Open Access Article
Open Access ArticleMorphology-controlled synthesis of MoS2 using citric acid as a complexing agent and self-assembly inducer for high electrochemical performance†
Mingmin Bai *a,
Weixin Lib,
Hu Yanga,
Weixia Donga,
Qinyu Wanga and
Qibing Changa
*a,
Weixin Lib,
Hu Yanga,
Weixia Donga,
Qinyu Wanga and
Qibing Changa
aSchool of Materials Science and Engineering, Jingdezhen Ceramic University, Jingdezhen, 333403, PR China. E-mail: bellebai2010@126.com
bDepartment of Humanities, Jingdezhen University, Jingdezhen, 333499, PR China
First published on 5th October 2022
Abstract
Two-dimensional MoS2 with a controllable morphology was prepared via a simple one-step hydrothermal method. Citric acid was used as a complexing agent and self-assembly inducer. The morphology of MoS2 changed from clusters to nanosheets, and, eventually, to stacked nanorods. A formation mechanism is proposed for the observed evolution of the morphology. The nanosheet structure presents a relatively large specific surface area, more exposed active sites and greater 1T phase content compared to the other morphologies. The electrochemical performance tests show that the MoS2 nanosheets exhibit excellent electrochemical behavior. Their specific capacitance is 320.5 F g−1, and their capacitance retention is up to 95% after 5000 cycles at 5 mA cm−2. This work provides a feasible approach for changing the morphology of MoS2 for high efficiency electrode materials for supercapacitors.
Introduction
Supercapacitors have attracted intense attention due to their excellent attributes, such as high power density, fast charge/discharge rates, and higher magnitudes of energy density. Supercapacitors are considered to be the best option for storing renewable energy.1–3 Suitable electrode materials are important for achieving high-performance supercapacitors. To date, a number of materials have been developed for use as electrode materials in supercapacitors.4–8MoS2 is a common electrode material in supercapacitors. It can provide two-dimensional permeable channels for ion adsorption and transport, which is attributed to weak interlayer van der Waals interactions between the individual molecular layers.9 In application, the influence of the morphology of MoS2 on the resulting performance cannot be ignored. In the past decades, various types of MoS2 nanostructures, including nanosheets,10 quantum dots,11 nanoflowers,12 and nanomeshes,13 have been fabricated. Among these nanostructures, layered MoS2 nanosheets are expected to act as excellent electrode materials due to their significantly enlarged specific surface areas.
Many works have reported attempts to prepare nanosheet MoS2. Alkali-metal intercalation–exfoliation14 and CVD15 are considered to be effective preparation methods for the synthesis of nanosheet MoS2. However, these approaches are complex and difficult to use in the fabrication of large-scale devices because of their low yields. The hydrothermal method is a common method for preparing MoS2, but the MoS2 prepared by this method is clustered and poorly dispersed, which reduces the specific surface area and active sites of MoS2.16,17 Therefore, surfactants or template agents are selectively added to the solvent to improve the agglomeration or change the morphology of MoS2.
Citric acid is a common organic acid and is often used as complexing agent or capping agent.18 For example, F. A. Deorsola et al.19 synthesized MoS2 via a hydrothermal method and added citric acid as a reductant/complexing agent to obtain MoS2 nanoparticles for lubricant applications. X. D. Zheng et al.20 prepared MoS2 with flower-like hollow microsphere and blocky structures with citric acid as a surfactant.
In addition to its morphology, the phase composition of MoS2 is also crucial to its electrochemical performance. The metastable trigonal phase (1T) of MoS2 presents excellent conductivity and electrochemical properties.21 In the process of preparing MoS2 via a hydrothermal method as mentioned above, it has been proved that MoS2 with a high 1T phase content can be prepared without introducing any surfactant.22 However, the introduction of a surfactant will affect the crystallinity of the MoS2, and the content of the 1T phase will be reduced. Determining how to manage the trade-off between nanosheet morphology and high 1T phase content is an area that requires attention.
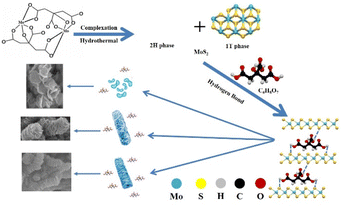
Herein, we report a novel approach for the controlled synthesis of high-1T-phase MoS2 clusters, nanosheets and nanorods with citric acid as a complexing agent and self-assembly inducer. It involves an easy and economical one-pot hydrothermal method for the preparation of morphology-controlled MoS2. The experimental results show that the MoS2 nanosheets have the best electrochemical performance because of their sufficient exposed active sites. The specific capacitance of the MoS2 nanosheets is 320.5 F g−1, and their capacitance retention is up to 95% after 5000 cycles at 5 mA cm−2. This work may attract the attention of peers regarding the role of citric acid in the synthesis of nano-powder and provide an idea for the fabrication of MoS2 nanomaterials.
Experimental
Synthesis of different morphologies of MoS2
MoS2 was synthesized via a one-step hydrothermal method using ammonium molybdate ((NH4)6Mo7O24·4H2O) and thiourea as the molybdenum and sulfur sources. First, 2.472 g of ammonium molybdate and 2.394 g thiourea were dissolved in 40 mL deionized water, and different amounts of citric acid (0, 0.005, 0.015, 0.02, and 0.03 mol, respectively) were added to the transparent solution. After 0.5 h of stirring, the transparent solution was transferred to a 100 mL Teflon-lined reactor and reacted at 200 °C for 24 h. After being cooled to room temperature, the black products were vacuum-filtered and washed with deionized water and ethanol. Finally, the black products were dried at 70 °C for 5 h and labelled as MoS2-CA(No.), in which CA means citric acid and No. is the number of moles of citric acid. For example, MoS2-CA(0.02) means the sample prepared using 0.02 mol citric acid.Characterization
The XRD data of the MoS2 samples were collected using a Rigaku D/max-(β) X-ray diffractometer with a graphite monochromator and Cu Kα radiation (λ = 0.15418 nm) with a scanning range (2θ) of 5–80°. The microstructure and morphology of the samples were observed using a scanning electronic microscope (SEM, JEOM-JMS-6700F, Japan) and a transmission electron microscope (TEM) as well as high resolution TEM (HRTEM) (Titan G260-300). The Raman spectra of the samples were recorded using a Raman spectrometer (HR800, HoribaJobinYvon). The X-ray photoelectron spectroscopy tests were performed using a Thermo Scientific K-Alpha equipped with PerkinElmer instruments using an Al Kα monochromatic X-ray source. The photoelectron spectra were calibrated according to the binding energy of C1s at 284.5 eV. The specific surface areas of the MoS2 samples were determined using the Brunauer–Emmett–Teller (BET) method, and the pore sizes were determined with the Barrett–Joyner–Halenda (BJH) method using the nitrogen desorption branches of the isotherms measured at 77 K (Micromeritics TriStar II 3020). Infrared spectra of the samples were recorded using a Nicolet Nexus 470 FT-IR spectrometer.The electrochemical performances of the MoS2 samples were tested using a three-electrode workstation (CHI660E, Shanghai Chenhua Instruments) at room temperature. 80 wt% MoS2, 10 wt% acetylene black and 10 wt% polyvinylidene difluoride (PVDF, Aldrich) were mixed uniformly and spread onto a nickel foam substrate with a size of 1.5 × 1.5 cm to prepare the working electrodes. The active materials loaded on the substrate weighed about 1–1.5 mg per cm2 per electrode. A platinum plate was used as a counter electrode and a saturated calomel electrode (SCE) as a reference electrode. 3 M KOH was chosen as the aqueous electrolyte solution. The CV curves were measured between −1.0 V and −0.3 V (vs. SCE). The galvanostatic charge/discharge (GCD) curves were recorded at 5 mA cm−2, 10 mA cm−2, 30 mA cm−2 and 50 mA cm−2.
Results and discussion
In this work, different morphologies of MoS2 were obtained by adjusting the amount of citric acid. Fig. 1 shows the SEM images of the samples synthesized with different mole contents of citric acid. As presented in Fig. 1a, MoS2-CA(0) without citric acid shows a flower-like morphology, as has been reported elsewhere.23,24 When the citric acid content is 0.005 mol, the resulting MoS2-CA(0.005) is no longer clustered and forms MoS2 nanosheets (Fig. 1b). The size of the MoS2 nanosheets is about 250 nm. As the citric acid content is increased to 0.015 mol, the resulting MoS2-CA(0.015) nanosheets show orderly stacking and begin to form self-assembled nanorods (Fig. 1c); the nanosheets of MoS2 are approximately 150 nm. With further increasing the citric acid content, the order of the MoS2 nanosheet stacking gradually increases (Fig. 1d and e). As presented in Fig. S1,† a hole is observed in the cross section of some MoS2-CA(0.02), and hollow nanorod structure is observed. The size of the nanosheets is about 100–150 nm. Fig. S2† shows that the MoS2-CA(0.03) nanorods are composed of small nanoparticles, and the size of the nanoparticles decreases to 80 nm. This demonstrates that the addition of citric acid will induce directional self-arrangement of MoS2 and reduce its size from nanosheets to nanoparticles. | ||
| Fig. 1 SEM images of (a) MoS2-CA(0), (b) MoS2-CA(0.005), (c) MoS2-CA(0.015), (d) MoS2-CA(0.02), and (e) MoS2-CA(0.03). | ||
Fig. 2 shows the TEM images, corresponding SAED patterns, and HRTEM images of the MoS2-CA samples. Fig. 2a reveals that the curved nanosheets of MoS2-CA(0) stacked into a flower-like structure. The HRTEM image (Fig. 2b) shows that the interlayer spacings are about 0.9 nm and 0.24 nm, corresponding to (002) and (100).25,26 It can be seen that the 1T phase coexists with the 2H phase in a basal plane, suggesting atomic lateral heterostructures in MoS2.27 The diffraction rings in the selected area electron diffraction patterns (Fig. 2c) correspond to the (100), (103), and (110) planes,25 demonstrating that polycrystalline MoS2 was obtained. Fig. 2d shows that the MoS2-CA(0.005) sample exhibits irregular nanosheets. From the HRTEM image of MoS2-CA(0.005) (Fig. 2e), the interlayer spacing of (002) is about 0.9 nm, and coexistence of the 1T phase and 2H phase can also be observed. Fig. 2f reveals that the MoS2-CA(0.015) nanosheets stack regularly into rods, and that certain gaps can be observed between the nanosheets, which present a loose stacking. For a CA content of 0.02 mol, a hollow nanorod of MoS2 was selected; the TEM image shows a hollow rod that consists of stacked circular nanosheets (Fig. 2g). The SAED pattern (Fig. 2h) reveals distinct diffraction rings and/or bright ambiguous areas, indicating the existence of both polycrystalline and amorphous components in the sample.28 This demonstrates that increasing the citric acid content makes MoS2 tend to be amorphous. Fig. S3† reveals the coexistence of the 1T and 2H phases in MoS2-CA(0.02), which proves that citric acid does not change the phase of MoS2. Fig. 2i reveals a regular dense nanorod structure for MoS2-CA(0.03), which corresponds with the SEM image (Fig. 1e).
Fig. 3a presents the XRD patterns of all the samples. As depicted in Fig. 3a, compared with the standard card no. 73-1539, the typical (002) peaks are located at 9.2°, indicating an interlayer expansion. The interlayer spacing calculated using the Scherrer equation is about 0.95 nm, which is close to the observed value in the HRTEM images (Fig. 2b and e), and represents an enlargement of about 0.33 nm compared to the theoretical value of 0.62 nm. The expansion should be ascribed to the intercalation of ammonium ions (NH4+) with a radius of 0.35 nm.29–31 The ammonium ions should come from the raw material (NH4)6Mo7O24. The intensity of the diffraction peaks decreases with the addition of CA, especially for MoS2-CA(0.005) and MoS2-CA(0.015). This result illustrates that the citric acid has a negative effect on the crystallinity of MoS2, which can be proven by the SAED pattern of MoS2-CA(0.02) (Fig. 2h).
 | ||
| Fig. 3 (a) XRD patterns of MoS2-CA, (b) FT-IR spectrum of MoS2-CA, and (c) Raman spectra of MoS2-CA. | ||
Fig. 3b presents the FTIR spectra of all the MoS2-CA samples. The vibrational modes at ∼3138 and 1670 cm−1 were assigned to the stretching vibration and bending vibration of H2O absorbed on the MoS2 nanosheets.32,33 The peaks observed at 1595 cm−1 were attributed to the presence of COO.34,35 The vibrational peaks at 1403 cm−1 should be assigned to the ammonium group.29,36 Furthermore, the vibration modes in the region 1203–1031 cm−1 were attributed to the stretching modes of C–OH.34,35 Additionally, the peaks at 912 and 760 cm−1 could be attributed to the Mo–O stretching vibration in MoO3, illustrating that part of the MoS2 was oxidized.37,38 The weak peak at 455 cm−1 corresponds to the characteristic vibrational mode of Mo–S in MoS2.33,38 The amount of citric acid did not affect the functional groups on the surface of MoS2.
The Raman spectra of MoS2 are shown in Fig. 3c. It can be observed that there are five peaks at 405, 377, 335, 284 and 236 cm−1. The characteristic peaks at 284 cm−1 (E1g), 377 cm−1 (E12g) and 405 cm−1 (A1g) correspond to 2H MoS2.39,40 Additionally, the vibration peaks emerging at 236 cm−1 (J2) and 335 cm−1 (J3) are attributed to the superlattice distortion in the basal plane of the 1T phase of MoS2,41 suggesting the coexistence of the 1T phase and 2H phase in all samples. This proved that MoS2 with the 1T phase can be synthesized via the simple one-step hydrothermal method in this paper.
Energy-dispersive X-ray spectroscopy (EDX) mappings of MoS2-CA(0.02) were taken from the area shown in Fig. 4, and illustrate the uniform distribution of Mo, S and N in MoS2. The element N is considered to be derived from NH4+.
The phase identification of the synthesized MoS2-CA(0) and MoS2-CA(0.02) was further studied by X-ray photoelectron spectroscopy. The two polymorphs of MoS2 can be identified from the XPS of the Mo 3d and S 2p regions, as shown in Fig. 5. As shown in Fig. 5a and d, there are four peaks at 225.1, 228, 231, and 235.5 eV. The peak at 235.5 eV is due to the Mo 3d5/2 of Mo6+, which is derived from the oxidation of Mo4+ in the air atmosphere.42 The peak centered at 225.1 eV is due to S 2s in MoS2.16 According to the literature,43–45 the binding energy of 1T-MoS2 is lower than that of 2H-MoS2. The peaks at 232.4 and 228.5 eV represent the binding energies of Mo 3d3/2 and Mo 3d5/2 for 2H-MoS2,30 while the peaks at 231.1 and 227.8 eV correspond to the Mo 3d binding energy of 1T-MoS2.46 Similarly, the S 2p peaks of 1T-MoS2 are located at ∼160.5 and ∼162 eV, corresponding to S 2p3/2 and S 2p1/2, respectively. The corresponding S 2p peaks in 2H-MoS2 are centered at ∼161 and ∼163.3 eV, which are slightly higher energies than those for 1T-MoS2 (Fig. 5b and e).47 The XPS results further demonstrate the co-existence of the 1T phase and 2H phase in MoS2-CA(0) and MoS2-CA(0.02). Based on the deconvolution of the Mo 3d and S 2p regions in the MoS2-CA(0) and MoS2-CA(0.02) spectra, the relative contents of the 1T phase were estimated to be 69.1% and 49.2%, respectively. In this paper, MoS2 with a high content of the 1T phase was prepared via a simple hydrothermal method. From the estimated data, the addition of citric acid decreases the content of the 1T phase in MoS2-CA(0.02). Fig. 5c and f show the characteristic peaks of N 1s for MoS2-CA(0) and MoS2-CA(0.02), demonstrating the presence of the element N. The peak located at ∼401 eV should be attributed to the intercalation of NH4+,29,30 which is consistent with the XRD and EDS results.
 | ||
| Fig. 5 XPS spectra: (a) Mo 3d of MoS2-CA(0), (b) S 2p of MoS2-CA(0), (c) N 1s of MoS2-CA(0), (d) Mo 3d of MoS2-CA(0.02), (e) S 2p of MoS2-CA(0.02), and (f) N 1s of MoS2-CA(0.02). | ||
The formation mechanism of the 1T phase of MoS2 could be related to the insertion of NH4+ in the lattice (see Fig. 6). NH4+, which has a large radius, enlarges the interlayer distance (see Fig. 2b and e), leading to a distortion in the 2H phase. This kind of distortion would provide the driving energy for the emergence of the 1T phase. Dezhi Wang et al. discovered this phenomenon when ammonia or ammonium bicarbonate was added to the solution.29,30
Fig. 7 depicts the mechanism by which citric acid controls the morphology of MoS2. The hydrolysis and reaction of (NH4)2MoO4 and CH4N2S resulted in the formation of MoS2. During the hydrothermal process, the reaction is usually rapid, and MoS2 rapidly grows into clusters at the crystal nuclei that are initially formed. When citric acid is added, it acts as a complexing agent, stabilizing the metal species via the formation of metal citrate complexes48 and preventing the interaction between the Mo atoms and S atoms. This effect leads to deterioration of the crystallinity of MoS2, as observed in the XRD patterns, and slowing of the growth rate of MoS2.49,50 The effect of complexation on size is more obvious, and the size of nanosheets decreases from 250 nm to 80 nm (see Fig. 1). At the same time, citric acid acts as a self-assembly inducer. Citric acid has three carboxyl (–COOH) groups, and these three –COOH functional groups may also selectively bind onto the planes of MoS2 and form hydrogen bonding between hydrogen and sulfur.
When the amount of citric acid added is 0.005 mol, there is not enough citric acid to complex with the Mo atoms and prevent the growth of MoS2. Additionally, the hydrogen bonds between citric acid and nanosheets are weak, so MoS2 nanosheets are formed. With increasing the citric acid to 0.015 mol, the size of the obtained nanosheets decreases, and due to the strong hydrogen bonding, the nanosheets self-assemble into nanorods with warping and distortion (Fig. 2f). With further increasing the citric acid, the hydrogen bonding effect becomes more obvious, and MoS2 finally stacks to form a regular rod shape. From the SEM and TEM images, the size of MoS2-CA(0.03) is about 80 nm. Regular nanorods are obtained by the synergistic effect of hindrance and hydrogen bonding.
The adsorption–desorption isotherms and pore size distribution curves of MoS2-CA are shown in Fig. 8. Fig. 8a–e shows that the BET surface areas of MoS2-CA(0), MoS2-CA(0.005), MoS2-CA(0.015), MoS2-CA(0.02) and MoS2-CA(0.03) were 13.81 m2 g−1, 21.89 m2 g−1, 12.63 m2 g−1, 13.05 m2 g−1 and 30.20 m2 g−1, respectively. The BET surface area of MoS2-CA(0.005) was higher than that of MoS2-CA(0); this effect is due to the addition of citric acid, which changes the stacking form of MoS2. MoS2-CA(0.005) exhibits a nanosheet structure and has more surfaces. As the amount of citric acid increased, the surface areas of MoS2-CA(0.015) and MoS2-CA(0.02) decreased. This can be attributed to the increase in hydrogen bonding between MoS2 nanosheets, and the MoS2 gradually stacked from the nanosheet structure into a rod shape. Some MoS2 nanosheets are covered by adjacent MoS2 nanosheets, reducing the specific surface area. MoS2-CA(0.03) has the largest surface area, which is due to the smaller size of the MoS2 nanoparticles. The adsorption–desorption isotherms have H3 hysteresis loops, which is a typical loop with unevenly distributed holes. Therefore, the obtained pore diameter represents slit holes formed by the accumulation of nanosheets. The BJH adsorption average pore diameters of MoS2-CA(0), MoS2-CA(0.005), MoS2-CA(0.015), MoS2-CA(0.02) and MoS2-CA(0.03) were 25.76 nm, 27.52 nm, 25.20 nm, 28.30 nm and 14.78 nm, respectively. The pore size of MoS2-CA(0.015) is lower than that of MoS2-CA(0.005) due to the close packing of MoS2 nanosheets and small slit holes. The MoS2-CA(0.02) has a higher pore size due to the formation of hollow nanorods (see Fig. S1† and 2g). The MoS2-CA(0.03) has the smallest pore size due to the extensive hydrogen bonding between MoS2 nanoparticles and smaller slit holes compared to MoS2-CA(0.015) and MoS2-CA(0.02). The higher level of pore diameter indicates adequate contact between the active sites and electrolyte.
 | ||
| Fig. 8 N2 adsorption–desorption isotherms and pore diameter distributions of the samples: (a) MoS2-CA(0), (b) MoS2-CA(0.005), (c) MoS2-CA(0.015), (d) MoS2-CA(0.02), and (e) MoS2-CA(0.03). | ||
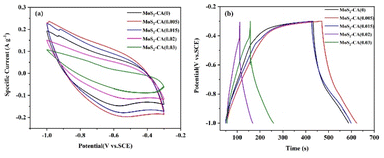
To evaluate the electrochemical performance of the MoS2-CA samples, cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) tests were performed using three-electrode systems. Fig. 9a shows the CV curves of the MoS2-CA samples at a scanning rate of 100 mVs−1 in the potential window from −1.0 to −0.3 V (vs. SCE). The integral area enclosed by the CV curve of the MoS2-CA(0.005) electrode is found to be the largest of all the electrodes, showing that the MoS2-CA(0.005) electrode has the maximum specific capacitance. This is attributed to the fact that, due to the small amount of citric acid, MoS2 exhibits dispersed monolayer sheets, exposing more planes and boundaries able to be in contact with the electrolyte, which facilitates the ion intercalation and deintercalation reactions. The MoS2-CA(0.015) electrode and MoS2-CA(0) electrode have similar CV curve areas. The CV areas of MoS2-CA(0.02) and MoS2-CA(0.03) are smaller than that of MoS2-CA(0). It is noteworthy that due to the large amount of citric acid, the amorphous carbon formed after hydrothermal reaction can no longer be ignored. The generated amorphous carbon reduces the conductivity of MoS2.
Fig. 9b shows the galvanostatic charge–discharge curves of the MoS2-CA electrodes at a current density of 5 mA cm−2. There are noticeable differences among the MoS2-CA electrodes in terms of charge–discharge time. For MoS2-CA(0.005), the time for the charge and discharge is higher than that for MoS2-CA(0) and MoS2-CA(0.015), which is attributed to the high BET surface area and edge defect supplying high electrochemical activity for ion intercalation–deintercalation in electrode. In accordance with the CV results, MoS2-CA(0.02) and MoS2-CA(0.03) show poor charge and discharge performance. The specific capacitance based on GCD can be calculated using eqn (1):51,52
| C = IΔt/mΔV | (1) |
The specific capacitance of composites is an important performance parameter for supercapacitors.53 Fig. 10a shows the specific capacitance of MoS2-CA(0), MoS2-CA(0.005) and MoS2-CA(0.015). When the current density increased, the specific capacitance of all three samples decreased. At all current densities, the specific capacitance of MoS2-CA(0.005) is higher than those of MoS2-CA(0) and MoS2-CA(0.015), which can be attributed to the fact that its dispersed single nanosheets of MoS2 and high content of the 1T phase provide high surface area for ion transition and high conductivity between the electrode and the electrolyte. The EIS measurements of MoS2-CA(0), MoS2-CA(0.005) and MoS2-CA(0.015) were performed in the frequency range of 0.01 Hz to 100 kHz at the open-circuit potential with an amplitude of 5 mV. Fig. 10b shows the Nyquist plots of the three samples. The intercept of the EIS curve at the Z′ axis represents the internal resistance of the electrode and electrolyte. The diameter of the semicircle at high frequency represents the charge transfer resistance. Additionally, the straight line at low frequency relates to the Warburg impedance.51,52,54 The impedance data of MoS2-CA(0.005) were fitted using the software ZView to generate an equivalent electrical circuit (Fig. 10c inset). From Fig. 10b and combined with the fitting results, the internal resistance of MoS2-CA is about 0.3 Ω, which reveals that the MoS2-CA electrode has low internal resistance. The charge transfer resistances of MoS2-CA(0.005) and MoS2-CA(0.015) are approximately 0.1 Ω, which is smaller than that of MoS2-CA(0) (0.28 Ω), which indicates that MoS2-CA(0.005) and MoS2-CA(0.015) exhibit favorable charge transport. In the low-frequency region, the slope of MoS2-CA(0.005) is close to 90°, which suggests the ideal kinetic behavior of ionic diffusion.53,54
The electrochemical properties of MoS2-CA(0.005) were further analysed. Fig. 10d shows the typical CV curves of the MoS2-CA(0.005) electrode at different scan rates ranging from 10 mV s−1 to 100 mV s−1. The shape of the CV curve is quasi-rectangular when the scan rate is low, showing no redox reactions. However, weak redox peaks appear in the range from −0.6 V to −0.7 V. During charging, K+ in the electrolyte can absorb onto the surface of MoS2 and participate in the reverse reaction process to form MoS–SK. The redox reaction can be expressed by eqn (2):
| MoS2 + K+ + e− → MoS−SK | (2) |
Moreover, the high content of the 1T phase in MoS2-CA(0.005) boosts the electrochemical reaction because of the excellent conductivity of the metastable phase. The GCD curves of the MoS2-CA(0.005) electrode are shown in Fig. 10e. The shape of the GCD curves is a quasi-isosceles triangle at high current density (30 mA cm−2 and 50 mA cm−2), which indicates good reversibility during the charge/discharge process. At low current density, the GCD curves exhibit a near-triangle shape, and deviate slightly from linearity, which is more noticeable for the charging process. This behavior is related to the redox reaction as described in eqn (2). To further analyse the performance of MoS2-CA(0.005) for electrochemical energy storage, its cycling stability was investigated via repeated charge/discharge processes at 5 mA cm−2. Fig. 10f shows the cycling capacitance retention of MoS2-CA(0.005) at 5 mA cm−2 over 5000 cycles. The MoS2-CA(0.005) exhibits excellent cycle stability with almost 95% capacitance retention. This is attributed to the nanosheets having a high surface area and allowing abundant adsorption of ions, as well as efficient ion migration and charge transportation.
A comparison of the electrochemical properties of MoS2 electrode materials with different morphologies used to build supercapacitors that have been reported in the literature and in this study was conducted. Compared with those in many previous reports, the MoS2-CA(0.005) nanosheet electrode has high capacitance and long cycle life (Table 1).
| Electrode material | Method of preparation | Specific capacitance | Cycle life (%) | Ref |
|---|---|---|---|---|
| MoS2 nanosheets | Hydrothermal | 129.2 F g−1 @ 1 A g−1 | 85 @ 500 cycles | 55 |
| MoS2 nanostructure | Hydrothermal | 106 F g−1 @ 5 mV s−1 | 93.8 @ 1000 cycles | 56 |
| MoS2 microspheres | Biopolymer-assisted hydrothermal | 145 F g−1 @ 3 A g−1 | 100 @ 500 cycles | 57 |
| 3D-MoS2 nanosheets | Hydrothermal | 683 F g−1 @ 1 A g−1 | 85.1 @ 10000 cycles | 58 |
| Few-layered MoS2 | Ball milling | 14.7 F g−1 @ 0.75 A g−1 | 91.2 @ 5000 cycles | 59 |
| MoS2 nanoworms | Hydrothermal | 138 F g−1 @ 1 A g−1 | 86 @ 5000 cycles | 60 |
| MoS2 nanosheet | Hydrothermal | 320.5 F g−1 @ 5 mA cm−2 | 95 @ 5000 cycles | This work |
Conclusion
In summary, MoS2 with different morphologies was synthesized via a one-step hydrothermal method with the addition of citric acid. Flower-like cluster, nanosheet and nanorod MoS2 were obtained. During the formation of MoS2, citric acid acted as a complexing agent, preventing the growth and deteriorating the crystallinity of MoS2. At the same time, the hydrogen bonding introduced by citric acid promoted the establishment of nanorods. The electrochemical properties of these structures were investigated. MoS2-CA(0.005) exhibited the optimum electrochemical performance with a larger specific capacitance and smaller charge transfer resistance than the other MoS2-CA composites due to its large specific surface area, more abundant exposed active sites and high 1T phase content. MoS2-CA(0.02) and MoS2-CA(0.03) showed poor electrochemical properties due to their low crystallinity and small specific surface area after the hydrothermal reaction with citric acid. For supercapacitors, the specific capacities of MoS2-CA(0), MoS2-CA(0.005), MoS2-CA(0.015), MoS2-CA(0.02) and MoS2-CA(0.03) were 268.3 F g−1, 320.5 F g−1, 261.9 F g−1, 114.8 F g−1 and 112.6 F g−1 at a current density of 5 mA cm−2, respectively. This work will provide a method for the morphology design and performance modification of MoS2 for supercapacitors.Author contributions
Mingmin Bai: Conceptualization, Methodology, Writing – original draft, Data curation. Weixin Li: Software, Validation, Formal analysis, Visualization. Hu Yang: Investigation, Methodology, Software. Weixia Dong: Writing – review & editing. Qinyu Wang: Investigation, Methodology. Qibing Chang: Writing – review & editing.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the Education Project of Jiangxi Province in China [Grant No. GJJ201301, GJJ211309], National Natural Science Foundation of China [Grant No. 51772136] and 2021 Open Project “State Key Laboratory of Silicon Materials” [Grant No. SKL2021-04].Notes and references
- Z. Song, L. Miao, L. Li, D. Zhu, L. Gan and M. Liu, Carbon, 2021, 180, 135–145 CrossRef CAS.
- Q. Yang, M. Liang, M. Mansuer, C. M. Hu, Y. K. Lv, L. H. Gan and M. X. Liu, ACS Appl. Mater. Interfaces, 2022, 14, 33328–33339 CrossRef.
- Z. Y. Song, L. Miao, H. Duan, L. Ruhlmann, Y. K. Lv, D. Z. Zhu, L. C. Li, L. H. Gan and M. X. Liu, Angew. Chem., Int. Ed., 2022, 61, e202208821 CAS.
- R. Abazari, S. Sanati, A. Morsali and D. P. Dubal, J. Mater. Chem. A, 2021, 9, 11001 RSC.
- I. S. Imaduddin, S. R. Majid, S. B. Aziz, I. Brevik, S. N. F. Yusuf, M. A. Brza, S. R. Saed and M. F. Z. A. Kadir, Materials, 2021, 14, 573 CrossRef CAS PubMed.
- F. Azimov, J. Kim, S. M. Choi and H. M. Jung, Nanomaterials, 2021, 11, 1557 CrossRef CAS PubMed.
- G. Singh, R. Bahadur, A. M. Ruban, J. M. Davidraj, D. Su and A. Vinu, Green Chem., 2021, 23, 5571 RSC.
- Q. Liu, A. R Zhao, X. X. He, Q. Li, J. Sun, Z. B. Lei and Z. H. Liu, Adv. Funct. Mater., 2021, 31, 2010944 CrossRef CAS.
- O. Samy, S. Zeng, M. D. Birowosuto and A. El Moutaouakil, Crystals, 2021, 11, 355 CrossRef CAS.
- D. Voiry, M. Salehi, R. Silva, T. Fujita, M. Chen, T. Asefa, V. B. Shenoy, G. Eda and M. Chhowalla, Nano Lett., 2013, 13, 6222e6227 CrossRef PubMed.
- X. Li, X. Lv, N. Li, J. Wu, Y.-Z. Zheng and X. Tao, Appl. Catal. B Environ., 2019, 243, 76e85 Search PubMed.
- J. M. Wu, W. E. Chang, Y. T. Chang and C. K. Chang, Adv. Mater., 2016, 28, 3718e3725 Search PubMed.
- Y. Li, K. Yin, L. Wang, X. Lu, Y. Zhang, Y. Liu, D. Yan, Y. Song and S. Luo, Appl. Catal. B Environ., 2018, 239, 537e544 Search PubMed.
- X. Fan, P. Xu, Y. C. Li, D. Zhou, Y. Sun, M. A. T. Nguyen, M. Terrones and T. E. Mallouk, J. Am. Chem. Soc., 2016, 138, 5143 CrossRef CAS PubMed.
- J. Jeon, S. K. Jang, S. M. Jeon, G. Yoo, Y. H. Jang, J. H. Park and S. Lee, Nanoscale, 2015, 7, 1688 RSC.
- Y. Shi, Y. Zhou, D. R. Yang, W. X. Xu, C. Wang, F. B. Wang, J. J. Xu, X. H. Xia and H. Y. Chen, J. Am. Chem. Soc., 2017, 139, 15479 CrossRef CAS PubMed.
- M. D. Sharma, C. Mahala, B. Modak, S. Pande and M. Basu, Langmuir, 2021, 4847 CrossRef CAS PubMed.
- N. Reddy and Y. Yang, Food Chem., 2010, 118, 702–711 CrossRef CAS.
- F. A. Deorsola, N. Russo, G. A. Blengini and D. Fino, Chem. Eng. J., 2012, 195–196, 1–6 CrossRef CAS.
- X. Zheng, Y. Zhu, Y. Sun and J. Qingjie Jiao, J. Power Sources, 2018, 395, 318–327 CrossRef CAS.
- L. N. Wang, X. Wu, F. T. Wang, X. Chen, J. Xu and K. J. Huang, J. Colloid Interface Sci., 2021, 583, 579–585 CrossRef CAS PubMed.
- C. Tan, Z. Luo, A. Chaturvedi, Y. Cai, Y. Du, Y. Gong, Y. Huang, Z. Lai, X. Zhang, L. Zheng, X. Qi and M. Hao, Adv. Mater., 2018, 1705509 CrossRef PubMed.
- Z. Feng, P. Yang, G. Wen, H. Li, Y. Liu and X. Zhao, Appl. Surf. Sci., 2020, 502, 144129 CrossRef CAS.
- L. Pan, C. Jiao, Y. Liang, J. Xiong, S. Wang, H. Zhu, G. Chen and H. Song, New J. Chem., 2021, 45, 5645 RSC.
- G. Suo, J. Zhang, D. Li, Q. Yu, M. He, L. Feng, X. Hou, Y. Yang, X. Ye, L. Zhang and W. Wang, J. Colloid Interface Sci., 2020, 566, 427–433 CrossRef CAS PubMed.
- H. Xu, J. Yi, X. She, Q. Liu, L. Song, S. Chen, Y. Yang, Y. Song, R. Vajtai, J. Lou, H. Li, S. Yuan, J. Wu and P. M. Ajayan, Appl. Catal., B, 2018, 220, 379 CrossRef CAS.
- W. Liu, C. Luo, S. Zhang, B. Zhang, J. Ma, X. Wang, W. Liu, Z. Li, Q. H. Yang and W. Lv, ACS Nano, 2021, 15, 7491 CrossRef CAS PubMed.
- H. Ren, Z. Xiong, E. Wang, Z. Yuan, Y. Sun, K. Zhu, B. Wang, X. Wang, H. Ding, P. Liu, L. Zhang, J. Wu, S. Fan, X. Li and K. Liu, ACS Nano, 2019, 13, 3106 CrossRef CAS PubMed.
- D. Wang, X. Zhang, S. Bao, Z. Zhang, H. Fei and Z. Wu, J. Mater. Chem. A, 2017, 5, 2681 RSC.
- D. Wang, B. Su, Y. Jiang, L. Li, B. K. Ng, Z. Wu and F. Liu, Chem. Eng. J., 2017, 330, 102 CrossRef CAS.
- J. Dai, Y. Lv, J. Zhang, D. Zhang, H. Xie, C. Guo, A. Zhu, Y. Xu, M. Fan, C. Yuan and L. Dai, J. Colloid Interface Sci., 2021, 590, 591 CrossRef CAS PubMed.
- A. T. Massey, R. Gusain, S. Kumari and O. P. Khatri, Ind. Eng. Chem. Res., 2016, 55, 7124–7131 CrossRef CAS.
- S. Kumari, R. Gusain, N. Kumar and O. P. Khatri, J. Ind. Eng. Chem., 2016, 42, 87 CrossRef CAS.
- A. Santra, M. Kayal, S. Tiwari, D. Jana and B. Ghosh, J. Basic Appl. Eng. Res., 2015, 2, 1309 Search PubMed.
- S. Mishra, P. K. Maurya and A. K. Mishra, Mater. Chem. Phys., 2020, 255, 123551 CrossRef CAS.
- Z. Guo, Q. Ma, Z. Xuan, F. Du and Y. Zhong, RSC Adv., 2016, 6, 16730 RSC.
- L. Ye, W. Guo, Y. Yang, Y. Du and Y. Xie, Chem. Mater., 2007, 19, 6331 CrossRef CAS.
- G. Nagaraju, C. N. Tharamani, G. T. Chandrappa and J. Livage, Nanoscale Res. Lett., 2007, 2, 461 CrossRef CAS PubMed.
- J. Kibsgaard, Z. Chen, B. N. Reinecke and T. F. Jaramillo, Nat. Mater., 2012, 11, 963–969 CrossRef CAS PubMed.
- X. Fan, P. Xu, D. Zhou, Y. Sun, Y. C. Li, M. T. Nguyen, M. Terrones and T. E. Mallouk, Nano Lett., 2015, 15, 5956 CrossRef CAS PubMed.
- F. Ma, Y. Liang, P. Zhou, F. Tong, Z. Wang, P. Wang, Y. Liu, Y. Dai, Z. Zheng and B. Huang, Mater. Chem. Phys., 2020, 244, 122642 CrossRef CAS.
- B. B. Fan, H. N. Fan, X. H. Chen, X. W. Gao, S. Chen, Q. L. Tang, W. B. Luo, Y. Deng, A. P. Hu and W. Hu, ACS Appl. Mater. Interfaces, 2021, 13, 19894 CrossRef CAS PubMed.
- G. Eda, H. Yamaguchi, D. Voiry, T. Fujita, M. Chen and M. Chhowalla, Nano Lett., 2011, 11, 5111 CrossRef CAS PubMed.
- M. Acerce, D. Voiry and M. Chhowalla, Nat. Nanotechnol., 2015, 10, 313–318 CrossRef CAS PubMed.
- Y. Qi, Q. Xu, Y. Wang, B. Yan, Y. Ren and Z. Chen, ACS Nano, 2016, 10, 2903–2909 CrossRef CAS PubMed.
- X. Chen, Z. Wang, Y. Wei, X. Zhang, Q. Zhang, L. Gu, L. Zhang, N. Yang and R. Yu, Agnew. Chem., 2019, 131, 1 Search PubMed.
- H. Zhao, S. Cui, G. Li, N. Li and X. Li, J. Colloid Interface Sci., 2020, 567, 10 CrossRef CAS.
- M. Dong, J. Wang and Y. Sun, Microporous Mesoporous Mater., 2001, 43, 237 CrossRef CAS.
- B. Lertpanyapornchai, T. Yokoi and C. Ngamcharussrivichai, Microporous Mesoporous Mater., 2016, 226, 505 CrossRef CAS.
- R. Rai and M. Molli, Bull. Mater. Sci., 2021, 44, 34 CrossRef CAS.
- S. Moghimian and P. Sangpour, J. Appl. Electrochem., 2020, 50, 71 CrossRef CAS.
- J. Dai, Y. Lv, J. Zhang, D. Zhang, H. Xie, C. Guo, A. Zhu, Y. Xu, M. Fan, C. Yuan and L. Dai, J. Colloid Interface Sci., 2021, 590, 591–600 CrossRef CAS PubMed.
- A. Gigot, M. Fontana, M. Serrapede, M. Castellino, S. Bianco, M. Armandi, B. Bonelli, C. F. Pirri, E. Tresso and P. Rivolo, ACS Appl. Mater. Interfaces, 2016, 8, 32842 CrossRef CAS.
- Z. Qua, M. Shia, H. Wua, Y. Liua, J. Jianga and C. Yan, J. Power Sources, 2019, 410, 179 CrossRef.
- A. Ramadoss, T. Kim, G. S. Kima and S. J. Kim, New J. Chem., 2014, 38, 2379 RSC.
- C. Zhang, J. Ning, B. Wang, H. Guo, X. Feng, X. Shen, Y. Jia, J. Dong, D. Wang, J. Zhang and Y. Hao, Nano Res., 2021, 14, 114 CrossRef CAS.
- A. Laheäär, P. Przygocki, Q. Abbas and F. Béguin, Electrochem. Commun., 2015, 60, 21 CrossRef.
- K. J. Huang, J. Z. Zhang, G. W. Shi and Y. M. Liu, Electrochim. Acta, 2014, 4, 7 Search PubMed.
- K. Krishnamoorthy, G. K. Veerasubramani, S. Radhakrishnan and S. J. Kim, Mater. Res. Bull, 2014, 50, 499–502 CrossRef CAS.
- L. Ma, L. M. Xu, X. P. Zhou and X. Y. Xu, Mater. Lett., 2014, 132, 291–294 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra05351a |
| This journal is © The Royal Society of Chemistry 2022 |