 Open Access Article
Open Access ArticleEnhancing mechanism of arsenic(III) adsorption by MnO2-loaded calcined MgFe layered double hydroxide†
Mingqi Xie ab,
Xiangping Luoab,
Chongmin Liu
ab,
Xiangping Luoab,
Chongmin Liu *ab,
Shaohong Youab,
Saeed Radab,
Huijun He
*ab,
Shaohong Youab,
Saeed Radab,
Huijun He ab,
Yongxiang Huangab and
Zhihong Tuabc
ab,
Yongxiang Huangab and
Zhihong Tuabc
aCollege of Environmental Science and Engineering, Guilin University of Technology, Guilin 541004, China. E-mail: chongmin@glut.edu.cn
bGuangxi Key Laboratory of Theory & Technology for Environmental Pollution Control, Guilin University of Technology, Guilin 541004, China
cCAS Key Laboratory of Mineralogy and Metallogeny, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou 510640, China
First published on 12th September 2022
Abstract
The use of MnO2/MgFe-layered double hydroxide (MnO2/MgFe-LDH) and MnO2/MgFe-layered double oxide (MnO2/MgFe-LDO400 °C) for arsenic immobilization from the aqueous medium is the subject of this research. Fourier transform infrared spectroscopy, X-ray diffraction, X-ray photoelectron spectroscopy, scanning electron microscopy, and transmission electron microscopy were used to characterise MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C. Based on our developed method, MnO2 was spread on the clay composites' surfaces in the form of a chemical bond. The clay composite exhibited a good adsorption effect on arsenic. The experimental findings fit the pseudo-second-order model well, indicating that the chemisorption mechanism played a significant role in the adsorption process. Furthermore, the Freundlich model suited the adsorption isotherm data of all adsorbents well. The recycling experiment showed that MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C exhibited good stability and reusability. In summary, MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C are promising for developing processes for efficient control of the pollutant arsenic.
1. Introduction
As one of the most dangerous elements in wastewater effluents, arsenic is an extremely toxic metal described as a potent class-one non-threshold carcinogen.1 Arsenic poisoning can harm plant growth, development, and metabolism, resulting in yield loss. In addition, arsenic levels in the human body rise as a result of drinking arsenic-contaminated water or consuming foods with high arsenic contents. Increased arsenic levels are linked to various acute and chronic human health issues, including malignancies and adverse effects on the cardiovascular, neurological, hematological, renal, reproductive, and respiratory systems.2,3 The World Health Organization has set the maximum safe limit of arsenic in drinking water to 10 μg L−1 because of its extreme toxicity and carcinogenicity. However, several areas worldwide, including China, still suffer from the threat of arsenic contamination.4,5 Approximately 20 million Chinese people are possibly exposed to drinking water contaminated with arsenic. Therefore, there is an urgent need to explore environmentally friendly ways to remove arsenic from the aquatic environment.Extensive studies have been conducted on the treatment of arsenic using biological methods,6 coagulation,7 adsorption,8 and the reverse osmosis method.9 Among there, adsorption is one of the most promising technologies for arsenic removal from aqueous solutions because of its simple operation and broad applicability. In addition, the adsorption method can achieve a high removal efficiency and recover arsenic from aqueous solutions with different arsenic concentrations over a wide range of pH values. Various materials for arsenic removal are continuously being evaluated by researchers worldwide, including activated carbon,10 biochar-based sorbents,11 organic polymers,12 and Al2O3.13 However, the reported adsorbents could adsorb arsenate (As(V)) more easily, but experienced difficulty attaching arsenite (As(III)). Arsenic occurs in two main states in the natural aquatic environment: trivalent (As(III)) and pentavalent (As(V)). The toxicity of As(III) is 20–60 times higher than that of As(V).14 In addition, compared to As(V), As(III) is more difficult to be removed from water than As(V), is easier to transfer in the environment, and has a stronger ability to enter biological cells under neutral and acidic conditions.15 Therefore, we focused on evaluating an efficient adsorption material for As(III) contamination.
Manganese is one of the most widely distributed elements in the environment, and manganese oxides showed a high affinity to various pollutants.16 Among these Mn-oxides, manganese dioxide (MnO2) is non-toxic and has extensive sources, and has been applied in the removal of various pollutants from the aquatic environment.17 Owning to its oxidation, MnO2 showed excellent superiority as an adsorbent for As(III) removal. Wei et al. reported that MnO2 could remove As(III) by absorption and oxidisation.18 Jian et al. used β-MnO2 composite materials to adsorb arsenic(III), indicating that MnO2 oxidises As(III) to As(V), resulting in an easier removal of As(III) from water.19 However, this material is difficult to crystallise during synthesis, undergoes reduction easily, and releases manganese ions during the process of As(III) oxidation. Some precursor studies have proven that loading on a suitable carrier, to form the compound materials could be an efficient way to overcome the disadvantages of MnO2 and improve its application.20,21
Layered double hydroxides (LDHs), known as anionic clays or hydrotalcites, are a kind of materials that have attracted attention worldwide owing to their low cost, high anion exchange capacity, large specific surface area, and excellent interlayer intercalation. Moreover, their calcined products, namely layered double oxides (LDOs), have many advantages, including uniform dispersion, excellent thermal stability, huge specific surface areas, and synergistic action between elements.22 Benefiting from excellent characteristics, these two types of materials have been extensively used as precursors for catalyzers23 and absorbents.24 Dai et al. developed a novel two-dimensional magnetic NiFe LDH nanosheet grown on diatomite with superior adsorption performance for anionic, cationic dyes, As(III), and As(V) from wastewater.25 Long et al. synthesized polyaniline/Mg–Al layered double oxides which the composites showed highly efficient removal of hexavalent chromium from aqueous solution. Furthermore, “memory effect” of LDOs performed favorable ability for arsenic removal.26 The research of Lv et al. revealed that controlling the calcination temperature to 500 °C could enhance the As(V) adsorption capacity of NiAL-LDO composite.27 Also Mubarak et al. reported that the MgFe-LDO hollow nanospheres showed high adsorption effectiveness for As(V).28 Thus there are reasons to believe the combination of MnO2 and LDO to form composites can provide potential adsorbents to enhance As(III) oxidation and adsorption activity through the synergistic effects between MnO2 and LDO. However, to our knowledge, little research has been done in this aspect. Meanwhile, the related arsenic removal mechanism is worth exploring.
In this study, MnO2-doped MgFe LDH and LDO were successfully synthesised to efficiently remove arsenate from aqueous solutions. This research aimed to investigate the adsorption behaviour and preliminary mechanism of clay composites (MnO2/MgFe-LDH, MnO2/MgFe-LDO400 °C) for As(III) removal.
2. Materials and methods
2.1 Chemicals
The chemicals Mg(NO3)2·6H2O, Fe(NO3)3·9H2O, KMnO4, MnCl2·4H2O, NaAsO2, NaOH, Na2CO3, NaCl, HCl, H2SO4, H2SO4, and HNO3 were purchased from Xilong Scientific Co., Ltd. The chemical reagents used in this study were of analytical grade, and solutions were prepared using deionised (DI) water.2.2 Material synthesis methods
The co-precipitation approach was used to prepare MnO2/MgFe-LDH. To prepare the mixed aqueous solution, 4.615 g of Mg(NO3)2·6H2O and 2.424 g of Fe(NO3)3·9H2O were dissolved in 300 mL of DI water. Under vigorous stirring, NaOH was slowly added dropwise to the mixed solution. The pH of the reaction solution was maintained at 11 ± 0.5 by controlling the lowering speed of the NaOH solution. After 48 h of ageing at 60 °C, the KMnO4 and MnCl2·4H2O were carefully added, followed by 4 h of stirring at 30 °C. After ageing for 12 h, the slurry was centrifuged and rinsed numerous times with DI water until the supernatant was neutral. The MnO2/MgFe-LDH was then dried at 70 °C, and crushed into a powder. Finally, a portion of the MnO2/MgFe-LDH powder was calcined at 400 °C for 5 h in a tube furnace to produce MnO2/MgFe-LDO400 °C.2.3 Characterisation and analysis
The surface physical morphology and microstructure of the clay composites were determined by a scanning electron microscopy (SEM). The transmission electron microscopy (TEM) was employed for microstructural and compositional analyses of the clay composites. The specific surface areas were determined using the Brunauer–Emmett–Teller (BET) method. A Nano ZS 90 type nanoparticle size and zeta potential analyser was used to analyse and test the zeta potential of the materials. X-ray diffraction (XRD) patterns were obtained using an X'Pert 3 Powder diffractometer (PNAlytical, Holland) with copper Kα radiation (λ = 1.54059 Å). Fourier transform infrared spectroscopy (FT-IR) was used to observe the structural changes in the particle surface. The surface elements species were analysed using X-ray photoelectron spectroscopy (XPS).2.4 Adsorption experiments
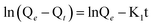 | (1) |
 | (2) |
 | (3) |
 | (4) |
3. Results and discussion
3.1 Selection of materials
Through a series of adsorption experiments, the materials of MnO2/MgFe-LDO400 °C exhibited the best adsorption performance, whereas MnO2 had the lowest arsenic adsorption capacity. Among the LDH materials, MnO2/MgFe-LDH displayed a slightly better adsorption capacity compared with that of MgFe-LDH (Fig. 1), with an initial arsenic concentration of 50 mg L−1. Therefore, modification by calcination for MnO2/MgFe-LDH at the temperatures of 400 °C, 500 °C, and 600 °C was carried out to improve the adsorption of As(III). The results showed that the adsorption capacity of As(III) by MnO2/MgFe-LDO400 °C reaches 35.93 mg g−1, which is greater than that of MnO2/MgFe-LDO500 °C (30.09 mg g−1) and MnO2/MgFe-LDO600 °C (20.82 mg g−1). These phenomena can be illustrated by the irreversible oxide formation and elimination of the “memory effect” in the structure of LDH at high calcination temperatures.27 Consequently, MnO2/MgFe-LDO400 °C presents the best removal performance for As(III) among the selected calcination temperatures.3.2 Structure characterization
The structures of the clay composites were studied using XRD. The XRD spectrum of MgFe-LDH revealed a succession of peaks 2 = 11.341°, 22.783°, 34.142°, 38.542° and 59.388°, which correspond to (003), (006), (012), (015), and (110) of the hydrotalcite-like structure.31 As shown in Fig. 2(a), diffraction peaks with hydrotalcite structures were also visible in the MnO2/MgFe-LDH spectra. Furthermore, the characteristic MnO2 peaks occurred at 2θ = 12.183° (002) and 18.618° (101), indicating that the loading of MnO2 did not disrupt the hydrotalcite structure and that the MnO2/MgFe-LDH composite material was successfully produced. The XRD pattern of MnO2/MgFe-LDO400 °C revealed the obvious absence of several diffraction peaks, indicating that the material lost some interlayer molecules and anions, such as water molecules and CO32−, and the layered structure was disrupted, resulting in a magnesium-iron mixed metal oxide. | ||
| Fig. 2 (a) XRD spectra of MgFe-LDH, MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C. (b) FT-IR of MgFe-LDH, MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C. | ||
The FT-IR spectra are shown in Fig. 2(b). The three materials displayed prominent absorption peaks between 3400–3500 cm−1, which were assigned with the O–H bending vibration peak of the interlayer water molecules and the stretching vibration of O–H on the laminate.32 From the spectral point of view, the absorption peak of approximately 1630 cm−1 corresponded to the O–H vibration peak in crystallisation water.33 The absorption peaks in the low-frequency region (less than 1000 cm−1) are caused by O-M or O-M-O (M = Mg, Fe, Mn) bending vibrations.34,35 The absorption peak in the spectra of MgFe-LDH and MnO2/MgFe-LDH was induced by the interlayer CO32− ion asymmetric tensile vibration at 1386 cm−1. The absorption peak at 3699 cm−1 in the MnO2/MgFe-LDH spectrum corresponded to the hydroxyl vibration on the sample surface, where the calcined MnO2/MgFe-LDH material did not show an O–H peak at 3699 cm−1. In addition, at 1386 cm−1, CO32− ions did not cause a vibration peak, but a new absorption peak caused by the vibration of HCO3− ions appeared at 1440 cm−1, indicating that water loss and dehydroxylation during the calcination process, and due to decomposition, CO32− transformed to HCO3−.36 In addition, the absorption peak at 1012 cm−1 in the MnO2/MgFe-LDH spectrum should be the C–O vibration in C–O-M (M = Mg, Fe, Mn), which appears at approximately 1006 cm−1 after calcination, indicating that MnO2 is chemically bonded the way is loaded to LDH.37
The micromorphologies of three materials were studied using SEM, and the results for MgFe-LDH, MnO2/MgFe-LDH, and MnO2/MgFe-LDO400 °C are presented in Fig. 3(a–c). The MgFe-LDH material has a plate-like structure with rough flake fragments on its surface, a typical anionic clay structure. The rough and uneven surface of the MnO2/MgFe-LDH, with layered and scaly particles, improved the specific surface area and void ratio. The MnO2/MgFe-LDO400 °C surface had crumbled, leaving uneven shards. Many adsorption sites were found on this rough, uneven surface.
TEM was used to explore the microstructures of the materials. As displayed by the TEM image in Fig. 3(d), MnO2 has a certain thickness, and it is clear that its surface is not perfectly smooth. MgFe LDH had sheet-like shape, composed of thin nano-scale curved platelets (Fig. 3(e)). We can see MnO2/MgFe-LDH (Fig. 3(f)) and MnO2/MgFe-LDO400 °C (Fig. 3(g)) composites, their surface showed a morphology similar to that of MnO2, element mapping of MnO2/MgFe-LDH (Fig. 3(h)) indicated that the elements of Mg, Fe, and Mn were distributed uniformly in the structure. The Mn–K signal is attributed to the loading of MnO2, which showed that MnO2 binds to MgFe-LDH uniformly. Combined with XRD, manganese oxide was successfully loaded on MgFe-LDH.
According to BET results in Table 1, the specific surface area of MgFe-LDH after loaded MnO2 is significantly larger than that of MgFe-LDH. While calcination could can result in the reduction of specific surface area of MnO2/MgFe-LDH.
| Sample | SBET/(m2 g−1) | Vtotal/(cm2 g−1) | DBET/nm |
|---|---|---|---|
| MgFe-LDH | 154.005 | 0.390 | 10.135 |
| MnO2/MgFe-LDH | 226.794 | 0.388 | 6.841 |
| MnO2/MgFe-LDHO400 °C | 153.455 | 0.589 | 15.349 |
3.3 Effect of pH on adsorption
The pH of the materials has a significant impact on their adsorption process. The adsorption capacity of MnO2/MgFe-LDH for As(III) diminished when the pH value increased, as illustrated in Fig. 4(a). This phenomenon can be explained by the electrostatic repulsion between As(III) and the MnO2/MgFe-LDH. At a pH lower than 9, As(III) exists in the solution in the form of H3AsO3.38 The surface of MnO2/MgFe-LDH becomes negatively charged as the pH rises(Fig. 4(b)), and the H3AsO3 in the solution dissociates into H2AsO3−, HAsO32−, and AsO33−.39 The adsorption capacity of MnO2/MgFe-LDO400°C for As(III) improves with increasing pH, and is superior to that of MnO2/MgFe-LDH in the pH range of 3–12. Arsenic generates Fe–O–As, Mn–O–As, and other complexes with the surface metal of the material because the metal active sites of LDO are relatively uniformly spread and have a large specific surface area. When the pH value was high, As(III) anions were incorporated into the interlayer structure due to the “memory effect” of the LDO material.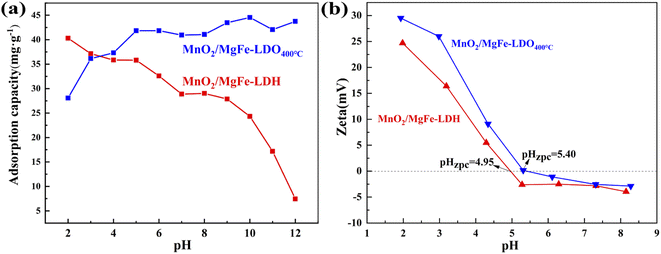 | ||
| Fig. 4 (a) Effect of initial pH on the adsorption of arsenic, (b) pH-zeta of MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C composites. | ||
3.4 Adsorption kinetic
As shown in Fig. 5, the adsorption kinetics of the As(III) solutions showed that the amount of As(III) adsorbed increased with increasing of As(III) concentration. The MnO2/MgFe-LDO400 °C adsorption capacity increased with time and gradually tended to equilibrium. The equilibrium times were 2,520, 2,880, and 3240 minutes for the initial As(III) concentration of 30, 40, and 50 mg L−1, respectively. Meanwhile, the adsorption effect of MnO2/MgFe-LDO400 °C was more significant than that of MnO2/MgFe-LDH. The MnO2/MgFe-LDH adsorption capacity increased with time and gradually reached equilibrium, and the equilibrium times were 1,800, 2,160, and 2520 minutes for the initial As(III) concentrations at 30, 40, and 50 mg L−1, respectively.Two kinetic models of pseudo-first-order and pseudo-second-order equations were used to investigate the adsorption behavior of MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C on As(III) during the adsorption process. The fitted results are shown in Fig. S1,† and the relevant parameters for each kinetic model are listed in Table 2. The pseudo-second-order model was more suitable than pseudo-first-order model for fitting the experimental data of MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C at the initial concentrations of 30, 40, and 50 mg L−1 As(III), where the correlation coefficients (R2) were >0.999 and >0.996, respectively. The results showed that the adsorption of As(III) by the two composite materials was a chemical adsorption process.
| Materials | C0 (mg L−1) | Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|---|
| Qe(mg g−1) | K1 | R2 | Qe(mg g−1) | K2 | R2 | ||
| MnO2/MgFe-LDH | 30 | 5.05 | 4.8389 × 10−4 | 0.9815 | 16.40 | 1.079 × 10−3 | 0.9994 |
| 40 | 7.20 | 7.2183 × 10−4 | 0.9937 | 20.89 | 1.001 × 10−3 | 0.9996 | |
| 50 | 10.21 | 8.8669 × 10−4 | 0.9946 | 24.85 | 8.045 × 10−4 | 0.9996 | |
| MnO2/MgFe-LDO400 °C | 30 | 15.15 | 7.9165 × 10−4 | 0.9900 | 22.94 | 3.676 × 10−4 | 0.9982 |
| 40 | 19.47 | 7.3657 × 10−4 | 0.9875 | 29.16 | 2.701 × 10−4 | 0.9979 | |
| 50 | 25.78 | 6.4443 × 10−4 | 0.9798 | 36.58 | 1.727 × 10−4 | 0.9967 | |
3.5 Adsorption isotherm
Langmuir and Freundlich adsorption isotherm models were used to fit the adsorption behaviours of MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C composites under different initial As(III) concentrations. The results and related parameters are presented in Fig. S2† and Table 3, respectively. Better fitting data for As(III) adsorption on the two composite materials were obtained using the Freundlich isotherm model. The adsorption capacity of the material for As(III) increased with the increasing of temperature, indicating that the adsorption process is an endothermic process. When the temperature increased, the movement of solute molecules in the system accelerates, and the mass transfer rate and diffusion coefficient increase, thereby increasing the adsorption capacity.40| Materials | TEMP(°C) | Langmuir isotherm | Freundlich isotherm | ||||
|---|---|---|---|---|---|---|---|
| Qm(mg g−1) | KL | R2 | KF | 1/n | R2 | ||
| MnO2/MgFe-LDH | 25 | 53.793 | 0.0356 | 0.9846 | 3.794 | 0.57684 | 0.9994 |
| 35 | 55.843 | 0.04107 | 0.9837 | 4.402 | 0.5656 | 0.9991 | |
| 45 | 52.522 | 0.04839 | 0.9804 | 4.985 | 0.53171 | 0.9992 | |
| MnO2/MgFe-LDO400 °C | 25 | 51.028 | 0.06605 | 0.9765 | 6.834 | 0.46887 | 0.9913 |
| 35 | 50.360 | 0.09817 | 0.9655 | 9.379 | 0.41053 | 0.9904 | |
| 45 | 58.939 | 0.11574 | 0.9655 | 11.785 | 0.40909 | 0.9920 | |
For MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C, under the optimal experimental conditions, the maximum adsorption capacity of As(III) reached 53.8 mg L−1 and 51.0 mg L−1, respectively (Table 3). Compared with the maximum adsorption capacities of various materials in the Table 4, MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C present good advantages in arsenic removal.
| Adsorbents | Adsorption capacities (mg g−1) | Ref. |
|---|---|---|
| MgAl–CO3-LDH | 44.66 | Wu et al.41 |
| MgFeLa-CLDHs | 47.40 | Jun et al.42 |
| Mg–Al–Cl | 36.00 | Pigna et al.43 |
| Activated carbon | 30.50 | Rojas et al.44 |
| HT-Zn-MOF-74 | 48.70 | Mahmoodi et al.45 |
| UiO-66-(SH)2 | 40.00 | Cox et al.46 |
| MnO2/MgFe-LDH | 53.79 | This study |
| MnO2/MgFe-LDO400 °C | 51.03 | This study |
3.6 Regeneration and reuse
Six solutions of NaOH, Na2CO3, NaCl, HCl, HNO3, and H2SO4 with an initial concentration of 0.1 mol L−1 were selected as desorbents to explore the recycling performance of the material on As(III). The desorption effects of the six adsorbents are shown in Fig. 6(a). The desorption effect of 0.1 mol L−1 NaOH was the most superior, with the adsorption capacity can reaching 25.5 and 30.1 mg g−1, respectively. We selected 0.1 mol L−1 NaOH as the desorbents to study the regeneration performance of the adsorbent on As(III), and the results are shown in Fig. 6(b). The figure shows that after five times of adsorption–desorption of As(III) by MnO2/MgFe-LDH and MnO2/MgFe-LDO400°C, the adsorption capacity of the composite still reached 28.5 and 28.9 mg g−1, and the desorption rates were as high as 99% and 96% respectively. This demonstrates that the material can be used repeatedly in treating of As(III), and thus is a renewable and environmentally friendly material that will not cause secondary pollution. | ||
| Fig. 6 (a) The influence of six analytical agents on the desorption effect of As(III), (b) the influence of the number of regenerations by the two materials on the adsorption capacity. | ||
4. Adsorption mechanisms
As shown in Fig. 7(a), before and after the adsorption of As(III) by MnO2/MgFe-LDH, the peak intensities at 38.087° and 58.722° were weaker than those before adsorption, and the adsorbed material was AsMn2O7 (ICSD card No.00-042-0035). Combined with the kinetic and isothermal adsorption results, it could be concluded that the adsorption process was chemical-based multilayer adsorption, and it was determined that As(III) and manganese complexed on the surface of the composite material to form a ternary complex to be removed.47,48 After MnO2/MgFe-LDO400 °C adsorbed As(III), the characteristic peaks of AsMn2O7 (ICSD card No. 00-042-0035) appeared at 2θ = 20.629° (020), 34.118° (220), and 59.096° (003). This demonstrates that manganese participated in the removal reaction of As(III) through surface complexation. In addition, the XRD characteristic peaks of MnO2/MgFe-LDO400 °C + As were similar to those of MnO2/MgFe-LDH, indicating that structural reconstruction of MnO2/MgFe-LDO400 °C had occurred due to the “memory effect”.49 | ||
| Fig. 7 (a) XRD pattern of MnO2/MgFe-LDH and MnO2/MgFe-LDO400°C before and after As(III) adsorption. (b) FT-IR of MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C before and after As(III) adsorption. | ||
As shown in Fig. 7(b), the tensile vibration absorption peak near 3430 cm−1 was obviously enhanced. However, the flexural vibration absorption peak of crystal water between 1632 and 1634 cm−1 was still retained after the adsorption As(III) by the two composite materials, indicate that water molecules are absorbed by the adsorbent during the adsorption process. It may also be that As(V) ions have reacted with the hydroxyl groups on the laminate, resulting in interlayer water.50 The absorption peaks at 626 and 854 cm−1 were caused by O-M or O-M-O (M = Mg, Fe, Mn) bending vibrations. After As(III) absorption, the absorption peak intensity weakened or even disappeared. This may be due to the coordination complexation reaction between As(III) and M–O on the laminate material, which produced a number of complexes.51 The 1386 cm−1 peak representing Mn–O shifted to 1397 cm−1, further confirming that Mn–O participated in the reaction. Combined with the adsorption reaction model and XRD analysis, As(III) was partially oxidised to As(V), forming As–O or As–O–As.52
The MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C samples after the As(III) adsorption were analysed for microscopic morphology and elements using SEM-EDS. The results are shown in Fig. 8. The surfaces of the two materials were covered with scale-like substances. Compared with Fig. 3(a–c), after the adsorption of As(III), the surface of the material was rougher, and more granular and scaly substances were present, indicating that new substances of complexes were attached to the material. This can be reciprocally verified with the XRD results.
The surface compositions and valence states of MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C were analysed by XPS. The high-resolution O 1s spectra are shown in Fig. 9(a). Before the reaction, the O 1s spectra can be deconvoluted into three peaks at approximately 531.1, 531.7, and 532.8 eV, corresponding to the lattice oxygen M–O, –OH and adsorbed-water, respectively. However, after the reaction, the content of the –OH decreased, and a new peak appeared at 531.2 eV, which can be assigned to As–O.53 It can be concluded that arsenic species achieved the complexation of –OH on the adsorbent surface. Fig. 9(b) shows the As 3d spectra after adsorption of arsenic, indicating that the adsorbed inorganic arsenic species for the two adsorbents were almost As(V) after the reaction.54 Compared to MnO2/MgFe-LDH, the As 3d signal of MnO2/MgFe-LDO400 °C after adsorption was stronger than that of MnO2/MgFe-LDH. Therefore, it can be inferred that MnO2/MgFe-LDO400 °C favoured the uptake of the arsenic to form As–O-M species.23,55 As shown in Fig. 9(c–d), for MnO2/MgFe-LDH before the reaction, the peaks of Mn 2p3/2 located at 643.81 eV and 642.09 eV were ascribed to Mn(IV) (50.62%) and Mn(II) (49.38%), respectively. After the reaction, the relative proportion of Mn(II) increased from 49.38% to 57%. For MnO2/MgFe-LDO400 °C, the relative proportion of Mn(II) increased from 51% to 53%. These results suggest the reduction of Mn(IV) to Mn(II), indicating that Mn(IV) participates in electron transfer.
 | ||
| Fig. 9 XPS spectrum of (a) O 1s of MnO2/MgFe-LDH before and after reaction, (b) As 3d fitting and (c) Mn 2p of MnO2/MgFe-LDH and (d) MnO2/MgFe-LDO400 °C after As(III) adsorption. | ||
In summary, the main adsorption mechanisms for As(III) removal for MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C including complexation, oxidation, and electrostatic attraction, as shown in Fig. 10. The results of XRD, FT-IR, and SEM indicated that As(III) might combine with functional groups of Mn–O or Fe–O on the surface of MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C to form complexes, shown in Fig. 10(a). Inner-sphere bidentate surface complex with iron ions on the hydroxide material surface can be formed from H2AsO3− and H2AsO4−.38,56 As shown in Fig. 10(b), some AsO2− and H3AsO3 were oxidised to H2AsO4− by MnO2, and MnO2 mainly acted as an oxidant for oxidation.57 According to the XPS analysis, electron transfer occurs during adsorption, and the valence states of manganese and arsenic are changed. In addition, the pH adsorption experiment proved the electrostatic attraction between the sorbents and As(III)/As(V) (Fig. 10(c)). At pH < 4.95, the electrostatic attraction was present between the positively charged MnO2/MgFe-LDH and negatively charged AsO33−/AsO43− (Fig. 4(a)).39 Similarly, for MnO2/MgFe-LDO400 °C, electrostatic attraction could occur in the arsenic removal process when the solution pH was below 5.40. Besides, As(III) also can be absorbed through the “memory effect” of MnO2/MgFe-LDO400°C (Fig. 10(d)).
5. Conclusion
In summary, the focus of this study was to investigate the adsorption behaviour and preliminary mechanism of heavy metal arsenic in water using two materials MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C. According to the XRD, FT-IR spectrum, XPS, and SEM-EDS characterisation results, the mechanism of MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C mediated adsorption of As(III) was inferred. The adsorption mechanisms of MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C adsorbents for As(III) were mainly involved complexation, oxidation, electrostatic attraction, and “memory effect”. After the materials adsorbed As(III), it was adsorbed and desorbed by 0.1 mol L−1 NaOH for five times, with no significant loss of As(III) adsorption capacity. Therefore, MnO2/MgFe-LDH and MnO2/MgFe-LDO400 °C composite materials are potential renewable adsorption materials that can effectively adsorb As(III) in water.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was funded by the Science & Technology Program of Guangxi (Grant No. Guike AD19110007, Guike AD19110105 and Guike AD19245065), the Research funds of The Guangxi Key Laboratory of Theory and Technology for Environmental Pollution Control (Grant No. Guikeneng 1801K010), Research Foundation of Guilin University of Technology (Grant No. GUTQDJJ201808), GuangDong Basic and Applied Basic Research Foundation (Grant No. 2021A1515012207).References
- Z. Li, D. Zhang, X. Xiong, B. Yan, W. Xie, J. Sheen and J. F. Li, Nat. Plants, 2017, 3, 930–936 CrossRef CAS PubMed
.
- P. K. Sahoo and K. Kim, Geosci. J., 2013, 17, 107–122 CrossRef CAS
.
- M. Jaishankar, T. Tseten, N. Anbalagan, B. B. Mathew and K. N. Beeregowda, Interdiscip. Toxicol, 2014, 7, 60–72 CrossRef PubMed
.
- D. Mohan and C. U. Pittman, J. Hazard. Mater., 2007, 142, 1–53 CrossRef CAS PubMed
.
- H. Ba Su, R. K. Singhal, M. V. Pimple and A. Reddy, Water, Air, Soil Pollut., 2015, 226, 22 CrossRef
.
- D. Lievremont, P. N. Bertin and M. C. Lett, Biochimie, 2009, 91, 1229–1237 CrossRef CAS
.
- T. Yuan, Q. F. Luo, J.-Y. Hu, S.-L. Ong and W. J. Ng, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng. Part A, 2003, 38, 1731–1744 CrossRef PubMed
.
- F. Peng, T. Luo and Y. Yuan, New J. Chem., 2014, 38, 4427–4433 RSC
.
- T. F. Lin and J. K. Wu, Water Res., 2001, 35, 2049–2057 CrossRef CAS PubMed
.
- X. J. Gong, Y. S. Li, Y. Q. Dong and W. G. Li, Chemosphere, 2020, 250, 126275 CrossRef CAS PubMed
.
- R. Amen, H. Bashir, I. Bibi, S. M. Shaheen, N. K. Niazi, M. Shahid, M. M. Hussain, V. Antoniadis, M. B. Shakoor, S. G. Al-Solaimani, H. Wang, J. Bundschuh and J. Rinklebe, Chem. Eng. J., 2020, 396, 125195 CrossRef CAS
.
- C. M. Iesan, C. Capat, F. Ruta and I. Udrea, Water Res., 2008, 42, 4327–4333 CrossRef CAS PubMed
.
- P. Hu, S. Wang and Y. Zhuo, Chem. Eng. J., 2022, 431, 134204 CrossRef CAS
.
- X. Cai, Y. Li, J. Guo, S. Liu and P. Na, Chem. Eng. J., 2014, 248, 9–17 CrossRef CAS
.
- R. Shabnam, M. A. Rahman, M. A. J. Miah, M. K. Sharafat, H. M. T. Islam, M. A. Gafur and H. Ahmad, Ind. Eng. Chem. Res., 2017, 56, 7747–7756 CrossRef CAS
.
- J. H. Park, I. Jang, K. Song and S. G. Oh, J. Phys. Chem. Solids, 2013, 74, 1056–1062 CrossRef CAS
.
- M. Li, S. Kuang, Y. Kang, H. Ma, J. Dong and Z. Guo, Sci. Total Environ., 2022, 819, 153157 CrossRef CAS PubMed
.
- Z. Wei, Z. Wang, J. Yan, Y. Liu, Y. Wu, Y. Fang, L. Yu, G. Cheng, Z. Pan and G. Hu, J. Hazard. Mater., 2019, 373, 232–242 CrossRef CAS
.
- M. Jian, H. Wang, R. Liu, J. Qu, H. Wang and X. Zhang, Environ. Sci.: Nano, 2016, 3, 1186–1194 RSC
.
- S. Panimalar, S. Logambal, R. Thambidurai, C. Inmozhi, R. Uthrakumar, A. Muthukumaran, R. A. Rasheed, M. K. Gatasheh, A. Raja, J. Kennedy and K. Kaviyarasu, Environ. Res., 2022, 205, 112560 CrossRef CAS
.
- L. M. Camacho, R. R. Parra and S. Deng, J. Hazard. Mater., 2011, 189, 286–293 CrossRef CAS
.
- J. Li, H. Yu, T. Yan, M. Sun, X. Li, W. Song and L. Yan, Colloids Surf., A, 2022, 634, 128021 CrossRef CAS
.
- C. Ye, J. Deng, L. Huai, A. Cai, X. Ling, H. Guo, Q. Wang and X. Li, Sci. Total Environ., 2022, 806, 150379 CrossRef CAS PubMed
.
- X. Feng, R. Long, L. Wang, C. Liu, Z. Bai and X. Liu, Sep. Purif. Technol., 2022, 284, 120099 CrossRef CAS
.
- X. Dai, W. Yi, C. Yin, K. Li, L. Feng, Q. Zhou, Z. Yi, X. Zhang, Y. Wang, Y. Yu, X. Han and Y. Zhang, Appl. Clay Sci., 2022, 229, 106664 CrossRef CAS
.
- F. L. Long, C. G. Niu, N. Tang, H. Guo, Z. W. Li, Y. Y. Yang and L. S. Lin, Chem. Eng. J., 2021, 404, 127084 CrossRef CAS
.
- Z. Lv, S. Yang, H. Zhu, L. Chen, N. S. Alharbi, M. Wakeel, A. Wahid and C. Chen, Appl. Surf. Sci., 2018, 448, 599–608 CrossRef CAS
.
- M. Mubarak, H. Jeon, M. S. Islam, C. Yoon, J. S. Bae, S. J. Hwang, W. S. Choi and H. J. Lee, Chemosphere, 2018, 201, 676–686 CrossRef CAS PubMed
.
- Y. S. Ho and G. McKay, Chem. Eng. J., 1998, 70, 115–124 CrossRef CAS
.
- B. Zhou, Y. Wu, J. Chan, S. Wang and S. Hu, Bull. Environ. Contam. Toxicol., 2019, 103, 75–81 CrossRef CAS PubMed
.
- F. Cheng, H. Guo, J. Cui, B. Hou, H. Xi, L. Jia and D. Li, Reaction Kinetics, Mechanisms and Catalysis, 2019, 126, 119–136 CrossRef CAS
.
- J. Zhang, F. Zhang, L. Ren, D. G. Evans and X. Duan, Mater. Chem. Phys., 2004, 85, 207–214 CrossRef CAS
.
- P. x. Wu, W. Li, Y.-j. Zhu, Y. n. Tang, N. w. Zhu and C. l. Guo, Appl. Clay Sci., 2014, 100, 76–83 CrossRef CAS
.
- I. Lagadic, A. Léaustic and R. Clément, J. Chem. Soc., Chem. Commun., 1992, 19, 1396–1397 RSC
.
- M. K. Titulaer, J. B. H. Jansen and J. W. Geus, Clays Clay Miner., 1994, 42, 249–258 CrossRef CAS
.
- M. T. Hasan, B. J. Senger, C. Ryan, M. Culp, R. Gonzalez-Rodriguez, J. L. Coffer and A. V. Naumov, Sci. Rep., 2017, 7, 6411 CrossRef PubMed
.
- H. Fu, Z. j. Du, W. Zou, H. q. Li and C. Zhang, Carbon, 2013, 65, 112–123 CrossRef CAS
.
- P. K. Raul, R. R. Devi, I. M. Umlong, A. J. Thakur, S. Banerjee and V. Veer, Mater. Res. Bull., 2014, 49, 360–368 CrossRef CAS
.
- Y. Y. Wang, H.-Y. Ji, H. H. Lu, Y. X. Liu, R. Q. Yang, L. L. He and S. M. Yang, RSC Adv., 2018, 8, 3264–3273 RSC
.
- M. F. Torres, J. M. González, M. R. Rojas, A. J. Müller, A. E. Sáez, D. Löf and K. Schillén, J. Colloid Interface Sci., 2007, 307, 221–228 CrossRef CAS PubMed
.
- X. Wu, X. Tan, S. Yang, T. Wen, H. Guo, X. Wang and A. Xu, Water Res., 2013, 47, 4159–4168 CrossRef CAS PubMed
.
- H. Jun, Z. Zhiliang, L. Hongtao and Q. Yanling, RSC Adv., 2014, 4, 5156–5164 RSC
.
- M. Pigna, J. J. Dynes, A. Violante, A. Sommella and A. G. Caporale, Environ. Eng. Sci., 2015, 33, 98–104 CrossRef
.
- S. Rojas and P. Horcajada, Chem. Soc. Rev., 2020, 120, 8378–8415 CrossRef CAS PubMed
.
- N. M. Mahmoodi, M. Oveisi, A. Taghizadeh and M. Taghizadeh, J. Hazard. Mater., 2019, 368, 746–759 CrossRef CAS PubMed
.
- C. S. Cox, V. Cossich Galicia and M. Lessio, J. Phys. Chem. C, 2021, 125, 3157–3168 CrossRef CAS
.
- J. Wu, D. Huang, X. Liu, J. Meng, C. Tang and J. Xu, J. Hazard. Mater., 2018, 348, 10–19 CrossRef CAS PubMed
.
- S. Hu, L. Yan, T. Chan and C. Jing, Environ. Sci. Technol., 2015, 49, 5973–5979 CrossRef CAS PubMed
.
- C. T. Vu and T. Wu, Water Res., 2020, 175, 115679 CrossRef CAS PubMed
.
- W. Xu, J. Wang, L. Wang, G. Sheng, J. Liu, H. Yu and X. J. Huang, J. Hazard. Mater., 2013, 260, 498–507 CrossRef CAS PubMed
.
- R. Dou, J. Ma, D. Huang, C. Fan, W. Zhao, M. Peng and S. Komarneni, Appl. Clay Sci., 2018, 161, 235–241 CrossRef CAS
.
- X. Ge, J. Liu, X. Song, G. Wang, H. Zhang, Y. Zhang and H. Zhao, Chem. Eng. J., 2016, 301, 139–148 CrossRef CAS
.
- B. Hudcová, V. Veselská, J. Filip, S. Číhalová and M. Komárek, Chemosphere, 2017, 168, 539–548 CrossRef
.
- D. Kang, X. Yu, S. Tong, M. Ge, J. Zuo, C. Cao and W. Song, Chem. Eng. J., 2013, 228, 731–740 CrossRef CAS
.
- S. Ploychompoo, J. Chen, H. Luo and Q. Liang, J. Environ. Sci., 2020, 91, 22–34 CrossRef PubMed
.
- P. R. Grossl, M. Eick, D. L. Sparks, S. Goldberg and C. C. Ainsworth, Sci. Technol., 1997, 31, 321–326 CrossRef CAS
.
- Q. Zheng, J. Hou, W. Hartley, L. Ren, M. Wang, S. Tu and W. Tan, Chem. Eng. J., 2020, 389, 124470 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra04805a |
| This journal is © The Royal Society of Chemistry 2022 |





