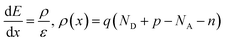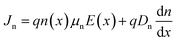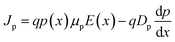Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Simulation and analysis of lead-free perovskite solar cells incorporating cerium oxide as electron transporting layer
Ali K. Al-Mousoia,
Mustafa K. A. Mohammed *b,
Rahul Pandey
*b,
Rahul Pandey c,
Jaya Madanc,
Davoud Dastand,
G. Ravi
c,
Jaya Madanc,
Davoud Dastand,
G. Ravi e,
P. Sakthivele and
G. Anandha babuf
e,
P. Sakthivele and
G. Anandha babuf
aDepartment of Radiology and Ultrasonography Techniques, College of Medical Techniques, Al-Farahidi University, 10011 Baghdad, Iraq
bRadiological Techniques Department, Al-Mustaqbal University College, 51001 Hillah, Babylon, Iraq. E-mail: mustafa_kareem97@yahoo.com
cVLSI Centre of Excellence, Chitkara University Institute of Engineering and Technology, Chitkara University, 140417 Rajpura, Punjab, India
dDepartment of Materials Science and Engineering, Cornell University, Ithaca, NY 14850, USA
eDepartment of Physics, Alagappa University, Karaikudi 630003, Tamil Nadu, India
fDepartment of Physics, Bannari Amman Institute of Technology, Tamil Nadu, India
First published on 11th November 2022
Abstract
The great demand for renewable energy has greatly contributed to the development of the solar cell industry. Recently, silicon solar cells have dominated the world market. The ease of processing gives perovskite solar cells (PSCs) an advantage over conventional silicon solar cells. Regular silicon photovoltaics require expensive, multi-step processes accomplished in a specialized ultraclean-chamber facility at an elevated temperature (>1000 °C) and highly vacuumed workspace. Hence, researchers and the solar cell industry have focused on PSC as a great rival to silicon solar cells. Despite this, the highest efficiency was obtained from lead-based PSC, which has a considerably high toxicity issue and low stability related to lead content, so the research field pays attention to lead-free perovskite solar cells. In this digital simulation, tin-based perovskite in this paper, methylammonium tin iodide (MASnI3) with the use of cerium oxide (CeOx) as an electron transporting layer (ETL) with varying percentages of oxygen, which means different shallow donor densities (ND). The optimum value for the thickness of the absorber layer (perovskite) was made, and the current–voltage characteristics and efficiency calculations were also accomplished for the best cell. Then an improvement was made by changing the ND value of CeOx, and the best-optimized cell parameters were: open circuit voltage (VOC) of 0.92 V, short circuit current density (JSC) of 30.79 mA cm−2, power conversion efficiency (PCE) of 17.77%, and fill factor (FF) of 62.86%.
1. Introduction
The production of electricity is crucial for world development and is unquestionably the main driver of economic growth in both industrialized and emerging economies. A huge increase in energy demand is being fueled by both a high population growth rate and increased per capita energy consumption.1–4 Fossil fuel-based energy sources currently meet the majority of the world's energy needs. However, fossil fuel supplies are being depleted more quickly as energy use rises. Renewable energy solutions must be created in order to address these concerns and the growing need for energy. Solar energy, photovoltaic cells that can be used to convert directly into electricity, is the most abundant renewable energy source.5–11Perovskite has gained popularity as a light-harvesting material for photovoltaic applications. Perovskite has a distinct set of optoelectrical properties, including adjustable band gaps, a high absorption coefficient, long carrier diffusion lengths, and high charge carrier mobilities.12–16 In 2009, Kojima and colleagues reported a power conversion efficiency (PCE) of 3.8% for perovskite solar cells (PSC).17 After a decade, Cui et al. reported a 20.8% PCE for methylammonium lead iodide PSC.18 CITY U HK/UW established the most recent approved efficiency of close to 25.8% in 2021.19 Regardless of the rapid evolution of PCE, the present stability of solar cells makes mass production of PSC difficult.20–22
The lead content of lead perovskite solar cells is one of their main disadvantages.23 Due to lead's toxicity, the Restriction of Hazardous Substances directive of the European Union forbids its use in any electronics or electrical equipment. Alternatives to lead as the metal cation in the perovskite photo-absorber have thus become a significant area of research.24 Stepping up or down within group IV of the periodic table of elements provides an easy way to replace lead. The recently found element, scarcely radioactively stable flerovium, lies in the row below lead. Due to its radioactivity, it may not be a suitable replacement for lead.25
Due to its lower toxicity, tin (Sn), which is on the same row as lead, would be an excellent replacement for lead in PSCs. Organic tin halide synthesis has been going on for as long as lead halide synthesis.26 The MASnI3 perovskite, with an energy gap (Eg) of 1.3 eV, has been reported to be used in solar cells and produced a PCE of 6.4%. The +2 oxidation state of Sn, which is necessary for the formation of a perovskite, is unstable, and the metal quickly oxidizes to the +4 state when exposed to oxygen or air humidity,27,28 this affects both the conditions under which the device operates and the technique used to make solar cells. It has only been capable of determining the cell performance of the pure tin halide PSCs with strong sealing of the devices because any interaction with oxygen may instantaneously cause the oxidation of tin.29
CeOx is regarded as one of the most significant infrequent oxides because of its wide bandgap, high dielectric constant, strong ionic conductivity, high thermal and chemical stability, matching lattice parameters with silicon, and exceptional capacity to store and release oxygen.30,31 Wang et al. prepared CeOx (x = 1.87) films by a facile sol–gel approach at a low temperature (150 °C) and used them as an alternative to the high-temperature annealing processed TiO2 ETL. The optimized PSC achieved a champion PCE of 14.32% by adjusting the CeOx precursor solution.32 Yang et al. used CeOx as ETL in an inverted structure of PSC, utilizing CsPbIBr2 perovskite as light harvesting material. The all-inorganic PSC recorded a maximum efficiency of 5.6% with improved stability.33 Jien et al. reported CeOx film as a protection layer for perovskite against humidity and metal reactions with the electrode. The device with CeOx as the ETL had a PCE of 17.47%.34
In this simulation, CeOx is used as an ETL material due to its tunable broad band gap (3.0–3.6 eV) and outstanding optical and dielectric properties.35,36 Moreover, CeOx may enhance the stability of the PSC against moisture and oxygen.37 A numerical simulation of the solar cell would be necessary to figure out the best set of parameters and the physical parameters for prediction accuracy. The device is simulated using the SCAPS-1 D software and the influence of different properties of the MASnI3 material and CeOx layer on the efficiency of a tin-PSC is estimated. A unique device structure (fluoride tin oxide (FTO)/CeOx/MASnI3/2,2′,7,7′-tetrakis[N,N-di(4-ethoxyphenyl)amino]-9,9′-spirobifluorene (Spiro-MeTAD)/Au) of a tin-based PSC using the Spiro-OMeTAD as a hole transporting layer (HTL) has been suggested for modeling. The aim of this simulation is to illustrate that the efficiency of lead-free PSCs may be enhanced by varying the ND value of the CeOx and the thickness of the absorber layer (MASnI3).
2. Architecture and materials properties of suggested PCS
For PV modelling, a one-dimensional SCAPS simulation software was employed. The SCPAS program employs Poisson's equation, which defines the relationship between the photocarrier and the semiconductor's electrostatic potential, and continuity equations, which represent charge generation and recombination kinetics in materials.38 Solving both Poisson's equation and the continuity equation gives us the QE and J–V properties.39 Using Poisson's equation and the continuity equation, it is possible to figure out the density of electrons and holes.40 Poisson's equation can be used to determine the distribution of the electric field E(x):Drift and diffusion current densities control the transportation properties of charge carriers in semiconductor. The following equations describe the drift and diffusion current densities for electrons and holes.38
where μn and μp are to electron hole mobility, respectively, and Dn, and Dp are electron and hole diffusion coefficients respectively. ρ(x), ε, and q refer to space charge distribution, dielectric permittivity, and charge of electron, respectively. n(x) and Jn represent the concentration and current density of electrons. p(x) and Jp represent the concentration and current density of hole. Seven layers, consisting of perovskite absorber, ETL, HTL, and electrodes, can be constructed into a HPSC using the SCAPS software. All PV computations in this study are performed under AM 1.5G (100 mW cm−2) conditions.
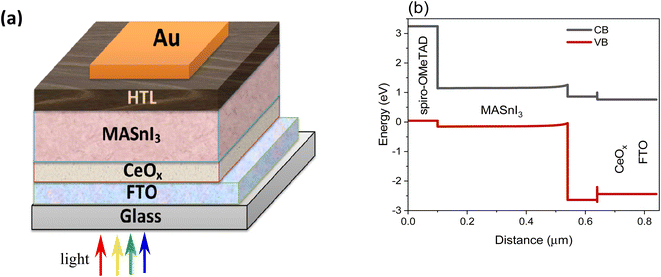
The structure for the proposed PSC, FTO/CeOx/MASnI3/Spiro-OMeTAD/Au, is shown in Fig. 1a. The FTO layer, which is deposited on a glass substrate, acts as a front transparent contact. The CeOx layer represents the crucial layer that determines the performance of the device since the transport of electrons is a major factor affecting the PCE. MASnI3 perovskite is the absorber material that is responsible for charge carrier generation through light absorption. Spiro-OMeTAD material acts as HTL. The energy band graph of the suggested structure (Fig. 1b) clearly shows that the conduction bands of the absorbing material MASnI3 are smaller than those of electron transporting material CeOx, and the mismatch between the conduction band (CB) of MASnI3 and the CeOx material is very slight. As a direct consequence, electrons can easily pass from MASnI3 to FTO via CeOx. Electrons can thus move freely through CeOx from MASnI3 to FTO. A very large valence band (VB) offset (VBO) exists between the absorbing material MASnI3 and the ETL material CeOx. Therefore, the positive charge (h+) in the ETL material CeOx will be sealed. As shown in Fig. 1b, the valence bands of the hole transporting material Spiro-OMeTAD are greater than those of the absorbing material MASnI3, and the valence band mismatch among those two materials is significantly small. Furthermore, the offsetting in the conduction bands between the HTL material Spiro-OMeTAD and the absorbing material MASnI3 is very considerable, forbidding the electrons from MASnI3 from reaching the back contact.
The physical parameters for the simulations of the FTO/CeOx/MASnI3/Spiro-OMeTAD/Au heterostructure solar cell are listed in Table 1,41 whereas the physical properties of the defects density in MASnI3 are listed in Table 2. All simulations are achieved at a temperature of 300 K.
| Material | Spiro-OMeTAD | MASnI3 | CeOx | FTO |
| Thickness of layer (μm) | 0.1 | 0.44 | 0.1 | 0.2 |
| Energy band gab (eV) | 3.2 | 1.3 | 3.5 | 3.2 |
| Electron affinity (eV) | 2.1 | 4.2 | 4.6 | 4.4 |
| Relative permittivity | 3 | 10 | 9 | 9 |
| CB effective densities of states (cm−3) | 2.50 × 1018 | 1 × 1018 | 1 × 1020 | 2.2 × 1018 |
| VB effective densities of states (cm−3) | 1.8 × 1019 | 1 × 1018 | 2 × 1021 | 1.8 × 1019 |
| Electron thermal velocity (cm s−1) | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 |
| Hole thermal velocity (cm s−1) | 1 × 107 | 1 × 107 | 1 × 107 | 1 × 107 |
| Mobility of electron (cm2 V−1 s−1) | 2 × 104 | 1.6 | 100 | 20 |
| Mobility of hole (cm2 V−1 s−1) | 2 × 104 | 1.6 | 25 | 10 |
| Uniform donor densities ND (cm−3) | — | — | 1 × 1021 | 1 × 1021 |
| Uniform acceptor densities NA (cm−3) | 1 × 1020 | 3.20 × 1015 | — | — |
| Defect | 1 × 1014 | 4.5 × 1016 | 1 × 1015 | 1 × 1015 |
| Parameters | Spiro-OMeTAD/MASnI3 interface | CeOx/MASnI3 interface |
| Defects type | Neutral | Neutral |
| Capture cross-section for electron (cm2) | 1 × 10−19 | 1 × 10−19 |
| Capture cross-section for hole (cm2) | 1 × 10−19 | 1 × 10−19 |
| Energetic distributions | Single | Single |
| Defects energy level Et | Up the maximum Ev | Up the maximum Ev |
| Reference for defect energy level Et | 0.06 | 0.06 |
| Total density (integrated over all energies) (cm−2) | 1 × 1010 | 1 × 1010 |
3. Results and discussion
3.1 Optimization of MASnI3 thickness
The thickness of the absorbing material plays a significant role in solar cell efficiency since it is related to the absorption of the incident light, which leads to enhancing the I–V characteristics.42–44 In this study, all the parameters of the materials in Tables 1 and 2 are kept fixed, and different values of the thickness of MASnI3 are selected in order to estimate the optimum thickness value of the absorber material. Fig. 2 shows the parameters of the suggested PSC as a function of MASnI3 thickness. As Fig. 2a illustrates the quantum efficiency of the suggested PSC, we can see the influence of the absorbing material thickness on the QE value. As expected, by increasing the value of the thickness, the QE value increases. Fig. 2b demonstrates the J–V curve of the PSC under the illumination of 100 mW cm−2 (air mass AM 1.5G). Fig. 2c–f shows the VOC, JSC, FF, and, PCE respectively. From Fig. 2b, the VOC is slightly decreased with thickness increases, while Fig. 2c shows a significant change in the value of JSC. Besides the variant JSC with the thickness of the absorbing material, the FF is also highly affected by the thickness, as shown in Fig. 2d. As a consequence of the change in the last mentioned parameters, the PCE varies with changing the thickness, as illustrated in Fig. 2e. Table 3 shows the calculated parameters that are used to select the optimum thickness.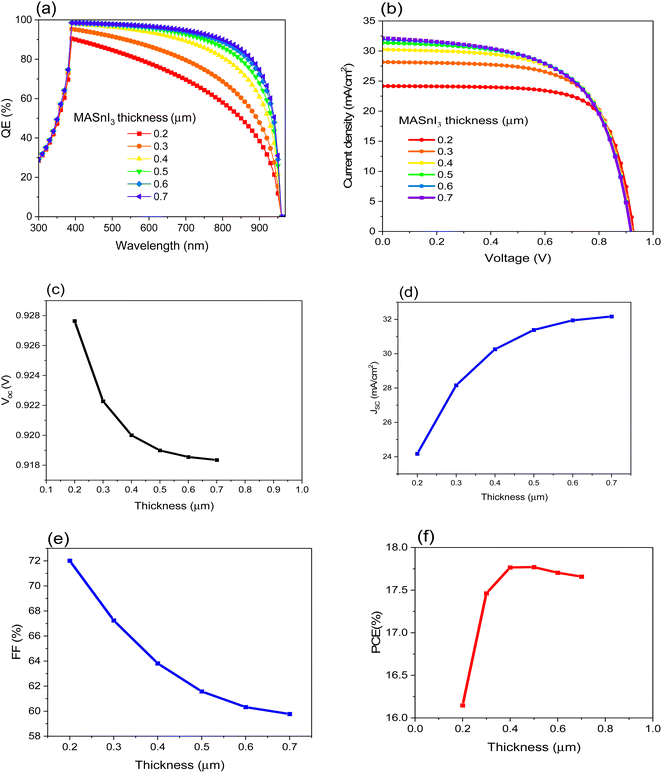 | ||
| Fig. 2 . Displays the impact of the MASnI3 thickness on the efficiency of FTO/CeOx/MASnI3/Spiro-OMeTAD/Au PSC where (a) QE, (b) J–V curve, (c) VOC, (d) JSC, (e) FF and (f) PCE. | ||
| MASnI3 thickness (μm) | VOC (V) | JSC (mA cm−2) | FF (%) | PCE (%) | Vm (V) | Jm (mA cm−2) |
|---|---|---|---|---|---|---|
| 0.2 | 0.928 | 24.172 | 72.010 | 16.147 | 0.754 | 21.406 |
| 0.3 | 0.922 | 28.158 | 67.243 | 17.463 | 0.726 | 24.056 |
| 0.4 | 0.920 | 30.260 | 63.815 | 17.766 | 0.711 | 24.993 |
| 0.5 | 0.919 | 31.386 | 61.573 | 17.770 | 0.704 | 25.217 |
| 0.6 | 0.919 | 31.948 | 60.327 | 17.703 | 0.702 | 25.218 |
| 0.7 | 0.918 | 32.169 | 59.776 | 17.659 | 0.701 | 25.185 |
3.2 Optimization of donor density (ND) of the CeOx
The percentage of oxygen content in CeOx determines the value of ND.45 In this simulation, different values of ND are chosen in order to understand their effect on the PSC performance. Fig. 3a–e shows the variation of the solar cell parameters as a function of log(ND). All the parameter values increase as the value of ND increases. This is attributed to the enhancement of the conductivity, which makes the movement of the electrons through the ETL much easier. ETL's primary function is to give electrons a low-resistive route so that they can be collected. Additionally, it must prevent electrons from stacking up close to the interface. On the other hand, if this happens because of lower conductivity, unrestricted electrons and holes interact together on the interfacial side, resulting in a decreased generated current. Low conductivity causes a higher series resistance (Rs). The high value of resistance in the cell causes the FF to drop. The influence of low conductivity in this case caused by low ND will affect both the FF and VOC, as shown in Fig. 3b and d, while the JSC is not significantly influenced. Table 4 summarizes the obtained results when varying the ND value. It is obvious that the best PCE obtained of 17.77% at ND is equal to 1 × 1021 cm−3. As the ND of CeOx increases to a certain point, the PCE also increases. After this point, the ND of the cerium oxide has no effect on the PCE because the CeOx has degenerated (practically). Furthermore, once the ND reaches a certain value where the CeOx serves as a hole-blocking and electron-transporting layer, any further increase in the ND value is unnecessary.46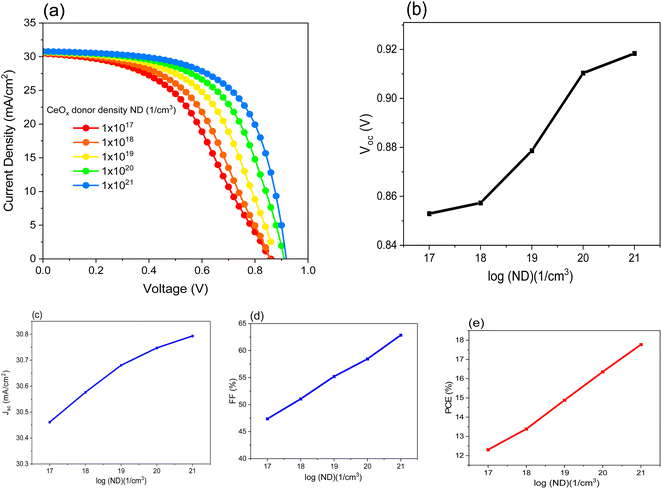 | ||
| Fig. 3 Shows the effect of the ND of CeOx on performance of FTO/CeOx/MASnI3/Spiro-OMeTAD/Au PSC where: (a) (J–V) curve, (b), (c) JSC, (d) FF and (e) PCE. | ||
| ND (cm−3) | VOC (V) | JSC (mA cm−2) | FF (%) | PCE (%) | Vm (V) | Jm (mA cm−2) |
|---|---|---|---|---|---|---|
| 1 × 1017 | 0.85 | 30.46 | 47.37 | 11.82 | 0.52 | 23.58 |
| 1 × 1018 | 0.86 | 30.58 | 51.07 | 13.37 | 0.56 | 24.05 |
| 1 × 1019 | 0.88 | 30.68 | 55.21 | 14.87 | 0.61 | 24.49 |
| 1 × 1020 | 0.91 | 30.75 | 58.45 | 16.35 | 0.66 | 24.83 |
| 1 × 1021 | 0.92 | 30.79 | 62.86 | 17.77 | 0.71 | 25.13 |
3.3 Effect of the series and shunt resistance (Rs & Rsh)
The Rs and Rsh have significant control over how well solar cells work. It comes from the metal connections on the solar cell and layer surfaces. The device's efficiency was assessed by changing the value of Rs from 0 to 10 (Ω cm2). Fig. 4a–d show that as Rs increases, the FF and PCE decrease, resulting in leakage currents, while the VOC and JSC remain unchanged.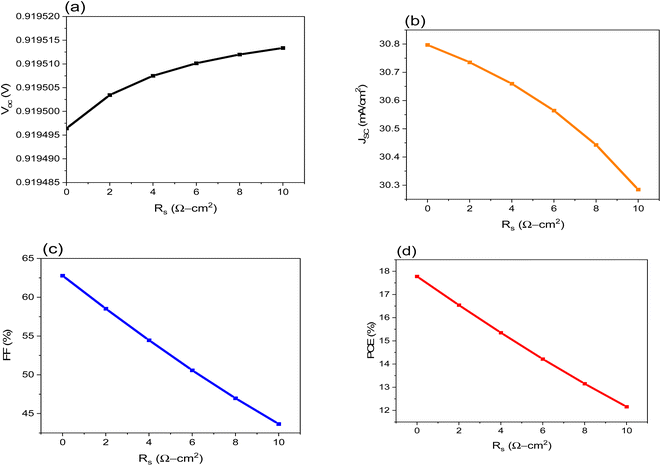 | ||
| Fig. 4 Shows the influence of Rs on the performance of FTO/CeOx/MASnI3/Spiro-OMeTAD/Au PSC where: (a) VOC, (b), JSC, (c) FF and (d) PCE. | ||
Poor shunt resistance increases power loss in the solar cell by enabling the current produced by light to take an alternative path. A similar deflection drops the voltage produced by the photovoltaic cell and minimizes the current flowing through the photovoltaic junction. Fig. 5a–d shows that the FF is the most affected parameter, resulting in a decrease in the value of PCE, while the VOC and JSC are almost unchanged.
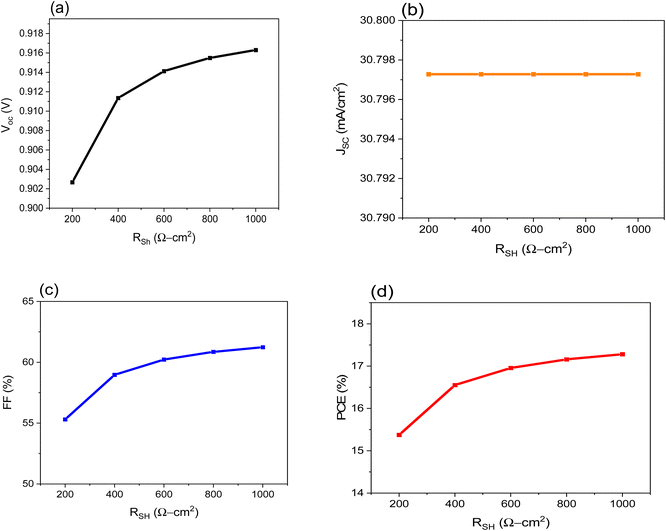 | ||
| Fig. 5 Shows the impact of Rsh of the on performance of FTO/CeOx/MASnI3/Spiro-OMeTAD/Au PSC where: (a) VOC, (b), JSC, (c) FF and (d) PCE. | ||
Notably, the results showed that PSCs designed with a room-temperature CeOx layer could improve the PCE of MASnI3-based photovoltaics, which is a significant step toward PSC industrialization. Table 5 shows the results of a comparison between the various structures, and it can be stated that adding CeOx into the perovskite-based devices is the most beneficial method for using it in PSCs.
| Author | Structure | Perovskite thickness (nm) | Total defect density (cm−3) | PCE (%) |
|---|---|---|---|---|
| Anu et al.47 | FTO/CeOx/PCBM/MAPbI3/Spiro-OMeTAD/Au | 250 | 5 × 1014 | 18.36 |
| Pandey et al.45 | FTO/CeOx/PCBM/MAPbI3/CNTs/Spiro-OMeTAD/Ag | 250 | 2.14 × 1017 | 18.20 |
| Raoui et al.48 | FTO/CeOx/PCBM/CsSn0.5Ge0.5I3/PTAA/Spiro-OMeTAD/Au | 200 | 1 × 1011 | 24.20 |
| Ahmmed et al.49 | ITO/CeOx/CsBi3I10/NiOx/Au | 1000 | 1 × 1014 | 19.16 |
| Moiz et al.50 | FTO/CeOx/Cs2TiBr6/N,N′-bis(naphthalen-1-yl)-N,N′-bis(phenyl)-benzidine/Au | 150 | 1 × 1015 | 20.40 |
| This work | FTO/CeOx/MASnI3/Spiro-OMeTAD/Au | 600 | 4.5 × 1016 | 17.77 |
4. Conclusions
The performance of the suggested cell structure FTO/CeOx/MASnI3/Spiro-OMeTAD/Au PSC has been examined using the SCAPS 1D simulation software. Different CeOx and MASnI3 parameters are tuned in order to investigate the behavior of the device. The open-circuit voltage and efficiency received a significant contribution from the CeOx layer. Cell performance was shown to be enhanced by the CeOx ETL's higher ND. Cell performance appears to be significantly influenced by the MASnI3 layer's thickness. The density of the MASnI3/CeOx/MASnI3 interfacial defect and the MASnI3/Spiro-OMeTAD interfacial defect were determined. The highest PCE ever calculated was 17.77%, with a JSC value of 30.79 mA cm−2, a VOC value of 0.919 V, and a fill factor of 62.78%. Our findings indicate that future research may show that the suggested device structure involving both CeOx and MASnI3 is an effective device for making thin-film solar cells that are both inexpensive and efficient.Data availability
Data will be available based on reasonable request.Ethics approval and consent to participate
We comply with the ethical standards. We provide our consent to take part.Conflicts of interest
There are no conflicts to declare.References
- K. Brinkmann, T. Becker, F. Zimmermann, C. Kreusel, T. Gahlmann, M. Theisen, T. Haeger, S. Olthof, C. Tückmantel and M. Günster, Perovskite–organic tandem solar cells with indium oxide interconnect, Nature, 2022, 604(7905), 280–286 CrossRef CAS PubMed.
- Y. Ding, B. Ding, H. Kanda, O. J. Usiobo, T. Gallet, Z. Yang, Y. Liu, H. Huang, J. Sheng and C. Liu, Single-crystalline TiO2 nanoparticles for stable and efficient perovskite modules, Nat. Nanotechnol., 2022, 1–8 Search PubMed.
- R. York and S. E. Bell, Energy transitions or additions?: why a transition from fossil fuels requires more than the growth of renewable energy, Energy Res. Social Sci., 2019, 51, 40–43 CrossRef.
- A. J. Chapman, B. C. McLellan and T. Tezuka, Prioritizing mitigation efforts considering co-benefits, equity and energy justice: fossil fuel to renewable energy transition pathways, Appl. Energy, 2018, 219, 187–198 CrossRef.
- K. A. S. Singh, M. K. Mohammed and A. E. Shalan, Effect of 2D perovskite layer and multivalent defect on the performance of 3D/2D bilayered perovskite solar cells through computational simulation studies, Solar Energy, 2021, 223, 193–201 CrossRef.
- A. Al-Mousoi, M. Mehde and A. Al-Gebori, Annealing temperature effects on the performance of the perovskite solar cells, IOP Conference Series: Materials Science and Engineering, IOP Publishing, 2020, p. 012039 Search PubMed.
- M. K. Mohammed and A. K. Al-Mousoi, Deposition of multi-layer graphene (MLG) film on glass slide by flame synthesis technique, Optik, 2016, 127(20), 9848–9852 CrossRef CAS.
- J.-P. Correa-Baena, M. Saliba, T. Buonassisi, M. Grätzel, A. Abate, W. Tress and A. J. S. Hagfeldt, Promises and challenges of perovskite solar cells, Science, 2017, 358(6364), 739–744 CrossRef CAS PubMed.
- M. D. Humadi, H. T. Hussein, M. S. Mohamed, M. K. Mohammed and E. Kayahan, A facile approach to improve the performance and stability of perovskite solar cells via FA/MA precursor temperature controlling in sequential deposition fabrication, Opt. Mater., 2021, 112, 110794 CrossRef CAS.
- M. J. Kadhim and M. K. Mohammed, Fabrication of Efficient Triple-Cation Perovskite Solar Cells Employing Ethyl Acetate as an Environmental-Friendly Solvent Additive, Mater. Res. Bull., 2022, 112047 Search PubMed.
- M. K. Mohammed, A. K. Al-Mousoi, S. M. Majeed, S. Singh, A. Kumar, R. Pandey, J. Madan, D. S. Ahmed and D. Dastan, Stable Hole-Transporting Material-Free Perovskite Solar Cells with Efficiency Exceeding 14% via the Introduction of a Malonic Acid Additive for a Perovskite Precursor, Energy Fuel., 2022, 36(21), 13187–13194 CrossRef CAS.
- S. H. Kareem, M. H. Elewi, A. M. Naji, D. S. Ahmed and M. K. Mohammed, Efficient and stable pure α-phase FAPbI3 perovskite solar cells with a dual engineering strategy: additive and dimensional engineering approaches, Chem. Eng. J., 2022, 136469 CrossRef.
- M. K. Mohammed, M. S. Jabir, H. G. Abdulzahraa, S. H. Mohammed, W. K. Al-Azzawi, D. S. Ahmed, S. Singh, A. Kumar, S. Asaithambi and M. Shekargoftar, Introduction of cadmium chloride additive to improve the performance and stability of perovskite solar cells, RSC Adv., 2022, 12(32), 20461–20470 RSC.
- M. M. Moharam, A. N. El Shazly, K. V. Anand, D. E. Rayan, M. K. Mohammed, M. M. Rashad and A. E. Shalan, Semiconductors as effective electrodes for dye sensitized solar cell applications, Top. Curr. Chem., 2021, 379(3), 1–17 CrossRef PubMed.
- A. K. Al-Mousoi and M. K. Mohammed, Engineered surface properties of MAPI using different antisolvents for hole transport layer-free perovskite solar cell (HTL-free PSC), J. Sol-Gel Sci. Technol., 2020, 96(3), 659–668 CrossRef CAS.
- M. K. Mohammed, A. K. Al-Mousoi, S. Singh, U. Younis, A. Kumar, D. Dastan and G. Ravi, Ionic Liquid Passivator for Mesoporous Titanium Dioxide Electron Transport Layer to Enhance the Efficiency and Stability of Hole Conductor-Free Perovskite Solar Cells, Energy Fuel., 2022, 36(19), 12192–12200 CrossRef CAS.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Organometal halide perovskites as visible-light sensitizers for photovoltaic cells, J. Am. Chem. Soc., 2009, 131(17), 6050–6051 CrossRef CAS PubMed.
- P. Cui, D. Wei, J. Ji, H. Huang, E. Jia, S. Dou, T. Wang, W. Wang and M. J. N. E. Li, Planar p–n homojunction perovskite solar cells with efficiency exceeding 21.3%, Nat. Energy, 2019, 4(2), 150–159 CrossRef CAS.
- H. Min, D. Y. Lee, J. Kim, G. Kim, K. S. Lee, J. Kim, M. J. Paik, Y. K. Kim, K. S. Kim and M. G. J. N. Kim, Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes, Nature, 2021, 598(7881), 444–450 CrossRef CAS PubMed.
- M. S. Mehde, A. M. Al-Gebori and A. K. Hantoosh, The effect of the spinning speed variation on the perovskite solar cell efficiency, IOP Conference Series: Materials Science and Engineering, IOP Publishing, 2020, p. 012071 Search PubMed.
- M. K. Mohammed, A. K. Al-Mousoi, M. S. Mehde and A. M. Al-Gebori, Engineered electronic properties of the spin-coated MAPI for hole-transport-free perovskite solar cell (HT-free PSC): spinning time and PSC performance relationship, Chem. Phys. Lett., 2020, 754, 137718 CrossRef CAS.
- D. S. Ahmed, B. K. Mohammed and M. K. Mohammed, Long-term stable and hysteresis-free planar perovskite solar cells using green antisolvent strategy, J. Mater. Sci., 2021, 56(27), 15205–15214 CrossRef CAS.
- J. Cao, F. J. E. Yan and E. Science, Recent progress in tin-based perovskite solar cells, Energy Environ. Sci., 2021, 14(3), 1286–1325 RSC.
- G. Schileo and G. Grancini, Lead or no lead? Availability, toxicity, sustainability and environmental impact of lead-free perovskite solar cells, J. Mater. Chem. C, 2021, 9(1), 67–76 RSC.
- S. F. Hoefler, G. Trimmel and T. Rath, Progress on lead-free metal halide perovskites for photovoltaic applications: a review, Monatshefte für Chemie-Chemical Monthly, 2017, 148(5), 795–826 CrossRef CAS PubMed.
- N. Sun, W. Gao, H. Dong, Y. Liu, X. Liu, Z. Wu, L. Song, C. Ran and Y. Chen, Architecture of pin Sn-based perovskite solar cells: characteristics, advances, and perspectives, ACS Energy Lett., 2021, 6(8), 2863–2875 CrossRef CAS.
- X. Liu, Z. Yang, C.-C. Chueh, A. Rajagopal, S. T. Williams, Y. Sun and A. Jen, Improved efficiency and stability of Pb–Sn binary perovskite solar cells by Cs substitution, J. Mater. Chem. A, 2016, 4(46), 17939–17945 RSC.
- M. E. Kayesh, K. Matsuishi, R. Kaneko, S. Kazaoui, J.-J. Lee, T. Noda and A. J. A. E. L. Islam, Coadditive engineering with 5-ammonium valeric acid iodide for efficient and stable Sn perovskite solar cells, ACS Energy Lett., 2018, 4(1), 278–284 CrossRef.
- F. Hao, C. C. Stoumpos, D. H. Cao, R. P. Chang and M. Kanatzidis, Lead-free solid-state organic–inorganic halide perovskite solar cells, Nat. Photon., 2014, 8(6), 489–494 CrossRef CAS.
- R. Fang, S. Wu, W. Chen, Z. Liu, S. Zhang, R. Chen, Y. Yue, L. Deng, Y.-B. Cheng and L. Han, [6, 6]-Phenyl-C61-butyric acid methyl ester/cerium oxide bilayer structure as efficient and stable electron transport layer for inverted perovskite solar cells, ACS Nano, 2018, 12(3), 2403–2414 CrossRef CAS PubMed.
- A. Pang, J. Li, X.-F. Wei, Z.-W. Ruan, M. Yang and Z.-N. Chen, UV–O3 treated annealing-free cerium oxide as electron transport layers in flexible planar perovskite solar cells, Nanoscale Adv., 2020, 2(9), 4062–4069 RSC.
- X. Wang, L.-L. Deng, L.-Y. Wang, S.-M. Dai, Z. Xing, X.-X. Zhan, X.-Z. Lu, S.-Y. Xie, R.-B. Huang and L.-S. Zheng, Cerium oxide standing out as an electron transport layer for efficient and stable perovskite solar cells processed at low temperature, J. Mater. Chem. A, 2017, 5(4), 1706–1712 RSC.
- J. Yang, Q. Zhang, J. Xu, H. Liu, R. Qin, H. Zhai, S. Chen and M. Yuan, All-inorganic perovskite solar cells based on CsPbIBr2 and metal oxide transport layers with improved stability, Nanomaterials, 2019, 9(12), 1666 CrossRef CAS.
- J. Yang, J. Xu, Q. Zhang, Z. Xue, H. Liu, R. Qin, H. Zhai and M. Yuan, An efficient and stable inverted perovskite solar cell involving inorganic charge transport layers without a high temperature procedure, RSC Adv., 2020, 10(32), 18608–18613 RSC.
- A. S. Thill, F. O. Lobato, M. O. Vaz, W. P. Fernandes, V. E. Carvalho, E. A. Soares, F. Poletto, S. R. Teixeira and F. Bernardi, Shifting the band gap from UV to visible region in cerium oxide nanoparticles, Appl. Surf. Sci., 2020, 528, 146860 CrossRef CAS.
- C. Rodwihok, D. Wongratanaphisan, T. V. Tam, W. M. Choi, S. H. Hur and J. S. Chung, Cerium-oxide-nanoparticle-decorated zinc oxide with enhanced photocatalytic degradation of methyl orange, Appl. Sci., 2020, 10(5), 1697 CrossRef CAS.
- T. Hu, S. Xiao, H. Yang, L. Chen and Y. Chen, Cerium oxide as an efficient electron extraction layer for p–i–n structured perovskite solar cells, Chem. Commun., 2018, 54(5), 471–474 RSC.
- I. Elango, M. Selvamani, P. C. Ramamurthy and A. V. Kesavan, Studying VOC in lead free inorganic perovskite photovoltaics by tuning energy bandgap and defect density, Ceram. Int., 2022, 48(19), 29414–29420 CrossRef CAS.
- F. Jannat, S. Ahmed and M. A. Alim, Performance analysis of cesium formamidinium lead mixed halide based perovskite solar cell with MoOx as hole transport material via SCAPS-1D, Optik, 2021, 228, 166202 CrossRef CAS.
- H.-J. Du, W.-C. Wang and J.-Z. Zhu, Device simulation of lead-free CH3NH3SnI3 perovskite solar cells with high efficiency, Chin. Phys. B, 2016, 25(10), 108802 CrossRef.
- P. Tiwari, M. F. Alotaibi, Y. Al-Hadeethi, V. Srivastava, B. Arkook, P. Lohia, D. K. Dwivedi, A. Umar, H. Algadi and S. Baskoutas, Design and Simulation of Efficient SnS-Based Solar Cell Using Spiro-OMeTAD as Hole Transport Layer, Nanomaterials, 2022, 12(14), 2506 CrossRef CAS PubMed.
- S. M. Majeed, D. S. Ahmed and M. K. Mohammed, Anti-solvent engineering via potassium bromide additive for highly efficient and stable perovskite solar cells, Org. Electron., 2021, 99, 106310 CrossRef CAS.
- M. R. Mohammad, D. S. Ahmed and M. K. Mohammed, ZnO/Ag nanoparticle-decorated single-walled carbon nanotubes (SWCNTs) and their properties, Surf. Rev. Lett., 2020, 27(03), 1950123 CrossRef CAS.
- M. K. Mohammed, G. Sarusi, P. Sakthivel, G. Ravi and U. Younis, Improved stability of ambient air-processed methylammonium lead iodide using carbon nanotubes for perovskite solar cells, Mater. Res. Bull., 2021, 137, 111182 CrossRef CAS.
- R. Pandey, A. P. Saini and R. Chaujar, Numerical simulations: toward the design of 18.6% efficient and stable perovskite solar cell using reduced cerium oxide based ETL, Vacuum, 2019, 159, 173–181 CrossRef CAS.
- A. Walsh, J. L. Da Silva and S.-H. Wei, Origins of band-gap renormalization in degenerately doped semiconductors, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78(7), 075211 CrossRef.
- A. Singla, R. Pandey, R. Sharma, J. Madan, K. Singh, V. K. Yadav and R. Chaujar, Numerical Simulation of CeOx ETL based Perovskite Solar Cell: An Optimization Study for High Efficiency and Stability, 2018, IEEE Electron Devices Kolkata Conference (EDKCON), IEEE, 2018, pp. 278–282 Search PubMed.
- Y. Raoui, S. Kazim, Y. Galagan, H. Ez-Zahraouy and S. Ahmad, Harnessing the potential of lead-free Sn–Ge based perovskite solar cells by unlocking the recombination channels, Sustainable Energy Fuels, 2021, 5(18), 4661–4667 RSC.
- S. Ahmmed, M. A. Karim, M. H. Rahman, A. Aktar, M. R. Islam, A. Islam and A. B. M. Ismail, Performance analysis of lead-free CsBi3I10-based perovskite solar cell through the numerical calculation, Sol. Energy, 2021, 226, 54–63 CrossRef CAS.
- S. A. Moiz, Optimization of hole and electron transport layer for highly efficient lead-free Cs2TiBr6-based perovskite solar cell, Photonics, 2021, 23 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |