Zhou, Preparation of ionic liquid modified magnetic metal-organic frameworks composites for the solid-phase extraction of α-chymotrypsin, Talanta, 2018, 182, 484–491 CrossRef CAS PubMed.
- M. A. Habila, B. Alhenaki, A. El-Marghany, M. Sheikh, A. A. Ghfar, Z. A. ALOthman and M. Soylak, Metal Organic Framework-Based Dispersive Solid-Phase Microextraction of Carbaryl from Food and Water Prior to Detection by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry, J. Sep. Sci., 2020, 43, 3103–3109 CrossRef CAS PubMed.
- S. Bourrelly, P. L. Llewellyn and C. Serre, Different Adsorption Behaviors of Methane and Carbon Dioxide in the Isotypic Nanoporous Metal Terephthalates MIL-53 and MIL-47, J. Am. Chem. Soc., 2005, 127(39), 13519–13521 CrossRef CAS PubMed.
- J. L. C. Rowsell and O. M. Yaghi, Metal–organic frameworks: a new class of porous materials, Microporous Mesoporous Mater., 2004, 73(1), 3–14 CrossRef CAS.
- Y. Shen, Z. Li and L. Wang, Cobalt-citrate framework armored with graphene oxide exhibiting improved thermal stability and selectivity for biogas decarburization, J. Mater. Chem. A, 2015, 3(2), 593–599 RSC.
- P. Thangavel, M. Ha and S. Kumaraguru, Graphene-nanoplatelets-supported NiFe-MOF: high-efficiency and ultra-stable oxygen electrodes for sustained alkaline anion exchange membrane water electrolysis, Energy Environ. Sci., 2020, 13(10), 3447–3458 RSC.
- A. Jarrah and S. Farhadi, Preparation and characterization of novel polyoxometalate/CoFe2O4/metal–organic framework magnetic core-shell nanocomposites for the rapid removal of organic dyes from water, RSC Adv., 2020, 10(65), 39881–39893 RSC.
- S. Zheng, J. Hu and X. Cui, In situ Growth of a Cobalt-based Metal-organic Framework on Multi-walled Carbon Nanotubes for Simultaneously Detection of Hydroquinone and Catechol, Electroanalysis, 2020, 32(9), 2010–2017 CrossRef CAS.
- L. Huang, W. Huang, R. Shen and S. A. Qin, Chitosan/thiol functionalized metal-organic framework composite for the simultaneous determination of lead and cadmium ions in food samples, Food Chem., 2020, 330, 127212 CrossRef CAS PubMed.
- A. Yohannes, X. Feng and S. Yao, Dispersive solid-phase extraction of racemic drugs using chiral ionic liquid-metal-organic framework composite sorbent, J. Chromatogr. A, 2020, 1627, 461395 CrossRef CAS PubMed.
- J.-H. Qin, Y.-D. Huang, M.-Y. Shi, H.-R. Wang, M. L. Han, X. G. Yang, F. F. Li and L. F. Ma, Aqueous-phase detection of antibiotics and nitroaromatic explosives by an alkali-resistant Zn-MOF directed by an ionic liquid, RSC Adv., 2020, 10(3), 1439–1446 RSC.
- M. Sarker, I. Ahmed and S. H. Jhung, Adsorptive removal of herbicides from water over nitrogen-doped carbon obtained from ionic liquid@ZIF-8, Chem. Eng. J., 2017, 323, 203–211 CrossRef CAS.
- H. Ji, K. Naveen and W. Lee, Pyridinium-Functionalized Ionic Metal-Organic Frameworks Designed as Bifunctional Catalysts for CO2 Fixation into Cyclic Carbonates, ACS Appl. Mater. Interfaces, 2020, 12(22), 24868–24876 CrossRef CAS PubMed.
- F. Tian, C. Qiao, R. Zheng, R. Y. Zheng, Q. F. Ru, X. Sun, Y. F. Zhan and C. G. Meng, Synthesis of bimetallic-organic framework Cu/Co-BTC and the improved performance of thiophene adsorption, RSC Adv., 2019, 9(27), 15642–15647 RSC.
- Z. Guo, W. Zheng, X. Yan, D. Yan, X. H. Ruan, X. H. Yang, X. C. Li, N. Zhang and G. H. He, Ionic liquid tuning nanocage size of MOFs through a two-step adsorption/infiltration strategy for enhanced gas screening of mixed-matrix membranes, J. Membr. Sci., 2020, 605, 118101–118109 CrossRef CAS.
- Z. Lotfi, H. Mousavi and M. S. Sajjadi, A hyperbranched polyamidoamine dendrimer grafted onto magnetized graphene oxide as a sorbent for the extraction of synthetic dyes from foodstuff, Microchim. Acta, 2017, 184(11), 4503–4512 CrossRef CAS.
- J. Zhang, C. Zhang and X. Duo, Self-assembled acicular CuCo-MOF for enhancing oxygen evolution reaction, Ionics, 2020, 26(10), 5123–5132 CrossRef CAS.
- M. Kamalabadi, M. M. Razavi-Mashouf and T. Madrakian, Electrochemically controlled solid phase microextraction based on nanostructured polypyrrole film for selective extraction of sunset yellow in food samples, J. Iran. Chem. Soc., 2021, 18(11), 3127–3135 CrossRef CAS.
- R. Khani and M. Irani, Mixed Magnetic Dispersive Micro-Solid Phase-Cloud Point Extraction of Sunset Yellow in Food and Pharmaceutical Samples, ChemistrySelect, 2021, 6(3), 273–278 CrossRef CAS.
- L. Floriano, L. C. Ribeiro, N. Saibt, N. M. G. Bandeira, O. D. Prestes and R. Zanella, Determination of Six Synthetic Dyes in Sports Drinks by Dispersive Solid-Phase Extraction and HPLC-UV-Vis, Soc. Bras. Quim., 2018, 29, 602–608 CAS.
- W. J. Li, Q. Jia, Z. Y. Zhang and Y. P. Wang, Preparation of γ-alumina nanoparticle modified polyacrylamide composite and study on its solid phase extraction of Sunset Yellow, Korean J. Chem. Eng., 2016, 33, 1337–1344 CrossRef CAS.
- J. Yi, L. Zeng and Q. Wu, Sensitive Simultaneous Determination of Synthetic Food Colorants in Preserved Fruit Samples by Capillary Electrophoresis with Contactless Conductivity Detection, Food Anal. Methods, 2018, 11(6), 1608–1618 CrossRef.
- B. Hatamluyi, R. Sadeghian, F. Malek and M. T. Boroushaki, Improved solid phase extraction for selective and efficient quantification of sunset yellow in different food samples using a novel molecularly imprinted polymer reinforced by Fe3O4@UiO-66-NH2, Food Chem., 2021, 357, 129782–129789 CrossRef CAS PubMed.
- T. A. Saleh, A. Sarı and M. Tuzen, Effective adsorption of antimony(III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent, Chem. Eng. J., 2017, 307, 230–238 CrossRef CAS.
- J. Fu, Z. Chen and M. Wang, Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis, Chem. Eng. J., 2015, 259, 53–61 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |