Open Access Article
Open Access ArticleIdentification of the effect of N-glycan modification and its sialylation on proteolytic stability and glucose-stabilizing activity of glucagon-like peptide 1 by site-directed enzymatic glycosylation†
Huan Liu‡
a,
Zengwei Liang‡a,
Yu Wang‡a,
Yingze Lia,
Ya Wanga,
Xin Guocd,
Wanyi Guan *b,
Wei Zou*a and
Zhigang Wu
*b,
Wei Zou*a and
Zhigang Wu *a
*a
aCollege of Food and Biology, Hebei University of Science and Technology, Shijiazhuang, Hebei 050018, China. E-mail: wuzhigang0204@163.com; zw000@hotmail.com
bMinistry of Education Key Laboratory of Molecular and Cellular Biology, Hebei Key Laboratory of Molecular and Cellular Biology, College of Life Sciences, Hebei Normal University, Shijiazhuang, Hebei 050024, China. E-mail: guanwanyi@hebtu.edu.cn
cResearch Center, Hebei Province Hospital of Chinese Medicine, Affiliated Hospital of Hebei University of Traditional Chinese Medicine, Shijiazhuang, Hebei 050011, China
dDepartment of Pathology and Laboratory Medicine, Department of Pathology, Kanazawa Medical University, Uchinada, Ishikawa 920-0293, Japan
First published on 7th November 2022
Abstract
In this study, an approach to prepare long-acting glucagon-like peptide 1 (GLP-1) by site-directed enzymatic glycosylation with homogeneous biantennary complex-type N-glycan has been developed. All the N-glycan-modified GLP-1 analogues preserved an unchanged secondary structure. The glycosylated GLP-1 analogues with sialyl complex-type N-glycan modified at Asn26 and Asn34 exhibited a 36.7- and 24.0-fold in vitro half-life respectively when incubated with dipeptidyl peptidase-IV (DPP-IV), and 25.0- and 13.9-fold respectively when incubated with mouse serum. Compared to native GLP-1, both glycosylated GLP-1 analogues modified at Asn34 by asialyl and sialyl N-glycan demonstrated lower maximum blood glucose levels, as well as more rapid and more persistent glucose-stabilizing capability in type 2 diabetic db/db mice. Our results indicated that the selection of an appropriate position (to avoid hindering the peptide-receptor binding) is crucial for N-glycan modification and its sialylation to improve the therapeutic properties of the modified peptides. The information learned would facilitate future design of therapeutic glycopeptides/glycoproteins with N-glycan to achieve enhanced pharmacological properties.
1. Introduction
Peptides and protein-based drugs have promising therapeutic applications because of their multiple therapeutically favourable properties including high activity and better target specificity.1,2 However, their clinical use has been limited by their poor physiochemical and pharmacological properties, such as short metabolic stability and rapid clearance.3 Numerous attempts, including but not limited to glycosylation, PEGylation and lipidation, have been made to improve the pharmacological profile of peptide/protein drugs.1,4,5 Among them, glycosylation is a promising strategy, since it can enhance the metabolic stability of peptides/proteins, reduce the clearance rate, and weaken antigenicity.6,7 Among all the attempts at glycosylation of peptide/protein drugs, some attached a sugar moiety to Asn, Ser/Thr, or Cys to form N–, O– or S–linked glycosylation, mimicking the glycosylation site in natural glycopeptides/glycoproteins,8–17 whereas some conjugated carbohydrates to the N-terminal amino acid residue of the peptides/proteins.18,19 In addition,a non-natural amino acid bearing bioorthogonal group that facilitates site-specific glycosylation has been incorporated into proteins to enable the semi-synthesis of complex therapeutic glycoproteins.20 As for glycoforms, some efforts modified the peptides/proteins with sugar entities having relatively simple structures (e.g. mono-, di- or trisaccharides),8,9,18,21 while some incorporated complex-type N-glycans as in natural eukaryotic glycopeptides/glycoproteins.11,12,14–17,22 N-glycosylation with complex-type N-glycan is a post-translational modification for natural glycoproteins, but fine choice of position of glycosylation site within a peptide/protein drug for this modification and the subsequent evaluation of therapeutic properties were rarely reported.Glucagon-like peptide-1 (GLP-1) is a peptide hormone that behaves as a promising therapeutic candidate for treatment of type 2 diabetes.23 However, it is easy to be degraded by enzymes in serum, leading to its short serum half-life (∼2 min) and restricting its potential usage for diabetes treatment.24 Glycosylation has been reported to improve its proteolytic stability as well as in vivo activity. For instance, site-specific glycosylation on Asn26, Asn34 or/and Asn37 with sialylated N-acetyllactosamine (LacNAc) moiety improved its proteolytic stability and extended its in vivo glucose-lowering activity.9 GLP-1 analogue with O-GlcNAcylation on Ser18 demonstrated a small change of structure, increased serum half-life, and improved in vivo glucose-controlling activity.21 S-glycosylation on Cys18 with N-acetylglucosamine (GlcNAc) showed unchanged secondary structure and enhanced glucose-stabilizing activity.25 All of these modifications were with relatively simple carbohydrate moieties, including mono-, di- and trisaccharides. Compared to simple sugar moieties, N-glycan is the modification of natural glycoprotein, and acts better for improvement of half-life and activity. In our previous work, a sialylated complex-type N-glycan was conjugated to GLP-1 at Ser26, and the modification resulted in unremarkable change in the secondary structure of GLP-1,10 but the biological activity of the generated glycopeptide was not characterized. Hence, it is still unclear how humanized glycosylation, i.e. the biantennary complex-type N-glycan attached to the consensus sequence for N-glycosylation, can affect the serum half-life and the biological activity of GLP-1.
Under these circumstances, to better understand how the position of glycosylation site and the sialylation status of glycan can affect the half-life and bioactivity of glycoengineered therapeutic peptides, herein, taking GLP-1 as a model, we explored the effect of N-glycan modification and its sialylation at different positions of GLP-1 on its secondary structure, in vitro proteolytic stability against dipeptidyl peptidase-IV (DPP-IV) and mouse serum, and blood glucose-stabilizing activity in diabetic db/db mice.
2. Experimental
2.1 Materials
GLP-1 analogues with pre-designed N-glycosylation site were synthesized by Chinapeptides Co., Ltd. Dipeptidyl peptidase-IV (DPP-IV) was purchased from R&D Systems Co., Ltd. Eclipse XDB-C18 column (5 μm, 4.6 mm × 250 mm) and Xbridge Peptide BEH C18 column (10 μm, 10 mm × 250 mm) were from Agilent Technologies Inc. and Waters Corp., respectively. BKS-Leprem2Cd479/Jpt db/db mice (7–10 weeks of age) were purchased from GemPharmatech Co., Ltd.2.2 Preparation of enzymes used in this study
Endo-β-N-acetylglucosaminidase M (EndoM)26 and sialidase BiNanH2 from Bifidobacterium longum subsp. infantis ATCC15697 (ref. 27) were prepared following the previously reported method.The Q469A mutant of N-glucosyltransferase derived from A. pleuropneumoniae (ApNGTQ469A),28 and the N180H mutant of endoglycosidase from C. cinerea (EndoCCN180H)29 were codon-optimized and synthesized by Genewiz Co. (Suzhou, China) and were inserted into pET22b vector, respectively. Both recombinant enzymes were over-expressed with a C-terminal His-tag in Escherichia coli BL21 (DE3) cells and purified by Ni-NTA affinity column.
2.3 Preparation of sialylglycopeptide (SGP), complex-type glycan oxazoline (SCT-oxa) and desialylated complex-type glycan oxazoline (CT-oxa)
SGP was prepared according to the previously reported method.30 Sequential digestion of SGP by EndoM and BiNanH2 provided sialyl-complex-type glycan (SCT) and desialylated complex-type glycan (CT), respectively. SCT-oxa and CT-oxa were then prepared following the previously reported method.312.4 Two-step chemoenzymatic preparation of glycan-GLP-1 analogues
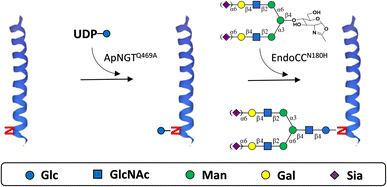
Glycan-GLP-1 analogues containing homogeneous N-glycan were prepared by ApNGTQ469A-catalyzed glucosylation and EndoCCN180H-catalyzed transglycosylation sequentially.To prepare Glc-GLP-1 analogues, reaction mixtures containing 200 mM Tris–HCl (pH 8.0), 6 nM GLP-1 analogues as acceptor, 30 nM UDP-Glc as donor and 20 μg ApNGTQ469A were incubated at 37 °C overnight. The reaction was terminated by boiling for 5 min and then analysed by reverse-phase HPLC (RP-HPLC) through an Eclipse XDB-C18 column (5 μm, 4.6 mm × 250 mm) and electrospray ionization mass spectrometry (ESI-MS). The synthesized Glc-GLP-1 analogues were purified from the mixture through Xbridge Peptide BEH C18 column (10 μm, 10 mm × 250 mm). The purified compounds were analysed by ESI-MS.
Subsequently, to prepare glycan-GLP-1 analogues, 200 mM PBS (pH 7.5), 6 nM Glc-GLP-1 analogues as acceptor, 60 nM SCT-oxa (or CT-oxa) as donor, and 20 μg EndoCCN180H were mixed and incubated at 30 °C for 15 min. The reaction was terminated by boiling for 5 min and then analysed by RP-HPLC through an Eclipse XDB-C18 column (5 μm, 4.6 mm × 250 mm) and ESI-MS. The synthesized glycan-GLP-1 analogues were purified from the mixture through Xbridge Peptide BEH C18 column (10 μm, 10 mm × 250 mm). The purified compounds were analysed by ESI-MS.
2.5 ESI-MS analysis
High resolution MS assays were performed on an Impact HD Q-ToF mass spectrometer (Bruker Daltonics) equipped with heated-ESI source and UltiMate 3000 high performance liquid chromatography system (Thermo Fisher). HPLC purified samples were assayed through an autosampler without separation. Full-scan positive MS experiments (m/z range from 300 to 2000; ESI voltage at 4500 V; Nebulizer gas pressure at 0.4 bar; dry gas flow of 4.0 L min−1 at 180 °C) were performed with the Impact HD mass spectrometer, using sodium formate as internal mass calibration standard.2.6 Analysis of secondary structures of glycan-GLP-1 analogues by circular dichroism (CD) spectroscopy
Secondary structures of native GLP-1 and glycan-GLP-1 analogues were analysed by far-UV (195–260 nm) CD spectroscopy by Beijing Bio-Tech Pack Technology Company Ltd. The spectra were recorded for 120 μM GLP-1 or glycan-GLP-1 analogues in 10 mM PB (pH 6.5) on a J-810 spectropolarimeter (Jasco) at ambient temperature (25 °C) using a 0.1 cm-pathlength quartz cuvette. All spectra presented were averaged for at least 3 scans, and the background signal was from the buffer.2.7 Evaluation of proteolytic stability of Glc-GLP-1 and glycan-GLP-1 analogues against DPP-IV and incubation with mouse serum
Evaluation of proteolytic stability of GLP-1, Glc-GLP-1 and glycan-GLP-1 analogues was performed against DPP-IV and incubation with mouse serum in vitro. The DPP-IV was diluted with 50 mM Tris–HCl (pH 7.4) to a final concentration of 10 ng μL−1. To ensure accurate half-life measurement, the amount of DPP-IV required was first optimized so that the in vitro half-life of GLP-1 was around 10 min. To this end, 0.3 nmol (i.e. 1 μg) of native GLP-1 was incubated with various amount of DPP-IV at 37 °C to determine appropriate amount of DPP-IV. Then, the same amount of DPP-IV was used to incubate with 0.3 nmol of each Glc-GLP-1 or glycan-GLP-1 analogue to determine the in vitro half-life. Each sample was analysed in triplicates. All incubation mixtures were boiled for 5 min to terminate the proteolysis, and the supernatants obtained after centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min were analysed by RP-HPLC. The proteolytic half-life of GLP-1, Glc-GLP-1 or glycan-GLP-1 analogues was calculated based on the peak area. For incubation with mouse serum, 15 μL (containing 6 nmol (i.e. 20 μg)) of GLP-1, Glc-GLP-1 or glycosylated GLP-1 analogues were mixed with the same volumes of mouse serum, and incubated at 37 °C. Each sample was analyzed in triplicates. The reactions were quenched by adding twice volume of ice-cold methanol and centrifuged at 12
000 rpm for 30 min were analysed by RP-HPLC. The proteolytic half-life of GLP-1, Glc-GLP-1 or glycan-GLP-1 analogues was calculated based on the peak area. For incubation with mouse serum, 15 μL (containing 6 nmol (i.e. 20 μg)) of GLP-1, Glc-GLP-1 or glycosylated GLP-1 analogues were mixed with the same volumes of mouse serum, and incubated at 37 °C. Each sample was analyzed in triplicates. The reactions were quenched by adding twice volume of ice-cold methanol and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 60 min. Supernatants were then analysed by RP-HPLC. The proteolytic half-life of GLP-1, Glc-GLP-1 or glycan-GLP-1 analogues was calculated based on the peak area.
000 rpm for 60 min. Supernatants were then analysed by RP-HPLC. The proteolytic half-life of GLP-1, Glc-GLP-1 or glycan-GLP-1 analogues was calculated based on the peak area.
2.8 Evaluation of glucose-stabilizing capability in type 2 diabetic db/db mice
BKS-Leprem2Cd479/Jpt db/db mice (7–10 weeks of age) were fasted for 18 h and received an i.p. injection of saline, GLP-1, or glycan-GLP-1 analogues (10 nmol kg−1, n = 6–8), respectively at 30 min before glucose administration. At 0 min, a 1.0 g kg−1 dose of glucose was administered orally to each mouse. At predetermined times (15 min, 30 min, 60 min, 90 min, 120 min, and 180 min), a drop of blood was drawn from the tail vein, and the blood glucose level was measured by using a one-touch blood glucose meter (ACCU-CHEK® Sensor, Roche Diagnostics Corp., USA). Three factors, including (i) maximum blood glucose level (BGLmax), (ii) required time to lower the blood glucose level to below 10 mmol L−1 (TBGL<10mmolL−1), and (iii) total hypoglycaemic degree (HGD%total) were considered to assess the glucose-stabilizing capability. The total hypoglycaemic degree (%versus saline group) was calculated as follows: [(AUCsaline,0–180min − AUCtest,0–180min)/AUCsaline,0–180min] × 100. (AUC: area under the curve.) All experiments were approved by Institutional Animal Care and Use Committee of Hebei University of Science & Technology (Protocol code: 2021–0901).2.9 Statistical analysis
Results are expressed as means ± S.D. Significant differences were analysed using the Student's t-test where appropriate. Values of p < 0.05 were considered to be statistically significant.3. Results and discussion
3.1 Design and preparation of site-directed glycosylated GLP-1 analogues
To better understand how the position of glycosylation site can affect the half-life and bioactivity of glycoengineered therapeutic peptides, we designed the glycosylation regions of the α-helix spanning from Thr13 to Val33 of GLP-1, which is critical for the binding of GLP-1 to its receptor:32 (1) inside the α-helix and close to the N-terminus (peptides 1 and 2), (2) in the middle the α-helix (peptide 3), and (3) adjacent to the C-terminus of the α-helix (peptides 4 and 5) (Table 1). Since the six amino acids (HDEFER) at the N-terminus of native GLP-1 were absent in its biologically active form,23 the truncated GLP-1 (amino acids 7–37) was used in this study.| Peptide ID | Peptide sequence (amino acids 7–37) | Glycosylation site | Yielda (%) | ||
|---|---|---|---|---|---|
| ApNGTQ469A | EndoCCN180H | ||||
| Glc | CT | SCT | |||
| a Yields were determined by peak ratio of HPLC analysis of reaction mixtures.b N. D.: Not detected. | |||||
| 1 | HAEGTFTS![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) VSSYLEGQAAKEFIAWLVKGRG VSSYLEGQAAKEFIAWLVKGRG |
15N | 97.13 | 46.33 | 66.73 |
| 2 | HAEGTFTSD![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) SSYLEGQAAKEFIAWLVKGRG SSYLEGQAAKEFIAWLVKGRG |
16N | N. D.b | — | — |
| 3 | HAEGTFTSDVSSYLEGQAA![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) ESIAWLVKGRG ESIAWLVKGRG |
26N | 96.74 | 68.57 | 68.83 |
| 4 | HAEGTFTSDVSSYLEGQAAKEFIAWLV![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) GSG GSG |
34N | N. D. | — | — |
| 5 | HAEGTFTSDVSSYLEGQAAKEFIAWLV![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) GTG GTG |
34N | 94.44 | 63.49 | 61.19 |
The glycosylated GLP-1 analogues were prepared by Q469A mutant of N-glucosyltransferase derived from Actinobacillus pleuropneumoniae (ApNGTQ469A)-catalysed glucosylation28 followed by N180H mutant of endoglycosidase from Coprinopsis cinerea (EndoCCN180H)-catalysed transglycosylation29 (Scheme 1). In the first step, ApNGTQ469A requires the presence of a conserved Asn-X-Ser/Thr (X ≠ Pro) sequon28 which does not naturally exist in GLP-1. Meanwhile, some amino acids in GLP-1 were found not essential for its affinity to receptor or activity.32 Therefore, we introduced the consensus N-glycosylation site into GLP-1 by substituting a few replaceable residues with Asn when designing the sequences of GLP-1 analogues to be glycosylated (Table 1, peptides 1–5). In addition, Thr at position +2 was shown to be preferred over Ser by ApNGT.33 Hence, a pair of candidate GLP-1 analogues were designed with the only difference at their +2 positions (peptides 4 & 5) for comparison.
After the glucosylation catalysed by ApNGTQ469A, three out of five peptides provided Glc-GLP-1 peptide products with over 90% yields (Table 1, peptides 1, 3 and 5), whereas no products were detected for the other two peptides (Table 1, peptides 2 and 4). The successful glucosylation of peptides 1, 3 and 5 demonstrated that ApNGTQ469A could transfer Glc residue efficiently to the peptides regardless of which portion of the α-helix the glycosylation site located. However, failure of glucosylation of peptides 2 and 4 indicated that the local environment surrounding the glucosylation sequon did influence the activity of ApNGTQ469A. Compared to peptide 1, peptide 2 has the same NXS sequon but its location has only one amino acid difference, resulting in different surrounding amino acids. As for peptide 4, its only difference with peptide 5 is that the amino acid at +2 position is Ser instead of Thr, suggesting that for specific glycosylation site, NXT sequon is favoured by ApNGTQ469A than NXS, consistent with previous report.33
Next, to generate glycopeptides whose glycoforms are asialylated complex-type N-glycan or its sialylated version for functional evaluation, both asialylated and sialylated biantennary complex-type N-glycans (CT and SCT) were used as glycan donor for the subsequent glycan elaboration of the 3 Glc-GLP-1 peptides, catalysed by EndoCCN180H. Moderate yields (over 60%) were achieved except for Glc-GLP-1-1 with CT as glycan donor (Table 1), which means the overall yields of the two-step enzymatic glycosylation for most GLP-1 analogues we designed were over 50%. For Glc-GLP-1 peptides 3 and 5, sialylation state of donor glycan did not affect the transglycosylation yields. Yet for Glc-peptide-1, the transglycosylation yield with CT as glycan donor was only 46.33%, much less than the yield when SCT was the glycan donor (66.73%). This implies that different glycan donors may result in considerably different transglycosylation efficacy when the position of glycosylation site varies. All 6 glycan-GLP-1 analogues were purified by reverse phase-HPLC (RP-HPLC) to a purity of over 95% for subsequent evaluation and characterized by electrospray ionization mass spectrometry (ESI-MS) (Table S1, Fig. S1†).
3.2 Secondary structure analysis of glycosylated GLP-1 analogues
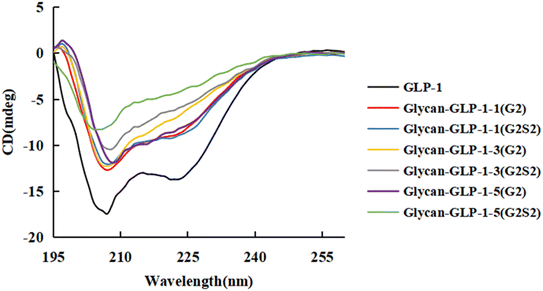
Glycosylation of some peptides was reported to result in altered structure of peptide chain and hence lead to loss of bioactivity.1 Therefore, after purification of the glycosylated GLP-1 analogues, their secondary structures were assayed by circular dichroism (CD) spectroscopy in the far-UV (195–260 nm) range and compared to native GLP-1. No dramatic differences were observed for the CD spectra of GLP-1 and the 6 glycan-GLP-1 analogues (Fig. 1, Table S2†), demonstrating that the N-glycan modification has no influence on the secondary structure of GLP-1.3.3 In vitro proteolytic stability of glycosylated GLP-1 analogues against DPP-IV and mouse serum
Short serum half-life has been a significant drawback that limits the therapeutic application of GLP-1. Degradation by DPP-IV is one major factor that inactivates GLP-1.23 Hence, the proteolytic stability against DPP-IV and mouse serum was then assessed for glycosylated GLP-1 analogues. Compared to native GLP-1, the Glc-GLP-1 analogues showed slightly prolonged in vitro half-lives by incubation both with DPP-IV and with mouse serum. Glc-GLP-1-3, which is glycosylated at Asn26, the same position as for Liraglutide (a GLP-1 analogue in clinical use with fatty acid modification at Lys26),34 showed the most increase of in vitro half-life, whereas Glc-GLP-1-1 showed the least increase (Table 2, Fig. S2 and S3†). This illustrates that glycosylation at different positions has diverse effects on enhancement of half-life. All glycan-GLP-1 analogues exhibited much longer (3 ∼ 10 fold) in vitro half-life compared to the corresponding Glc-GLP-1 analogues (Table 2, Fig. S2 and S3†), indicating that modification with N-glycan has better stabilizing effect on GLP-1 peptides against peptidase degradation than with a single Glc residue. In addition, sialylation contributed greatly to proteolytic stability. This could be attributed to the negative charges carried by sialic acid that prevented the binding between the peptide and DPP-IV. Sialylated glycan-GLP-1 analogues displayed 1.3- to 3-fold half-life of that of their asialylated counterparts when incubating with DPP-IV, and approximately 2-fold when incubating with mouse serum. Of all the 6 glycan-GLP-1 peptides, the sialylated glycan-GLP-1-3 (named as glycan-GLP-1-3(G2S2)) and sialylated glycan-GLP-1-5 (named as glycan-GLP-1-5(G2S2)) showed the longest half-lives (374.7 min and 245.3 min, respectively), which were 36.7- and 24.0-fold of the native GLP-1 (10.2 min) for DPP-IV incubation, and were 25.0- and 13.9-fold (1393.1 min and 777.0 min, respectively, compared to 55.7 min) for serum incubation. Collectively, N-glycan modification, especially with sialylated glycoforms, has great potential in enhancing the proteolytic stability of GLP-1.| Variants | In vitro half-life (min) | |
|---|---|---|
| DPP-IV | Serum | |
| a Data are presented as means ± S.D. of triplicates. | ||
| GLP-1 | 10.2 ± 0.4 | 55.7 ± 12.1 |
| Glc-GLP-1-1 | 30.2 ± 2.4 | 94.1 ± 7.6 |
| Glc-GLP-1-3 | 46.1 ± 3.5 | 138.8 ± 14.5 |
| Glc-GLP-1-5 | 32.5 ± 1.4 | 113.3 ± 11.8 |
| Glycan-GLP-1-1(G2) | 49.1 ± 5.7 | 355.5 ± 9.3 |
| Glycan-GLP-1-1(G2S2) | 145.7 ± 4.3 | 736.6 ± 12.3 |
| Glycan-GLP-1-3(G2) | 185.3 ± 1.1 | 738.6 ± 9.7 |
| Glycan-GLP-1-3(G2S2) | 374.7 ± 1.8 | 1393.1 ± 8.9 |
| Glycan-GLP-1-5(G2) | 182.0 ± 0.1 | 390.4 ± 13.5 |
| Glycan-GLP-1-5(G2S2) | 245.3 ± 1.3 | 777.0 ± 10.2 |
3.4 In vivo glucose-stabilizing activity of glycosylated GLP-1 analogues
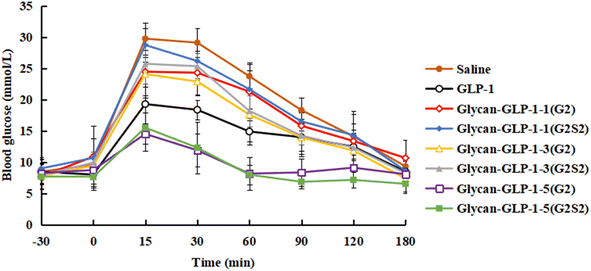
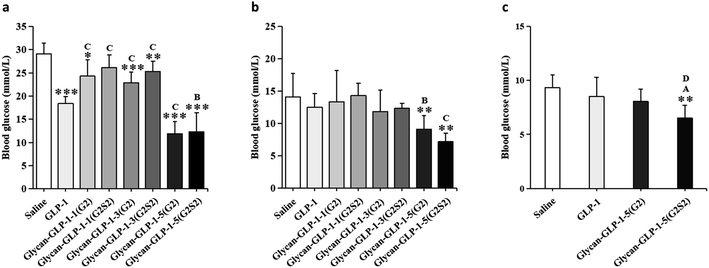
The glucose-stabilizing capability of the N-glycan modified GLP-1 analogues was evaluated and compared with native GLP-1 by performing an oral glucose tolerance test (OGTT) on type 2 diabetic db/db mice. Saline, GLP-1 and glycan-GLP-1 analogues were intraperitoneal administrated to mice at 30 min before glucose was fed orally. Three factors, including (i) maximum blood glucose level (BGLmax), (ii) required time to lower the blood glucose level to below 10 mmol L−1 (TBGL<10mmolL−1), and (iii) total hypoglycaemic degree (HGD%total), were considered to evaluate their glucose-stabilizing capability (Table 3). Glycan-GLP-1-5 peptides (both G2 and G2S2 glycoforms) were found to have the most significant glucose-stabilizing capability in all factors evaluated (Table 3, Fig. 2 and 3). The BGLmax values of glycan-GLP-1-5(G2) (14.50 ± 1.62 mmol L−1) and glycan-GLP-1-5(G2S2) (15.50 ± 3.74 mmol L−1) were obviously lower than those of saline (29.73 ± 2.56 mmol L−1) and GLP-1 (19.27 ± 1.41 mmol L−1). Glycan-GLP-1-5(G2) and glycan-GLP-1-5(G2S2) lowered the blood glucose level (BGL) more rapidly (at 30 min) than saline (both P < 0.001) and GLP-1 (P < 0.001 and P < 0.01, respectively) (Fig. 2 and 3a), and could lower the BGL to <10 mmol L−1 within 60 min after administration, whereas the TBGL<10mmolL−1 of the saline and GLP-1 were 180 min or longer (Table 3, Fig. 2). Moreover, the glucose-stabilizing capability of glycan-GLP-1-5 analogues was persistent, showing significantly better effect at 120 min (glycan-GLP-1-5(G2) and glycan-GLP-1-5(G2S2)) and 180 min (glycan-GLP-1-5(G2S2)) compared to native GLP-1 (Fig. 3b and c). On the contrary, the glucoregulatory activity of glycan-GLP-1-1 and glycan-GLP-1-3 analogues were not improved at all. Their BGLmax were higher than that of native GLP-1 group, and their TBGL<10mmolL−1 were the same or even longer than that of native GLP-1 group (Table 3). These results clearly indicated that N-glycan modification could improve the therapeutic efficacy of peptide drugs, but the modification must be incorporated into the peptides at the proper position.| Variants | Glucose-stabilizing capability | ||
|---|---|---|---|
| BGLmaxa (mmol L−1) | TBGL<10mmolL−1b (min) | HGD%totalc (vs. saline) | |
| a BGLmax: maximum blood glucose level. Data are presented as means ± S.D. of six to eight determinations.b TBGL<10mmolL−1: required time to lower the blood glucose level below 10 mmol L−1.c HGD%total: total hypoglycaemic degree. The total hypoglycaemic degree (%versus saline group) was calculated as follows: [(AUCsaline,0–180min − AUCtest,0–180min)/AUCsaline,0–180min] ×100. AUC: area under the curve.d N. A.: not applicable. | |||
| Saline | 29.73 ± 2.56 | 180 | N. A.d |
| GLP-1 | 19.27 ± 1.41 | 180 | 27.00 |
| Glycan-GLP-1-1(G2) | 24.44 ± 2.37 | >180 | 9.57 |
| Glycan-GLP-1-1(G2S2) | 28.71 ± 2.63 | 180 | 6.03 |
| Glycan-GLP-1-3(G2) | 24.07 ± 3.79 | 180 | 20.93 |
| Glycan-GLP-1-3(G2S2) | 25.71 ± 3.19 | 180 | 16.24 |
| Glycan-GLP-1-5(G2) | 14.50 ± 1.62 | 60 | 49.29 |
| Glycan-GLP-1-5(G2S2) | 15.50 ± 3.74 | 60 | 54.29 |
According to the current binding model for GLP-1 and GLP-1 receptor (GLP-1R) and the crystal structure of receptor-bound GLP-1 (Fig. S4†), the C-terminal fragment of GLP-1 (Ala24–Val33) forms a stable α-helical conformation and binds the extracellular domain (ECD) of GLP-1R. The α-helical conformation of C-terminal segment may further facilitate the formation of a stable α-helical conformation in the N-terminal segment of GLP-1 (Thr13–Glu21) and the latter part binds the extracellular loops and transmembrane α-helices (TM domain) of GLP-1R, thus activating the receptor.32 This suggests that stable binding between the C-terminal part of GLP-1 and the ECD of GLP-1R is an important prerequisite for GLP-1R activation. In our trials, glycan-GLP-1-1 peptides were glycosylated at position 15, in the region within positions 7–17, which is suggested to be essential for activity of GLP-1.23 The loss of biological activity of glycosylated peptide 1 could be because the glycan linked to position 15 impedes the binding between the N-terminal region of GLP-1 and the TM domain of GLP-1R, hence impairing the receptor activation. When the complex-type N-glycan was attached to position 26, glycan-GLP-1-3 peptides exhibited weakened glucoregulatory effect compared to native GLP-1, although their in vitro stability was enhanced. The results were contrary to the results when GLP-1 was modified at the same position by acylation (Liraglutide at Lys26),34 PEGylation (at Lys26)35 or glycosylation with simple sugar moieties (at Asn26).9 One possible explanation for this disagreement could be that the biantennary complex-type N-glycan moieties (nonasaccharide or undecasaccharide) used in this study can take up more space than the fatty acid, the PEG or the sialyl-LacNAc moiety in those work, hence block the interaction between the N-glycan modified GLP-1 to its receptor for its bioactivity. The glycosylation at position 34 has provided glycan-GLP-1-5 peptides with both increased in vitro half-life and greatly enhanced glucose stabilizing capability, indicating that bulky complex-type N-glycan linked to position 34 did not hamper the receptor binding and receptor activating. Therefore, for glycoengineering of therapeutic peptides/proteins with N-glycan, the glycosylation site should be carefully considered to avoid impeding the peptide/protein-receptor binding.
In addition, sialylation of N-glycan displayed considerable influence on the bioactivity of the engineered peptides. For glycan-GLP-1-5 analogues, glycan-GLP-1-5(G2S2) showed clearly better glucose-stabilizing effect than glycan-GLP-1-5(G2) at 120 and 180 min (Fig. 3b and c). The difference in the pharmacokinetics of the two glycosylated peptides may be explained by the longer half-life of sialylated glycopeptides. Similar results were also found for recombinant human erythropoietin (rHuEPO), as the desialylation of rHuEPO led to quicker serum clearance rate and drastically decreased biological activity.3 However, for peptides 1 and 3, the sialylated glycopeptides exhibited even worse glucoregulatory properties compared to the asialylated version (Table 3, Fig. 2 and 3). Our speculation is that the N-glycan modification of the two peptides were already at the positions that hampered the binding between the peptide and its receptor, hence the negatively charged sialic acid further weakened the binding. The decreased in vitro bioactivity and in vitro receptor binding affinity observed for human interleukin-17A modified with sialyl complex-type N-glycan36 might also support this view. Recently, the Dong group37 reported the chemical synthesis of a few GLP-1 analogues containing sialylated complex-type N-glycan, in which Ala8 was substituted with unnatural amino acid 2-aminoisobutyric acid. Sialyl N-glycosylated GLP-1 analogues modified relatively close to N-terminal showed decreased activity, whereas the other analogues exhibited improved hypoglycaemic activity in vivo. From all the above-mentioned results, we can speculate that a proper position is critical for sialylation of N-glycan to exert positive effects on peptide/protein-receptor binding and the corresponding bioactivity.
4. Conclusions
In this work, we have developed an approach to generate long-acting GLP-1 by chemoenzymatic synthesis of site-directed monoglycosylated GLP-1 analogues with homogeneous biantennary complex-type N-glycan. The glycosylated GLP-1 analogues exhibited unchanged secondary structure but greatly prolonged proteolytic stability. Furthermore, since N-glycan moiety is bulky, the improvement of bioactivity of the modified peptides upon N-glycan modification requires appropriate position to be selected. Sialylation of N-glycan should be considered in conjunction with the location of the N-glycan modification. The information learned from this study would be useful for future research on rational design of therapeutic glycopeptides/glycoproteins with N-glycan to achieve improved pharmacological properties.Author contributions
H. L. synthesized, purified and analysed glycopeptides. Z. L. and Y. L. prepared SCT and CT. Yu Wang expressed and purified enzymes. Ya Wang prepared SGP. H. L., Z. L., Yu Wang and X. G. performed OGTT experiment. X. G. analysed data. W. G. designed the work and wrote the original draft of the manuscript. W. Z. and Z. W. designed and supervised the work. All the authors contributed review and editing of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for financial support from Hebei Provincial Department of Human Resources and Social Security grants (C201812, C20190348 and E2018100010), Foundation of Hebei Educational Committee (QN2019217), Natural Science Foundation of Hebei Province (C2020205049 and H2021208001). We thank Dr Jingyao Qu (Shandong University) for spectrometric assistance. We thank Dr Jiansong Cheng (Nankai University) for discussions.Notes and references
- S. V. Moradi, W. M. Hussein, P. Varamini, P. Simerska and I. Toth, Chem. Sci., 2016, 7, 2492–2500 RSC.
- R. J. Sola and K. Griebenow, BioDrugs, 2010, 24, 9–21 CrossRef CAS PubMed.
- A. M. Sinclair and S. Elliott, J. Pharm. Sci., 2005, 94, 1626–1635 CrossRef CAS PubMed.
- S. Frokjaer and D. E. Otzen, Nat. Rev. Drug Discovery, 2005, 4, 298–306 CrossRef CAS PubMed.
- R. Vaishya, V. Khurana, S. Patel and A. K. Mitra, Expert Opin. Drug Delivery, 2015, 12, 415–440 CrossRef CAS PubMed.
- A. Varki, Glycobiology, 2017, 27, 3–49 CrossRef CAS PubMed.
- A. Kuriakose, N. Chirmule and P. Nair, J. Immunol. Res., 2016, 2016, 1298473 Search PubMed.
- L. Q. Wan, X. Zhang, Y. Zou, R. Shi, J. G. Cao, S. Y. Xu, L. F. Deng, L. Zhou, Y. Gong, X. Shu, G. Y. Lee, H. Ren, L. Dai, S. Qi, K. N. Houk and D. Niu, J. Am. Chem. Soc., 2021, 143, 11919–11926 CrossRef CAS.
- T. Ueda, K. Tomita, Y. Notsu, T. Ito, M. Fumoto, T. Takakura, H. Nagatome, A. Takimoto, S. Mihara, H. Togame, K. Kawamoto, T. Iwasaki, K. Asakura, T. Oshima, K. Hanasaki, S. Nishimura and H. Kondo, J. Am. Chem. Soc., 2009, 131, 6237–6245 CrossRef CAS PubMed.
- Z. Wu, K. Jiang, H. Zhu, C. Ma, Z. Yu, L. Li, W. Guan, Y. Liu, H. Zhu, Y. Chen, S. Li, J. Li, J. Cheng, L. Zhang and P. G. Wang, Bioconjugate Chem., 2016, 27, 1972–1975 CrossRef CAS PubMed.
- S. K. Prabhu, Q. Yang, X. Tong and L. X. Wang, Bioorg. Med. Chem., 2021, 33, 116037 CrossRef CAS PubMed.
- J. D. Valderrama-Rincon, A. C. Fisher, J. H. Merritt, Y. Y. Fan, C. A. Reading, K. Chhiba, C. Heiss, P. Azadi, M. Aebi and M. P. DeLisa, Nat. Chem. Biol., 2012, 8, 434–436 CrossRef CAS.
- S. Mezzato, M. Schaffrath and C. Unverzagt, Angew. Chem., Int. Ed., 2005, 44, 1650–1654 CrossRef CAS PubMed.
- N. Yamamoto, Y. Tanabe, R. Okamoto, P. E. Dawson and Y. Kajihara, J. Am. Chem. Soc., 2008, 130, 501–510 CrossRef CAS.
- P. Wang, S. Dong, J. H. Shieh, E. Peguero, R. Hendrickson, M. A. S. Moore and S. J. Danishefsky, Science, 2013, 342, 1357–1360 CrossRef CAS.
- M. Murakami, R. Okamoto, M. Izumi and Y. Kajihara, Angew. Chem., Int. Ed. Engl., 2012, 51, 3567–3572 CrossRef CAS.
- M. Murakami, T. Kiuchi, M. Nishihara, K. Tezuka, R. Okamoto, M. Izumi and Y. Kajihara, Sci. Adv., 2016, 2, e1500678 CrossRef PubMed.
- J. P. Bapst, M. Calame, H. Tanner and A. N. Eberle, Bioconjugate Chem., 2009, 20, 984–993 CrossRef CAS.
- S. V. Moradi, F. M. Mansfeld and I. Toth, Bioorg. Med. Chem., 2013, 21, 4259–4265 CrossRef CAS PubMed.
- K. Streichert, C. Seitz, E. Hoffmann, I. Boos, W. Jelkmann, T. Brunner, C. Unverzagt and M. Rubini, Chembiochem, 2019, 20, 1914–1918 CrossRef CAS PubMed.
- P. M. Levine, A. T. Balana, E. Sturchler, C. Koole, H. Noda, B. Zarzycka, E. J. Daley, T. T. Truong, V. Katritch, T. J. Gardella, D. Wootten, P. M. Sexton, P. McDonald and M. R. Pratt, J. Am. Chem. Soc., 2019, 141, 14210–14219 CrossRef CAS PubMed.
- Y. Xu, Z. Wu, P. Zhang, H. Zhu, H. Zhu, Q. Song, L. Wang, F. Wang, P. G. Wang and J. Cheng, Chem. Commun., 2017, 53, 9075–9077 RSC.
- D. Donnelly, Br. J. Pharmacol., 2012, 166, 27–41 CrossRef CAS PubMed.
- B. Ahrén, Drug Discovery Today: Ther. Strategies, 2004, 1, 207–212 Search PubMed.
- G. Li, Y. Dao, J. Mo, S. Dong, S.-I. Shoda and X.-S. Ye, CCS Chem., 2021, 3, 2316–2323 CrossRef.
- M. Umekawa, C. Li, T. Higashiyama, W. Huang, H. Ashida, K. Yamamoto and L. X. Wang, J. Biol. Chem., 2010, 285, 511–521 CrossRef CAS.
- Z. Wu, Y. Liu, C. Ma, L. Li, J. Bai, L. Byrd-Leotis, Y. Lasanajak, Y. Guo, L. Wen, H. Zhu, J. Song, Y. Li, D. A. Steinhauer, D. F. Smith, B. Zhao, X. Chen, W. Guan and P. G. Wang, Org. Biomol. Chem., 2016, 14, 11106–11116 RSC.
- Q. Song, Z. Wu, Y. Fan, W. Song, P. Zhang, L. Wang, F. Wang, Y. Xu, P. G. Wang and J. Cheng, J. Biol. Chem., 2017, 292, 8856–8863 CrossRef CAS PubMed.
- Y. Higuchi, Y. Eshima, Y. Huang, T. Kinoshita, W. Sumiyoshi, S. I. Nakakita and K. Takegawa, Biotechnol. Lett., 2017, 39, 157–162 CrossRef CAS.
- Y. Zou, Z. G. Wu, L. L. Chen, X. W. Liu, G. F. Gu, M. Y. Xue, P. G. Wang and M. Chen, J. Carbohydr. Chem., 2012, 31, 436–446 CrossRef CAS.
- W. Huang, Q. Yang, M. Umekawa, K. Yamamoto and L. X. Wang, Chembiochem, 2010, 11, 1350–1355 CrossRef CAS.
- C. R. Underwood, P. Garibay, L. B. Knudsen, S. Hastrup, G. H. Peters, R. Rudolph and S. Reedtz-Runge, J. Biol. Chem., 2010, 285, 723–730 CrossRef CAS.
- A. Naegeli, C. Neupert, Y. Y. Fan, C. W. Lin, K. Poljak, A. M. Papini, F. Schwarz and M. Aebi, J. Biol. Chem., 2014, 289, 2170–2179 CrossRef CAS PubMed.
- K. B. Degn, C. B. Juhl, J. Sturis, G. Jakobsen, B. Brock, V. Chandramouli, J. Rungby, B. R. Landau and O. Schmitz, Diabetes, 2004, 53, 1187–1194 CrossRef CAS PubMed.
- S. H. Lee, S. Lee, Y. S. Youn, D. H. Na, S. Y. Chae, Y. Byun and K. C. Lee, Bioconjugate Chem., 2005, 16, 377–382 CrossRef CAS PubMed.
- H. Li, J. Zhang, C. An and S. Dong, J. Am. Chem. Soc., 2021, 143, 2846–2856 CrossRef CAS PubMed.
- Q. Wei, J. Zhang, Y. Dao, M. Ye, D. Liu, W. Dong, N. Yuan, H. Li, C. Song, M. Li, X. Shi and S. Dong, CCS Chem., 2022 DOI:10.31635/ccschem.022.202202122.
Footnotes |
| † Electronic supplementary information (ESI) available: Characterization data including HPLC chromatographs, ESI-MS spectra, secondary structure, degradation profiles against DPP-IV and mouse serum (PDF). See DOI: https://doi.org/10.1039/d2ra05872c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |