Open Access Article
Open Access ArticleGenetic encoding of isobutyryl-, isovaleryl-, and β-hydroxybutryl-lysine in E. coli†
Jayani A. Christopher,
Sahan A. Galbada Liyanage,
Eve M. Nicholson,
William D. Kinney and
T. Ashton Cropp *
*
Department of Chemistry, Virginia Commonwealth University, Richmond, VA 23284, USA. E-mail: tacropp@vcu.edu
First published on 29th November 2022
Abstract
Here we report the synthesis and genetic encoding of the lysine post translational modifications, β-hydroxybutyryl-lysine, isobutyryl-lysine and isovaleryl-lysine. The ability to obtain a homogenous protein samples with site-specific incorporation of these acylated lysine residues can serve as a powerful tool to study the biological role of lysine post translational modifications.
Lysine residues undergo several different types of post-translational modifications (PTMs) such as methylation, SUMOylation, ubiquitination, glycation, and acylation.1 Lysine acylation is the most common type of PTM that has been studied extensively in the past due to its direct impact on cell functions such as gene transcription,2,3 energy metabolism,2,4 nuclear transport,4 sub-cellular protein localization,5 protein–DNA interactions,5 apoptosis,6 and cell metabolism.7–10 Acylation occurring in lysine residues at the tails of the histone proteins can have a profound effect on the regulation of gene transcription.11–13 At physiological pH, lysine residues of histones carry positive charges due to the protonation of epsilon nitrogen, and hence leads to the tight binding with negatively charged DNA backbone in chromatin structure. By undergoing acylation, these amine groups lose the positive charge, thereby minimizing the tightly bound interactions giving fluidity to the DNA strands. This can increase gene expression because the DNA becomes more accessible to be transcription.13–15
The lysine PTM, β-hydroxybutyryl-lysine, (HBK) (Fig. 1), was discovered in 2016 as a modification of histone proteins.9 It was shown that this modification is directly related to the concentration of the corresponding ketone bodies available in the cell, and is thus heavily influenced by metabolism. Interestingly, HBK not only quenches the lysine positive charge, but the presence of a hydroxyl group introduces the potential for additional hydrogen bonding, that could contribute to the alteration of protein–protein or protein DNA interactions.8 Interestingly a similar structure, isobutyryl-lysine (IBK), was also recently discovered as a histone modification mark.16 Indeed, many other acylations that are derived from the corresponding acyl-CoAs have been observed, including isovaleryl-lysine (IVK).17 Given the structural similarity of these amino acids, and the importance of understanding epigenetic regulation, we attempted to add these three amino acids to the genetic code.
Previously, we reported the successful incorporation of a different lysine PTM, 2-hydroxyisobutyryl lysine PTM into the genetic code of E. coli using the pyrrolysyl-tRNA synthetase (PylRS) and the orthogonal tRNACUA originally from Methanosarcina mazei species.18 Xiao and co-workers also successfully incorporated this PTM into recombinant histone proteins in E. coli and mammalian cells using an evolved PylRS/tRNACUA pair which was initially obtained from Methanosarcina barkeri (Mb) species.19 Here we show that this pair can also be used for the successful genetic encoding of these related PTMs. These analogues have been identified as potential lysine PTMs due to the availability of the corresponding acyl-CoAs from metabolic pathways and similar counter parts have been discovered in different proteins.17,20
We first prepared the HBK amino acid in several steps from commercially available benzoyl protected Boc-lysine (Nε-Cbz-Boc-lysine). The acylating agent, ethyl-3-hydroxybutyrate was protected by a TBDMS group to avoid reactivity of the 3-hydroxyl group, which we found critical for success (Scheme S2†). The sequence of our synthesis allowed us to produce the amino acid as a methyl ester (HBK-OMe), which was expected to be more bioavailable than the free amino acid. Methyl esters of amino acids can be hydrolysed in the cell,21 and we found that in the case of HBK, esterification was essential for successful protein production. Both IVK and IBK were synthesized as free amino acids using a similar synthetic route starting with Boc-lysine and isovaleryl chloride and isobutyl chloride for acylation, respectively (Scheme S3†). It was not necessary to use these as methyl esters.
As an initial approach, we performed a genetic selection against a Methanosarcina mazei PylRS (MbPylRS) active site library using HBKOMe. Surprisingly, the vast majority of enriched clones from this library consisted of wild-type active PylRS sequences. Importantly, these gene sequences consisted of many variants of degenerate codons that encoded the wild-type residues, suggesting that the wild-type sequence was enriched from the selection. To confirm the substrate specificity of the synthetase, a screening was performed by using a reporter plasmid that expresses GFPuv from a T7 promoter, and a T7-RNA polymerase gene containing an in-frame amber stop codon. This reporter is quite sensitive as a single amber suppression even can lead to multiple copies of GFPuv being expressed. As shown in Fig. 1, fluorescence was observed only in the presence of the acylated lysines, with little to no background expression in the absence of supplemented amino acids. The observed activity was similar to a positive control, Nε-Boc-Lys, which is often used as a benchmark in unnatural amino acid mutagenesis studies. This indicates that all three amino acids serve as substrates for the wild-type MbPylRS.
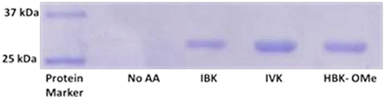
To isolate protein containing these amino acids, we performed an expression of superfolder GFP (His6-sfGFP) containing an amber stop codon mutation in place of Y151, and an N-terminal 6X-histidine tag. The expression was performed by growing the cultures in the presence of 2 mM IBK, IVK and HBK-OMe and purified the His tagged proteins with Ni2+ affinity chromatography under native purification conditions. Protein expression was only observed in the presence of the amino acids (Fig. 2), again confirming that endogenous amino acids are not substrates for the synthetase. The ESI-MS mass spectra of the intact proteins confirmed incorporation of the correct amino acid (Fig. S1†). The mass spectra did indicate the presence of some free lysine residues, perhaps the result of hydrolysis reactions in vivo, or during protein purification (Fig. S1†).
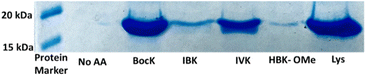
Finally, to demonstrate expression of the proteins most likely to be studied using these amino acids, we expressed human histone H3 protein containing the amber stop codon in place of the codon for lysine 9. This position has been observed to contain lysine modifications, including HBK, in cellular studies. The protein expression was performed with 4 mM BocK, IBK, IVK and HBK-OMe cultures alongside a wild-type histone H3 containing a native K9 residue. H3-proteins were purified by Ni2+ affinity chromatography under denaturing conditions, which has been used in the past to successfully re-fold functional protein.3 The protein expression was observed only in the presence of the acylated and wildtype lysines. The expression with IVK showed the highest yield when, whereas HBK-OMe expression was observed to be the lowest (Fig. 3). Nevertheless, as seen before, no expression was observed in the absence of exogenous amino acids indicating efficient site-specific incorporation. While HBK expression was low in this example, in other protein expressions, yields are comparable.
In summary, we were able to successfully synthesize and add new lysine PTMs to the genetic code of E. coli using the Mb PylRS/PylRS tRNACUA orthogonal pair. This leverages this system and enables the synthesis of natural human histone proteins containing newly discovered PTMs at site-specific locations. These protein products could serve as unique substrates to be used in deciphering the substrate specificity of enzymes that act on PTMs such as histone deacetylases. Moreover, this approach should enable the in-depth study of the consequences of these modifications with residue-level precision.
Autor contributions
J. Christopher, S. Liyanage, and T. A. Cropp conceived and designed the experiments. J. Christopher, S. Liyanage, E. Nicholson, and W. Kinney performed experiments and analysed data. J. Christopher and T. A. Cropp prepared the manuscript.Conflicts of interest
The authors indicate there are no conflicts to declare.Acknowledgements
The authors acknowledge NSF CHE-2109051 for support of this work.Notes and references
- C. Kontaxi, P. Piccardo and A. C. Gill, Front. Mol. Biosci., 2017, 4, 56 CrossRef PubMed.
- X. J. Yang and E. Seto, Mol. Cell, 2008, 31, 449–461 CrossRef CAS PubMed.
- B. J. Wilkins, L. E. Hahn, S. Heitmuller, H. Frauendorf, O. Valerius, G. H. Braus and H. Neumann, ACS Chem. Biol., 2015, 10, 939–944 CrossRef CAS.
- S. Lee, Toxicol. Res., 2013, 29, 81–86 CrossRef CAS.
- S. C. Kim, R. Sprung, Y. Chen, Y. Xu, H. Ball, J. Pei, T. Cheng, Y. Kho, H. Xiao, L. Xiao, N. v. Grishin, M. White, X. J. Yang and Y. Zhao, Mol. Cell, 2006, 23, 607–618 CrossRef CAS.
- T. Yanagisawa, T. Umehara, K. Sakamoto and S. Yokoyama, ChemBioChem, 2014, 15, 2181–2187 CrossRef CAS.
- D. Zhang, Z. Tang, H. Huang, G. Zhou, C. Cui, Y. Weng, W. Liu, S. Kim, S. Lee, M. Perez-Neut, J. Ding, D. Czyz, R. Hu, Z. Ye, M. He, Y. G. Zheng, H. A. Shuman, L. Dai, B. Ren, R. G. Roeder, L. Becker and Y. Zhao, Nature, 2019, 574, 575–580 CrossRef CAS PubMed.
- B. R. Sabari, D. Zhang, C. D. Allis and Y. Zhao, Nat. Rev. Mol. Cell Biol., 2017, 18, 90–101 CrossRef CAS.
- Z. Xie, D. Zhang, D. Chung, Z. Tang, H. Huang, L. Dai, S. Qi, J. Li, G. Colak, Y. Chen, C. Xia, C. Peng, H. Ruan, M. Kirkey, D. Wang, L. M. Jensen, O. K. Kwon, S. Lee, S. D. Pletcher, M. Tan, D. B. Lombard, K. P. White, H. Zhao, J. Li, R. G. Roeder, X. Yang and Y. Zhao, Mol. Cell, 2016, 62, 194–206 CrossRef CAS.
- M. D. Hirschey and Y. Zhao, Mol. Cell. Proteomics, 2015, 14, 2308–2315 CrossRef CAS.
- Z. A. Wang, Y. Zeng, Y. Kurra, X. Wang, J. M. Tharp, E. C. Vatansever, W. W. Hsu, S. Dai, X. Fang and W. R. Liu, Angew. Chem., Int. Ed., 2017, 56, 212–216 CrossRef CAS PubMed.
- L. Dai, C. Peng, E. Montellier, Z. Lu, Y. Chen, H. Ishii, A. Debernardi, T. Buchou, S. Rousseaux, F. Jin, B. R. Sabari, Z. Deng, C. D. Allis, B. Ren, S. Khochbin and Y. Zhao, Nat. Chem. Biol., 2014, 10, 365–370 CrossRef CAS.
- B. R. Sabari, Z. Tang, H. Huang, V. Yong-Gonzalez, H. Molina, H. E. Kong, L. Dai, M. Shimada, J. R. Cross, Y. Zhao, R. G. Roeder and C. D. Allis, Mol. Cell, 2015, 58, 203–215 CrossRef CAS.
- T. Narita, B. T. Weinert and C. Choudhary, Nat. Rev. Mol. Cell Biol., 2019, 20, 156–174 CrossRef CAS PubMed.
- M. J. Gattner, M. Vrabel and T. Carell, Chem. Commun., 2013, 49, 379–381 RSC.
- Z. Zhu, Z. Han, L. Halabelian, X. Yang, J. Ding, N. Zhang, L. Ngo, J. Song, H. Zeng, M. He, Y. Zhao, C. H. Arrowsmith, M. Luo, M. G. Bartlett and Y. G. Zheng, Nucleic Acids Res., 2021, 49, 177–189 CrossRef CAS PubMed.
- T. Baldensperger, S. di Sanzo, A. Ori and M. A. Glomb, Anal. Chem., 2019, 91, 12336–12343 CrossRef CAS.
- W. A. Knight and T. A. Cropp, Org. Biomol. Chem., 2015, 13, 6479–6481 RSC.
- H. Xiao, W. Xuan, S. Shao, T. Liu and P. G. Schultz, ACS Chem. Biol., 2015, 10, 1599–1603 CrossRef CAS.
- J. Baeza, M. J. Smallegan and J. M. Denu, Trends Biochem. Sci., 2016, 41, 231–234 CrossRef CAS.
- H. Zhou, J. W. Cheung, T. Carpenter, S. K. Jones, N. H. Luong, N. C. Tran, S. E. Jacobs, S. A. Galbada Liyanage, T. A. Cropp and J. Yin, Bioorg. Med. Chem. Lett., 2020, 30, 126876 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra04898a |
| This journal is © The Royal Society of Chemistry 2022 |