Open Access Article
Open Access ArticleA stable Fe/Co bimetallic modified biochar for ofloxacin removal from water: adsorption behavior and mechanisms†
Jiajie Haoa,
Lieshan Wu *a,
Xiaowei Lua,
Yalin Zenga,
Bing Jiaa,
Tingting Luoa,
Shixing Hea and
Liuling Liangb
*a,
Xiaowei Lua,
Yalin Zenga,
Bing Jiaa,
Tingting Luoa,
Shixing Hea and
Liuling Liangb
aGuangxi University, School of Resources Environment and Materials, Nanning, 530004, China. E-mail: wuls312@163.com
bGuangxi Zhuang Autonomous Region Ecological and Environmental Monitoring Centre, Nanning 530028, China
First published on 4th November 2022
Abstract
In this study, Fe–Co-modified biochar (FMBC) loaded with iron (Fe) and cobalt (Co) bimetals after NaOH activation was prepared by pyrolysis using forestry waste cedar bark as a raw material to study its properties and the adsorption of ofloxacin (OFX). The surface structure and chemical properties were analyzed by BET, SEM-EDS, XRD, XPS, and FTIR characterization, and the results showed that the FMBC possessed a larger specific surface area and abundant surface functional groups. FMBC conformed to pseudo-second-order kinetic and Langmuir isotherm models, indicating that the OFX adsorption process on FMBC was a monolayer adsorption process and controlled by chemisorption. The saturation adsorption capacity of FMBC was 10 times higher than that of cedar bark biochar (BC). In addition, the effects of initial pH and coexisting ions on the adsorption process were investigated, and FMBC showed good adsorption, with the best adsorption capacity at pH = 7. Multiple adsorption mechanisms, including physical and chemical interactions, were involved in the adsorption of OFX by FMBC. TG, metal leaching, different water sources, and VSM tests showed that FMBC had good stability and was easily separated from water. Finally, the reusability performance of FMBC was investigated by various methods, and after five cycles it could still reach 75.78–89.31% of the adsorption capacity before recycling. Therefore, the FMBC synthesized in this study is a promising new adsorbent.
1. Introduction
With the development of scientific technology, antibiotics were widely and heavily used in both medical and animal husbandry and aquaculture, leading to their frequent detection in the environment. Mainly, antibiotics enter the environment through four pathways: the process of antibiotic production, sewage, land application of municipal biosolids, and incorrect disposal of expired drugs.1 Although their concentrations may be low in these environments, residual antibiotics could cause serious environmental and health problems due to cumulative effects, such as disruption of the human immune system, growth and escalation of antibiotic resistance genes, and damage to aquatic ecosystems.2 The UK Antibiotic Crisis Survey analysis stated that approximately 700, thousand people worldwide died each year from drug-resistant bacterial infections and this number was expected to reach 10 million by 2050.3Fluoroquinolone antibiotics, one of the most commonly used antibacterial drugs in the world, as the third largest sales of antibiotics, its global sales in 2009 reached 7.1 billion US dollars.4 Ofloxacin (OFX) was one of the most heavily used fluoroquinolone antibiotics because it had a broad spectrum and high efficiency in treating infectious diseases in urinary and respiratory systems. It was reported that about 60–90% of OFX was not completely metabolized by the human body and was excreted through feces and urine,5 and subsequently discharged into hospital or municipal wastewater, however, wastewater treatment plants could not completely remove it and the remaining OFX could reach surface water, groundwater and be deposited as biosolids.6 Many studies in recent years had reported the presence of OFX in fresh water at concentrations ranging from 0.05 μg L−1 to 17.7 μg L−1,7 and OFX was detected in the effluent of a hospital and a sewage treatment plant in France at an average daily amount of 3.7 g and 0.09 g, respectively.8 Thus OFX also became one of the most frequently detected antibiotics in various water bodies (rivers, domestic sewage, and hospital wastewater).
To remove fluoroquinolone antibiotics from water, the main techniques available were adsorption,9 oxidation,10 photocatalysis,11 and biodegradation.12 Among them, adsorption had the advantages of simple operation, low cost, high efficiency, and environmental friendliness, and had great potential for large-scale applications.13 Common adsorbent materials could be classified as activated carbon, graphene oxide, carbon nanomaterials, biochar, and clay minerals.14–18 Among them, biochar was a solid material rich in carbon, obtained by thermochemical transformation of biomass under anaerobic/oxygen-limited conditions,19 which was mainly applied for soil improvement, waste resource utilization, climate change mitigation, and energy production in environmental management, with good social and financial benefits.20 The conversion of waste biomass into biochar had received increasing attention due to its potential for resource utilization and waste management. Biochar, a new low-cost adsorbent material, was well demonstrated in the scientific literature, possessing many reviews,21,22 which had a large specific surface area, porous structure, rich surface functional groups, and mineral composition.23
The main biomass sources used for biochar were from agriculture (crops, herbaceous plants, and plantations), forestry (logging residues, wood processing by-products), and organic wastes (food industry). The International Renewable Energy Agency estimated that the global biomass supply potential would reach 147 EJ in the next 10 years, of which about 24–43 EJ would come from forestry, and the highest supply potential would amount to about 43–77 EJ per year in Asia and Europe.24 From the perspective of sustainable development and comprehensive resource utilization, recycling forestry waste that was often discarded to decompose naturally or burned by factories could effectively reduce some of the environmental impacts caused by its combustion. Numerous studies conducted showed that bark was one of the most widely used adsorbent materials in studies of pollutant removal from water.25 Bark, as a waste produced in large quantities from forest harvesting and wood processing, was estimated to total no less than 100 million m3 per year in industrially developed countries worldwide, and a total of more than 3 million m3 per year in China.26 Currently, barks were mainly used as an energy source for direct combustion in sawmills and pulp mills, or as compost for horticultural usage,27 while most of them were disposed of in the natural environment. In southern China, cedar trees were widely planted, while the abundant cedar bark resources had been neglected; therefore, the preparation of biochar from cedar bark could reduce its production cost and was of great practical significance to realize the resource utilization of forestry waste.
Since the adsorption capacity of biochar obtained by direct pyrolysis of biomass was low, it was often enhanced by physical and chemical modification of biochar to enrich the functional groups on its surface and increase its specific surface area and porosity. The addition of activation media (such as KOH, and NaOH) during pyrolysis could lead to higher porosity and the addition of metal salts could help to improve the properties of the prepared materials.28,29 In addition, the rapid separation of biochar from water not only reduced the risk of releasing adsorbate after adsorption, but also solved the problem of difficult separation and recovery of powder adsorbent from solution during adsorption. Thus, biochar loaded with magnetic metal elements offered the possibility to achieve separation and recycling.22
As far as we know, there has not been a study to evaluate the adsorption of magnetic biochar prepared from cedar bark as biomass modified with Fe and Co bimetals for the removal of antibiotics from water. Therefore, in this study, Fe–Co-modified biochar (FMBC) was prepared by pyrolysis using low-cost cedar bark as raw material, which was further modified with iron (Fe) and cobalt (Co) bimetals after NaOH activation. The objectives of this study were to: (1) investigate the main adsorption mechanism of FMBC on OFX by characterization, kinetic, and isothermal analysis. (2) The effects of initial pH and coexisting ions on the adsorption capacity of FMBC were systematically investigated. (3) The stability and regeneration performance of FMBC were explored to evaluate its practical application.
2. Materials and methods
2.1 Materials
The cedar bark was collected from a wood processing plant (Guangxi Province, China); sodium hydroxide (purity ≥ 98.0%), ferric nitrate nonahydrate (purity ≥ 98.5%), and cobalt nitrate hexahydrate (purity ≥ 98.5%) were purchased from Guangdong Chemical Reagent Engineering Technology Research and Development Center (Guangdong Province, China), and OFX (purity = 98.0%) was purchased from Shanghai Maclean Biochemical Co. All chemicals in this study were of analytical purity grade, and deionized water (DI water) was used for all solutions.2.2 Preparation of biochars and characterization
A schematic illustration of the preparation process of biochar was shown in Fig. S1.† Cedar bark biochar (BC), alkali modified biochar (MBC) and Fe–Co-modified biochar (FMBC) were all prepared by nitrogen pyrolysis, the typical preparation process and characterization details of samples were provided in the Text S1 and S2.†2.3 Adsorption experiment
Unless otherwise stated, all adsorption experiments were performed by putting 0.05 g of biochar into a 500 mL OFX solution with a mass concentration of 20 mg L−1 in a 500 mL conical flask, shaking it for 12 h in a water bath thermostatic oscillator at 160 rpm and 25 °C. The pH of the OFX solution was adjusted to a range of 3 to 11 using 0.1 mol per L NaOH or HCl solution in the FMBC initial pH experiment. All experiments conducted in this study were carried out three times, and OFX solutions were filtered through 0.45 μm microporous filter membranes before measurement.The detailed experimental methods of adsorption kinetics, isotherms, recycling experiment, and data analysis were summarized in Text S3 and S4.†
3. Results and discussion
3.1 Characterization of biochar
| Biochar | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore diameter (nm) |
|---|---|---|---|
| BC | 45.993 | 0.051 | 4.402 |
| MBC | 416.859 | 0.185 | 3.951 |
| FMBC | 496.423 | 0.498 | 4.012 |
 | ||
| Fig. 2 SEM images of (a) BC, (b) MBC, (c) FMBC at 10 K of magnification, and (d and e) FMBC at 15 K of magnification. | ||
EDS was used to examine the elemental composition of the materials, and Table S1† listed the elemental composition of BC, MBC, and FMBC. The main component of biochars was C. Before modification, BC had a high content of C thus forming a relatively flat carbon surface structure, which could be clearly seen in the SEM image. After NaOH alkali modification, the C content of MBC decreased from 95.83% to 88.04%, which was due to the loss of CO, CO2 and other gases generated at high temperatures through the pores, resulting in the formation of more micropores on the surface of MBC and a lower carbon content. After doping of metals, the percentage of C content was further reduced due to the increase of O content and the presence of Fe and Co elements, so FMBC had the highest O/C ratio, indicating that the modified FMBC had more oxygen-containing functional groups.35 From the EDS spectra of material FMBC in Fig. 3, it was clear that the main components of FMBC were C, O, Ca, Fe and Co, and Fe and Co elements were uniformly distributed on the surface of the material, indicating that the preparation of material FMBC was successful.
Fig. S2(a)–(d)† showed the C 1s and O 1s spectra of BC and FMBC, the peaks in the C 1s spectra corresponded to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O, and O–C
C, C–O, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds.37 The peaks in the O 1s spectra corresponded to C–O and C
O bonds.37 The peaks in the O 1s spectra corresponded to C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively. While the peak fitted at 530.27 eV in the FMBC corresponded to O–M bonds,38 indicating that metal oxides were produced during the modification process, which was consistent with the presence of CoFe2O4 in the above SEM and XRD. Fig. S2(e) and (f)† showed the Fe 2p and Co 2p spectra of FMBC, respectively. The Fe 2p spectra showed peaks corresponded to Fe 2p3 and Fe 2p1, respectively, where the peaks at 711.45 eV and 724.25 eV were attributed to Fe2+, the peaks at 714.87 eV and 727.67 eV were attributed to Fe3+, the peaks at 719.23 eV and 733.03 eV were attributed to the satellite peaks of Fe3+.32,33 The Co 2p spectra showed peaks corresponded to Co 2p3 and Co 2p1, respectively, where the peaks at 781.37 eV and 796.06 eV were attributed to Co3+ and the peaks at 789.45 eV and 804.14 eV were attributed to the satellite peaks of Co2+, the peaks at 786.14 eV and 800.83 eV peaks were attributed to Co2+.39 The X-ray photoelectron spectroscopy (XPS) results further confirmed that the FMBC surface had abundant functional groups, Fe and Co were present in it in various valence forms.
O bonds, respectively. While the peak fitted at 530.27 eV in the FMBC corresponded to O–M bonds,38 indicating that metal oxides were produced during the modification process, which was consistent with the presence of CoFe2O4 in the above SEM and XRD. Fig. S2(e) and (f)† showed the Fe 2p and Co 2p spectra of FMBC, respectively. The Fe 2p spectra showed peaks corresponded to Fe 2p3 and Fe 2p1, respectively, where the peaks at 711.45 eV and 724.25 eV were attributed to Fe2+, the peaks at 714.87 eV and 727.67 eV were attributed to Fe3+, the peaks at 719.23 eV and 733.03 eV were attributed to the satellite peaks of Fe3+.32,33 The Co 2p spectra showed peaks corresponded to Co 2p3 and Co 2p1, respectively, where the peaks at 781.37 eV and 796.06 eV were attributed to Co3+ and the peaks at 789.45 eV and 804.14 eV were attributed to the satellite peaks of Co2+, the peaks at 786.14 eV and 800.83 eV peaks were attributed to Co2+.39 The X-ray photoelectron spectroscopy (XPS) results further confirmed that the FMBC surface had abundant functional groups, Fe and Co were present in it in various valence forms.
3.2 Adsorption kinetics
In this study, the obtained kinetic experimental data were fitted nonlinearly using the pseudo-first-order model, pseudo-second-order model, and intraparticle diffusion model. From Fig. S3(a) and (b),† it was seen that the adsorption amounts of BC, MBC, and FMBC increased rapidly within the initial 5, 10, and 30 min, then gradually slowed down, and reached the equilibrium basically at 20, 180, and 360 min, respectively. The equilibrium adsorption capacity of biochar for OFX was FMBC > MBC > BC. Both the alkali and metal modification improved the ability of BC to adsorb OFX, suggesting that surface oxygen-containing functional groups and small metal particles play an important role in the adsorption of OFX. As shown in Table 2, the pseudo-first-order model was more consistent with the process of OFX removal by BC (R12 = 0.9981 > R22 = 0.8611), indicating that the physical adsorption mechanism dominated the process of OFX adsorption on BC. While the pseudo-second-order model was more consistent with the process of OFX removal by MBC and FMBC (R22 = 0.9728–0.9781 > R12 = 0.9008–0.9539). Furthermore, the qe values of 11.9012 and 60.2306 mg g−1 obtained from the pseudo-second-order model were closer to the experimental values of 12.3862 and 58.0110 mg g−1. These results confirmed that the adsorption of OFX on MBC and FMBC was dominated by a chemisorption mechanism,40 which was consistent with the kinetic behavior of adsorption reported by Yongfei Ma et al.41| Biochar | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|
| qe (mg g−1) | k1 | R12 | qe (mg g−1) | k2 (g mg−1 min−1) | R22 | |
| BC | 3.1348 | 2.2419 | 0.9981 | 3.1509 | 2.9702 | 0.8611 |
| MBC | 11.1028 | 0.0662 | 0.9008 | 11.9012 | 0.0079 | 0.9728 |
| FMBC | 53.9685 | 0.0135 | 0.9539 | 60.2306 | 0.0003 | 0.9781 |
To further identify the possible rate-limiting step and diffusion mechanisms of OFX adsorption onto BC, MBC and FMBC, thus intraparticle diffusion model was adopted to fit the experimental data, the fitted results were in Table S2.† However, it failed to demonstrate a good agreement with the experimental data since the non-linear regression coefficient (R2) of BC was significantly lower compared to pseudo-first-order and pseudo-second-order models. Thus, intraparticle diffusion was unlikely to be the rate controlling step in the sorption of OFX onto BC. As shown in Fig. S3(c),† the adsorption process could be divided into three phases. The first stage was liquid film diffusion,42 in which OFX molecules were transferred from the bulk solution toward the adsorbent surface. The diffusion rate constant (K1 = 2.7939 mg (g min1/2)−1 of OFX adsorption onto FMBC was greater than those of MBC and BC (K1 = 1.9758 mg (g min1/2)−1 and K1 = 0.2795 mg (g min1/2)−1). It suggested that the liquid film diffusion on FMBC was more distinct which was due to its larger specific surface area than the two.43 The second stage was intraparticle diffusion, where OFX molecules diffused from the outer surface to the inner structure of the adsorbent through the rich pores of MBC and FMBC, and then combined with the adsorption sites in mesopores and micropores. The third stage was equilibrium adsorption, which took a long time to reach and was mainly limited by the reduction of the available active sites on adsorbents. C value could be used to evaluate the diffusion resistance. The lower C1 values described that weak liquid diffusion resistance in the initial stage due to the existence of large numbers of unoccupied active sites. After that, the active sites were occupied by OFX, and the migration of OFX was hindered, the larger C value indicated a greater diffusion resistance of OFX in mesopores and microporous relative to the diffusion resistance of liquid membranes. A higher C value illustrated a thicker boundary layer, which demonstrated the main control for OFX adsorption on FMBC was intraparticle diffusion.44 Furthermore, a non-zero C value meant that the curve did not pass through the origin, demonstrating that intraparticle diffusion was not the only rate-limiting step for adsorption and liquid film diffusion controlled the adsorption process simultaneously.45
3.3 Adsorption isotherms
In this study, the obtained experimental data were fitted nonlinearly using Langmuir, Freundlich, and Temkin isotherms model (Fig. S3(d)–(f)†). Langmuir isotherms model was usually to estimate the theoretical maximum adsorption amounts of adsorbents, also it assumed that the adsorption process was monolayer chemisorption and adsorbates were homogeneously distributed on the surface of the adsorbent without horizontal/longitudinal interaction. Freundlich isotherms model described that the adsorption process was a multilayer that occurred on the heterogeneous surface of the adsorbent.46 Temkin isotherm model assumed a uniform distribution of the binding energies and the adsorption free energy as a function of the surface coverage. This model mainly described the adsorption process as chemisorption through electrostatic interaction.47The model parameters in Table S3† showed that BC and MBC were more consistent with the Freundlich model, and the adsorption of OFX was non-homogeneous multilayer adsorption. On the basis of its high correlation coefficient (R2), the Langmuir model suited better the adsorptive mechanism of OFX on FMBC than the Freundlich and Temkin models, indicating that the adsorption behavior observed was largely monolayer adsorption. The KF value in Freundlich represented the strength of the interaction between the adsorbent and the adsorbate, the larger the KF value, the greater the affinity,48 and the order in Table S3† was FMBC > MBC > BC, indicating that FMBC had the highest affinity for OFX. 1/n value was related to the ease of adsorption reaction, all 1/n values were between 0 and 1, further confirming that the adsorption was favorable.49 In this study, the Temkin model for FMBC was also well fitted owing to excellent (R2 = 0.9085), indicating electrostatic interaction to be significantly responsible for the adsorption mechanism of OFX using FMBC.47 The bT values of MBC and FMBC (4.9763 and 13.8008 kJ mol−1) were higher than 4.2 kJ mol−1, demonstrating that chemical adsorption was the key process in the adsorption of OFX on biochars and therefore might have an existence of endothermic adsorption,2 which was consistent with the results of the kinetic analysis described above. According to the Langmuir model, the saturation adsorption capacity (qm) of FMBC was 142.1914 mg g−1 for OFX, which was about 5 and 10 times higher than those of MBC (26.4475 mg g−1) and BC (14.2864 mg g−1), respectively. Studies had shown that the doping of Fe and Co acted synergistically to improve the adsorption capacity of the material.50 Therefore, in this study, Fe/Co doping further enhanced the adsorption capacity of FMBC for OFX on the basis of alkali modification.
3.4 Adsorption process influencing factors
As shown in Fig. 6(a), in terms of coexisting anions, the addition of SO42− had a small enhancement on the adsorption, which may be due to ionic hydrolysis reactions and the formation of a small amount of hydrogen bonds.53,56 The addition of Cl− had little effect on the adsorption capacity, and when the Cl− concentration was 0.01 mol L−1, it promoted the adsorption process, probably because the solubility of OFX was reduced by salting out, and therefore the adsorption capacity was enhanced.56 However, the addition of NO3−, CO32−, and PO43− showed a significant decrease in the adsorption capacity. As the adsorbent preferred to adsorb the less hydrated anions, and the affinity of anions to adsorbent followed the order: OH− < PO43− < CO32− < NO3−,57 which explained why the inhibition of NO3− on OFX adsorption was the most. In terms of coexisting cations (Fig. 6(b)), the addition of Na+ and K+ had a small effect on the adsorption capacity, while the addition of Ca2+ and Mg2+ showed a significant decrease in adsorption with increasing concentration. This was because the priority of cations binding to organic matter usually was expressed as follows: alkaline-earth metal cations (Ca2+ and Mg2+) > alkali-metal cations (Na+ and K+).58 Therefore, during adsorption, Ca2+ and Mg2+ bounded more strongly to the adsorption sites. Besides, the hydrated radius of divalent Ca2+ was the largest,48 and thus the inhibition was the most pronounced.
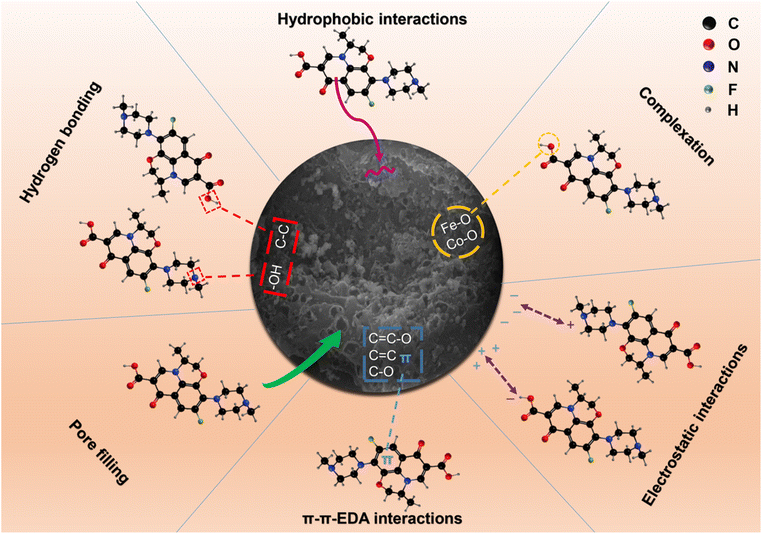
3.5 Adsorption mechanism
Fig. 7 showed that schematic diagram of the adsorption removal mechanism of OFX by FMBC. The discussion on the effect of initial solution pH showed that electrostatic interactions played an important role in the adsorption process.The SEM and BET analyses revealed that after alkali modification and metal modification of FMBC, the specific surface area and total pore volume highly increased, resulting in a significant increase in OFX adsorption capacity. The average pore size and pore size distribution graphs indicated that FMBC was mainly mesoporous in structure, it could be speculated that this porosity structure of FMBC not only afforded more active sites for OFX but also made it easier for smaller OFX molecules to migrate into porous surroundings, causing a pore filling effect.
As shown in Fig. 8, the peaks observed at 3434/3426 cm−1, 2923/2920/2857/2828 cm−1, 1638/1635 cm−1, 1403/1400 cm−1, and 1084/1069 cm−1 were associated with the –OH, C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, and C–O bond stretching vibration,59–62 and obvious sharp peaks M–O at 590 cm−1 were caused by the stretching vibration of the Fe (Co)–O bond.42 It was clear that some changes took place in the characteristic peaks of Fourier transform infrared spectroscopy (FTIR) of FMBC before and after OFX adsorption, a new peak was found at 1446 cm−1 corresponding to a lower intensity C–H bond stretching vibration, which could be attributed to the CH2 group on OFX,63 indicating that OFX was successfully adsorbed by FMBC. The positions of functional groups on FMBC all changed, and the intensity of the characteristic peaks were all weakened, it could be inferred that the corresponding functional groups were involved in the adsorption reaction of OFX. The –OH and C–C bonds on FMBC could act as hydrogen bond donors and form hydrogen bonds with the hydrogen bond acceptors (–OH and –NH2) on the OFX benzene ring. Studies confirmed that hydrogen bonds can promote the adsorption of polar organic compounds on biochar.64 The OFX could serve as a π-electron acceptor due to the F group of the benzene ring's strong electron-withdrawing ability, and the C
C–O, and C–O bond stretching vibration,59–62 and obvious sharp peaks M–O at 590 cm−1 were caused by the stretching vibration of the Fe (Co)–O bond.42 It was clear that some changes took place in the characteristic peaks of Fourier transform infrared spectroscopy (FTIR) of FMBC before and after OFX adsorption, a new peak was found at 1446 cm−1 corresponding to a lower intensity C–H bond stretching vibration, which could be attributed to the CH2 group on OFX,63 indicating that OFX was successfully adsorbed by FMBC. The positions of functional groups on FMBC all changed, and the intensity of the characteristic peaks were all weakened, it could be inferred that the corresponding functional groups were involved in the adsorption reaction of OFX. The –OH and C–C bonds on FMBC could act as hydrogen bond donors and form hydrogen bonds with the hydrogen bond acceptors (–OH and –NH2) on the OFX benzene ring. Studies confirmed that hydrogen bonds can promote the adsorption of polar organic compounds on biochar.64 The OFX could serve as a π-electron acceptor due to the F group of the benzene ring's strong electron-withdrawing ability, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and oxygen-containing functional groups (C
C bond and oxygen-containing functional groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O and C–O) on the surface of FMBC readily acted as a π-electron donor, thus forming π–π EDA interactions and enhancing the adsorption of OFX.43 The peak position of M–O stretching vibrations was also shifted to some extent, indicating that Fe (Co)–O may provide more adsorption sites for OFX adsorption.
C–O and C–O) on the surface of FMBC readily acted as a π-electron donor, thus forming π–π EDA interactions and enhancing the adsorption of OFX.43 The peak position of M–O stretching vibrations was also shifted to some extent, indicating that Fe (Co)–O may provide more adsorption sites for OFX adsorption.
Fig. 9(a)–(d) showed the XPS patterns of C 1s, O 1s, Fe 2p, and Co 2p of FMBC before and after the adsorption of OFX. After adsorption of OFX, for C1s, the peak area ratio of C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C decreased from 66% to 57.09% for a significant decrease, the peak area ratio of O–C
C decreased from 66% to 57.09% for a significant decrease, the peak area ratio of O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O decreased from 8.46% to 7.86%, and the peak area ratio of C–O increased from 24.78% to 35.05% for a significant increase. Probably due to that they combined with the groups in OFX through hydrogen bonding and π–π EDA interactions,65 which was consistent with the FTIR results. For O 1s, the peak area ratio of C
O decreased from 8.46% to 7.86%, and the peak area ratio of C–O increased from 24.78% to 35.05% for a significant increase. Probably due to that they combined with the groups in OFX through hydrogen bonding and π–π EDA interactions,65 which was consistent with the FTIR results. For O 1s, the peak area ratio of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O increased from 29.97% to 31.66%, which might be a result of its serving as electron donors during OFX adsorption.66 The peak area ratio of M–O in O 1s increased from 24.72% to 25.82%, and the peak intensities and binding energies of both Fe 2p and Co 2p also changed, indicating that Fe and Co may be engaged in the adsorption of OFX through surface complexation, which improved the adsorption capacity of FMBC.67
O increased from 29.97% to 31.66%, which might be a result of its serving as electron donors during OFX adsorption.66 The peak area ratio of M–O in O 1s increased from 24.72% to 25.82%, and the peak intensities and binding energies of both Fe 2p and Co 2p also changed, indicating that Fe and Co may be engaged in the adsorption of OFX through surface complexation, which improved the adsorption capacity of FMBC.67
In summary, multiple adsorption mechanisms, among physical and chemical interactions, were involved in the adsorption of OFX by FMBC, including pore filling, electrostatic interactions, hydrogen bonding, hydrophobic interactions, complexation, and π–π EDA interactions.
3.6 Material stability and recycling
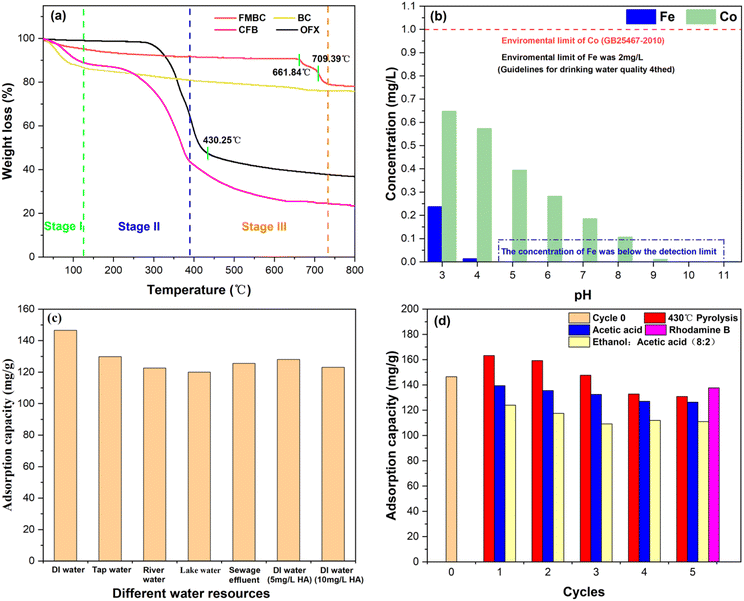
Thermal regeneration was an effective method to achieve desorption. From the above TG analysis, it could be seen that the temperature of thermal regeneration should not be higher than 661.84 °C to ensure that the internal structure of FMBC was not destroyed. From the TG curve of OFX in Fig. 10(a), OFX basically finished the pyrolysis at 430.25 °C, and the weight loss remained basically constant for the subsequent increase in temperature. Therefore, the temperature of thermal regeneration in the recycling experiment was determined to be 430 °C. Solvent regeneration was often used in chemical regeneration, according to the theory of similar mutual solubility, OFX was readily soluble in organic solvents because it was an organic compound.70
From the comparison of the three different desorption methods in Fig. 10(d), it could be shown that thermal desorption at 430 °C was the best. The adsorption capacity in the first three cycles was better than that before the cycle, and the highest adsorption capacity was 163.26 mg g−1, which was 111.44% of the adsorption capacity before the cycle (146.50 mg g−1). This could be that the calcination process promoted further formation of pores inside the FMBC, thus increasing the adsorption active sites. And it gradually decreased from the 4th cycle, which may be due to the disruption of the internal pore structure of FMBC caused by repeated pyrolysis and the occupancy or blockage of the pores by the residual ash from the decomposition of OFX.44 Desorption by acetic acid was superior to the mixture (alcohol: acetic acid), probably due to the physical and chemical properties of OFX, which was soluble in acetic acid and able to desorb from FMBC into the acetic acid solution, and then gradually decreased adsorption may be due to the residual OFX on the surface of the biochar, thus occupying part of the active site and inhibiting the reactivation.71 After five cycles, the three methods were still able to reach 89.31% (130.84 mg g−1), 86.24% (126.34 mg g−1) and 75.78% (111.02 mg g−1) of the adsorption capacity before recycling, respectively. In addition, FMBC showed a high sorption capability of Rhodamine B (137.67 mg g−1) even after 5 cycles in OFX removal, it was higher than the nanosorbent material prepared by Shaswat.72 In a word, it showed that FMBC had high reusability and improved the utilization of biochar.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe, and the hysteresis loops were shown in Fig. S4.† The saturation magnetization (Ms) of FMBC, FMBC-OFX, and FMBC-OFX (5 times desorption at 430 °C) were 14.02, 10.00, and 10.98 emu g−1, remnant magnetization (Mr) were 4.68, 2.55, and 4.85 emu g−1, while coercive force (Hc) were 437.76, 218.88, and 802.55 Oe, respectively. Based on the high Hc of the three, it was shown that FMBC was a hard magnetic property and could maintain its magnetic properties for a long time after magnetization,42 and this study showed that FMBC could maintain high magnetic properties after five times thermal regeneration. Fig. S5† exhibited the magnetic attraction effect of FMBC. The adsorbents were tightly adsorbed towards the bottle wall for 1 minute, verifying again that FMBC had excellent magnetism and could be easily separated, recovered, and reused from the aqueous solution.
000 Oe, and the hysteresis loops were shown in Fig. S4.† The saturation magnetization (Ms) of FMBC, FMBC-OFX, and FMBC-OFX (5 times desorption at 430 °C) were 14.02, 10.00, and 10.98 emu g−1, remnant magnetization (Mr) were 4.68, 2.55, and 4.85 emu g−1, while coercive force (Hc) were 437.76, 218.88, and 802.55 Oe, respectively. Based on the high Hc of the three, it was shown that FMBC was a hard magnetic property and could maintain its magnetic properties for a long time after magnetization,42 and this study showed that FMBC could maintain high magnetic properties after five times thermal regeneration. Fig. S5† exhibited the magnetic attraction effect of FMBC. The adsorbents were tightly adsorbed towards the bottle wall for 1 minute, verifying again that FMBC had excellent magnetism and could be easily separated, recovered, and reused from the aqueous solution.3.7 Comparison with other adsorbents
The capacity of the adsorbent FMBC prepared towards the adsorption of OFX was compared with that of other adsorbents already reported in the literature and the data was shown in Table 3. The comparison of Table 3 showed that the adsorbent FMBC prepared in this study was superior to previous studies on the adsorption performance of OFX compared to those that had been reported on the adsorbent performance of OFX in recent years.4. Conclusions
The residual antibiotics in water could cause potential harm to the environment and human beings, and it was of practical significance to find an efficient, green, and inexpensive adsorbent to remove the antibiotics from water. In this study, FMBC was effective in removing OFX from water. The BET showed that FMBC possessed a higher specific surface area, nearly 11 times higher than BC, and the pore structure was predominantly mesoporous. SEM-EDS observed the successful loading of Fe and Co on the biochar surface, and XRD further confirmed the presence of Fe and Co in the form of metal oxides and alloys. XPS and FTIR analyses showed that FMBC possessed abundant surface functional groups. The saturation adsorption capacity of FMBC (142.19 mg g−1) was 10 times higher than that of BC (14.2864 mg g−1). Pore filling, electrostatic interactions, hydrogen bonding, hydrophobic interactions, complexation, and π–π electron donor–acceptor interactions were possible mechanisms for OFX adsorption by FMBC. In different environmental conditions (coexisting ions, different water resources) and tests (TG, metal leaching, VSM), it showed good thermal stability, the leaching concentrations of both Fe and Co were well below the environmental limits, and the good magnetic properties made it easy to be separated from water. The FMBC was desorbed using thermal regeneration, acetic acid, and (alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid) mixture, and after five cycles it still achieved a 75.78%–89.31% of adsorption capacity before recycling. Consequently, the FMBC synthesized in this study was an efficient, inexpensive, environmentally safe, and reusable adsorbent with great potential for OFX removal application in actual water bodies.
acetic acid) mixture, and after five cycles it still achieved a 75.78%–89.31% of adsorption capacity before recycling. Consequently, the FMBC synthesized in this study was an efficient, inexpensive, environmentally safe, and reusable adsorbent with great potential for OFX removal application in actual water bodies.
Author contributions
Jiajie Hao: investigation, writing – original draft, writing – review & editing. Lieshan Wu: conceptualization, supervision. Xiaowei Lu: methodology. Yalin Zeng: data curation. Bing Jia: data curation. Tingting Luo: validation. Shixing He: visualization. Liuling Liang: resources.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by grant from Science and Technology Research and Development Program of Guangxi Zhuang Autonomous Region of China (no. AB21196039).References
- M. Malakootian, A. Nasiri and H. Mahdizadeh, Water Sci. Technol., 2018, 78, 2158–2170 CrossRef CAS PubMed.
- T. Nguyen, Q. Truong, C. Chen, R. Doong, W. Chen and C. Dong, Bioresour. Technol., 2022, 346, 126351 CrossRef CAS.
- C. Ratia, R. G. Soengas and S. M. Soto, Front. Microbiol., 2022, 13, 846959 CrossRef PubMed.
- Y. Deng, A. Debognies, Q. Zhang, Z. Zhang, Z. Zhou, J. Zhang, L. Sun, T. Lu and H. Qian, Aquat. Toxicol., 2022, 244, 106084 CrossRef CAS.
- H. Zhang, Y. Jia, S. K. Khanal, H. Lu, H. Fang and Q. Zhao, Environ. Sci. Technol., 2018, 52, 6476–6486 CrossRef CAS PubMed.
- P. Kovalakova, L. Cizmas, T. J. McDonald, B. Marsalek, M. Feng and V. K. Sharma, Chemosphere, 2020, 251, 126351 CrossRef CAS PubMed.
- G. Zhang, X. Liu, S. Lu, J. Zhang and W. Wang, Chemosphere, 2020, 242, 125269 CrossRef CAS PubMed.
- Q. Dinh, E. Moreau-Guigon, P. Labadie, F. Alliot, M. Teil, M. Blanchard, J. Eurin and M. Chevreuil, Sci. Total Environ., 2017, 575, 758–766 CrossRef PubMed.
- Y. Ma, P. Li, L. Yang, L. Wu, L. He, F. Gao, X. Qi and Z. Zhang, Ecotoxicol. Environ. Saf., 2020, 196, 110550 CrossRef CAS PubMed.
- R. Kaur, J. P. Kushwaha and N. Singh, J. Electrochem. Soc., 2019, 166, H250–H261 CrossRef CAS.
- R. Kaur, J. P. Kushwaha and N. Singh, Sci. Total Environ., 2019, 677, 84–97 CrossRef CAS PubMed.
- A. S. Maia, P. M. L. Castro and M. E. Tiritan, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1029–1030, 174–183 CrossRef CAS PubMed.
- L. Yan, Y. Liu, Y. Zhang, S. Liu, C. Wang, W. Chen, C. Liu, Z. Chen and Y. Zhang, Bioresour. Technol., 2020, 297, 122381 CrossRef CAS PubMed.
- A. Arabpour, S. Dan and H. Hashemipour, Arab. J. Chem., 2021, 14, 103003 CrossRef CAS.
- E. D. D. Freitas, H. J. D. Almeida and M. G. A. Vieira, Appl. Clay Sci., 2017, 146, 503–509 CrossRef.
- V. K. Gupta, S. Agarwal, A. K. Bharti and H. Sadegh, J. Mol. Liq., 2017, 230, 667–673 CrossRef CAS.
- L. Li, D. Zou, Z. Xiao, X. Zeng, L. Zhang, L. Jiang, A. Wang, D. Ge, G. Zhang and F. Liu, J. Clean. Prod., 2019, 210, 1324–1342 CrossRef CAS.
- Z. Ren, B. Jia, G. Zhang, X. Fu, Z. Wang, P. Wang and L. Lv, Chemosphere, 2021, 262, 127895 CrossRef CAS PubMed.
- K. N. Palansooriya, Y. S. Ok, Y. M. Awad, S. S. Lee, J. Sung, A. Koutsospyros and D. H. Moon, J. Environ. Manage., 2019, 234, 52–64 CrossRef CAS PubMed.
- J. Lehmann and S. Joseph, Biochar for Environmental Management Science & Technology, 2009, vol. 25, pp. 15801–15811 Search PubMed.
- C. E. R. Barquilha and M. C. B. Braga, Bioresour. Technol. Rep., 2021, 15, 100728 CrossRef CAS.
- N. Cheng, B. Wang, P. Wu, X. Lee, Y. Xing, M. Chen and B. Gao, Environ. Pollut., 2021, 273, 116448 CrossRef CAS PubMed.
- X. Zhu, C. Li, J. Li, B. Xie, J. Lü and Y. Li, Bioresour. Technol., 2018, 263, 475–482 CrossRef CAS PubMed.
- E. Butnaru, D. Pamfil, E. Stoleru and M. Brebu, Biomass Bioenergy, 2022, 159, 106413 CrossRef CAS.
- J. O. Ighalo and A. G. Adeniyi, Journal of Water Process Engineering, 2020, 35, 101228 CrossRef.
- S. Liang, PhD thesis, Central South University, 2012.
- S. Feng, S. Cheng, Z. Yuan, M. Leitch and C. C. Xu, Renewable Sustainable Energy Rev., 2013, 26, 560–578 CrossRef CAS.
- X. Zhang, Y. Li, M. Wu, Y. Pang, Z. Hao, M. Hu, R. Qiu and Z. Chen, Bioresour. Technol., 2021, 320, 124264 CrossRef CAS PubMed.
- J. Zhou, F. Ma and H. Guo, Chem. Eng. J., 2020, 384, 123290 CrossRef CAS.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- Y. Sun, L. Zheng, X. Zheng, D. Xiao, Y. Yang, Z. Zhang, B. Ai and Z. Sheng, Front. Chem., 2022, 9 Search PubMed.
- X. Wang, Y. Lu, T. Zhu, S. Chang and W. Wang, Chem. Eng. J., 2020, 388, 124317 CrossRef CAS.
- Q. Ye, J. Wu, P. Wu, S. Rehman, Z. Ahmed and N. Zhu, Chem. Eng. J., 2021, 417, 129111 CrossRef CAS.
- X. Luo, H. Ma, H. Ren, X. Zou, Y. Wang, X. Li, Z. Shen, Y. Wang and L. Cui, J. Colloid Interface Sci., 2021, 590, 622–631 CrossRef CAS PubMed.
- A. Herath, C. A. Layne, F. Perez, E. B. Hassan, C. U. Pittman and T. E. Mlsna, Chemosphere, 2021, 269, 128409 CrossRef CAS PubMed.
- Z. Zheng, B. Zhao, Y. Guo, Y. Guo, T. Pak and G. Li, Sci. Total Environ., 2021, 787, 147397 CrossRef CAS PubMed.
- S. Han and P. Xiao, Sep. Purif. Technol., 2022, 287, 120533 CrossRef CAS.
- Y. Wen, M. Liu, S. Li, L. Su, Y. Wang, Z. Zhou, N. Zhou and R. Li, Chem. Eng. J., 2022, 446, 137188 CrossRef CAS.
- J. Deng, Y. Zhou, Y. Zhao, L. Meng, T. Qin, X. Chen, K. Li and S. Yuan, Energy, 2022, 244, 122602 CrossRef CAS.
- Z. Wang and H. M. Jang, Bioresour. Technol., 2022, 351, 127025 CrossRef CAS PubMed.
- Y. Ma, T. Lu, L. Yang, L. Wu, P. Li, J. Tang, Y. Chen, F. Gao, S. Cui, X. Qi and Z. Zhang, Environ. Pollut., 2022, 298, 118833 CrossRef CAS PubMed.
- J. Zhang, W. Lu, S. Zhan, J. Qiu, X. Wang, Z. Wu, H. Li, Z. Qiu and H. Peng, Sep. Purif. Technol., 2021, 276, 119296 CrossRef CAS.
- X. Geng, S. Lv, J. Yang, S. Cui and Z. Zhao, J. Environ. Manage., 2021, 280, 111749 CrossRef CAS PubMed.
- S. Zeng, Y. Choi and E. Kan, Sci. Total Environ., 2021, 750, 141691 CrossRef CAS PubMed.
- A. Nasiri, S. Rajabi, M. Hashemi and H. Nasab, Sep. Purif. Technol., 2022, 296, 121366 CrossRef CAS.
- Z. Liu, X. Fang, L. Chen, B. Tang, F. Song and W. Li, Sustainability, 2022, 14, 5892 CrossRef CAS.
- W. A. Shaikh, R. U. Islam and S. Chakraborty, J. Environ. Chem. Eng., 2021, 9, 104982 CrossRef CAS.
- T. Nguyen, Q. Truong, C. Chen, W. Chen and C. Dong, Bioresour. Technol., 2022, 351, 127043 CrossRef CAS PubMed.
- J. Song, S. A. Messele, L. Meng, Z. Huang and M. Gamal El-Din, Water Res., 2021, 194, 116930 CrossRef CAS PubMed.
- Y. Zhou, X. Hu, Y. Zhang, X. Chen, H. Zhao, Q. Fu, F. Xu and Y. Gao, Appl. Surf. Sci., 2021, 566, 150657 CrossRef CAS.
- V. Singh and V. C. Srivastava, Biomass Conversion and Biorefinery, 2022 Search PubMed.
- Y. Zhou, S. Cao, C. Xi, X. Li, L. Zhang, G. Wang and Z. Chen, Bioresour. Technol., 2019, 292, 121951 CrossRef CAS PubMed.
- S. He, Q. Chen, G. Chen, G. Shi, C. Ruan, M. Feng, Y. Ma, X. Jin, X. Liu, C. Du, C. He, H. Dai and C. Cao, Microporous Mesoporous Mater., 2022, 335, 111848 CrossRef CAS.
- H. M. Hamadeen and E. A. Elkhatib, Environ. Res., 2022, 210, 112929 CrossRef CAS PubMed.
- Z. Wen, J. Xi, J. Lu, Y. Zhang, G. Cheng, Y. Zhang and R. Chen, J. Hazard. Mater., 2021, 411, 124909 CrossRef CAS PubMed.
- Y. Zhang, C. Zhu, F. Liu, Y. Yuan, H. Wu and A. Li, Sci. Total Environ., 2019, 646, 265–279 CrossRef CAS PubMed.
- K. N. Palansooriya, S. Kim, A. D. Igalavithana, Y. Hashimoto, Y. Choi, R. Mukhopadhyay, B. Sarkar and Y. S. Ok, J. Hazard. Mater., 2021, 415, 125464 CrossRef CAS PubMed.
- S. Yang, P. Zong, X. Ren, Q. Wang and X. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 6891–6900 CrossRef CAS PubMed.
- G. Chen, H. Wang, L. Han, N. Yang, B. Hu, M. Qiu and X. Zhong, J. Mol. Liq., 2021, 327, 114807 CrossRef CAS.
- J. Dobrzyńska, A. Wysokińska and R. Olchowski, J. Environ. Manage., 2022, 316, 115260 CrossRef PubMed.
- G. Kaur, N. Singh and A. Rajor, Environ. Technol. Innovation, 2022, 25, 102176 CrossRef CAS.
- X. Zhou, L. Shi, T. B. Moghaddam, M. Chen, S. Wu and X. Yuan, J. Hazard. Mater., 2022, 425, 128003 CrossRef CAS PubMed.
- X. Wang, Z. Meng, X. Liu, T. Wang, X. Hu and X. Sun, Environ. Sci., 2021, 42, 2334–2342 Search PubMed.
- R. R. Solis, O. Dinc, G. Fang, M. N. Nadagouda and D. D. Dionysiou, Environ. Sci.: Nano, 2021, 8, 960–977 RSC.
- C. Xu, X. Tan, J. Zhao, J. Cao, M. Ren, Y. Xiao and A. Lin, J. Hazard. Mater., 2021, 416, 125785 CrossRef CAS PubMed.
- J. Wu, T. Wang, Y. Zhang and W. Pan, Bioresour. Technol., 2019, 291, 121859 CrossRef CAS PubMed.
- P. Wang, D. Zhang, H. Tang, H. Li and B. Pan, J. Hazard. Mater., 2019, 371, 513–520 CrossRef CAS PubMed.
- M. Zahoor, M. Wahab, S. M. Salman, A. Sohail, E. A. Ali and R. Ullah, Bioinorg. Chem. Appl., 2022, 2022, 1–17 CrossRef PubMed.
- S. F. Lütke, A. V. Igansi, L. Pegoraro, G. L. Dotto, L. A. A. Pinto and T. R. S. Cadaval, J. Environ. Chem. Eng., 2019, 7, 103396 CrossRef.
- R. Li, Y. Zhang, W. Chu, Z. Chen and J. Wang, RSC Adv., 2018, 8, 13546–13555 RSC.
- A. Nasiri, M. Malakootian, M. R. Heidari and S. N. Asadzadeh, J. Polym. Environ., 2021, 29, 2660–2675 CrossRef CAS.
- S. V. Gupta and M. Ahmaruzzaman, Int. J. Environ. Anal. Chem., 2022, 1–20 CrossRef.
- A. Jaswal, M. Kaur, S. Singh, S. K. Kansal, A. Umar, C. S. Garoufalis and S. Baskoutas, J. Hazard. Mater., 2021, 417, 125982 CrossRef CAS.
- Y. Yang, Z. Zhong, J. Li, H. Du and Z. Li, J. Hazard. Mater., 2022, 430, 128500 CrossRef CAS PubMed.
- X. Chen, M. Zhang, H. Qin, J. Zhou, Q. Shen, K. Wang, W. Chen, M. Liu and N. Li, Sep. Purif. Technol., 2022, 280, 119751 CrossRef CAS.
- G. Kaur, N. Singh and A. Rajor, J. Contam. Hydrol., 2021, 236, 103737 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra05334a |
| This journal is © The Royal Society of Chemistry 2022 |