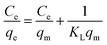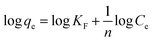Open Access Article
Open Access ArticleMagnetic nanocomposites based on Zn,Al-LDH intercalated with citric and EDTA groups for the removal of U(VI) from environmental and wastewater: synergistic effect and adsorption mechanism study†
Natalia Kobylinska *,
Liubov Puzyrnaya
*,
Liubov Puzyrnaya and
Galina Pshinko
and
Galina Pshinko
Dumansky Institute of Colloid and Water Chemistry, National Academy of Sciences of Ukraine, 42 Akad. Vernadsky Blvd., Kyiv, 03142, Ukraine. E-mail: kobilinskaya@univ.kiev.ua
First published on 9th November 2022
Abstract
The efficient removal of U(VI) ions from contaminated natural waters and wastewaters of industrial processing plants by novel magnetic nanocomposites based on magnetite and Zn,Al-layered double hydroxides intercalated with citric and EDTA groups (Fe3O4/Zn,Al-LDH/Cit and Fe3O4/Zn,Al-LDH/EDTA) was shown. These adsorbents were obtained using co-precipitation and ion-exchange techniques. The infrared spectroscopy confirmed the existence of O-containing groups on the surfaces of Fe3O4/Zn,Al-LDH/Cit and Fe3O4/Zn,Al-LDH/EDTA, which could provide active sites in the interlayer of the adsorbents for the pollutants removal. The intercalation of Zn,Al-LDH with chelating EDTA-groups significantly increased the adsorption capacity toward U(VI) ions (131.22 mg g−1) compared to citric moieties in a wide range of pH (3.5–9.0). The maximum adsorption capacities of U(VI) at pH 7.5 were 81.12 mg g−1 for Fe3O4/Zn,Al-LDH/EDTA and 21.6 mg g−1 for Fe3O4/Zn,Al-LDH/Cit. The higher adsorption capacity of Fe3O4/Zn,Al-LDH/EDTA vs. the citric sample might be explained by high affinity of LDH-supports and high-activity of the chelating groups in formation of the complexes in the interlayer space of the magnetic nanocomposite. The removal of U(VI) by the magnetic nanocomposites occurred due to interlayer complexation and electrostatic interactions. The cations (Na+, K+, Ca2+), HCO3− and fulvic acid anions being typical for natural waters were practically not affected upon the removal of U(VI) from aqueous media. The adsorption performance of Fe3O4/Zn,Al-LDH/EDTA nanocomposites was evaluated in the analysis of environmental and wastewater samples with recoveries in the range of 95.8–99.9%. This superior intercalation performance of LDH-supports provides simple and low-cost adsorbents, providing a strategy for decontamination of radionuclides from wastewater.
1. Introduction
Over the last 50 years, radionuclides have been among the most dangerous pollutants of the environment.1 The major sources of radionuclide (235,238U, 137Cs, 90Sr, 60Co, 152+154Eu and 241Am) contaminations of the last decades originated from nuclear power plants, nuclear weapon productions, nuclear fuel recycling units, and were released from nuclear cement repositories and various nuclear accidents. Radionuclides can be found in ambient air, soil, and aquatic environments after their discharge from corresponding sources especially upon accidental releases of radionuclides to the environment from Nuclear Power Plants (i.e. Fukushima (Japan) in 2011, Chernobyl (Ukraine) in 1986,2 etc.), and leakages of liquid nuclear waste storage. Furthermore, accidents at Nuclear Power Plants and nuclear waste repositories leading to radioactive fall-outs with contamination to vegetation and soil, for example, in the Red forest in the Chernobyl Exclusion Zone district of Ukraine,2 can cause subsequent transfer of radionuclides to the ground and river water. U(VI) is the most common radionuclide in nuclear wastes. At the same time, U(VI) is a heavy actinide with a long half-life of radioactive decay, high chemical and biological toxicity, and a non-biodegradable and carcinogenic nature which constitutes a great threat to human health.3,4 To reduce these health risks, both World Health Organization (WHO) and the United States Environmental Protection Agency (USEPA) set a guideline limit for total U(VI) content in drinking water at 30 ppb.5 Uranium concentrations in the groundwater of the contaminated zone were much higher than the safety limit.2 Thus, monitoring and subsequent removal of U(VI) from contaminated water including U(VI)-containing nuclear waste is one of the major environmental remediation problems of today.4 Numerous studies have shown6 that U(VI) is present in environmental water and wastewater mainly in the soluble anionic carbonate forms such as UO2(CO3)22− and UO2(CO3)34− which are not sufficiently eliminated by mineral suspensions and clay components of soils.Numerous methods have been proposed to remove U(VI) from waste and contaminated environmental water7 such as solvent extraction,8 distillation,9 coagulation,10 reverse osmosis,11 precipitation,12 adsorption/ion exchange,13 membrane filtration and biological technologies.14 Each of these methods has its advantages and disadvantages. For example, coagulation using ferric sulfate, ferrous sulfate, or aluminum is an efficient method but the efficiency greatly depends on the pH value of water. Removal efficiencies of more than 80% can be achieved only at higher pH (near 10) values. This means that at pH values typical of natural waters, coagulation and lime softening is not effective. The long precipitation time that is required makes the process impractical with large volumes of wastewater. The important way for the removal of U(VI) is the reductive precipitation in anaerobic conditions, in which U(VI) is reduced to U(IV), which is insoluble and thus its can be removed from water by precipitation. Usually, the zero-valent iron (Fe0)15 and denitrifying bacteria16 were used as reducing agents. In water and especially water containing dissolved oxygen, Fe0 is quickly oxidized to Fe(II)/Fe(III), which leads to a rapid loss of the reducing capacity.17 Other common dissolved solutes such as bicarbonate/carbonate and oxygen from water medium also cause corrosion of Fe0.18 U(VI) was removed by Fe0 systems down to concentrations <10 μg L−1 (recovery >98%), and partial chemical reduction of U(VI) to U(IV) concurrent with Fe oxidation was confirmed.19 In contrast under these conditions, Fe3O4 sample achieved U(VI) removal >20% from the carbonate-rich environmental water. Both nanomaterials are ineffective at timescales >1 week contact because of uranium re-release into water. This behavior is attributed to the high stability of uranium complexes in the presence of carbonate or other ligands present in the natural water. Consequently, further research is required to develop materials that exhibit extended retention of inorganic contaminants including uranium from chemically complex environmental waters. Reverse osmosis is very effective (removal ≥ 99%),11 however, this method is pretty expensive and causes big changes in water composition, which makes these devices not applicable for drinking water treatment.11 Among published approaches to removal of U(VI) from aqueous solutions, the adsorption technique is considered as the most popular pathway due to economy and efficiency in purification of U(VI)-contaminated wastes. Previously, U(VI) ion removal by adsorption was studied on several adsorbents such as activated carbon,20,21 hydrogels,22 ion-imprinted polymers,23 and various composite materials,24–26 etc.
During recent years, a lot of scientific studies have been focused on the removal of uranium(VI) from contaminated water using layered double hydroxides (LDH) and corresponding composites.25–28 This tremendous attention may be attributed to a good combination of physicochemical properties such as high stability, excellent anion exchange capacity, and relative easiness of modification of the surface by selective functional groups to increase surface concentration of active adsorptive centers towards U(VI) ions. For example, Ma S. et al.29 applied Mg,Al-LDH modified by polysulfide to remove uranium efficiently from environmental water and specifically seawater. However, the authors described sorption of UO22+ species without taking into account uranium–carbonate complexes which prevail in water solution. It was reported30 that phytic acid modified Zn–Al–Ti metal oxide was used to remove U(VI) from wastewater. Phosphate functionalized LDH were synthesized for efficient U(VI) uptake from polluted solutions,31 but in this case it is necessary to take precautions to avoid the eutrophication of water bodies. Linghu W. highlighted the application of Mg,Al-LDH/GO-based composites for removal of U(VI) in environmental cleanup.26 The magnetic separation technique was shown to be a promising method for solid–liquid phase separation of sorbent from water matrices with an external magnetic field particularly for the removal of radionuclides including U(VI) ions.32 Most of these adsorbents were magnetite-based, while other magnetic materials such as Fe0 (ref. 17 and 19) could be also applicable. The sorption of U(VI) on pristine magnetite nanoparticles with relatively small adsorption capacity at pH 7.0 was demonstrated by Das et al.33 Compared to the pristine magnetite, the Mn-doped ferrite nanomaterials exhibited generally superior adsorption performance towards U(VI) uptake in the pH range from 4 to 7.34 In order to impart improved adsorption abilities towards U(VI) to magnetite, it is modified via introduction of specific functional groups or its combination in composite materials with different inorganic or organic compounds.33,35,36 The S-doped magnetite hollow spheres used for the removal of UO22+ with a large adsorption capacity up to 450.0 mg U(VI) per g was reported.37
Recently, high potential of chelating groups intercalated in the LDH-based materials was used for removal of U(VI) ions from an aqueous solution (removal > 70%).38,39 Since the interlayer of functional groups is easily formed from coordination compounds with various U(VI) forms, it makes LDH useful for the adsorption applications. Previously, many studies were published about adsorption of various metal ions including U(VI) ions by materials containing functional ethylenediaminetetraacetic (EDTA) groups.40,41 On the other hand, synthesis of the EDTA-intercalated LDH-based magnetic nanocomposites with high adsorption capacity and selectivity towards U(VI) ions has not been performed until today.
This work is devoted to preparation of EDTA and citric acids intercalated Zn,Al-layered double hydroxide magnetic nanocomposites (Fe3O4/Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/Cit) via a facile and simple step-by-step co-precipitation method and ion-exchange techniques. These adsorbents were applied for effective, high selective removal of U(VI) from environmental and wastewaters. Various characterization techniques were used to investigate the interaction mechanism of the anionic radionuclide forms with Fe3O4/Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/Cit. The uptake performance and selectivity of the obtained adsorbents towards U(VI) ions were minutely studied under influence of various experimental factors (pH and HCO3−/CO32− effects, contact time, adsorption capacity, coexisting ions, etc.). Finally, a possible use of the synthesized magnetic nanocomposites as adsorbents for the removal of U(VI) from environmental and industrial wastewaters was demonstrated.
2. Experimental
2.1. Preparation of adsorbents
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(Fe2+) = 2
n(Fe2+) = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After adding 400 mL of CO2-free distilled water the obtained mixture was mechanically stirred, and, then, a 30 wt% aqueous solution of NaOH was added with constant stirring to pH 10.0 ± 0.5. The mixture was heated up to 70 °C with constant stirring for further 2 hours under N2 atmosphere. The precipitate was washed with deionized water several times until the negative reaction of sulfate-ions and stored in ethanol solution (400 mL) without drying.
1. After adding 400 mL of CO2-free distilled water the obtained mixture was mechanically stirred, and, then, a 30 wt% aqueous solution of NaOH was added with constant stirring to pH 10.0 ± 0.5. The mixture was heated up to 70 °C with constant stirring for further 2 hours under N2 atmosphere. The precipitate was washed with deionized water several times until the negative reaction of sulfate-ions and stored in ethanol solution (400 mL) without drying.The intercalation compounds (Zn,Al-LDH/Cit and Zn,Al-LDH/EDTA) were prepared from Zn,Al-LDH/CO3 by anion-exchange reaction. Typically, Zn,Al-LDH/CO3 (20 g) solid was added to 400 mL of distillated water at temperature 50 °C to obtain a suspension. To the resultant suspension at constant stirring was added solution of 44.1 g (0.21 mol) of citric acid (or 0.015 M Na2H2EDTA) by dropwise (at 5 mL min−1) under nitrogen atmosphere. The products were washed with distilled water, followed by being dried for 24 h at 50 °C.
2.2. Characterization of the adsorbents
For the quantification of the main elements present in the samples, the solids (0.1000 g) were dissolved in 15–20 mL of diluted HCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and CO2-free distilled water (total 100 mL), and analyzed in duplicate with the atomic absorption spectroscopy (C-115-M1 spectrometer) at 213.9 nm (Zn), 309.3 nm (Al) and 248.3 nm (Fe). The content of organic functional motives was determined using total organic carbon analysis.42 The obtained data were used in the compounds' formulations.
1) and CO2-free distilled water (total 100 mL), and analyzed in duplicate with the atomic absorption spectroscopy (C-115-M1 spectrometer) at 213.9 nm (Zn), 309.3 nm (Al) and 248.3 nm (Fe). The content of organic functional motives was determined using total organic carbon analysis.42 The obtained data were used in the compounds' formulations.
Temperature and field dependent magnetic measurements of obtained samples were measured with EV9 vibrating sample magnetometer under the high sensitivity reciprocal space option. The size and morphology of the nanoparticles were examined using transmission electron microscopy (TEM), on JEM-2100F (JEOL, Japan) operated at 200 kV and equipped with an ultra-high resolution pole-piece. The samples were sprayed on a carbon coated copper grid, and then exposed to air-drying. The surface topography of the nanoparticles was studied using scanning electron microscopy (SEM), JSM-6100 (JEOL, Japan). To increase the optical contrast, the samples were covered with gold on superfine spraying instrument JFC-1600 Auto Fine Coater (JEOL, Japan). The chemical composition of the nanoparticles was determined by energy dispersive X-ray analysis (EDX) on Microanalysis INCA Energy 200 equipped with an Oxford PentaFET ultrathin window detector attached to a scanning electron microscope. The powder X-ray diffraction (XRD) data were collected using PANalytical X'Pert Pro diffractometer with CuKα radiation (λ = 1.5405 Å). Measurements over the range 5° ≤ 2θ ≤ 120° with a step size of 0.040° were performed at room temperature. The thermal analysis measurements (TG/DTG and DTA curves) were obtained in Mettler Toledo TGA/SDTA851 equipment, using platinum crucibles, flow of nitrogen, and heating rate of 10 min−1. The total amount of intercalated organic groups (CL, mmol g−1) for LDH-based samples was determined from TG curves. Assuming that the initial samples and intercalated samples after 1000 °C treatment have the same extent of surface hydroxylation, the following formula was used for the calculations
| CL = Δm200−500minitial/100M | (1) |
The N2 adsorption/desorption isotherms at 77 K were performed using a KELVIN 1042 BET-Sorptometer (Costech International). Prior to measurements, the samples (50 mg) were outgassed at 110 °C for 4 h under a vacuum above 102 Pa. The porosity of the samples was analysed by applying the nonlocal density functional theory (NLDFT).43
The Fourier transform infrared spectra (FTIR) of the samples were recorded in the 4000–400 cm−1 range, on Spectrum BX FTIR spectrometer (PerkinElmer, USA). The sample was thoroughly ground with KBr (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), after that they were tableted under load 1.7 t cm−2.
10), after that they were tableted under load 1.7 t cm−2.
2.3. Batch adsorption experiments
The influence of pH on U(VI) removal was tested for all obtained adsorbents at room temperature. For the preparation of stock solutions was used UO2SO4·3H2O (≥98%).Typically, 100 mg of solids were mixed with 50 mL of the solution containing 0.1 mmol L−1 of U(VI) in the centrifuge tube; the suspension pH in the range of 2.0–10.0 was adjusted by adding HNO3 (0.1 M) or NaOH (0.1 M). The pH (±0.005) value of the solution before and after adsorption process was measured using an I-160 M ionometer. The mixtures were agitated by orbital shaker (AVU-6S) during 1 h. After equilibrium, the solid phase was magnetically separated from liquid phase. The concentration of U(VI) in supernatant was measured by spectrophotometry with Arsenazo III at λ = 656 nm in 5–7 M HNO3. The limit of determination of U(VI) by this method is 5 μg. All adsorption experiments were performed in triple parallel tests.
The adsorption kinetic experiments was carried out by changing the contact time from 0 to 120 min between the 0.10 g of solid and 50 mL of 0.1 mmol L−1 U(VI) solution at pHinitial = 5.0 ± 0.1. The aliquots were collected at the times 0, 5, 15, 30, 60, 90 and 120 min, respectively. The removal of U(VI) ions (R, %) and adsorption capacity (qe) were determined as follows:
| R(%) = C0 − Ce/C0 × 100% | (2) |
| qe (mg g−1) = (C0 − Ce)VM/g or qe (mmol g−1) = (C0 − Ce)V/g | (3) |
Adsorption isotherms were carried out by the batch equilibration technique at room temperature. The solids (0.1 g) were dispersed in 50 mL of U(VI) solution with concentration varying from 0.15 to 0.90 mmol L−1 U(VI) solutions were prepared in an appropriate range of concentrations to obtain a final volume of 50 mL after the addition of the adsorbent and the pH was fixed at 7.5. Then, suspension is shacked during 24 h. After that the adsorbent was magnetically separated and the remaining concentration of the metals in solution was determined by spectrophotometry. The qe value was determined from the eqn (2).
2.4. Competitive adsorption of metal ions
2.5. Genuine water assay
To demonstrate the applicability and reliability of the synthesized adsorbents for water treatment were used river water and wastewater. The real wastewater samples were collected from uranium processing State Enterprise “Eastern Mining and Processing Plant” (Zhovti Vody city, Dnipro region, Ukraine).Briefly, main parameters of the tested water samples:
• Environmental water: pH ≈ 7, alkalinity 8.5 mg-eq L−1, hardness 28 mg-eq L−1, NO3− 470 mg L−1, SO42− 269 mg L−1, Cl− 280 mg L−1, Na+ 90 mg L−1, total salt content ∼2820 mg L−1, chemical oxygen demand (COD) 1.6 mg O per L;
• Wastewater: pH ≈ 8, HOC3− 235 mg L−1, CO32− 29 mg L−1, SO42− 470 mg L−1, Cl− 280 mg L−1, Ca2+ 170 mg L−1, Na+ 241 mg L−1, Mg2+ 54 mg L−1, U(VI) 0.85 mg L−1, total salt content ∼1500 mg L−1, COD 59.0 mg O per L.
The real water samples were analyzed after spiking with 0.1 mmol L−1 of U(VI) ions for reliably determination of U(VI) ions in solution by spectrophotometry method.
3. Results and discussion
3.1. Preparation and characterisation of nanocomposites
In this work, the synthesis of Fe3O4/Zn,Al-LDH/Cit and Fe3O4/Zn,Al-LDH/EDTA nanocomposites was performed in a three-step process: (1) preparation of magnetite nanoparticles; (2) fabrication of Zn,Al-LDH/CO3 and its modification with citric (or EDTA) groups through co-precipitation and ion-exchange techniques; (3) formation of magnetic nanocomposite based on pristine Fe3O4 and Zn,Al-LDH/Cit (or Zn,Al-LDH/EDTA) samples. The whole routes were represented in Scheme 1.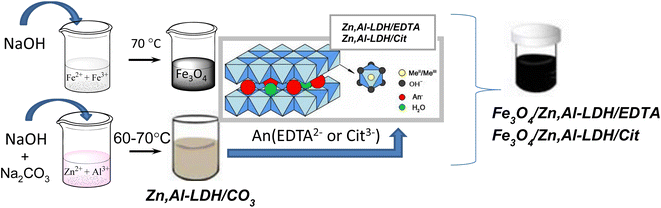 | ||
| Scheme 1 Schematic illustration of preparation of Fe3O4/Zn,Al-LDH/Cit and Fe3O4/Zn,Al-LDH/EDTA nanocomposites. | ||
The size, shape and chemical composition of the magnetic nanoparticles strongly depend on the preparation conditions, which affect the efficiency of the uptake U(VI). Therefore, all synthetic steps were controlled using instrumentation techniques.
According to SEM (Fig. S1†) and TEM data (Fig. 1), significant differences exist in morphology target magnetic nanocomposites and starting materials. The TEM image (Fig. 1a) show that the pure Fe3O4 nanoparticles present near-spherical form with rough surface and approximate diameter of ∼6–8 nm. Whereas Fig. 1b show that Zn,Al-LDH/Cit possess spherical and rod-like shapes, and the average diameter varied in the range of 24.7–82.4 nm. The Zn,Al-LDH/EDTA sample exhibit sieve-like structure. The TEM image of Fe3O4/Zn,Al-LDH/Cit and Fe3O4/Zn,Al-LDH/EDTA (Fig. 1c–e) exhibits the multiple layers of LDH around Fe3O4. The dark well-dispersed magnetite nanoparticles were interred consistently in LDH particles of the light grey color.
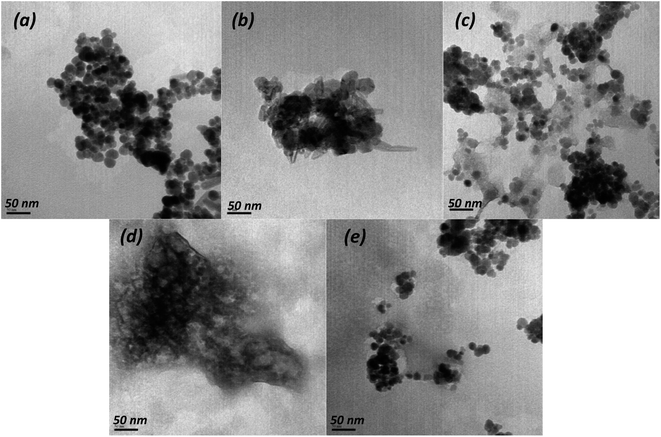 | ||
| Fig. 1 TEM images of pristine Fe3O4 (a), Zn,Al-LDH/Cit (b), Fe3O4/Zn,Al-LDH/Cit (c), Zn,Al-LDH/EDTA (d) and Fe3O4/Zn,Al-LDH/EDTA (e) samples. | ||
SEM images of Zn,Al-LDH/Cit and Zn,Al-LDH/EDTA reveal the lamellas shapes (Fig. S1b and d†). The flakes are about 1–2 μm in size and have sub-μm thickness.
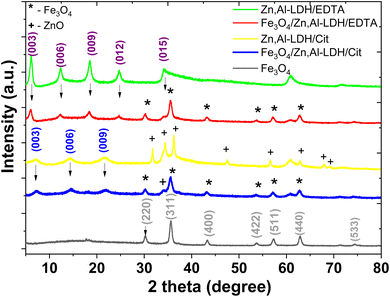
Powder XRD data was used to the phase identification of starting samples and resulted magnetic nanocomposites (Fig. 2). The XRD patterns of Fe3O4 show the diffraction peaks at 2θ = 30.1°, 35.5°, 43.1°, 53.4°, 57.0°, 62.5°, and 75.0° which related to the corresponding (220), (311), (400), (422), (511), (440) and (533) Bragg reflections, respectively. This data indicates that the resulted nanoparticles were magnetite (JCPDS Card No. 19-0629) with a face-centered cubic spinel crystalline structure. The average crystallite sizes calculated using Scherrer equation45 for the (311) reflection of magnetite are 22.5 nm, 13.9 nm and 15.4 nm for Fe3O4, Fe3O4/Zn,Al-LDH/Cit and Fe3O4/Zn,Al-LDH/EDTA, respectively.
A dominating feature powder XRD patterns of most layered compounds is the series of reflections corresponding to the basal plane d spacing (“harmonic” peaks). Furthermore, according to Bragg's law,46 the Zn,Al-LDH/CO3 powder XRD pattern displays first-, second, third-, and fourth-order peaks at 7.59 Å, 3.78 Å, 2.59 Å, and 2.29 Å, respectively (Fig. S2†). The intercalated samples and its starting samples (Zn,Al-LDH/Cit and Zn,Al-LDH/EDTA) appear basal reflections, indicating the presence of the layers sequences. The XRD patterns of Zn,Al-LDH/Cit displays first-, second-, and third-order peaks at 2θ = 7.21, 14.47, and 21.8° corresponds to the basal reflections (003), (006), and (009), respectively, matching of LDH compound,47 with several additional reflection peaks from ZnO impurity (JCPDS Card No. 36-1451) were found as result of the adverse reaction:
| 2ZnCl2 + 2C3H5O(COOH)3 + 9O2 → 2ZnO + 12CO2 + 6H2O + 4HCl |
The basal spacing (d003) for Zn,Al-LDH/Cit is 12.25 Å. This is indicative of the significantly (∼40%) increase basal spacing of Zn,Al-LDH/CO3 after intercalation. The XRD pattern of Zn,Al-LDH/EDTA display first-, second-, third- and fourth-order reflection peaks. As expected, the XRD patterns of Fe3O4/Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/Cit samples containing the peaks corresponding basal reflections LDH and Fe3O4, proves that both phases are present in the structure of magnetic layered nanocomposites (Fig. 2). Because of the intercalation of citric or EDTA groups in LDH compound, a shift (00L) of the basal reflections occurs towards lower angles with a corresponding increase in d-spacing. Comparison of the XRD patterns revealed that the XRD peaks for magnetic composites are weakened and broadened compared to those for Zn,Al-LDH/Cit and Zn,Al-LDH/EDTA samples. For Fe3O4/Zn,Al-LDH/Cit, incorporation of the magnetite in Zn,Al-LDH/Cit basal spacing is almost constant. Thus, compared to the initial sample d003 of the intercalated Fe3O4/Zn,Al-LDH/EDTA samples reached 14.57 Å, respectively. These results suggest that EDTA groups was intercalated into the interlayer space of the Fe3O4/Zn,Al-LDH/EDTA, which was attributed to its high specific surface area. Applying Debye–Scherrer equation for the most intense diffraction peaks, crystallite sizes of bare magnetite and derivated magnetic nanocomposites were estimated (Table 1).
| Parameters | Fe3O4 | Zn,Al-LDH/CO3 | Zn,Al-LDH/Cit | Fe3O4/Zn,Al-LDH/Cit | Zn,Al-LDH/EDTA | Fe3O4/Zn,Al-LDH/EDTA | |
|---|---|---|---|---|---|---|---|
| 2θ | (003) | — | 13.4 | 7.2 | 7.15 | 6.1 | 6.2 |
| (006) | — | 27.2 | 14.5 | 14.4 | 12.3 | 12.4 | |
| (009) | — | 40.1 | 21.8 | 21.5 | 19.4 | 19.5 | |
| Crystallite size, nm | 7.1 | — | — | 12.1 | — | 11.1 | |
| Particle size, nm | 6–8 | — | 24.7–82.4 | 14.5–22.1 | 12.7–14.4 | 12.8–25.6 | |
| S, m2 g−1 | 11.70 | 91.60 | 52.18 | 127.69 | 116.08 | ||
| Dpore, nm | 0.3 | — | 0.8 | 0.61 | 1.59 | 1.22 | |
| Ms, emu g−1 | 66.16 | — | — | 20.05 | — | 18.06 | |
| HC, Oe | 2.2 | — | — | 1.5 | — | 1.4 | |
| CL, mmol g−1 | — | — | 0.040 | 0.028 | 0.029 | 0.015 | |
| pHpzc | — | — | 8.53 | 6.91 | 6.93 | 7.34 | |
Generally, the average particle size estimated by TEM method have commensurate with crystallite size from XRD data.
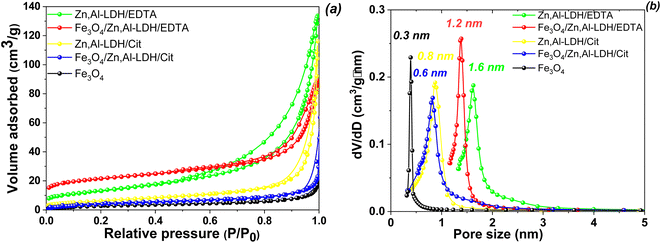
To characterize the surface properties of the magnetic nanocomposites studied N2 adsorption/desorption isotherm measurements were carried out for all synthesized materials and the experimental data are shown in Fig. 3. To ensure proper removal of physically adsorbed water before the adsorption measurements were taken, the layered structure were outgassed for 2 h at 120 °C under vacuum (10−4 Torr).
N2 isotherms adsorption/desorption of Fe3O4 sample exhibit the type I without hysteresis loops according to the IUPAC classification,48 which is typical for microporous material (Fig. 3a). The rest of the isotherms were type IV that possessed a characteristic H3-type hysteresis loop (p/p0 > 0.4), which indicated that the materials belongs to sheet-type materials with mesoporous structure.48 The specific surface area of obtained samples decrease in the following order: Zn,Al-LDH/EDTA (127.69 m2 g−1) >![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4/Zn,Al-LDH/EDTA (116.08 m2 g−1) >
Fe3O4/Zn,Al-LDH/EDTA (116.08 m2 g−1) >![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn,Al-LDH/Cit (91.60 m2 g−1) >
Zn,Al-LDH/Cit (91.60 m2 g−1) >![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4/Zn,Al-LDH/Cit (52.18 m2 g−1) > Fe3O4 (11.70 m2 g−1). The pores per size distribution were estimated by using the NLDFT model giving the average pore size values (Fig. 3b). Concurrently, the respective average pore diameters and pore volumes were 1.59 nm and 0.52 cm3 g−1 for Zn,Al-LDH/EDTA, 1.22 nm and 0.18 cm3 g−1 for Fe3O4/Zn,Al-LDH/EDTA, 0.8 nm and 0.14 cm3 g−1 for Zn,Al-LDH/Cit, 0.61 nm and 0.10 cm3 g−1 for Fe3O4/Zn,Al-LDH/Cit, and 0.29 nm and 0.09 cm3 g−1 for Fe3O4/Zn,Al-LDH/Cit, respectively. Clearly, the intercalation of samples by EDTA-groups leads to a larger specific surface area and pore volume than the intercalation by citric acid. Although the obtained magnetic nanocomposites induced smaller specific surface area than pure Zn,Al-LDH/EDTA and Zn,Al-LDH/Cit first ones still enables as an effective adsorbents.
Fe3O4/Zn,Al-LDH/Cit (52.18 m2 g−1) > Fe3O4 (11.70 m2 g−1). The pores per size distribution were estimated by using the NLDFT model giving the average pore size values (Fig. 3b). Concurrently, the respective average pore diameters and pore volumes were 1.59 nm and 0.52 cm3 g−1 for Zn,Al-LDH/EDTA, 1.22 nm and 0.18 cm3 g−1 for Fe3O4/Zn,Al-LDH/EDTA, 0.8 nm and 0.14 cm3 g−1 for Zn,Al-LDH/Cit, 0.61 nm and 0.10 cm3 g−1 for Fe3O4/Zn,Al-LDH/Cit, and 0.29 nm and 0.09 cm3 g−1 for Fe3O4/Zn,Al-LDH/Cit, respectively. Clearly, the intercalation of samples by EDTA-groups leads to a larger specific surface area and pore volume than the intercalation by citric acid. Although the obtained magnetic nanocomposites induced smaller specific surface area than pure Zn,Al-LDH/EDTA and Zn,Al-LDH/Cit first ones still enables as an effective adsorbents.
The magnetic behaviour of the magnetite and derived magnetic nanocomposites were studied at room temperature (Fig. 4).
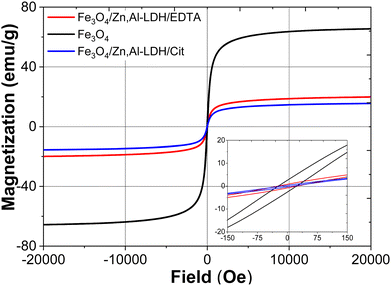 | ||
| Fig. 4 Wide and narrow (insert) magnetic hysteresis loops of bare Fe3O4, Fe3O4/Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/Cit samples at room temperature. | ||
Magnetization curves for pristine Fe3O4 and nanocomposites passed near the zero point of magnetization field (Fig. 4). The saturation magnetization (Ms) values of Fe3O4/Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/Cit samples are 20.05 and 18.06 emu g−1, respectively, which are lower than that of pristine magnetite nanoparticles (Ms = 66.16 emu g−1). The lower values saturation magnetization of both magnetic nanocomposites could be explained by the presence of diamagnetic Zn,Al-LDH in this samples. The magnetic hysteresis loops for these samples pointed to superparamagnetic behaviour in which remanent magnetization and coercivity (HC) parameters were close to zero (Table 1).
Finally, the saturation magnetisation value of magnetic nanocomposites enables to separate the adsorbents from liquids using an external magnet during magnetic solid phase extraction.
The zero-field cooled (ZFC) and field-cooled (FC) magnetization curves of obtained magnetic samples were measured at low magnetic field (80 Oe) in the temperature range of 5–380 K, on the pristine magnetic nanoparticles (Fig. 5a and b) as well as on the derivated magnetic nanocomposite (Fig. 5c and d).
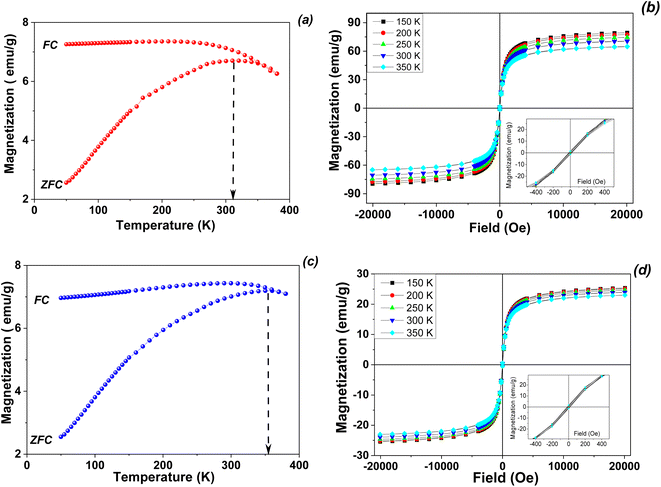 | ||
| Fig. 5 ZFC/FC magnetization curves (a and c) and temperature dependent hysteresis loops (b and d) for pristine magnetite (a and b) and Fe3O4/Zn,Al-LDH/EDTA nanocomposite (c and d). | ||
The main differences between the magnitude and temperature variations of the coercivities of the studied samples are reflected in the extent of the irreversibility appeared by the difference between the FC and ZFC curves (Fig. 5). The ZFC and FC curves of are typical for systems of magnetic monodomain magnetic nanoparticles presenting superparamagnetic behavior above a temperature which is called the blocking temperature. In the present case, the two curves show a divergence point very close to the maximum of the ZFC curve, suggesting both very weak interparticle magnetic interactions as well as a very narrow size distribution of the nanoparticles in the samples. On the other hand, the blocking temperature as estimated from the maximum of the ZFC curve is lower in the case of pristine magnetite nanoparticles (315 K) as compared to the case of the obtained nanocomposite (about 350 K). Keeping in mind that the blocking temperature, defined as a parameter which is dependent on the time window of the measuring method, is proportional to the anisotropy energy of the nanoparticle. It results directly that the anisotropy energy of Fe3O4/Zn,Al-LDH/EDTA magnetic nanocomposite is some 10% larger than of pristine magnetite in the nanosized level.
The formation of Fe3O4/Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/Cit were studied by FTIR technique (Fig. 6). The broad absorption band observed around 3400 cm−1 corresponds to the O–H stretching valence vibration of hydroxyl groups and/or interlayer-bonded water molecules. The absorption band observed around 570 cm−1 belongs to Fe–O stretching and torsional mode of Fe3O4. Other bands observed in the low-frequency region of the spectrum (<700 cm−1) are interpreted as the lattice vibration modes, such as the M–O (M = Zn, Al) vibration stretching of LDH. The peaks at 2850, 2930 and 1411 cm−1 are attributed to CH-stretching vibrations of intercalated functional groups. The FTIR spectra of LDH-based samples showing peaks at 1100 cm−1, 1393 cm−1, 1593 cm−1 indicating the presence of symmetrical and asymmetrical stretching vibrations of the carboxylic motives of intercalated functional groups. For Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/EDTA samples (Fig. 6b), the two low intensity bands of ν(C–N) are located at 1030–1270 cm−1. Also, the peak at 1175 cm−1 may be assigned to ν(C–O) vibration in C–O–N motives of EDTA-groups. In the Fe3O4/Zn,Al-LDH/Cit nanocomposite, the symmetric C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration peak at 1593 cm−1 shows a slight shift to higher frequency regions, when compared with those of the Zn,Al-LDH/Cit sample around 1581 cm−1 (Fig. 6a). This effect indicates that there is interaction between the intercalated citric groups and magnetite. Comparing the spectra of Fe3O4/Zn,Al-LDH/EDTA and Zn,Al-LDH/EDTA sample, the C
O vibration peak at 1593 cm−1 shows a slight shift to higher frequency regions, when compared with those of the Zn,Al-LDH/Cit sample around 1581 cm−1 (Fig. 6a). This effect indicates that there is interaction between the intercalated citric groups and magnetite. Comparing the spectra of Fe3O4/Zn,Al-LDH/EDTA and Zn,Al-LDH/EDTA sample, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching region did shift toward higher wavenumbers, indicating that intercalated functional groups are more free in the interfacial layer of Fe3O4/Zn,Al-LDH/EDTA. All these bands indicated the formation of chelating groups on the obtained solids and it could be concluded that the synthesis procedure had successfully. The introduction of magnetite into LDH structure and the change of CO32−-groups to chelating motives are beneficial to U(VI) adsorption from aqueous solutions.
O stretching region did shift toward higher wavenumbers, indicating that intercalated functional groups are more free in the interfacial layer of Fe3O4/Zn,Al-LDH/EDTA. All these bands indicated the formation of chelating groups on the obtained solids and it could be concluded that the synthesis procedure had successfully. The introduction of magnetite into LDH structure and the change of CO32−-groups to chelating motives are beneficial to U(VI) adsorption from aqueous solutions.
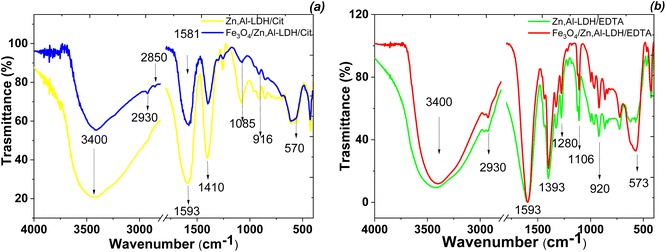 | ||
| Fig. 6 FTIR spectra of synthesized magnetic nanocomposites (Fe3O4/Zn,Al-LDH/Cit (a) and Fe3O4/Zn,Al-LDH/EDTA (b)) and its intermediates. | ||
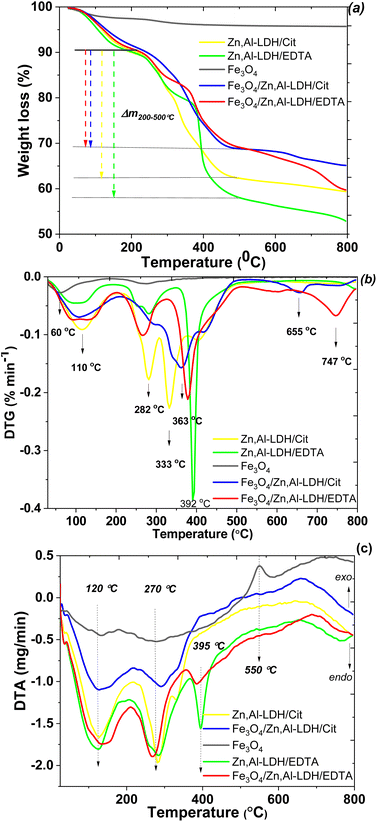
To evaluate the thermal stability of adsorbents was used thermogravimetric analysis data. In the TG/DTG and DTA, curves of studied samples (Fig. 7), there are three main steps of mass loss, which occur as endothermic processes. The water loss up to around 60 and 120 °C, due to different types physically bonded water molecules, attributed to Fe3O4 and LDH parts of the samples, respectively. The mass lost occurred at 120 °C was most likely due to the loss of interlayer water of LDH. The second mass loss of LDH-based samples was the largest. It occurred at 250–400 °C and accounted until 30% of total mass loss. Asymmetry of the corresponding peaks in the DTG curve indicates that several different processes are occurring simultaneously. Indeed the comparison of TG/DTG curves between the starting LDH and their corresponding intercalated magnetic composites demonstrate that the citric and EDTA groups decomposition observe around 280 °C shifted to 330 °C and up to 400 °C. Thus, the second major weight loss occurring in the range of 250 to 400 °C is related to the decarbonation of organic matters and LDH structure decomposition. Nearly 5.1% of weight loss was found from 500 to 660 °C, which was ascribed mostly to the dehydroxylation of the OH-groups of LDH. The last step at around 750 °C is attributed to the decomposition of sulfate as impurity, release of SO3 and formation of the respective oxides in the spinel structure. The total mass losses of the samples throughout to 800 °C are approximately 4.3 wt%, 40.6 wt%, 34.8 wt%, 47.1 wt% and 40.1 wt% for Fe3O4, Zn,Al-LDH/Cit, Fe3O4/Zn,Al-LDH/Cit, Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/EDTA, respectively. The comparison this data prove that magnetic composites have higher thermal stability than starting materials.
To determine the overall loading of the intercalated organic phase of the samples were used the value of weight loss at temperature range from 200 to 500 °C (Fig. 7a). According to the TG curves the amount of organic groups is about 0.040 mmol g−1 for Zn,Al-LDH/Cit, 0.028 mmol g−1 for Fe3O4/Zn,Al-LDH/Cit, 0.029 mmol g−1 for Zn,Al-LDH/EDTA and 0.015 mmol g−1 for Fe3O4/Zn,Al-LDH/EDTA. It can be seen that the amount of organic groups en Fe3O4/Zn,Al-LDH/Cit sample is higher than en Fe3O4/Zn,Al-LDH/EDTA.
It was ascertained from the EDX spectra (Fig. S4†) the presence of Al and Zn elements peaks with the other major peaks like C, O, N. The molar ratios of Zn/Al in the samples were observed to be 2.0–2.1. Using the atomic absorption spectrometry and total organic carbon analysis data, the chemical composition of intercalated compounds were determined. Atomic absorption spectroscopy gives weight percent on the order of 56% for Zn and 27% for Al. This result confirms the content of Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al with a molar ratio of 2
Al with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is in good agreement with the EDX data and ratio used in the synthesis. TGA measurements reveal water loss of about 10% up to 150 °C (Fig. 7a). A chemical composition of [Zn4Al2(OH)12](H2EDTA)·8H2O and [Zn4Al2(OH)12]Cit·8H2O are typical for obtained samples. The Fe3O4 content in the magnetic nanocomposite was 18.79 wt%. Similarly, the chemical composition for the obtained magnetic adsorbents were determined such as Fe3O4[Zn4Al2(OH)12]Cit·8H2O and Fe3O4[Zn4Al2(OH)12](H2EDTA)·8H2O.
1, which is in good agreement with the EDX data and ratio used in the synthesis. TGA measurements reveal water loss of about 10% up to 150 °C (Fig. 7a). A chemical composition of [Zn4Al2(OH)12](H2EDTA)·8H2O and [Zn4Al2(OH)12]Cit·8H2O are typical for obtained samples. The Fe3O4 content in the magnetic nanocomposite was 18.79 wt%. Similarly, the chemical composition for the obtained magnetic adsorbents were determined such as Fe3O4[Zn4Al2(OH)12]Cit·8H2O and Fe3O4[Zn4Al2(OH)12](H2EDTA)·8H2O.
3.2. Uranium(VI) adsorption onto obtained samples
The efficiency of magnetic nanocomposites and corresponding starting materials as adsorbents to remove U(VI) from water medium was evaluated by the impact of pH, contact time, porosity features and coexisted ions. The advantages and limitations several of obtained adsorbents discovered.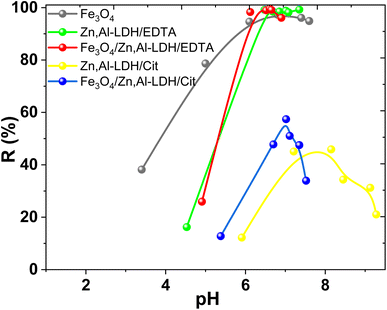 | ||
| Fig. 8 Effect of pH on U(VI) removal by obtained samples (conditions: C0(U(VI)) = 0.1 mmol L−1, V/m = 500 cm3 g−1, time 1 h). | ||
The increase of U(VI) adsorption on Zn,Al-LDH/Cit and Fe3O4/Zn,Al-LDH/Cit was observed at 4.5 < pH < 8.0 (Fig. 8). The maximum U(VI) uptake on Fe3O4/Zn,Al-LDH/Cit was demonstrated at pH 7.0. The high-level adsorption at pH 7.0–8.0 kept on Zn,Al-LDH/Cit sample, then whereas U(VI) uptake was significantly decreased at pH > 8.0. However, the removal percentage of U(VI) on Zn,Al-LDH/Cit and Fe3O4/Zn,Al-LDH/Cit samples was no more than 60%. Quantitative removal of U(VI) at 6.0 ≤ pH ≤ 8.0 was achieved for Fe3O4, Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/EDTA samples. For Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/EDTA samples, the significant increase of U(VI) uptake was observed at pH 4.0–6.0, and then remained at pH 6.0–8.0. The slight decrease of U(VI) adsorption was found at pH > 8.5. Similar pH removal range was observed for Fe3O4, Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/EDTA samples. These data demonstrated likeness of the samples surface properties. Besides, the EDTA intercalated samples and magnetite shows the highest adsorption performance toward U(VI) than for citrate-containing specimens. The reason for this phenomenon might be the interaction with functional groups with some hydroxides as agree with XRD or internal molecular interaction of adsorbents under various pH. Thus, the part of the surface groups (such as M–OH) probably were blocked by functional citric groups of Zn,Al-LDH/Cit and Fe3O4/Zn,Al-LDH/Cit, resulting in lower adsorption efficiency. Nevertheless, for all studied adsorbents the maximum removal of U(VI) was achieved from solution at pH range from 6.0 to 8.0.
In general, U(VI) ions forming of UO22+, (UO2)2(OH)22+, UO2OOH+, (UO2)3(OH)5+, (UO2)4(OH)7–, UO2(CO3)22− and UO2(CO3)34− species in aqueous solution upon contact with the air (Fig. S3†).6 The prevail UO22+ species in the acidic range (pH < 5) is observed. The pH in the environmental water is mainly 7.5–8.3, where the typically carbonate/bicarbonate is ubiquitous and often present relatively high concentration (near 5 × 10−3 mol L−1) both natural and wastewaters. Predominant species of U(VI) in carbonate-containing waters are UO2(CO3)34− (80–90%), while also including smaller amounts of UO2(CO3)22− and insignificant concentrations of uranyl hydroxide complexes. (e.g., ground water, uranium tailings wastewater).
It known, the adsorbent surface charges are influence the intensity and nature of interactions between the adsorption sites and U(VI) ions. The critical parameter of these features is the pHpzc value.44,49 The determination of pHpzc of obtained adsorbents was performed at pHinitial from 2.0 to 10.0 and the experimental data are presented in Fig. 9. The results of pHfinal have been reformatted as ΔpH (pHfinal − pHinitial, dot lines in the Fig. 9) versus pHinitial. Here the advantage of operating at similar adsorbents is obvious, because of virtually straight line of high slope extends well beyond of the x intercept.
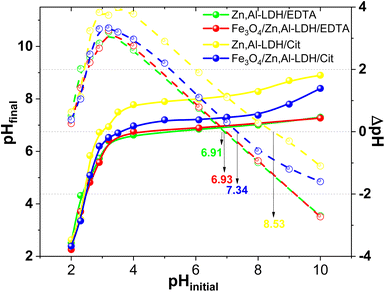 | ||
| Fig. 9 The determination of pHpzc of obtained adsorbents (conditions: m = 0.025 g, V = 50 mL, time 24 h, C(NaClO4) = 0.1 M). | ||
With the available experimental data, the pHpzc of Zn,Al-LDH/Cit, Zn,Al-LDH/EDTA, Fe3O4/Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/Cit samples was determined as 8.53, 6.93, 7.34 and 6.91, respectively (Fig. 8). In this line, compared to Zn,Al-LDH/Cit and Fe3O4/Zn,Al-LDH/Cit samples the pHpzc of Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/EDTA composites were observed in the lower pH range (Fig. 9). The difference in pHpzc values can be explained by the presence of available various acidic functional groups in the samples. At this state, the surface of samples were positive charged at pH < pHpzc. Therefore in this range pH, the charged magnetic composites and initial samples would attracted to cation-exchange and complexation reaction with cationic forms of U(VI), thus resulted in the great increased pH of suspensions (Fig. S4†). During the process of U(VI) removal at pH > pHpzc using obtained samples the pHinitial was decreased to pHfinal could be ascribed the anion-exchange between negative charged of adsorbents and the dominated at this pH negative charged of carbonate U(VI) complexes species.
The high-level uptake of U(VI) on magnetic composites and initial samples at pH 6.0–8.0 could be explain by complexation reaction of U(VI) with the Zn,Al-LDH/Cit, Zn,Al-LDH/EDTA, Fe3O4/Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/Cit samples. The magnetic composite based on Zn,Al-LDH with interlayer EDTA ions is more effective in this range of pH, in contrast to Zn,Al-LDH/Cit and Fe3O4/Zn,Al-LDH/Cit samples. This fact confirms the predominant role of the chelating ligands in the interlayer space of LDH-based materials and indicates a higher binding strength of U(VI) specifically with EDTA groups of the Fe3O4/Zn,Al-LDH/EDTA. These results suggest that EDTA groups were intercalated into the interlayer space of the Fe3O4/Zn,Al-LDH/EDTA, which was attributed to its high specific surface area, and were beneficial for the removal of U(VI) ions. In addition, it is very likely that U(VI) is bound by additional active sorption centers of magnetite (Fe–OH groups) too.
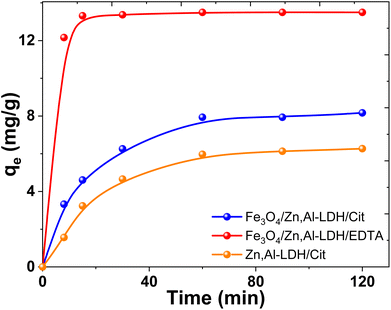 | ||
| Fig. 10 Effect of contact time on U(VI) adsorption on several obtained adsorbents (conditions: C0(U(VI)) = 0.1 mmol L−1, pH0 7.5, V/m = 500 mL g−1). | ||
The U(VI) uptake on the Fe3O4/Zn,Al-LDH/EDTA material increased dramatically in the first 5 min. While on the Fe3O4/Zn,Al-LDH/Cit the recovery of U(VI) increased slowly and attained plateau finally. In this case, the adsorption of U(VI) achieved equilibrium within more than 1 hour and the removal percentages does not exceed ∼30%. The slower kinetics of Fe3O4/Zn,Al-LDH/Cit is could be attributed to the intraparticle diffusion of uranium(VI) into the inner pores of adsorbent or the ion exchange in the interface layer of LDH. The fast adsorption on Fe3O4/Zn,Al-LDH/EDTA should be attributed to the features porous layered structure with high permeability, probably because the EDTA motifs in the interlayer space of the LDH facilitate the acceleration of the diffusion of U(VI) ions both within the pores as well as at the surface.
Lagergren method pseudo-first-order and pseudo-second-order kinetic models50,51 were applied to analyze the adsorption rate based on kinetic data (Fig. 10).52 The relative parameters calculated from the two models were listed in Table 2.
| Samples | qexp (mg g−1) | Pseudo first-order model | Pseudo second-order model | ||||
|---|---|---|---|---|---|---|---|
| qe (mg g−1) | K1 (min−1) | R2 | qe (mg g−1) | K2 (g (mg min)−1) | R2 | ||
| a qt and qe – adsorption capacity at time t and equilibrium conditions, respectively; K1 and K2 are the rate constants pseudo first-order and pseudo second-order models, respectively. | |||||||
| Zn,Al-LDH/Cit | 7.65 | 5.74 | 0.014 | 0.8716 | 6.85 | 0.013 | 0.9841 |
| Fe3O4/Zn,Al-LDH/Cit | 9.18 | 6.12 | 0.018 | 0.8687 | 8.61 | 0.016 | 0.9915 |
| Fe3O4/Zn,Al-LDH/EDTA | 14.55 | 2.61 | 0.011 | 0.2592 | 12.92 | 0.600 | 1.0000 |
It evidently manifest (Fig. S4†) that the experimental and calculated qe values fitted pretty well by the pseudo second-order model remaining nearly constant with the perfect linearity R2 (over 0.9841), demonstrating that the pseudo-second-order model can perfectly describe the uptake kinetics of U(VI) on the synthesized magnetic adsorbents. This effect further implies that the dominant mechanism for uranium(VI) removal on sample is chemisorption or strong complexation rather than mass transport.
Fig. 11 exhibited that the adsorption capacities for U(VI) adsorption improved remarkably with the increase of U(VI) concentrations. This result indicated that higher U(VI) concentrations can provide higher capacity to U(VI) transportation via solution to the adsorbents interfacial layer, and thereby lead to more interaction between U(VI) and active sites. According to Giles classification,53 obtained experimental isotherms adsorption of U(VI) can be described by various types of isotherms. Isotherm adsorption of UO22+ by Fe3O4/Zn,Al-LDH/EDTA can be considered as H-type, even though in this case a high slope was observed indicative of strong adsorbate–adsorptive interactions such as inner-sphere complexes. An Zn,Al-LDH/EDTA isotherm the slope initially increases with adsorptive concentration, but eventually stabilizes and becomes over increases as vacant adsorbent sites are filled. This type of isotherm indicates that at low concentrations the surface has a high affinity for the adsorptive, which significantly increases at higher concentrations. The adsorption isotherms of U(VI) on the other studied adsorbents may be assigned to L-type isotherms.53 The L-shaped (or Langmuir type) isotherm is characterized by a decreasing slope as concentration increases since vacant adsorption sites decrease as the adsorbent becomes covered. Such adsorption behavior could be explained by the high affinity of the adsorbent for the adsorptive at low concentrations, which then decreases as concentration increases. It can be seen that the adsorption ability of Fe3O4/Zn,Al-LDH/EDTA sample for U(VI) ions is higher than Fe3O4/Zn,Al-LDH/Cit. The adsorption capacity of pristine Fe3O4 nanoparticles should come from its porous microstructures, but its small surface area lead to its lower adsorption performance. The Zn,Al-LDH/CO3 show a better adsorption ability than pristine magnetite.
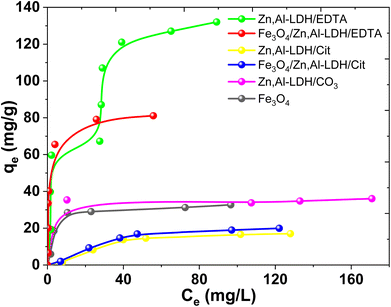 | ||
| Fig. 11 Isotherms adsorption of U(VI) by magnetic nanocomposites and starting materials (conditions: CU(VI) = 0.15–0.90 mmol L−1, рH 7.5, V/m = 500 mL g−1, contact time 1 h). | ||
According to the isotherm data the adsorption capacities is about 0.12 mmol g−1 (32.73 mg g−1) for Fe3O4, 0.13 mmol g−1 (36.05 mg g−1) for Zn,Al-LDH/CO3, 0.06 mmol g−1 (17.03 mg g−1) for Zn,Al-LDH/Cit, 0.45 mmol g−1 (131.22 mg g−1) for Zn,Al-LDH/EDTA, 0.07 mmol g−1 (19.95 mg g−1) for Fe3O4/Zn,Al-LDH/Cit and 0.29 mmol g−1 (81.12 mg g−1) for Fe3O4/Zn,Al-LDH/EDTA. The adsorption capacity for U(VI) on all obtained materials decreased in the following order: Zn,Al-LDH/EDTA > Fe3O4/Zn,Al-LDH/EDTA > Zn,Al-LDH/CO3 ∼ Fe3O4 > Fe3O4/Zn,Al-LDH/Cit > Zn,Al-LDH/Cit. This one is matches pretty well to the stability of the corresponding chelate U(VI) compounds in solution: the stability constants of U(VI) with citrate and EDTA groups are log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β(Cit) = 8.75 and log
β(Cit) = 8.75 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β(EDTA) = 19.7, respectively (Table S1†).54
β(EDTA) = 19.7, respectively (Table S1†).54
The adsorption data are usually interpreted using several adsorption equilibrium models such as the Langmuir and Freundlich.55,56 The Langmuir model is the most popular model to describe the adsorption properties of LDH based adsorbents.27 The fitting curves and calculation parameters of Langmuir and Freundlich models for the U(VI) adsorbed in the obtained adsorbents according to linear transformation of the isotherm data were presented in Fig. S6† and Table 3.
| Adsorbent | qexp, (mg g−1) | Langmuir isotherm model | Freundlich isotherm model | ||||
|---|---|---|---|---|---|---|---|
| qm, (mg g−1) | KL, (L μg−1) | R2 | KF, (mg1−1/n L1/n g−1) | 1/n | R2 | ||
| Fe3O4 | 32.73 | 33.98 | 0.23 | 0.9970 | 8.65 | 0.3357 | 0.6890 |
| Zn,Al-LDH/CO3 | 36.05 | 35.71 | 0.48 | 0.9984 | 20.57 | 0.4857 | 0.6323 |
| Zn,Al-LDH/Cit | 17.02 | 28.65 | 0.01 | 0.754 | 0.48 | 0.8076 | 0.8755 |
| Zn,Al-LDH/EDTA | 130.04 | 142.86 | 0.09 | 0.9217 | 28.77 | 0.3464 | 0.7629 |
| Fe3O4/Zn,Al-LDH/Cit | 19.95 | 33.56 | 0.01 | 0.7622 | 0.61 | 0.7903 | 0.8704 |
| Fe3O4/Zn,Al-LDH/EDTA | 81.12 | 112.36 | 0.11 | 0.8105 | 12.62 | 0.634 | 0.7958 |
By comparing the average values of R2 fitted by the Langmuir and Freundlich models, it can be found that the adsorption of U(VI) on obtained samples are mostly correlated with Langmuir isotherm than that for Freundlich model. The chemical nature of the sorption is actually in good agreement with mono-layered and uniform sorption indicated by Langmuir model, as well as our expectation that U(VI) ions are adsorbed through complexation with the EDTA-groups on the surface of adsorbents. For Zn,Al-LDH/Сit and Fe3O4/Zn,Al-LDH/Сit samples, the Freundlich model show better fitting with adsorption data.
The comparison of adsorption parameters of the adsorbents synthesized in this work with other published adsorbents for the removal U(VI) ions using magnetite-based adsorbents are summarized and tabulated in Table 4.
| Adsorbent | Preparation and modification of adsorbents | pH | Adsorption conditions | Adsorption mechanism and isotherm model | Ref. |
|---|---|---|---|---|---|
| a Sal-APS-FMNPS – magnetic nanoparticles with salicylaldehyde groups; rGO – reduced graphite oxide; CMLH – calcined magnetic Ca,Al layered double hydroxide/hydroxyapatite; HCC-Fe3O4 – magnetic hydrothermal cross-linking chitosan; Fe3O4@BP – bisphosphonate-modified magnetite nanoparticles. | |||||
| Fe3O4/Mg,Al-LDH/citrate | Co-precipitation; Mg–Al-LDH, citrate | 6 | qe 180 mg g−1, 4 h, 25 °C | Formation of chelate complex; Freundlich model | 39 |
| Sal-APS-FMNPS | Co-precipitation; salicylaldehyde | 7 | qe 49 mg g−1 | — | 57 |
| Fe3O4 | Co-precipitation; ammonium and phosphate | 9 | qe 70.7 mg g−1, 2 h | Electrostatic and chelating attraction | 58 |
| MnO2–Fe3O4–rGO | Hydrothermal, decorated graphene oxide | 6 | qe 95.24 mg g−1, 3 h | Surface complexation, electrostatic attraction and cation exchange | 59 |
| Ketoxime-Fe3O4@C | Solvothermal; carbon and ketoxime | 6 | qe 38.7 mg g−1, 2 h | 60 | |
| HCC-Fe3O4 | Hydrothermal; chitosan | 7 | qe 263.7 mg g−1, 3 h | Interaction with OH and NH2-groups | 61 |
| Fe3O4/C/Ni–Al-LDH | Co-precipitation; carbon, Ni–Al-LDH | 6 | qe 174 mg g−1, 3 h | Surface adsorption and intercalation; Langmuir model | 62 |
| Magnetic oxine | Solvothermal; oxine (8-hydroxyquinoline) | 7 | qe 125 mg g−1, >4 h | Inner-sphere complexation | 63 and 64 |
| Fe3O4@SiO2-AO | Hydrothermal; amidoxime | 5 | qe 105.5 mg g−1 T−1 298 K | — | 65 |
| Fe3O4@APTMS | Hydrothermal; aminopropyl groups | 6 | qe 151.8 mg g−1 | Complexation with NH2-groups | 66 |
| Fe3O4/Ca,Al-LDH (CMLH) | Co-precipitation; Са-Al-LDH, hydroxyapatite | 6 | qe 208 mg g−1, 1 h | Surface adsorption and complexation | 67 |
| GO@LDH | One-pot hydrothermal; Ni,Al-LDH and GO | 7–9 | qe 159, 7 mg g−1 | Surface complexation and electrostatic attraction | 68 |
| Zn,Al-LDH/EDTA, Fe3O4/Zn,Al-LDH/EDTA | Co-precipitation; Zn,Al-LDH, EDTA | 7.5 | qe 131.22 mg g−1, 5 min, 25 °C, qe 81.12 mg g−1, 5 min, 25 °C | Ion-exchange and complexation; Langmuir model | This work |
Obviously, the Fe3O4/Zn,Al-LDH/EDTA slightly lower adsorption capacity than the several previously reported adsorbents (Table 4). Most importantly, this adsorption technique with Fe3O4/Zn,Al-LDH/EDTA nanoadsorbent would enable simple magnetic separation and available instrumentation combine with the low sample and reagents consumption as well as pH values corresponding to uranium(VI)-polluted wastewater.
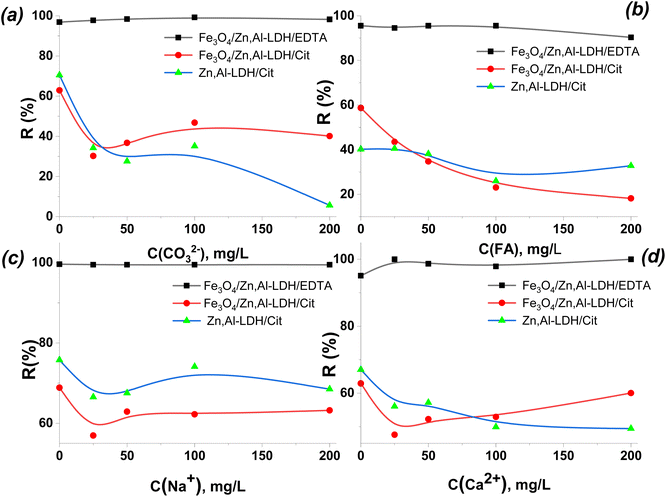
From Fig. 12, as the concentration of co-existing ion increased from 20 mg L−1 to 200 mg L−1, the effect of CO32−/HCO3−, FA, Na+ and Ca2+ ions on U(VI) adsorption was comparatively weak for Fe3O4/Zn,Al-LDH/EDTA, whereas the effect of these ions at using Zn,Al-LDH/Cit and Fe3O4/Zn,Al-LDH/Cit was strong in the wide range of their concentrations (up to 200 mg L−1). This result indicates that Fe3O4/Zn,Al-LDH/EDTA exhibits the high selectivity and its application would be expected to be feasible in most cases of environmental water treatments (with high concentration of CO32−/HCO3−).
The components typical of environmental and wastewaters aren't reduce the efficiency of removal of U(VI) from large volumes of humus-containing aqueous solution by the Fe3O4/Zn,Al-LDH/EDTA. Since, the stability constants of complexes of multiply charged U(VI) ions with EDTA groups are found the largest among one or two valence metal ions, which is the main reason for selectivity of Fe3O4/Zn,Al-LDH/EDTA towards U(VI) (Table S1†).41 Such phenomenon illustrated that the main mechanism for the uptake of radionuclide is complexation with the ligand of the inner surface of LDH space. So, Fe3O4/Zn,Al-LDH/EDTA could employed to efficiently remove U(VI) ions from multicomponent solution.
3.3. Effect of various factors on removal efficiency and adsorption mechanism
Characterization of both nanocomposites (as-prepared and metal-adsorbed) was done using FTIR spectra to identify the functional groups interaction of adsorbed metal ions with organic species on the adsorbents (Fig. 13).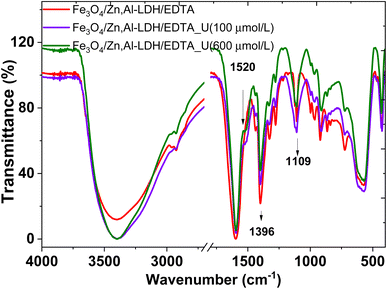 | ||
| Fig. 13 FTIR spectra of initial (Fe3O4/Zn,Al-LDH/EDTA) and resulting samples after adsorption U(VI) from solutions with various concentration. | ||
The new band at ∼1520 cm−1 in the metal-loaded sample (Fig. 13) is assigned to the antisymmetric vibration of C–O–[O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]+. This peak has a significant red-shift compared to the corresponding peak of aqueous UO22+ (∼963 cm−1), indicating the chemical bonding of UO22+ with the intercalated EDTA-groups.
O]+. This peak has a significant red-shift compared to the corresponding peak of aqueous UO22+ (∼963 cm−1), indicating the chemical bonding of UO22+ with the intercalated EDTA-groups.
Based on the above mentioned results, there are possible three main mechanisms for the removal of U(VI) forms (Fig. S3†) on magnetic nanocomposites (Scheme 2), which can be expressed as follows:
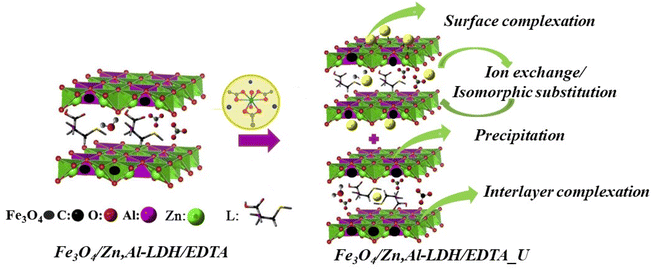 | ||
| Scheme 2 The schematic diagram of the main adsorption mechanisms for U(VI) ions removal using Fe3O4/Zn,Al-LDH/H2EDTA sample. | ||
(1) Surface complexation. It is a kind of complexation by intercalated groups in the inner- and outer-layers of magnetic composites with U(VI) ions (as evidenced by FTIR), which is mainly based on abundant hydroxyl groups of layered structure and intercalated chelating groups:
| Fe3O4/Zn,Al-LDH/H2EDTA + UO22+ → Fe3O4/Zn,Al-LDH/EDTA/UO2 + 2H+ |
| Fe3O4/Zn,Al-LDH/H2EDTA + (UO2)3(OH)5+ → Fe3O4/Zn,Al-LDH/HEDTA/(UO2)3(OH)5 + H+ |
| M–O–H + UO22+ → M–O···UO22+ + H+ |
| M–O–H + UO22+ → M–O–H···UO22+ |
Surface adsorption involves the bonding of U(VI) ion species to the hydroxyl units on the external surface of the LDH. So, interaction of the U(VI) ions species involves a network of hydrogen bonds between the metal ions species and the hydroxyl layers. By the synergism of the intercalation groups and the surface adsorption, a mass of U(VI) will be attracted to the surfaces of LDH and then adsorbed, leading to the high adsorption capacity. In the metal-adsorbed sample, the UO22+ binding with EDTA-groups – forms neutral salts inside the gallery of LDH-support.
(2) Ion-exchange. The Zn(II), Al(III) and Fe(III) surface sites on magnetic composites can react with U(VI) ions via ion exchange:
| M–O–H + UO22+ → M–O–UO2+ + H+ |
| M–O–H + UO2(CO3)22− → M–UO2(CO3)2– + OH− |
| Fe3O4/Zn,Al-LDH/H2EDTA + UO2(CO3)22− → Fe3O4/Zn,Al-LDH/UO2(CO3)2 + H2EDTA2– |
Since the interlayer anions are easily exchangeable, the anions in the interlayer can be exchanged by anions, i.e. so that it makes LDH useful for removal hazardous anionic forms of U(VI) from aqueous solution.
(3) Redox-precipitation. In this type of mechanism, a dissolution of the Fe3O4 loaded on LDH occurs, supplying the sorption system with EDTA groups which have high affinity for U(VI) ions, precipitating and forming thus a new phase UO2, which can be expressed as eqn:
| 2Fe2+ + UO22+ → 2Fe3+ + UO2 |
Therefore, U(VI) adsorption on the samples was mainly corelated to the ion-exchange and surface complexation due to the OH-groups from LDH, and complexation reaction with EDTA-ligands as well as redox reactions of iron(II) ions from magnetite, which indicated that the effective adsorption of Fe3O4/Zn,Al-LDH/EDTA was resulting to the synergetic effect all components of nanocomposite. Further studies are needed to more precisely characterize the detailed adsorption mechanism.
3.4. Analytical performance for real water samples
The effectiveness of the obtained magnetic nanocomposites was studied to water purification in real systems. The environmental and wastewater samples were analyzed using various adsorbent doses as influential parameters that directly affect the removal of pollutants from aqueous solution and cost effectiveness of obtained adsorbents for practical applications (Table 5). The effect of the adsorbent dose on the removal of U(VI) from water samples were studied during of 1 hour.| Adsorbent | Adsorbent dose, g L−1 | Recovery, % | |
|---|---|---|---|
| Environmental water | Wastewater | ||
| Fe3O4/Zn,Al-LDH/EDTA | 1 | 99.4 | 95.8 |
| 6 | 99.8 | 99.5 | |
| Fe3O4/Zn,Al-LDH/Cit | 1 | 28.8 | 10.3 |
| 6 | 40.9 | 22.7 | |
The adsorption percentage of U(VI) at adsorbents dose of 1 g L−1 is 99.4% and 28.8% for Fe3O4/Zn,Al-LDH/EDTA and Fe3O4/Zn,Al-LDH/Cit, respectively. Theoretically,25 the increase in adsorbent dosage results in enhanced percentage removal efficiency, i.e. this may be due to low adsorbent dosage, leading to the dispersal of magnetic nanocomposites into the aqueous solution. The increase in removal percentage of U(VI) from 95.8% to 99.5% into wastewater using Fe3O4/Zn,Al-LDH/EDTA are related to the increase in the from dose of 1 g L−1 to 6 g L−1. In this case, adsorbent active sites were completely exposed, which eases the adsorption capability of U(VI). In this process, the adsorbent surface sites are saturated quickly, leading to high adsorption capacity. Generally, the Fe3O4/Zn,Al-LDH/Cit sample is low effective for purification water even at adsorbent dose of 6 g L−1. Therefore, the optimum adsorbent dose is kept at 6 g L−1 for adsorption experiments.
Based on the high selectivity provided by using Fe3O4/Zn,Al-LDH/EDTA sample the recoveries of U(VI) in the 6 g L−1 ranged from 99.8% for environmental water, 99.5% for wastewater was satisfactory. Therefore, the studied sorption material with magnetic properties is highly efficient, highly selective and promising for the purification (post-purification) of aqueous media, including natural waters and waste water of uranium processing enterprises.
4. Conclusions
In this study, we prepared two magnetic nanocomposites based on magnetite and Zn,Al-layered double hydroxides intercalated with citric (or EDTA) groups and successfully applied their as effective adsorbent materials for the removal of U(VI) ions from various origin aqueous solution. The adsorption experiments were conducted by a batch technique, and the conditions have been optimized to be a pH value of 6.0–8.0, V/m 500 mL g−1 with high uptake time (5 min), which result was superior to the previously reported adsorbents. The maximum adsorption capacity of the Fe3O4/Zn,Al-LDH/EDTA nanocomposite for the uranium(VI) ion was 81.2 mg g−1 at pH 8.0, and high specificity and selectivity for U(VI) when coexisting with cations (such as Na(I) and Ca(II)), anions (HCO3−/CO32−) and dissolved organic motives. The adsorption followed pseudo-second-order kinetics and the equilibrium data of Fe3O4/Zn,Al-LDH/EDTA were well fitted with Langmuir isotherms. Also, the nanocomposite can be easily separated from the solution by an external magnetic field after the adsorption process. It provides a rapid and effective way for removing the nanoadsorbent from aqueous suspension after the adsorption process during environmental pollution clean-up treatment. Considering low-cost adsorbent synthesis environmental safety and high selectivity, as well as the possibility of complete automation of the technological process of extracting U(VI) from large volumes uranium-contaminated waters due to the use of magnetic separation, as well as reduce the cost of treatment and prevent secondary pollution of environmental objects. The obtained results illustrate that Fe3O4/Zn,Al-LDH/EDTA could be a perfect candidate as an adsorbent for the removal of U(VI) from wastewaters.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
Dr Natalia Kobylinska acknowledges the financial support given by the University of Oviedo (Spain) from the Spanish Ministry of Science and Innovation (PID 2020-113558RB-C41). The authors grateful Armed Forces of Ukraine for the service and sacrifice.References
- E. Wilds, Health Phys., 2013, 104, 232–233 CrossRef.
- V. Kashparov, B. Salbu, S. Levchuk, V. Protsak, I. Maloshtan, C. Simonucci, C. Courbet, H. L. Nguyen, N. Sanzharova and V. Zabrotsky, J. Environ. Radioact., 2019, 208–209, 106025 CrossRef CAS PubMed.
- L. S. Keith, O. M. Faroon and B. A. Fowler, Handb. Toxicol. Met., 2007, 881–903 Search PubMed.
- M. O. Barnett, P. M. Jardine, S. C. Brooks and H. M. Selim, Soil Sci. Soc. Am. J., 2000, 64, 908–917 CrossRef CAS.
- R. W. Herschy, Encycl. Earth Sci. Ser., 2012, 876–883 Search PubMed.
- D. Langmuir, Geochim. Cosmochim. Acta, 1978, 42, 547–569 CrossRef CAS.
- J. Li and Y. Zhang, Procedia Environ. Sci., 2012, 13, 1609–1615 CrossRef CAS.
- J. Wang and S. Zhuang, Rev. Environ. Sci. Bio/Technol., 2019, 18, 437–452 CrossRef.
- H. N. Bhatti and S. Hamid, Int. J. Environ. Sci. Technol., 2014, 11, 813–822 CrossRef CAS.
- A. Baeza, A. Salas and F. Legarda, Sci. Total Environ., 2008, 406, 24–34 CrossRef CAS PubMed.
- J. Shen and A. Schäfer, Chemosphere, 2014, 117, 679–691 CrossRef CAS PubMed.
- M. Dulama, M. Iordache and N. Deneanu, Inst. Nucl. Res., 2013, 80–87 Search PubMed.
- I. A. Katsoyiannis and A. I. Zouboulis, Desalin. Water Treat., 2013, 51, 2915–2925 CrossRef CAS.
- D. Rana, T. Matsuura, M. A. Kassim and A. F. Ismail, Desalination, 2013, 321, 77–92 CrossRef CAS.
- Y. Hua, W. Wang, X. Huang, T. Gu, D. Ding, L. Ling and W. xian Zhang, Chemosphere, 2018, 201, 603–611 CrossRef CAS PubMed.
- L. Newsome, K. Morris, D. Trivedi, N. Atherton and J. R. Lloyd, Appl. Geochem., 2014, 51, 55–64 CrossRef CAS.
- A. Liu, J. Liu, B. Pan and W. X. Zhang, RSC Adv., 2014, 4, 57377–57382 RSC.
- H. Paar, A. S. Ruhl and M. Jekel, Water Res., 2015, 68, 731–739 CrossRef CAS PubMed.
- R. A. Crane, M. Dickinson, I. C. Popescu and T. B. Scott, Water Res., 2011, 45, 2931–2942 CrossRef CAS PubMed.
- M. A. Abu-Dalo, S. Nevostrueva and M. Hernandez, Sci. Rep., 2020, 10, 1–13 CrossRef PubMed.
- N. Combernoux, L. Schrive, V. Labed, Y. Wyart, E. Carretier and P. Moulin, Water Res., 2017, 123, 311–320 CrossRef CAS PubMed.
- X. Yi, Z. Xu, Y. Liu, X. Guo, M. Ou and X. Xu, RSC Adv., 2017, 7, 6278–6287 RSC.
- V. V. Kusumkar, M. Galamboš, E. Viglašová, M. Daňo and J. Šmelková, Materials, 2021, 14, 1–29 CrossRef PubMed.
- S. J. Coleman, P. R. Coronado, R. S. Maxwell and J. G. Reynolds, Environ. Sci. Technol., 2003, 37, 2286–2290 CrossRef CAS PubMed.
- M. Khorshidi, S. Asadpour, N. Sarmast and M. Dinari, J. Mol. Liq., 2022, 348, 118399 CrossRef CAS.
- W. Linghu, H. Yang, Y. Sun, G. Sheng and Y. Huang, ACS Sustainable Chem. Eng., 2017, 1, 1–31 Search PubMed.
- M. Zubair, M. Daud, G. McKay, F. Shehzad and M. A. Al-Harthi, Appl. Clay Sci., 2017, 143, 279–292 CrossRef CAS.
- M. Grafe, K. G. Bunney, S. Cumberland and G. Douglas, ACS Omega, 2017, 2, 7112–7119 CrossRef CAS PubMed.
- S. Ma, L. Huang, L. Ma, Y. Shim, S. M. Islam, P. Wang, L. D. Zhao, S. Wang, G. Sun, X. Yang and M. G. Kanatzidis, J. Am. Chem. Soc., 2015, 137, 3670–3677 CrossRef CAS PubMed.
- Y. Cai, Y. Ma, J. Feng, M. Zhu, X. Wang, Z. Lv, M. Fang, X. Tan and X. Wang, Chem. Eng. J., 2020, 402, 125510 CrossRef CAS.
- X. Wang, Y. Cai, T. Han, M. Fang, K. Chen and X. Tan, J. Hazard. Mater., 2020, 399, 123081 CrossRef CAS PubMed.
- S. M. Husnain, W. Um, Woojin-Lee and Y. S. Chang, RSC Adv., 2018, 8, 2521–2540 RSC.
- D. Das, M. K. Sureshkumar, S. Koley, N. Mithal and C. G. S. Pillai, J. Radioanal. Nucl. Chem., 2010, 285, 447–454 CrossRef CAS.
- M. J. O'Hara, J. C. Carter, C. L. Warner, M. G. Warner and R. S. Addleman, RSC Adv., 2016, 6, 105239–105251 RSC.
- W. Li, L. D. Troyer, S. S. Lee, J. Wu, C. Kim, B. J. Lafferty, J. G. Catalano and J. D. Fortner, ACS Appl. Mater. Interfaces, 2017, 9, 13163–13172 CrossRef CAS PubMed.
- E. Calì, J. Qi, O. Preedy, S. Chen, D. Boldrin, W. R. Branford, L. Vandeperre and M. P. Ryan, J. Mater. Chem. A, 2018, 6, 3063–3073 RSC.
- X. M. Song, L. C. Tan, H. Y. Ma, Y. Guo, L. Zhu, X. Q. Yi, J. Y. Gao, R. J. Yang and Q. Dong, Dalton Trans., 2017, 46, 3347–3352 RSC.
- L. N. Puzyrnaya, G. N. Pshinko, B. Yatsik, V. Y. Zub and A. A. Kosorukov, Radiochemistry, 2020, 62, 50–61 CrossRef CAS.
- X. Zhang, L. Ji, J. Wang, R. Li, Q. Liu, M. Zhang and L. Liu, Colloids Surf., A, 2012, 414, 220–227 CrossRef CAS.
- N. G. Kobylinskaya, E. A. Khainakova, M. E. Diaz-Garcia and V. N. Zaitsev, Prot. Met. Phys. Chem. Surf., 2017, 53, 675–684 CrossRef CAS.
- S. Su, Q. Liu, J. Liu, H. Zhang, R. Li, X. Jing and J. Wang, Sci. Rep., 2017, 7, 1–9 CrossRef PubMed.
- L. A. Kaplan, Limnol. Oceanogr., 1992, 37, 1119–1125 CrossRef CAS.
- P. I. Ravikovitch, S. C. O'Domhnaill, A. V. Neimark, F. Schiith and K. K. Unger, Langmuir, 1995, 11, 4765–4772 CrossRef CAS.
- J. Park and J. R. Regalbuto, J. Colloid Interface Sci., 1995, 175, 239–252 CrossRef CAS.
- A. L. Patterson, Phys. Rev., 1939, 56, 978–982 CrossRef CAS.
- W. H. Bragg and W. L. Bragg, Proc. R. Soc. London, Ser. A, 1913, 88, 428–438 CAS.
- D. E. Bugaris and J. A. Ibers, Dalton Trans., 2010, 39, 5949–5964 RSC.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- M. Kosmulski, Adv. Colloid Interface Sci., 2016, 238, 1–61 CrossRef CAS PubMed.
- S. Azizian, J. Colloid Interface Sci., 2004, 276, 47–52 CrossRef CAS PubMed.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- W. Rudzinski and W. Plazinski, Langmuir, 2008, 24, 6738–6744 CrossRef CAS PubMed.
- C. H. Giles, D. Smith and A. Huitson, J. Colloid Interface Sci., 1974, 47, 755–765 CrossRef CAS.
- W. Verweij and J. P. Simonin, J. Solution Chem., 2020, 49, 1319–1327 CrossRef.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- H. Freundlich, Z. Physiol. Chem., 1907, 57U, 385–470 CrossRef.
- A. Rezaei, H. Khani, M. Masteri-Farahani and M. K. Rofouei, Anal. Methods, 2012, 4, 4107–4114 RSC.
- X. T. Chen, L. F. He, R. Z. Liu, C. Zhang, B. Liu and Y. P. Tang, RSC Adv., 2015, 5, 56658–56665 RSC.
- L. Tan, J. Wang, Q. Liu, Y. Sun, X. Jing, L. Liu, J. Liu and D. Song, New J. Chem., 2015, 39, 868–876 RSC.
- Q. Liu, W. Li, W. Zhao, L. Tan, X. Jing, J. Liu, D. Song, H. Zhang, R. Li, L. Liu and J. Wang, RSC Adv., 2016, 6, 22179–22186 RSC.
- S. Long Yu, Y. Dai, X. Hong Cao, Z. Bin Zhang, Y. Hai Liu, H. Jie Ma, S. Jin Xiao, Z. Jun Lai, H. Jun Chen, Z. Yang Zheng and Z. Gao Le, J. Radioanal. Nucl. Chem., 2016, 310, 651–660 CrossRef.
- X. Zhang, J. Wang, R. Li, Q. Dai, R. Gao, Q. Liu and M. Zhang, Ind. Eng. Chem. Res., 2013, 52, 10152–10159 CrossRef CAS.
- L. Tan, J. Wang, Q. Liu, Y. Sun, H. Zhang, Y. Wang, X. Jing, J. Liu and D. Song, Colloids Surf., A, 2015, 466, 85–91 CrossRef CAS.
- L. Tan, Y. Wang, Q. Liu, J. Wang, X. Jing, L. Liu, J. Liu and D. Song, Chem. Eng. J., 2015, 259, 752–760 CrossRef CAS.
- Y. Zhao, J. Li, L. Zhao, S. Zhang, Y. Huang, X. Wu and X. Wang, Chem. Eng. J., 2014, 235, 275–283 CrossRef CAS.
- X. Zhang, J. Wang, R. Li, Q. Dai and L. Liu, New J. Chem., 2013, 37, 3914–3919 RSC.
- S. Li, H. Bai, J. Wang, X. Jing, Q. Liu, M. Zhang, R. Chen, L. Liu and C. Jiao, Chem. Eng. J., 2012, 193–194, 372–380 CrossRef CAS.
- S. Yu, J. Wang, S. Song, K. Sun, J. Li, X. Wang, Z. Chen and X. Wang, Sci. China: Chem., 2017, 60, 415–422 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD patterns, scanning electron microscopy images, effect of pH, kinetic and isotherm fitting plots for the adsorption of uranium(VI) onto obtained samples, equilibrium constants of U(VI) in solution. See DOI: https://doi.org/10.1039/d2ra05503a |
| This journal is © The Royal Society of Chemistry 2022 |