DOI:
10.1039/D2RA04739J
(Paper)
RSC Adv., 2022,
12, 27281-27291
Acid-controlled multicomponent selective synthesis of 2,4,6-triaryl pyridines and pyrimidines by using hexamethyldisilazane as a nitrogen source under microwave irradiation†
Received
29th July 2022
, Accepted 20th September 2022
First published on 27th September 2022
Abstract
An efficient and general protocol for the synthesis of functionalized 2,4,6-triaryl pyridines and pyrimidines was developed from commercially available aromatic ketones, aldehydes and hexamethyldisilazane (HMDS) as a nitrogen source under microwave irradiation. In this multicomponent synthetic route, Lewis acids play an important role in selectively synthesizing six-membered heterocycles, including pyridines (1N) and pyrimidines (2N), by involving [2 + 1 + 2 + 1] or [2 + 1 + 1 + 1 + 1] annulated processes.
Over the past decade, multicomponent reactions (MCRs) have been recognized as a powerful synthetic tool for the establishment of diverse and complicated skeletons. By definition, these components have been assembled in protocols to form desired compound frameworks without adding any reagents, catalysts, solvents, or substrates.1 MCRs started from simple and readily available synthons to achieve facile execution, generate high productivities, increase transformation efficiency, and reduce lengthy pathways.2 The benefits of these combinatorial approaches are not only the systematic distribution in arrays of reactions to construct iterations but also the utilization of the natural characteristics of the chemical building blocks to connect chemical bonds on the common MCR-product skeletons.3 Some name reactions have been developed by Biginelli,4 Hantzsch,5 Mannich,6 Passerini,7 Povarov,8 Strecker9 and Ugi.10 These reactions are also broadly applied for synthetic and biological interests.
Nitrogen-containing heterocyclic compounds are identified as one of the privileged structural motifs in organic chemistry.11 Among them, the six-membered heterocyclic compounds such as pyridines and pyrimidines are representative compounds for their broad natural existence in natural products, synthetic intermediates, bioactive compounds, and functional materials.12 Furthermore, these heterocycles have also been applied in the pharmaceutical industry for their anticancer, antimycobacterial, antibacterial, antimalarial, anti-inflammatory, anticonvulsant, and antimicrobial activities.13 Since the first pyrimidine synthesis by Brugnatelli and later on by Kolbe, and the initial discovery of pyridine by Thomas Anderson, the various synthetic approaches of these two families have been an area of continuous active research and innovative methodologies and are frequently reported.14 As a consequence, providing a facile and efficient protocol for the controllable construction of these two azaaromaric skeletons is highly desired. In a comparison of the reported studies on pyridine synthesis,15 the synthetic protocols of the pyrimidine motif are relatively rarely developed.
Based on the reported literature, a variety of synthetic routes toward the preparation of substituted pyrimidines have been developed.16 The nitrogen source on the pyrimidine scaffold is an important issue in these methodologies, as shown in Scheme 1. Numerous commonly used protocols apply a nitrogen-fixed substrate as a starting material, including amidines,17 nitriles,18,19 and enamidines (enamidine)20 to construct the pyrimidine skeletons. Among them, substituted amidines are frequently applied as versatile nitrogen-containing blocks to react with various cyclization partners to form a pyrimidine ring to utilize the inherent nitrogen on the substrate.21 The MCRs for the preparation of pyrimidines from amidines have also been recognized as an efficient strategy.22 Some other synthetic routes utilized the extrinsic nitrogen-containing reagents, including ammonium acetate,23 ammonium iodide,24 hydroxylamine,25 and urea26 to generate in situ ammonia surrogate and condensed with carbonyl synthons to construct pyrimidine structures.
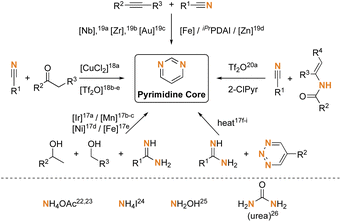 |
| | Scheme 1 Synthesis of 2,4,6-triarylpyrimidines. | |
Although many remarkable protocols have been reported for the construction of pyrimidines, the development of a facile synthetic protocol from simple and readily available starting materials under the MCRs strategy is of great interest.27 Recently, Li and Huang developed a concise and efficient protocol by controlling the carbon source to obtain diverse substituted pyridines and pyrimidines with high selectivity. This reaction was conducted from isopropene derivatives and the involvement of NH4I as the “N” source (Scheme 2A).24 In 2019, Deng and Zhang reported a synthetic route to selectively construct pyridines and pyrimidines by varying “N” sources in ammonium salts (NH4I and NH4OAc) under the DMSO/PhCl co-solvent system (Scheme 2B).22 Continuing our investigation of HMDS as a nitrogen source for the synthesis of N-heterocyclic compounds under a MW system,28 herein, we present a synthetic route to construct 1N or 2N six-membered ring pyrimidines 1 and pyridines 2 controlled by Lewis acids (BF3·OEt2 and TMSOTf) from commercially available acetophenones 3 and benzaldehydes 4 (Scheme 2C).
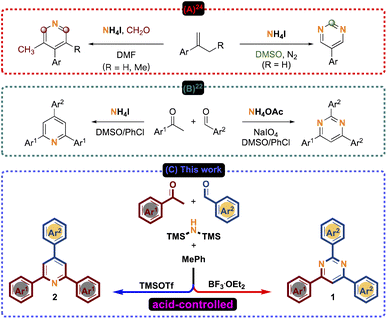 |
| | Scheme 2 Selective synthesis of pyridines and pyrimidines. | |
We started our investigation with commercially available acetophenone 3a, benzaldehyde 4a, hexamethyldisilazane (HMDS), and acids under microwave irradiation (MW) to explore the viability of controlling the selective formation of pyrimidine 3 and pyridine 4, as shown in Table 1. Based on our previous report, we first examined TMSOTf as Lewis acid for the synthesis of nitrogen-containing compounds. In entry 1, the reaction of 3a with 4a can provide 2aa in 92% yield in the presence of HMDS and toluene under MW at 150 °C in 0.5 h.28 Various metal triflates conducted the reaction well to obtain compound 2aa in approximate 80% yields (entries 2–6). Other Lewis acids, including AlCl3 and InCl3, give good to excellent yields of compound 2aa (entries 7 and 8). Brønsted acids were also investigated in this reaction, TfOH promotes the formation of 2aa in 82% yield but AcOH and p-TsOH did not (entries 9–11). Interestingly, BF3·OEt2 catalyzed the reaction of 3a and 4a in the presence of HMDS to obtain corresponding pyrimidine 1aa with 58% yield without the formation of pyridine 2aa (entry 12). When the reaction time was extended from 0.5 to 2 h, the yield of 1aa was increased, that of 2aa did not change (entries 13–14). The reaction proceeded well by changing reaction solvents such as DCM, THF and MeCN with lower isolated yields (entries 15–17). Therefore, an acid-controlled synthesis of pyrimidines and pyridines from commercially available 3a and 4a by using HMDS as a nitrogen source was established.
Table 1 Optimization of the reaction conditionsa

|
| Entry |
Acid |
Solvent |
Yieldsb (%) |
| 1aa |
2aa |
| Reaction conditions: 3a (4.0 mmol), 4a (4.0 mmol), HMDS (3.0 mL, 14.4 mmol), acid (0.5 mmol), solvent (2 mL), MW (150 °C), 0.5 h. Isolated yields. Reaction time: 2 h. |
| 1 |
TMSOTf |
Toluene |
0 |
92 |
| 2 |
In(OTf)3 |
Toluene |
0 |
80 |
| 3 |
Fe(OTf)3 |
Toluene |
0 |
80 |
| 4 |
Bi(OTf)3 |
Toluene |
0 |
81 |
| 5 |
Sc(OTf)3 |
Toluene |
0 |
82 |
| 6 |
Cu(OTf)2 |
Toluene |
0 |
76 |
| 7 |
AlCl3 |
Toluene |
0 |
91 |
| 8 |
InCl3 |
Toluene |
0 |
80 |
| 9 |
TfOH |
Toluene |
0 |
82 |
| 10 |
AcOH |
Toluene |
N.R. |
N.R. |
| 11 |
p-TsOH |
Toluene |
N.R. |
N.R. |
| 12 |
BF3·OEt2 |
Toluene |
58 |
0 |
| 13c |
BF3·OEt2 |
Toluene |
68 |
0 |
| 14c |
TMSOTf |
Toluene |
0 |
92 |
| 15c |
BF3·OEt2 |
DCM |
55 |
0 |
| 16c |
BF3·OEt2 |
THF |
62 |
0 |
| 17c |
BF3·OEt2 |
MeCN |
63 |
0 |
After screening the reaction conditions in Table 1, we examined various acetophenones 3 and benzaldehydes 4 for the synthesis of triaryl-substituted pyrimidines 1 by involving of the conditions mentioned in Table 1, entry 14. As shown in Table 2, the model reaction of 3a with 4a and HMDS in the presence of BF3·OEt2 provided 1aa in 78% yield. We first investigated the scope of the reaction with 3a and substituted benzaldehydes 4b–4k (4b, 3FPh; 4c, 4FPh; 4d, 3ClPh; 4e, 4ClPh; 4f, 4CF3Ph; 4g, 3MePh; 4h, 4MePh; 4i, 4OMePh; 4j, 34OMePh; 4k, 4PhPh, and 4l, furfural), coupling by HMDS/BF3·OEt2 system as nitrogen source under MW to provide the corresponding 1ab–1ak, with a yield between 58% and 74%. A lower yield of 1al was obtained when heterocyclic furfural 4l as aldehyde partner. We next screened the functionalized acetophenones 3b–3p (3b, 4FPh; 3c, 4FPh; 3d, 4MePh; 3e, 4OMePh; 3f, 4PhPh; 3g, 3FPh; 3h, 3BrPh; 3i, 3OMePh; 3j, 2FPh; 3j, 2ClPh; 3k, 2BrPh; 3m, 2MePh; 3n, 2OMePh; 3o, 4NO2Ph and 3p, 4CNPh) with 4a under optimized reaction conditions to afford corresponding pyrimidines 1ba–1pa in modest yields (see Table 3).
Table 2 Synthesis of triaryl-substituted pyrimidines 1ab
| Reaction conditions: 3 (4.0 mmol), 4 (4.0 mmol), HMDS (3.0 mL, 14.4 mmol), BF3·OEt2 (0.5 mmol), toluene (2 mL), MW (150 °C), 0.5 h. Isolated yields. |
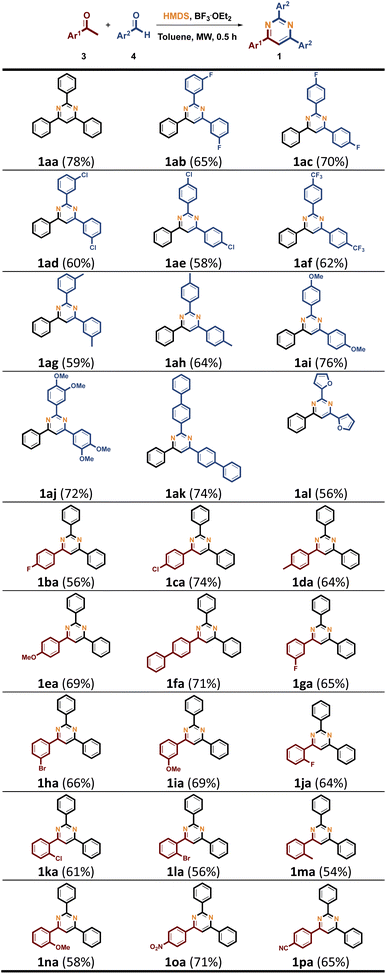 |
Table 3 Synthesis of triaryl-substituted pyridines 2a,b
| Reaction conditions: 3 (4.0 mmol), 4 (4.0 mmol), HMDS (3.0 mL, 14.4 mmol), TMSOTf (0.5 mmol), toluene (2 mL), MW (150 °C), 0.5 h. Isolated yields. |
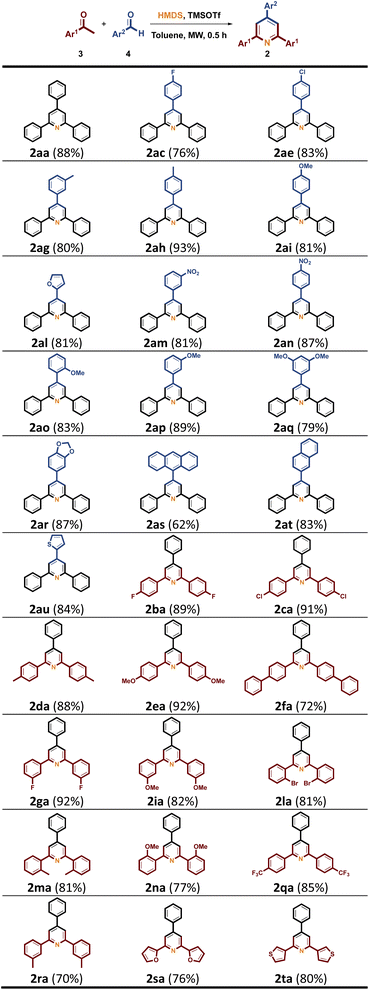 |
On the other hand, substituted pyrimidines are recognized as an important scaffold in pharmaceuticals.29 Among them, 2,4,6-triarlpyridines (TAPs) skeleton are also a practical synthon in the synthesis of drugs.30 In our previous work, we conducted the synthesis of TAPs by using HMDS/TMSOTf catalytic system under MW condition.28f Herein, we also describe a synthetic route to yield TAPs from the easily available acetophenones 3 and benzaldehydes 4 in the optimized reaction condition (Table 1, entry x1x). As shown in Scheme 2, we selected non-substituted 3a with some functionalized benzaldehydes 4c (4-FPh), 4e (4-ClPh), 4g (3-MePh), 4h (4-MePh), 4i (4-OMePh), 4l (2-furan), 4m (3-NO2Ph), 4n (4-NO2Ph), 4o (2-OMePh), 4p (3-OMePh), 4q (3,5-OMePh), 4r (3,5-CH2O2Ph), 4s (anthracene), 4t (2-naphthalene) and 4r (2-thiophene) under the optimized conditions to give desired pyridines 2ac–2au in 62–93% yields. Further investigation of 4a with diversified acetophenones 3b (4-FPh), 3c (4-ClPh), 3d (4-MePh), 3e (4-OMePh), 3f (4-PhPh), 3g (3-FPh), 3i (3-OMePh), 3l (2-BrPh), 3m (2-MePh), 3n (2-OMePh), 3q (4-CF3Ph), 3r (3-MePh), 3s (2-furan) and 3t (3-thiophene) in the optimized condition provided corresponding TAPs 2ba–2ta between 70% and 92% yields.
As shown in Scheme 3, non-aromatic benzaldehyde propionaldehyde 4v was also examined with 3a in this transformation to afford the desired pyridine 2av. It is possible that due to the low boiling point of 4v, only a 52% isolated yield of 2av was observed. Acetone 3u was also selected as aliphatic ketone with 4a to investigate this pathway to prepare desired pyridine 2ua in 88% yield.
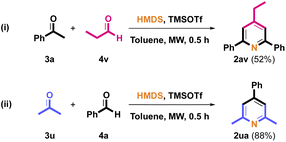 |
| | Scheme 3 Synthesis of compound 2av and 2ua. | |
On the basis of the above experimental results and the reported literature, a proposed reaction pathway is shown in Scheme 4. Claisen–Schmidt condensation of acetophenone 3 and benzaldehyde 4 occurred to give chalcone synthon I, which further reacts with HMDS to provide intermediate II under acidic conditions.28f Possibly, benzaldehyde 4 is transformed to intermediate III in BF3·OEt2/HMDS system, which then reacted with intermediate II to provide intermediate IV, and further aromatically converted to pyrimidine 1 under MW condition. Differently, TMSOTf acts not only as an acidic catalyst in the formation of II but also as a silylating agent to catalyze the conversion of acetophenone 3 to provide intermediate V. After the condensation of II with V, the intermediate VI was obtained, which further underwent aromatic cyclization to give corresponding pyridine 2. Therefore, we presume that the selective synthesis of skeletons 1 and 2 is based on the alternative pathway by involving BF3·OEt2 or TMSOTf.
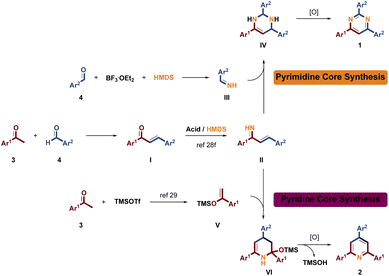 |
| | Scheme 4 Plausible reaction pathway. | |
Conclusions
In summary, we developed a strategy for the selective synthesis of six-membered heterocyclic compounds pyrimidines, and pyridines from commercially available acetophenones and benzaldehydes. In this protocol, same amounts of acetophenone and benzaldehyde were involved by using HMDS as a nitrogen source and control of Lewis acids under microwave irradiation. Acid catalysts TMSOTf and BF3·OEt2 played an important role in this selective transformation of corresponding pyrimidines and pyridines obtained in modest yields in this MCR method.
Experimental section
General information
All reagents and solvents were commercially available and used without further purification. Reactions were routinely performed using the discover SP system (CEM) in the sealed reaction vessels in standard mode with the temperature monitored using a vertically focused IR sensor. All reactions were monitored by thin-layer chromatography on silica gel 60 F254 (Merck) with detection by UV light. Column chromatography was performed using silica gel (200–300 mesh). Products in organic solvents were dried with anhydrous magnesium sulfate before concentration in vacuo. Melting points were determined with an MP-2D melting apparatus. 1H and 13C NMR spectra were recorded on a Bruker AVIII 500 spectrometer operating at 500 and 125 MHz, respectively. Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet, br = broad), coupling constants (Hz) and integration. HRMS were obtained on a Waters LCT Premier XE (Waters Corp., Manchester, UK) instrument equipped with an electrospray source. The X-ray intensity data were measured at a low temperature of 100 K using a Mo Kα radiation diffractometer equipped with a kappa geometry goniometer and corrected for absorption effects using the numerical method (SADABS).
General procedure for the synthesis of skeleton 1
A mixture of acetophenone 3 (4.0 mmol), benzaldehyde 4 (4.0 mmol), HMDS (14.4 mmol), BF3·OEt2 (0.5 mmol) and toluene (2 mL) in a dried 35 mL microwave vial at 25 °C. The mixture was subjected to a microwave irradiation instrument and stirred at 150 °C for 0.5 h. The consumption of the starting materials was confirmed by TLC. The reaction was cooled to 25 °C, the mixture of crude product was transferred to a 100 mL round bottom flask, and the solvent was concentrated. The residue was diluted with water (10 mL) and the mixture was extracted with CH2Cl2 (3 × 15 mL). The combined organic layers were washed with brine, dried, filtered and evaporated to afford crude product under reduced pressure. Purification on silica gel (hexanes/EtOAc = 8/1–3/1) afforded compounds 1aa–1pa.
2,4,6-Triphenylpyrimidine (1aa)22. Yield = 78% (481 mg); colorless solid; mp = 191–192 °C; HRMS (ESI, M+ + H) calcd for C22H17N2 309.1386, found 309.1395; 1H NMR (500 MHz, CDCl3): δ 8.76 (d, J = 8.0 Hz, 2H), 8.33–8.27 (m, 4H), 8.02 (s, 1H), 7.62–7.51 (m, 9H); 13C NMR (125 MHz, CDCl3): δ 164.70 (2x), 164.46, 138.13, 137.50 (2x), 130.74 (2x), 130.60, 128.87 (4x), 128.45 (2x), 128.41 (2x), 127.25 (4x), 110.25.
2,4-Bis(3-fluorophenyl)-6-phenylpyrimidine (1ab). Yield = 65% (447 mg); colorless solid; mp = 217–218 °C; HRMS (ESI, M+ + H) calcd for C22H15F2N2 345.1198, found 345.1200; 1H NMR (500 MHz, CDCl3): δ 8.49 (d, J = 7.5 Hz, 1H), 8.38 (d, J = 10.0 Hz, 1H), 8.29–8.22 (m, 2H), 8.04–7.97 (m, 2H), 7.95 (s, 1H), 7.59–7.47 (m, 5H), 7.27–7.19 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 165.04, 163.30 (d, J = 244.875 Hz), 163.17 (d, J = 243.125 Hz), 139.92 (d, J = 94.125 Hz), 139.86 (d, J = 93.875 Hz), 136.94, 131.05, 130.43 (d, J = 8.0 Hz), 129.89 (d, J = 7.875 Hz), 128.95 (2x), 127.23 (2x), 124.05 (d, J = 2.25 Hz), 122.74 (d, J = 2.375 Hz), 117.75 (d, J = 21.25 Hz, 2x), 117.58 (d, J = 21.25 Hz, 2x), 115.21 (d, J = 23.0 Hz), 114.19 (d, J = 22.75 Hz), 110.53.
2,4-Bis(4-fluorophenyl)-6-phenylpyrimidine (1ac)30a. Yield = 70% (482 mg); colorless solid; mp = 212–213 °C; HRMS (ESI, M+ + H) calcd for C22H15F2N2 345.1198, found 345.1196; 1H NMR (500 MHz, CDCl3): δ 8.71 (t, J = 7.5 Hz, 2H), 8.31–8.22 (m, 4H), 7.93 (s, 1H), 7.60–7.53 (m, 3H), 7.28–7.16 (m, 4H); 13C NMR (125 MHz, CDCl3): δ 164.73 (d, J = 248.75 Hz), 164.59 (d, J = 254.875 Hz), 164.85, 163.62, 137.28, 134.18 (d, J = 2.5125 Hz), 133.51 (d, J = 2.875 Hz), 130.89, 130.53 (d, J = 8.625 Hz, 2x), 129.25 (d, J = 8.625 Hz, 2x), 128.92 (2x), 128.70, 127.22 (2x), 115.93 (d, J = 21.625 Hz, 2x), 115.35 (d, J = 21.375 Hz, 2x), 109.72.
2,4-Bis(3-chlorophenyl)-6-phenylpyrimidine (1ad). Yield = 60% (451 mg); white solid; mp = 173–174 °C; HRMS (ESI, M+ + H) calcd for C22H15Cl2N2 377.0612, found 377.0608; 1H NMR (500 MHz, CDCl3): δ 8.66–8.62 (m, 1H), 8.56 (d, J = 7.5 Hz, 1H), 8.26–8.20 (m, 3H), 8.10 (d, J = 7.5 Hz, 1H), 7.92 (s, 1H), 7.58–7.53 (m, 3H), 7.52–7.43 (m, 4H); 13C NMR (125 MHz, CDCl3): δ 165.03, 163.31, 163.23, 139.66, 138.97, 136.86, 135.08, 134.57, 131.08, 130.81, 130.68, 130.14, 129.67, 128.94 (2x), 128.44, 127.31, 127.24 (2x), 126.57, 125.31, 110.55.
2,4-Bis(4-chlorophenyl)-6-phenylpyrimidine (1ae)23c. Yield = 58% (436 mg); white solid; mp = 200–201 °C; HRMS (ESI, M+ + H) calcd for C22H15Cl2N2 377.0612, found 377.0606; 1H NMR (500 MHz, CDCl3): δ 8.64 (d, J = 7.0 Hz, 2H), 8.26 (d, J = 2.5 Hz, 2H), 8.20 (d, J = 7.0 Hz, 2H), 7.97 (s, 1H), 7.57–7.50 (m, 7H); 13C NMR (125 MHz, CDCl3): δ 165.05, 163.62, 163.60, 137.15, 137.12, 136.94, 136.43, 135.73, 131.02, 129.79 (2x), 129.18 (2x), 128.98 (2x), 128.68 (2x), 128.53 (2x), 127.26, (2x) 110.13.
4-Phenyl-2,6-bis(4-(trifluoromethyl)phenyl)pyrimidine (1af)30a. Yield = 62% (551 mg); white solid; mp = 179–180 °C; HRMS (ESI, M+ + H) calcd for C24H15F6N2 445.1139, found 445.1137; 1H NMR (500 MHz, CDCl3): δ 8.80 (d, J = 8.0 Hz, 2H), 8.37 (d, J = 8.0 Hz, 2H), 8.31–8.26 (m, 2H), 8.07 (s, 1H), 7.83 (d, J = 8.0 Hz, 2H), 7.79 (d, J = 8.0 Hz, 2H), 7.62–7.56 (m, 3H); 13C NMR (125 MHz, CDCl3): δ 165.43, 163.48, 163.39, 141.03, 140.51, 136.78, 132.66 (q, J = 30.35 Hz, 2x), 136.42 (q, J = 30.35 Hz, 2x), 131.30, 129.07 (2x), 128.73 (2x), 127.62 (2x), 127.30 (2x), 125.93 (q, J = 3.65 Hz), 125.42 (q, J = 3.65 Hz), 124.17 (d, J = 272.30 Hz), 123.91 (d, J = 272.30 Hz), 111.20.
4-Phenyl-2,6-di-m-tolylpyrimidine (1ag). Yield = 59% (397 mg); white solid; mp = 107–108 °C; HRMS (ESI, M+ + H) calcd for C24H21N2 337.1699, found 337.1703; 1H NMR (500 MHz, CDCl3): δ 8.56–8.50 (m, 2H), 8.35 (dd, J = 1.5, 8.0 Hz, 2H), 8.10 (s, 1H), 8.08 (d, J = 7.5 Hz, 1H), 8.00 (s, 1H), 7.60–7.53 (m, 3H), 7.49–7.41 (m, 2H), 7.39–7.32 (m, 2H), 2.52 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 164.97, 164.65 (2x), 138.61, 138.12, 138.02, 137.63, 137.58, 131.51, 131.40, 130.69, 128.97, 128.89 (2x), 128.81, 128.35, 127.89, 127.29 (2x), 125.70, 124.47, 110.40, 21.60 (2x).
4-Phenyl-2,6-di-p-tolylpyrimidine (1ah)25. Yield = 64% (430 mg); colorless solid; mp = 167–168 °C; HRMS (ESI, M+ + H) calcd for C24H21N2 337.1699, found 337.1696; 1H NMR (500 MHz, CDCl3): δ 8.63 (d, J = 8.0 Hz, 2H), 8.29 (dd, J = 1.5, 8.5 Hz, 2H), 8.20 (d, J = 8.0 Hz, 2H), 7.96 (s, 1H), 7.59–7.52 (m, 3H), 7.34 (t, J = 8.0 Hz, 4H), 2.47 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 164.58, 164.50 (2x), 141.01, 140.69, 137.76, 135.58, 134.83, 130.58, 129.59 (2x), 129.15 (2x), 128.83 (2x), 128.41 (2x), 127.24 (2x), 127.15 (2x), 109.66, 21.52, 21.45.
2,4-Bis(4-methoxyphenyl)-6-phenylpyrimidine (1ai)25. Yield = 76% (560 mg); yellow solid; mp = 126–127 °C; HRMS (ESI, M+ + H) calcd for C24H21N2O2 369.1598, found 369.1598; 1H NMR (500 MHz, CDCl3): δ 8.68 (d, J = 8.5 Hz, 2H), 8.29–8.23 (m, 4H), 7.89 (s, 1H), 7.59–7.49 (m, 3H), 7.09–7.02 (m, 4H), 3.90 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 164.34, 164.11, 164.06, 161.82, 161.72, 137.84, 131.04, 130.52, 130.09, 130.03 (2x), 128.81 (2x), 128.72 (2x), 127.20 (2x), 114.19 (2x), 113.69 (2x), 108.76, 55.41, 55.35.
2,4-Bis(3,4-dimethoxyphenyl)-6-phenylpyrimidine (1aj). Yield = 72% (617 mg); white solid; mp = 184–185 °C; HRMS (ESI, M+ + H) calcd for C26H25N2O4 429.1809, found 429.1811; 1H NMR (500 MHz, CDCl3): δ 8.32 (d, J = 8.5 Hz, 1H), 8.26 (s, 1H), 8.25–8.21 (m, 2H), 7.90 (s, 1H), 7.85–7.81 (m, 1H), 7.80–7.75 (m, 1H), 7.57–7.49 (m, 3H), 7.02–6.94 (m, 2H), 4.04 (s, 3H), 4.02 (s, 3H), 3.96 (s, 3H), 3.94 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 164.20, 163.81 (2x), 151.31, 151.19, 149.14, 148.69, 137.61, 131.04, 130.49, 130.16, 128.72 (2x), 127.10 (2x), 121.67, 120.15, 111.03, 110.89, 110.62, 109.91, 108.84, 55.89, 55.84 (2x), 55.78.
2,4-Di([1,1′-biphenyl]-4-yl)-6-phenylpyrimidine (1ak). Yield = 74% (681 mg); white solid; mp = 215–216 °C; HRMS (ESI, M+ + H) calcd for C34H25N2 461.2012, found 461.2002; 1H NMR (500 MHz, CDCl3): δ 8.82 (d, J = 8.0 Hz, 2H), 8.40 (d, J = 8.0 Hz, 2H), 8.33 (d, J = 8.0 Hz, 2H), 8.07 (s, 1H), 7.80 (t, J = 8.0 Hz, 4H), 7.71 (t, J = 8.0 Hz, 4H), 7.63–7.56 (m, 3H), 7.53–7.48 (m, 4H), 7.44–7.37 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 164.78, 164.34, 164.32, 143.57, 143.27, 140.76, 140.33, 137.57, 137.15, 136.37, 130.79, 128.93 (8x), 128.83, 127.85, 127.73, 127.60 (2x), 127.30, 127.20 (4x), 127.18 (4x), 110.12.
2,4-Di(furan-2-yl)-6-phenylpyrimidine (1al). Yield = 56% (323 mg); white solid; mp = 108–109 °C; HRMS (ESI, M+ + H) calcd for C18H13N2O2 289.0972, found 289.0966; 1H NMR (500 MHz, CDCl3): δ 8.24–8.21 (m, 2H), 7.87 (s, 1H), 7.68 (s, 1H), 7.64 (s, 1H), 7.55–7.46 (m, 3H), 7.47 (d, J = 3.0 Hz, 1H), 7.42 (d, J = 3.0 Hz, 1H), 6.65–6.55 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 164.88, 157.93, 156.30, 152.53, 152.05, 144.95 (2x), 136.93, 130.92, 128.87 (2x), 127.24 (2x), 113.63, 112.50, 112.48, 112.05, 107.88.
4-(4-Fluorophenyl)-2,6-diphenylpyrimidine (1ba)22. Yield = 56% (365 mg); white solid; mp = 162–163 °C; HRMS (ESI, M+ + H) calcd for C22H16FN2 327.1292, found 327.1294; 1H NMR (500 MHz, CDCl3): δ 8.71 (d, J = 7.5 Hz, 2H), 8.31–8.28 (m, 4H), 7.97 (s, 1H), 7.60–7.51 (m, 6H), 7.27–7.23 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 164.86, 164.59 (d, J = 249.475 Hz), 164.51, 163.63, 138.01, 137.43, 133.65 (d, J = 2.75 Hz), 130.84, 130.71, 129.29 (d, J = 8.5 Hz, 2x), 128.93 (2x), 128.46 (2x), 128.44 (2x), 127.26 (2x), 115.93 (d, J = 21.5 Hz, 2x), 109.89.
4-(4-Chlorophenyl)-2,6-diphenylpyrimidine (1ca)22. Yield = 74% (506 mg); white solid; mp = 158–159 °C; HRMS (ESI, M+ + H) calcd for C22H16ClN2 343.0997, found 343.0987; 1H NMR (500 MHz, CDCl3): δ 8.71 (d, J = 7.5 Hz, 2H), 8.29 (d, J = 7.5 Hz, 2H), 8.25 (d, J = 7.0 Hz, 2H), 7.98 (s, 1H), 7.59–7.53 (m, 8H); 13C NMR (125 MHz, CDCl3): δ 164.98, 164.58, 163.52, 137.95, 137.36, 136.97, 135.95, 130.90, 130.75, 129.14 (2x), 128.94 (2x), 128.55 (2x), 128.47 (2x), 128.45 (2x), 127.28 (2x), 109.98.
2,4-Diphenyl-6-(p-tolyl)pyrimidine (1da)22. Yield = 64% (412 mg); colorless solid; mp = 146–147 °C; HRMS (ESI, M+ + H) calcd for C23H19N2 323.1543, found 323.1534; 1H NMR (500 MHz, CDCl3): δ 8.73 (d, J = 6.5 Hz, 2H), 8.29 (d, J = 6.5 Hz, 2H), 8.21 (d, J = 7.0 Hz, 2H), 8.00 (s, 1H), 7.57–7.52 (m, 6H), 7.37 (d, J = 8.0 Hz, 2H), 2.47 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 164.67, 164.60, 164.42, 141.13, 138.24, 137.64, 134.71, 130.67, 130.54, 129.63 (2x), 128.87 (2x), 128.44 (2x), 128.40 (2x), 127.25 (2x), 127.17 (2x), 109.93, 21.47.
4-(4-Methoxyphenyl)-2,6-diphenylpyrimidine (1ea)22. Yield = 69% (467 mg); colorless solid; mp = 136–137 °C; HRMS (ESI, M+ + H) calcd for C23H19N2O 339.1492, found 339.1494; 1H NMR (500 MHz, CDCl3): δ 8.72 (d, J = 8.0 Hz, 2H), 8.29 (d, J = 7.5 Hz, 4H), 7.96 (s, 1H), 7.57–7.51 (m, 6H), 7.07 (d, J = 8.5 Hz, 2H), 3.91 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 164.49, 164.35, 164.21, 161.92, 138.28, 137.71, 130.63, 130.52, 129.96, 128.87 (2x), 128.78 (2x), 128.42 (2x), 128.40 (2x), 127.24 (2x), 114.25 (2x), 109.42, 55.44.
4-([1,1′-Biphenyl]-4-yl)-2,6-diphenylpyrimidine (1fa)22. Yield = 71% (546 mg); colorless solid; mp = 174–175 °C; HRMS (ESI, M+ + H) calcd for C28H21N2 385.1699, found 385.1705; 1H NMR (500 MHz, CDCl3): δ 8.76 (d, J = 8.5 Hz, 2H), 8.39 (d, J = 8.5 Hz, 2H), 8.32 (d, J = 8.0 Hz, 2H), 8.06 (s, 1H), 7.80 (d, J = 8.0 Hz, 2H), 7.70 (d, J = 7.5 Hz, 2H), 7.61–7.53 (m, 6H), 7.52–7.48 (m, 2H), 7.44–7.39 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 164.75, 164.52, 164.31, 143.55, 140.32, 138.16, 137.55, 136.36, 130.76, 130.63, 128.91 (3x), 128.47 (2x), 128.44 (2x), 127.84, 127.71 (2x), 127.58 (2x), 127.28 (2x), 127.17 (2x), 127.12, 110.13.
4-(3-Fluorophenyl)-2,6-diphenylpyrimidine (1ga)27. Yield = 65% (424 mg); white solid; mp = 184–185 °C; HRMS (ESI, M+ + H) calcd for C22H16FN2 327.1292, found 327.1293; 1H NMR (500 MHz, CDCl3): δ 8.72 (d, J = 7.5 Hz, 2H), 8.30 (d, J = 7.5 Hz, 2H), 8.05 (d, J = 8.5 Hz, 2H), 7.99 (s, 1H), 7.59–751 (m, 7H), 7.24–7.22 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 165.06, 164.60, 163.39 (d, J = 2.5 Hz), 163.34 (d, J = 244.75 Hz), 139.88 (d, J = 7.375 Hz), 137.88, 137.30, 130.85 (d, J = 17.875 Hz, 2x), 130.42 (d, J = 8.0 Hz), 128.95 (2x), 128.48 (2x), 128.47 (2x), 127.28 (2x), 122.77 (d, J = 2.375 Hz), 117.62 (d, J = 21.125 Hz), 114.26 (d, J = 22.875 Hz), 110.27.
4-(3-Bromophenyl)-2,6-diphenylpyrimidine (1ha)30b. Yield = 66% (510 mg); white solid; mp = 128–129 °C; HRMS (ESI, M+ + H) calcd for C22H16BrN2 387.0491, found 387.0491; 1H NMR (500 MHz, CDCl3): δ 8.72 (d, J = 8.5 Hz, 2H), 8.44 (s, 1H), 8.29 (d, J = 8.0 Hz, 2H), 8.20 (d, J = 8.0 Hz, 1H), 7.97 (s, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.61–7.52 (m, 6H), 7.43 (t, J = 8.0 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 165.04, 164.61, 163.21, 139.60, 137.85, 137.24, 133.61, 130.95, 130.80, 130.40, 130.31, 128.94 (2x), 128.48 (4x), 127.29 (2x), 125.80, 123.20, 110.25.
4-(3-Methoxyphenyl)-2,6-diphenylpyrimidine (1ia)30c. Yield = 69% (467 mg); white solid; mp = 124–125 °C; HRMS (ESI, M+ + H) calcd for C23H19N2O 339.1492, found 339.1489; 1H NMR (500 MHz, CDCl3): δ 8.74 (d, J = 6.5 Hz, 2H), 8.30 (d, J = 6.0 Hz, 2H), 8.00 (s, 1H), 7.90 (s, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.60–7.50 (m, 6H), 7.48 (t, J = 8.0 Hz, 1H), 7.09 (dd, J = 1.5, 8.0 Hz, 1H), 3.95 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 164.71, 164.48, 164.41, 160.12, 138.98, 138.08, 137.46, 130.75, 130.61, 129.87, 128.87 (2x), 128.44, (2x) 128.41 (2x), 127.25 (2x), 119.59, 116.29, 112.77, 110.40, 55.43.
4-(2-Fluorophenyl)-2,6-diphenylpyrimidine (1ja)30d. Yield = 64% (417 mg); white solid; mp = 143–144 °C; HRMS (ESI, M+ + H) calcd for C22H16FN2 327.1292, found 327.1293; 1H NMR (500 MHz, CDCl3): δ 8.74–8.68 (m, 2H), 8.44–8.38 (m, 1H), 8.32–8.27 (m, 2H), 8.17 (d, J = 1.5 Hz, 1H), 7.60–7.47 (m, 7H), 7.40–7.34 (m, 1H), 7.25–7.22 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 164.54 (d, J = 23.125 Hz), 161.51 (d, J = 250.5 Hz), 160.90, 160.87, 138.04, 137.39, 132.05 (d, J = 8.875 Hz), 131.10 (d, J = 2.0 Hz), 130.82, 130.66, 128.90 (2x), 128.46 (2x), 128.38 (2x), 127.38 (2x), 125.63 (d, J = 10.375 Hz), 124.70 (d, J = 3.25 Hz), 116.54 (d, J = 0.375 Hz), 114.53 (d, J = 0.375 Hz).
4-(2-Chlorophenyl)-2,6-diphenylpyrimidine (1ka)22. Yield = 61% (417 mg); white solid; mp = 118–119 °C; HRMS (ESI, M+ + H) calcd for C22H16ClN2 343.0997, found 343.0996; 1H NMR (500 MHz, CDCl3): δ 8.69–8.66 (m, 2H), 8.30–8.27 (m, 2H), 8.03 (s, 1H), 7.89–7.82 (m, 1H), 7.59–7.50 (m, 7H), 7.49–7.41 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 164.69, 164.64, 163.89, 137.95, 137.58, 137.27, 132.44, 131.70, 130.89, 130.69, 130.66, 130.50, 128.94 (2x), 128.47 (4x), 127.38 (2x), 127.22, 115.16.
4-(2-Bromophenyl)-2,6-diphenylpyrimidine (1la)22. Yield = 56% (432 mg); white solid; mp = 130–131 °C; HRMS (ESI, M+ + H) calcd for C22H16ClN2 387.0491, found 387.0498; 1H NMR (500 MHz, CDCl3): δ 8.68 (d, J = 7.5 Hz, 2H), 8.29 (d, J = 7.0 Hz, 2H), 7.97 (s, 1H), 7.80–7.73 (m, 2H), 7.60–7.47 (m, 7H), 7.35 (t, J = 8.0 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 166.09, 164.55, 163.82, 139.59, 137.91, 137.22, 133.75, 131.57, 130.90, 130.72, 130.70, 128.94 (2x), 128.49 (2x), 128.47 (2x), 127.76, 127.36 (2x), 121.65, 115.07.
2,4-Diphenyl-6-(o-tolyl)pyrimidine (1ma)22. Yield = 54% (348 mg); white solid; mp = 59–60 °C; HRMS (ESI, M+ + H) calcd for C23H19N2 323.1543, found 323.1551; 1H NMR (500 MHz, CDCl3): δ 8.73–8.66 (m, 2H), 8.32–8.30 (m, 2H), 7.77 (s, 1H), 7.66–7.50 (m, 7H), 7.46–7.35 (m, 3H), 2.61 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 168.19, 164.16, 164.04, 138.65, 138.12, 137.37, 136.50, 131.27, 130.81, 130.61, 129.63, 129.41, 128.93 (2x), 128.46 (4x), 127.26 (2x), 126.15, 114.36, 20.71.
4-(2-Methoxyphenyl)-2,6-diphenylpyrimidine (1na)27. Yield = 58% (392 mg); white solid; mp = 67–68 °C; HRMS (ESI, M+ + H) calcd for C23H19N2O 339.1492, found 339.1500; 1H NMR (500 MHz, CDCl3): δ 8.70 (d, J = 7.0 Hz, 2H), 8.30 (s, 1H), 8.29–8.24 (m, 3H), 7.56–7.49 (m, 7H), 7.18 (t, J = 8.0 Hz, 1H), 7.08 (d, J = 8.0 Hz, 1H), 3.96 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 164.20, 163.60, 163.40, 158.06, 138.39, 137.91, 131.49, 131.33, 130.46, 130.36, 128.81 (2x), 128.36 (2x), 128.33 (2x), 127.34, 126.98 (2x), 121.17, 115.39, 111.60, 55.76.
4-(4-Nitrophenyl)-2,6-diphenylpyrimidine (1oa)22. Yield = 71% (501 mg); yellow solid; mp = 216–217 °C; HRMS (ESI, M+ + H) calcd for C22H16ClN2 354.1237, found 354.1231; 1H NMR (500 MHz, CDCl3): δ 8.71 (d, J = 5.0 Hz, 2H), 8.46 (d, J = 8.5 Hz, 2H), 8.41 (d, J = 9.0 Hz, 2H), 8.30 (d, J = 3.5 Hz, 2H), 8.06 (s, 1H), 7.59–7.56 (m, 6H); 13C NMR (125 MHz, CDCl3): δ 165.49, 164.86, 162.30, 149.17, 143.42, 137.53, 136.92, 131.24, 131.07, 129.05 (2x), 128.58 (2x), 128.48 (2x), 128.20 (2x), 127.32 (2x), 124.10 (2x), 110.84.
4-(2,6-Diphenylpyrimidin-4-yl)benzonitrile (1pa)22. Yield = 65% (433 mg); yellow solid; mp = 185–186 °C; HRMS (ESI, M+ + H) calcd for C23H16N3 334.1339, found 334.1334; 1H NMR (500 MHz, CDCl3): δ 8.71–8.64 (m, 2H), 8.33 (d, J = 8.5 Hz, 2H), 8.28–8.22 (m, 2H), 7.94 (s, 1H), 7.80 (d, J = 8.5 Hz, 2H), 7.60–7.51 (m, 6H); 13C NMR (125 MHz, CDCl3): δ 165.18, 164.59, 162.40, 141.46, 137.48, 136.82, 132.55 (2x), 131.12, 130.95, 128.94 (2x), 128.48 (2x), 128.37 (2x), 127.69 (2x), 127.20 (2x), 1218.47, 113.98, 110.40.
General procedure for the synthesis of skeleton 2
A mixture of acetophenone 3 (4.0 mmol), benzaldehyde 4 (4.0 mmol), HMDS (14.4 mmol), TMSOTf (0.5 mmol) and toluene (2 mL) in a dried 35 mL microwave vial at 25 °C. The mixture was subjected to a microwave irradiation instrument and stirred at 150 °C for 0.5 h. The consumption of the starting materials was confirmed by TLC. The reaction was cooled to 25 °C, the mixture of crude product was transferred to a 100 mL round bottom flask, and the solvent was concentrated. The residue was diluted with water (10 mL) and the mixture was extracted with CH2Cl2 (3 × 15 mL). The combined organic layers were washed with brine, dried, filtered and evaporated to afford crude product under reduced pressure. Purification on silica gel (hexanes/EtOAc = 8/1–3/1) afforded compounds 2aa–2av.
2,4,6-Triphenylpyridine (2aa)22. Yield = 92% (565 mg); colorless solid; mp = 138–139 °C; HRMS (ESI, M+ + H) calcd for C23H18N 308.1439, found 308.1447; 1H NMR (500 MHz, CDCl3): δ 8.35–8.31 (m, 4H), 7.96 (s, 2H), 7.82–7.80 (m, 2H), 7.66–7.57 (m, 6H), 7.57–7.51 (m, 3H); 13C NMR (125 MHz, CDCl3): δ 157.32 (2x), 149.99, 139.45 (2x), 138.87, 128.96 (2x), 128.93 (2x), 128.83, 128.58 (4x), 127.03 (6x), 116.93 (2x).
4-(4-Fluorophenyl)-2,6-diphenylpyridine (2ac)28f. Yield = 76% (535 mg); colorless solid; mp = 141–142 °C; HRMS (ESI, M+ + H) calcd for C23H17FN 326.1340, found 326.1337; 1H NMR (500 MHz, CDCl3): δ 8.22 (d, J = 8.0 Hz, 4H), 7.84 (s, 2H), 7.74–7.71 (m, 2H), 7.54 (t, J = 8.0 Hz, 4H), 7.49–7.46 (m, 2H), 7.23 (t, J = 8.5 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ 163.31 (d, J = 247.25 Hz), 157.49 (2x), 149.04, 139.36 (2x), 135.03 (d, J = 3.0 Hz), 129.08 (2x), 128.87 (d, J = 8.25 Hz, 2x), 128.68 (4x), 127.06 (4x), 116.83 (2x), 116.06 (d, J = 21.375 Hz, 2x).
4-(4-Chlorophenyl)-2,6-diphenylpyridine (2ae)28f. Yield = 83% (566 mg); yellow solid; mp = 119–120 °C; HRMS (ESI, M+ + H) calcd for C23H17ClN 342.1044, found 342.1048; 1H NMR (500 MHz, CDCl3): δ 8.20 (d, J = 8.0 Hz, 4H), 7.84 (s, 2H), 7.68 (d, J = 8.5 Hz, 2H), 7.56–7.49 (m, 6H), 7.48–7.44 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 157.66 (2x), 148.92, 139.38 (2x), 137.46, 135.17, 139.31 (2x), 129.15 (2x), 128.72 (4x), 128.43 (2x), 127.10 (4x), 116.79 (2x).
2,6-Diphenyl-4-(m-tolyl)pyridine (2ag)28f. Yield = 80% (514 mg); yellow solid; mp = 84–85 °C; HRMS (ESI, M+ + H) calcd for C24H20N 322.1590, found 322.1588; 1H NMR (500 MHz, CDCl3): δ 8.26–8.20 (m, 4H), 7.90 (s, 2H), 7.5–7.51 (m, 6H), 7.49–7.41 (m, 3H), 7.31 (d, J = 7.5 Hz, 1H), 2.49 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 157.42, 150.30, 139.61, 139.02, 138.80, 129.70, 128.99, 128.67, 127.86, 127.11, 124.27, 117.13, 21.52.
2,6-Diphenyl-4-(p-tolyl)pyridine (2ah)28f. Yield = 93% (597 mg); white solid; mp = 117–118 °C; HRMS (ESI, M+ + H) calcd for C24H20N 322.1590, found 322.1588; 1H NMR (500 MHz, CDCl3): δ 8.23 (d, J = 8.5 Hz, 4H), 7.90 (s, 2H), 7.68 (d, J = 8.5 Hz, 2H), 7.54 (t, J = 8.0 Hz, 4H), 7.47 (t, J = 7.5 Hz, 2H), 7.36 (d, J = 8.0 Hz, 2H), 2.46 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 157.42 (2x), 150.00, 139.64 (2x), 139.03, 136.03, 129.79 (2x), 128.95 (2x), 128.65 (4x), 127.10 (4x), 126.96 (2x), 116.85 (2x), 21.22.
4-(4-Methoxyphenyl)-2,6-diphenylpyridine (2ai)28f. Yield = 81% (546 mg); white solid; mp = 101–102 °C; HRMS (ESI, M+ + H) calcd for C24H20NO 338.1545, found 338.1541; 1H NMR (500 MHz, CDCl3): δ 8.22 (d, J = 7.5 Hz, 4H), 7.87 (s, 2H), 7.72 (d, J = 9.0 Hz, 2H), 7.57–7.50 (m, 4H), 7.49–7.43 (m, 2H), 7.06 (d, J = 9.0 Hz, 2H), 3.89 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.43, 157.41 (2x), 149.59, 139.68 (2x), 131.24, 128.93 (2x), 128.64 (4x), 128.28 (2x), 127.09 (4x), 116.57 (2x), 114.49 (2x), 55.38.
4-(Furan-2-yl)-2,6-diphenylpyridine (2al)28f. Yield = 81% (481 mg); brown solid; mp = 154–155 °C; HRMS (ESI, M+ + H) calcd for C21H16NO 298.1226, found 298.1236; 1H NMR (500 MHz, CDCl3): δ 8.26–8.18 (m, 4H), 7.95 (s, 2H), 7.61–7.46 (m, 7H), 6.99 (d, J = 3.0 Hz, 1H), 6.62–6.54 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 157.48 (2x), 151.95, 143.58, 139.44 (2x), 139.03, 129.05 (2x), 128.64 (4x), 127.04 (4x), 112.97 (2x), 112.08, 108.45.
4-(3-Nitrophenyl)-2,6-diphenylpyridine (2am)30e. Yield = 81% (570 mg); colorless solid; mp = 140–141 °C; HRMS (ESI, M+ + H) calcd for C23H17N2O2 353.1285, found 353.1280; 1H NMR (500 MHz, CDCl3): δ 8.59 (s, 1H), 8.33 (d, J = 8.0 Hz, 1H), 8.21 (d, J = 8.0 Hz, 4H), 8.05 (d, J = 7.5 Hz, 1H), 7.88 (s, 2H), 7.71 (t, J = 8.0 Hz, 1H), 7.57–7.51 (m, 4H), 7.50–7.44 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 157.92 (2x), 148.81, 147.60, 140.72, 138.98 (2x), 133.06, 130.14, 129.37 (2x), 128.77 (4x), 127.09 (4x), 123.61, 122.07, 116.70 (2x).
4-(4-Nitrophenyl)-2,6-diphenylpyridine (2an)28f. Yield = 87% (612 mg); white solid; mp = 174–175 °C; HRMS (ESI, M+ + H) calcd for C23H17N2O2 353.1290, found 353.1290; 1H NMR (500 MHz, CDCl3): δ 8.39 (d, J = 8.5 Hz, 2H), 8.24–8.18 (m, 4H), 7.90 (d, J = 8.5 Hz, 2H), 7.88 (s, 2H), 7.56–7.51 (m, 4H), 7.51–7.45 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 157.96 (2x), 148.14, 147.83, 145.44, 139.00 (2x), 129.42 (2x), 128.81 (4x), 128.16 (2x), 127.11 (4x), 124.35 (2x), 116.93 (2x).
4-(2-Methoxyphenyl)-2,6-diphenylpyridine (2ao)28f. Yield = 83% (560 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C24H20NO 338.1545, found 338.1541; 1H NMR (500 MHz, CDCl3): δ 8.20 (d, J = 7.5 Hz, 4H), 7.88 (s, 2H), 7.55–7.49 (m, 4H), 7.48–7.41 (m, 4H), 7.14–7.09 (m, 1H), 7.07 (d, J = 8.5 Hz, 1H), 3.88 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 156.74, 156.60, 147.87, 139.85, 130.50, 130.02, 128.78, 128.60, 128.46, 127.13, 121.06, 119.70, 111.44, 55.67.
4-(3-Methoxyphenyl)-2,6-diphenylpyridine (2ap)28f. Yield = 89% (600 mg); white solid; mp = 124–125 °C; HRMS (ESI, M+ + H) calcd for C24H20NO 338.1539, found 338.1538; 1H NMR (500 MHz, CDCl3): δ 8.23 (d, J = 7.5 Hz, 4H), 7.90 (s, 2H), 7.54 (t, J = 7.5 Hz, 4H), 7.50–7.43 (m, 3H), 7.35 (d, J = 7.5 Hz, 1H), 7.28 (t, J = 2.0 Hz, 1H), 7.03 (dd, J = 2.0, 8.0 Hz, 1H), 3.92 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.14 157.46 (2x), 150.06, 140.53, 139.53 (2x), 130.14, 129.02 (2x), 128.67 (4x), 127.10 (4x), 119.57, 117.13 (2x), 114.17, 112.99, 55.40.
4-(3,5-Dimethoxyphenyl)-2,6-diphenylpyridine (2aq). Yield = 79% (580 mg); colorless solid; mp = 101–102 °C; HRMS (ESI, M+ + H) calcd for C25H22NO2 368.1645, found 368.1644; 1H NMR (500 MHz, CDCl3): δ 8.26 (d, J = 7.0 Hz, 4H), 7.89 (s, 2H), 7.56 (t, J = 7.0 Hz, 4H), 7.49 (t, J = 7.0 Hz, 2H), 6.91 (s, 2H), 6.61 (s, 1H), 3.90 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 161.42 (2x), 157.51 (2x), 150.28, 141.32, 139.61 (2x), 129.14 (2x), 128.78 (4x), 127.21 (4x), 117.21 (2x), 105.57 (2x), 100.67, 55.57 (2x).
4-(Benzo[d][1,3]dioxol-5-yl)-2,6-diphenylpyridine (2ar)28f. Yield = 87% (611 mg); white solid; mp = 152–153 °C; HRMS (ESI, M+ + H) calcd for C24H18NO2 352.1332, found 352.1333; 1H NMR (500 MHz, CDCl3): δ 8.20–8.18 (m, 4H), 7.82 (s, 2H), 7.55–7.48 (m, 4H), 7.47–7.42 (m, 2H), 7.28–7.21 (m, 2H), 6.96 (d, J = 8.0 Hz, 1H), 6.06 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 157.46 (2x), 149.71, 148.47, 148.44, 139.55 (2x), 133.12, 129.02 (2x), 128.69 (4x), 127.09 (4x), 121.06, 116.76 (2x), 108.86, 107.45, 101.49. Single-crystal X-ray diagram: crystal of 2q was grown by slow diffusion of EtOAc into a solution of 2q in CH2Cl2 to yield colorless prisms. The compound crystallizes in the monoclinic crystal system, space group P21/n, a = 6.4058(2) Å, b = 12.1092(3) Å, c = 21.6453(7) Å, V = 1677.71(9) Å3, Z = 4, dcalcd = 1.391 mg m−3, F(000) = 736, 2θ range 1.927–27.102, R indices (all data) R1 = 0.0412, wR2 = 0.0956. CCDC number is 2085356.†
4-(Anthracen-9-yl)-2,6-diphenylpyridine (2as)28f. Yield = 62% (505 mg); yellow solid; mp = 222–223 °C; HRMS (ESI, M+ + H) calcd for C31H22N 408.1747, found 408.1745; 1H NMR (500 MHz, CDCl3): δ 8.58 (s, 1H), 8.28–8.22 (m, 4H), 8.10 (d, J = 8.5 Hz, 2H), 7.84 (s, 2H), 7.75 (d, J = 9.0 Hz, 2H), 7.54–7.48 (m, 6H), 7.47–7.43 (m, 2H), 7.42–7.37 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 157.03 (2x), 148.90, 139.23 (2x), 134.30, 131.29 (2x), 129.53 (2x), 129.20 (2x), 128.74 (4x), 128.53 (2x), 127.48, 127.13 (4x), 126.15 (2x), 126.07 (2x), 125.35 (2x), 121.39 (2x).
4-(Naphthalen-2-yl)-2,6-diphenylpyridine (2at)28f. Yield = 83% (593 mg); white solid; mp = 128–129 °C; HRMS (ESI, M+ + H) calcd for C27H20N 358.1590, found 358.1590; 1H NMR (500 MHz, CDCl3): δ 8.30–8.21 (m, 5H), 8.05–7.96 (m, 4H), 7.95–7.90 (m, 1H), 7.87 (dd, J = 1.5, 8.0 Hz, 1H), 7.60–7.52 (m, 6H), 7.51–7.45 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 157.56 (2x), 150.07, 139.59 (2x), 136.27, 133.49, 133.40, 129.05 (2x), 128.91, 128.70 (4x), 128.42, 127.74, 127.15 (4x), 126.74, 126.68, 126.45, 124.81, 117.28 (2x).
2,6-Diphenyl-4-(thiophen-2-yl)pyridine (2au)30e. Yield = 84% (526 mg); white solid; mp = 161–162 °C; HRMS (ESI, M+ + H) calcd for C21H16NS 314.0998, found 314.1006; 1H NMR (500 MHz, CDCl3): δ 8.25–8.19 (m, 4H), 7.89 (s, 2H), 7.64–7.61 (m, 1H), 7.55 (t, J = 7.5 Hz, 4H), 7.51–7.46 (m, 2H), 7.45 (dd, J = 1.0, 5.0 Hz, 1H), 7.19 (dd, J = 3.5, 5.0 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 157.64 (2x), 142.92, 141.88, 139.33 (2x), 129.09 (2x), 128.65 (4x), 128.32, 127.06 (4x), 126.85, 125.19, 115.25 (2x).
4-Ethyl-2,6-diphenylpyridine (2av)30h. Yield = 52% (270 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C19H18N 260.1434, found 260.1430; 1H NMR (500 MHz, CDCl3): δ 8.06 (d, J = 8.0 Hz, 2H), 7.74 (s, 1H), 7.69 (d, J = 8.0 Hz, 2H), 7.55–7.38 (m, 6H), 7.32 (s, 1H), 2.97 (q, J = 8.0 Hz, 2H), 1.43 (t, J = 8.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 163.94, 157.47, 149.57, 139.96, 139.08, 129.01 (2x), 128.78, 128.73, 128.67 (2x), 127.12 (4x), 118.58, 116.23, 31.66, 13.98.
2,6-Bis(4-fluorophenyl)-4-phenylpyridine (2ba)28f. Yield = 89% (611 mg); white solid; mp = 175–176 °C; HRMS (ESI, M+ + H) calcd for C23H16F2N 344.1245, found 344.1242; 1H NMR (500 MHz, CDCl3): δ 8.20–8.16 (m, 4H), 7.82 (d, J = 6.0 Hz, 2H), 7.73 (d, J = 8.0 Hz, 2H), 7.57–7.52 (m, 2H), 7.51–7.47 (m, 1H), 7.20 (t, J = 8.5 Hz, 4H); 13C NMR (125 MHz, CDCl3): δ 163.64 (d, J = 247.125 Hz, 2x), 156.46 (2x), 150.44, 138.84 (2x), 135.57 (d, J = 2.75 Hz, 2x), 129.15 (2x), 129.11, 128.89 (d, J = 8.2 Hz, 4x), 127.14 (2x), 116.67, 115.62 (d, J = 21.3625 Hz, 4x).
2,6-Bis(4-chlorophenyl)-4-phenylpyridine (2ca)28f. Yield = 91% (683 mg); colorless solid; mp = 186–187 °C; HRMS (ESI, M+ + H) calcd for C23H16Cl2N 376.0654, found 376.0654; 1H NMR (500 MHz, CDCl3): δ 8.12 (d, J = 8.5 Hz, 4H), 7.84 (s, 2H), 7.73–7.71 (m, 2H), 7.56–7.49 (m, 7H); 13C NMR (125 MHz, CDCl3): δ 156.31 (2x), 150.54, 138.67, 137.75 (2x), 135.27 (2x), 129.17 (2x), 128.90 (4x), 128.33 (4x), 127.14 (2x), 117.06 (2x).
4-Phenyl-2,6-di-p-tolylpyridine (2da)28f. Yield = 88% (590 mg); colorless solid; mp = 159–160 °C; HRMS (ESI, M+ + H) calcd for C25H22N 336.1747, found 336.1744; 1H NMR (500 MHz, CDCl3): δ 8.12 (d, J = 8.0 Hz, 4H), 7.85 (s, 2H), 7.76–7.74 (m, 2H), 7.55–7.52 (m, 2H), 7.49–7.47 (m, 1H), 7.33 (d, J = 8.0 Hz, 4H), 2.45 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 157.38 (2x), 149.99, 139.26, 138.93 (2x), 136.88 (2x), 129.38 (4x), 129.04 (2x), 128.83, 127.16 (2x), 126.97 (4x), 116.49 (2x), 21.30 (2x).
2,6-Bis(4-methoxyphenyl)-4-phenylpyridine (2ea)28f. Yield = 92% (676 mg); white solid; mp = 131–132 °C; HRMS (ESI, M+ + H) calcd for C25H22NO2 368.1645, found 368.1645; 1H NMR (500 MHz, CDCl3): δ 8.20 (d, J = 9.0 Hz, 4H), 7.79 (s, 2H), 7.75 (d, J = 7.5 Hz, 2H), 7.58.7.52 (m, 2H), 7.51–7.46 (m, 1H), 7.07 (d, J = 8.0 Hz, 4H), 3.89 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 160.40 (2x), 156.81 (2x), 149.84, 139.21, 132.23 (2x), 128.94 (2x), 128.72, 128.27 (4x), 127.05 (2x), 115.53 (2x), 113.93 (4x), 55.24 (2x)
2,6-Di([1,1′-biphenyl]-4-yl)-4-phenylpyridine (2fa)28f. Yield = 72% (661 mg); white solid; mp = 180–181 °C; HRMS (ESI, M+ + H) calcd for C35H26N 460.2060, found 460.2058; 1H NMR (500 MHz, CDCl3): δ 8.32 (d, J = 8.0 Hz, 4H), 7.95 (s, 2H), 7.82–7.75 (m, 6H), 7.70 (d, J = 8.0 Hz, 4H), 7.59–7.53 (m, 2H), 7.53–7.46 (m, 5H), 7.43–7.37 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 157.12 (2x), 150.23 (2x), 141.81 (2x), 140.68 (2x), 139.06, 138.47 (2x), 129.13 (2x), 129.00 (2x), 128.83 (4x), 127.52 (4x), 127.43 (4x), 127.20 (2x), 127.12 (4x), 117.04 (2x).
2,6-Bis(3-fluorophenyl)-4-phenylpyridine (2ga)28f. Yield = 92% (631 mg); white solid; mp = 147–148 °C; HRMS (ESI, M+ + H) calcd for C23H16F2N 344.1245, found 344.1254; 1H NMR (500 MHz, CDCl3): δ 7.98–7.92 (m, 4H), 7.89 (s, 2H), 7.77–7.72 (m, 2H), 7.58–7.53 (m, 2H), 7.52–7.45 (m, 3H), 7.18–7.13 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 163.35 (d, J = 243.75 Hz, 2x), 156.19 (d, J = 2.25 Hz, 2x), 150.62, 141.62 (d, J = 7.375 Hz, 2x), 138.59, 130.20 (d, J = 8.125 Hz, 2x), 129.22, 129.20 (2x), 127.15 (2x), 122.56 (d, J = 2.375 Hz, 2x), 117.62 (2x), 116.00 (d, J = 21.125 Hz, 2x), 114.06 (d, J = 22.75 Hz, 2x).
2,6-Bis(3-methoxyphenyl)-4-phenylpyridine (2ia)30f. Yield = 82% (602 mg); colorless gum; HRMS (ESI, M+ + H) calcd for C25H22NO2 368.1645, found 368.1640; 1H NMR (500 MHz, CDCl3): δ 7.89 (s, 2H), 7.82–7.81 (m, 2H), 7.79–7.73 (m, 4H), 7.57–7.52 (m, 2H), 7.51–7.46 (m, 1H), 7.44 (t, J = 8.0 Hz, 2H), 7.01 (dd, J = 2.5, 8.0 Hz, 2H), 3.93 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 160.03 (2x), 157.18 (2x), 150.17, 141.03 (2x), 138.98, 129.66 (2x), 129.10 (2x), 128.98, 127.16 (2x), 119.53 (2x), 117.39 (2x), 114.71 (2x), 112.66 (2x), 55.38, 55.36.
2,6-Bis(2-bromophenyl)-4-phenylpyridine (2la)28f. Yield = 61% (565 mg); white solid; mp = 150–151 °C; HRMS (ESI, M+ + H) calcd for C23H16Br2N 463.9644, found 463.9644; 1H NMR (500 MHz, CDCl3): δ 7.85 (s, 2H), 7.78–7.75 (m, 2H), 7.74–7.70 (m, 4H), 7.55–7.50 (m, 2H), 7.49–7.46 (m, 1H), 7.45–7.41 (m, 2H), 7.27 (td, J = 2.0, 8.0 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ 158.39 (2x), 148.20, 141.18 (2x), 138.11 (2x), 133.29 (2x), 131.75 (2x), 129.76 (2x), 129.13 (2x), 127.58 (2x), 127.21 (2x), 121.94 (2x), 121.41 (2x).
4-Phenyl-2,6-di-o-tolylpyridine (2ma)28f. Yield = 81% (543 mg); white solid; mp = 133–134 °C; HRMS (ESI, M+ + H) calcd for C25H22N 336.1747, found 336.1747; 1H NMR (500 MHz, CDCl3): δ 7.74–7.72 (m, 2H), 7.60 (s, 2H), 7.54–7.49 (m, 4H), 7.48–7.43 (m, 1H), 7.34–7.27 (m, 6H), 2.49 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 160.09 (2x), 148.79, 140.72 (2x), 138.49, 135.90 (2x), 130.68 (2x), 129.83 (2x), 129.13 (2x), 129.02, 128.24 (2x), 127.12 (2x), 125.83 (2x), 120.11 (2x), 20.62 (2x).
2,6-Bis(2-methoxyphenyl)-4-phenylpyridine (2na)28f. Yield = 77% (565 mg); white solid; mp = 133–134 °C; HRMS (ESI, M+ + H) calcd for C25H22NO2 368.1651, found 368.1642; 1H NMR (500 MHz, CDCl3): δ 8.00 (s, 2H), 7.96 (dd, J = 1.5, 7.5 Hz, 2H), 7.76–7.72 (m, 2H), 7.54–7.49 (m, 2H), 7.47–7.42 (m, 1H), 7.42–7.36 (m, 2H), 7.14–7.09 (m, 2H), 7.04 (d, J = 8.5 Hz, 2H), 3.90 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 157.08 (2x), 155.97 (2x), 147.66 (2x), 139.48, 131.56 (2x), 129.72 (2x), 129.62, 128.91 (2x), 128.51, 127.34 (2x), 121.41 (2x), 121.05 (2x), 111.41 (2x), 55.72 (2x).
4-Phenyl-2,6-bis(4-(trifluoromethyl)phenyl)pyridine (2qa)30f. Yield = 85% (753 mg); white solid; mp = 156–157 °C; HRMS (ESI, M+ + H) calcd for C25H16F6N 444.1182, found 444.1180; 1H NMR (500 MHz, CDCl3): δ 8.30 (d, J = 8.0 Hz, 4H), 7.99–7.92 (m, 2H), 7.78 (d, J = 8.0 Hz, 4H), 7.75 (d, J = 7.0 Hz, 2H), 7.60–7.50 (m, 3H); 13C NMR (125 MHz, CDCl3): δ 156.24, 150.87, 142.50 (2x), 138.34, 131.07 (q, J = 32.7 Hz, 2x), 129.40 (2x), 129.28 (2x), 127.38 (4x), 127.16 (2x), 125.71 (d, J = 3.5 Hz, 4x), 124.23 (q, J = 273.62 Hz, 2x), 118.18 (2x).
4-Phenyl-2,6-di-m-tolylpyridine (2ra)30g. Yield = 70% (469 mg); yellow solid; mp = 162–163 °C; (ESI, M+ + H) calcd for C25H22N 336.1747, found 336.1742; 1H NMR (500 MHz, CDCl3): δ 8.04 (s, 2H), 8.00 (d, J = 7.5 Hz, 2H), 7.89 (s, 2H), 7.77 (d, J = 7.5 Hz, 2H), 7.55 (t, J = 8.0 Hz, 2H), 7.50 (d, J = 7.5 Hz, 1H), 7.43 (t, J = 7.5 Hz, 2H), 7.29 (d, J = 7.5 Hz, 2H), 2.51 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 151.71 (2x), 150.02, 139.64 (2x), 139.11, 138.27 (2x), 129.75 (2x), 129.06 (2x), 128.89, 128.58 (2x), 127.83 (2x), 127.17 (2x), 124.30 (2x), 117.15 (2x), 21.59 (2x).
2,6-Di(furan-2-yl)-4-phenylpyridine (2sa)28f. Yield = 76% (436 mg); bown solid; mp = 118–119 °C; HRMS (ESI, M+ + H) calcd for C19H14NO2 288.1025, found 288.1033; 1H NMR (500 MHz, CDCl3): δ 7.81 (s, 2H), 7.78–7.74 (m, 2H), 7.58–7.55 (m, 2H), 7.54–7.50 (m, 2H), 7.49–7.44 (m, 1H), 7.20 (dd, J = 1.0, 3.0 Hz, 2H), 6.58–6.55 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 153.77 (2x), 149.77, 149.69 (2x), 143.28 (2x), 138.38, 129.11, 129.04 (2x), 127.03 (2x), 114.80 (2x), 112.05 (2x), 109.09 (2x).
4-Phenyl-2,6-di(thiophen-3-yl)pyridine (2ta)28f. Yield = 80% (510 mg); yellow solid; mp = 135–136 °C; HRMS (ESI, M+ + H) calcd for C19H14NS2 320.0562, found 320.0561; 1H NMR (500 MHz, CDCl3): δ 8.08 (dd, J = 1.5, 3.0 Hz, 2H), 7.84 (dd, J = 1.5, 5.0 Hz, 2H), 7.76–7.72 (m, 4H), 7.59–7.54 (m, 2H), 7.52–7.48 (m, 1H), 7.45 (dd, J = 3.0, 5.0 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ 153.64 (2x), 150.15, 142.44 (2x), 138.90, 129.09 (2x), 128.98, 127.10 (2x), 126.45 (2x), 126.14 (2x), 123.78 (2x), 116.60 (2x).
2,6-Dimethyl-4-phenylpyridine (2ua)31. Yield = 88% (322 mg); white solid; mp = 58–59 °C; HRMS (FAB, M+ + H) calcd for C13H14N 184.1126, found 184.1126; 1H NMR (500 MHz, CDCl3): δ 7.73–7.54 (m, 2H), 7.51–7.39 (m, 3H), 7.19 (s, 2H), 2.60 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 158.01, 149.35, 138.61, 128.99 (2x), 128.85 (2x), 127.05 (2x), 118.53 (2x), 24.41 (2x).
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank Ping-Yu Lin (IOC, Academia Sinica) for mass measurement, Ministry of Science and Technology (MOST 110-2811-M-001-644; MOST 111-2811-M-001-089 and 111-2113-M-001-043) and the Thematic Research Project, Academia Sinica Taiwan (AS-TP-111-M01) for financial support. Chieh-Kai Chan acknowledges Postdoctoral Scholar Program from Academia Sinica.
Notes and references
- J. Zhu and H. Bienaymé, Multicomponent Reactions, Wiley-VCH, Weinheim, Germany, 2005 Search PubMed.
-
(a) C. Allais, J.-M. Grassot, J. Rodriguez and T. Constantieux, Chem. Rev., 2014, 114, 10829–10868 CrossRef CAS PubMed;
(b) W. Guo, J. Liao, D. Liu, J. Li, F. Ji, W. Wu and H. Jiang, Angew. Chem., Int. Ed., 2017, 56, 1289–1293 CrossRef CAS PubMed;
(c) R. Echemendía, A. F. de La Torre, J. L. Monteiro, M. Pila, A. G. Corrêa, B. Westermann, D. G. Rivera and M. W. Paixão, Angew. Chem., Int. Ed., 2015, 54, 7621–7625 CrossRef;
(d) L. Reguera and D. G. Rivera, Chem. Rev., 2019, 119, 9836–9860 CrossRef CAS PubMed;
(e) P. Wu, M. Givskov and T. E. Nielsen, Chem. Rev., 2019, 119, 11245–11290 CrossRef CAS PubMed.
-
(a) B. H. Rotstein, S. Zaretsky, V. Rai and A. K. Yudin, Chem. Rev., 2014, 114, 8323–8359 CrossRef CAS PubMed;
(b) R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958–2975 RSC.
- P. Biginelli, Ber. Dtsch. Chem. Ges., 1891, 24, 1317–1319 CrossRef.
- A. Hantzsch, Ber. Dtsch. Chem. Ges., 1881, 14, 1637–1638 CrossRef.
- C. Mannich and W. Krösche, Arch. Pharm., 1912, 250, 647–667 CrossRef CAS.
- M. Passerini and L. Simone, Gazz. Chim. Ital., 1921, 51, 126–129 CAS.
- L. S. Povarov, Russ. Chem. Rev., 1967, 36, 656–670 CrossRef.
- A. Strecker, Justus Liebigs Ann. Chem., 1854, 91, 349–351 CrossRef.
-
(a) I. Ugi, R. Meyr, U. Fetzer and C. Steinbrückner, Angew. Chem., 1959, 71, 386 Search PubMed;
(b) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168–3210 CrossRef.
-
(a) A. R. Katrizky and C. W. Rees, Comprehensive Heterocyclic Chemistry, Pergamon Press, 1984 Search PubMed;
(b) J. A. Joule and K. Mills, Heterocyclic Chemistry, Wiley-Blackwell, Oxford, 5th edn, 2010 Search PubMed;
(c) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed.
-
(a) I. M. Lagoja, Chem. Biodiversity, 2005, 2, 1–50 CrossRef CAS PubMed;
(b) A. Basnet, P. Thapa, R. Karki, Y. Na, Y. Jahng, B.-S. Jeong, T. C. Jeong, C.-S. Lee and E.-S. Lee, Bioorg. Med. Chem., 2007, 15, 4351–4359 CrossRef CAS PubMed;
(c) S. R. Walker, E. J. Carter, B. C. Huff and J. C. Morris, Chem. Rev., 2009, 109, 3080–3098 CrossRef CAS PubMed;
(d) E. P. Aparna and K. S. Devaky, ACS Comb. Sci., 2019, 21, 35–68 CrossRef CAS PubMed.
-
(a) T. P. Selvam, C. R. James, P. V. Dniandev and S. K. Valzita, Res. Pharm., 2012, 2, 1–9 Search PubMed;
(b) S. P. Chan, K. Han, R. Qu, L. J. Tong, Y. J. Li, Z. Zhang, H. M. Cheng, X. J. Lu, A. Patterson, J. Smaill, X. M. Ren, J. Ding, H. Xie and K. Ding, Bioorg. Med. Chem. Lett., 2015, 25, 4277–4281 CrossRef CAS;
(c) O. S. Reddy, C. V. Suryanarayana, K. J. P. Narayana, V. Anuradha and B. H. Babu, Med. Chem. Res., 2015, 24, 1777–1788 CrossRef CAS;
(d) R. M. Okasha, F. F. Albalawi, T. H. Afifi, A. M. Fouda, A. A. M. Al-Dies and A. M. El-Agrody, Molecules, 2016, 21, 1450 CrossRef PubMed;
(e) M. Erra, J. Taltavull, A. Gréco, F. J. Bernal, J. F. Caturla, J. Gràcia, M. Domínguez, M. Sabaté, S. Paris, S. Soria, B. Hernández, C. Armengol, J. Cabedo, M. Bravo, E. Calama, M. Miralpeix and M. D. Lehner, ACS Med. Chem. Lett., 2017, 8, 118–123 CrossRef CAS PubMed;
(f) Z. Fang, S. Zheng, K.-F. Chan, W. Yuan, Q. Guo, W. Wu, H.-K. Lui, Y. Lu, Y.-C. Leung, T. H. Chan, K.-Y. Wong and N. Sun, Eur. J. Med. Chem., 2019, 161, 141–153 CrossRef CAS PubMed.
-
(a) G. Brugnatelli, Ann. Chim. Phys., 1818, 8, 201–206 Search PubMed;
(b) E. Frankland and H. Kolbe, Justus Liebigs Ann. Chem., 1848, 65, 269–287 CrossRef.
-
(a) G. Domínguez and J. Perez-Castells, Chem. Soc. Rev., 2011, 133, 12285–12292 CrossRef;
(b) J. A. Bull, J. J. Mousseau, G. Pelletier and A. B. Charette, Chem. Rev., 2012, 112, 2642–2713 CrossRef CAS PubMed;
(c) D. A. Shabalin, Org. Biomol. Chem., 2021, 19, 8184–8204 RSC.
-
(a) W. Guo, J. Liao, D. Liu, J. Li, F. Ji, W. Wu and H. Jiang, Angew. Chem., Int. Ed., 2017, 56, 1289–1293 CrossRef CAS PubMed;
(b) M. Mahfoudh, R. Abderrahim, E. Leclerc and J.-M. Campagne, Eur. J. Org. Chem., 2017, 2017, 2856–2865 CrossRef CAS.
-
(a) N. Deibl, K. Ament and R. Kempe, J. Am. Soc. Chem., 2015, 137, 12804–12807 CrossRef CAS PubMed;
(b) M. Mastalir, M. Glatz, E. Pittenauer, G. Allmaier and K. Kirchner, J. Am. Soc. Chem., 2016, 138, 15543–15546 CrossRef CAS PubMed;
(c) N. Deibl and R. Kempe, Angew. Chem., Int. Ed., 2017, 56, 1663–1666 CrossRef;
(d) G. Chakraborty, R. Sikari, R. Mondal, S. Mandal and N. D. Paul, Asian J. Org. Chem., 2020, 9, 431–436 CrossRef CAS;
(e) R. Mondal, S. Sinha, S. Das, G. Chakraborty and N. D. Paul, Adv. Synth. Catal., 2020, 362, 594–600 CrossRef CAS;
(f) E. D. Anderson and D. L. Boger, J. Am. Soc. Chem., 2011, 133, 12285–12292 CrossRef CAS PubMed;
(g) E. D. Anderson and D. L. Boger, Org. Lett., 2011, 13, 2492–2494 CrossRef CAS PubMed;
(h) E. D. Anderson and D. L. Boger, J. Am. Soc. Chem., 2011, 133, 12285–12292 CrossRef CAS PubMed;
(i) C. M. Glinkerman and D. L. Boger, Org. Lett., 2015, 17, 4002–4005 CrossRef CAS PubMed.
-
(a) L. Su, K. Sun, N. Pan, L. Liu, M. Sun, J. Dong, Y. Zhou and S.-F. Yin, Org. Lett., 2018, 20, 3399–3402 CrossRef CAS PubMed;
(b) A. Herrera, R. Martínez-Álvarez, P. Ramiro, M. Chioua and R. Torres, Tetrahedron, 2002, 58, 3755–3764 CrossRef CAS;
(c) A. Herrera, R. Martínez-Álvarez, M. Chioua, R. Chioua and Á. Sánchez, Tetrahedron, 2002, 58, 10053–10058 CrossRef CAS;
(d) Z. D. Pardo, G. L. Olsen, M. E. Fernández-Valle, L. Frydman, R. Martínez-Álvarez and A. Herrera, J. Am. Soc. Chem., 2012, 134, 2706–2715 CrossRef CAS PubMed;
(e) M. E. Fernández-Valle, R. Martínez-Álvarez, D. Molero-Vílchez, Z. D. Pardo, E. Sáez-Barajas and A. Herrera, J. Org. Chem., 2015, 80, 799–805 CrossRef.
-
(a) Y. Satoh, K. Yasuda and Y. Obora, Organometallics, 2012, 31, 5235–5238 CrossRef CAS;
(b) X. You, S. Yu and Y. Liu, Organometallics, 2013, 32, 5273–5276 CrossRef CAS;
(c) S. N. Karad and R.-S. Liu, Angew. Chem., Int. Ed., 2014, 53, 9072–9076 CrossRef CAS;
(d) T. K. Lane, M. H. Nguyen, B. R. D'Souza, N. A. Spahn and J. Louie, Chem. Commun., 2013, 49, 7735–7737 RSC.
-
(a) M. Movassaghi and M. D. Hill, J. Am. Chem. Soc., 2006, 128, 14254–14255 CrossRef CAS PubMed;
(b) O. K. Ahmad, M. D. Hill and M. Movassaghi, J. Org. Chem., 2009, 74, 8460–8463 CrossRef CAS PubMed;
(c) M. Movassaghi and M. D. Hill, Nat. Protoc., 2007, 2, 2018–2023 CrossRef CAS PubMed;
(d) H. B. Jalani, W. Cai and H. Lu, Adv. Synth. Catal., 2017, 359, 2509–2513 CrossRef CAS.
-
(a) X.-Q. Chu, W.-B. Cao, X.-P. Xu and S.-J. Ji, J. Org. Chem., 2017, 82, 1145–1154 CrossRef CAS PubMed;
(b) L.-Y. Zheng, W. Guo and X.-L. Fan, Asian J. Org. Chem., 2017, 6, 837–840 CrossRef CAS;
(c) C. Xu, S.-F. Jiang, X.-H. Wen, Q. Zhang, Z.-W. Zhou, Y.-D. Wu, F.-C. Jia and A.-X. Wu, Adv. Synth. Catal., 2018, 360, 2267–2271 CrossRef CAS;
(d) Q. Gao, M. Wu, K. Zhang, N. Yang, M. Liu, J. Li, L. Fang, S. Bai and Y. Xu, Org. Lett., 2020, 22, 5645–5649 CrossRef CAS PubMed;
(e) A. R. Romanov, A. Y. Rulev, A. V. Popov, E. V. Kondrashov and S. V. Zinchenko, Synthesis, 2020, 52, 1512–1522 CrossRef CAS;
(f) J. L. Zhan, M. W. Wu, F. Chen and B. Han, J. Org. Chem., 2016, 81, 11994–12000 CrossRef CAS;
(g) Y. Zhou, Z. H. Tang and Q. L. Song, Adv. Synth. Catal., 2017, 359, 952–958 CrossRef CAS.
- J. Chen, H. Meng, F. Zhang, F. Xiao and G.-J. Deng, Green Chem., 2019, 21, 5201–5206 RSC.
-
(a) T. Sasada, F. Kobayashi, N. Sakai and T. Konakahara, Org. Lett., 2009, 11, 2161–2164 CrossRef CAS;
(b) P. Wang, X. Zhang, Y. Liu and B. Chen, Asian J. Org. Chem., 2019, 8, 1122–1127 CrossRef CAS;
(c) P. Wu, X.-M. Cai, Q.-F. Wang and C.-G. Yan, Synth. Commun., 2007, 37, 223–229 CrossRef CAS;
(d) Y. Ding, R. Ma, R. C. Hider and Y. Ma, Asian J. Org. Chem., 2020, 9, 242–246 CrossRef CAS;
(e) C. Rakhi, K. Ramesh, M. P. Darbem, T. A. Branquinho, A. R. de Oliveira, P. S. Manjari and N. L. C. Domingues, Tetrahedron Lett., 2016, 57, 1656–1660 CrossRef CAS.
- J. Li, J. Li, R. He, J. Liu, Y. Liu, L. Chen, Y. Huang and Y. Li, Org. Lett., 2022, 24, 1620–1625 CrossRef CAS PubMed.
- M. Adib, N. Mahmoodi, M. Mahdavi and H. R. Bijanzadeh, Tetrahedron Lett., 2006, 47, 9365–9368 CrossRef CAS.
- Z. Hassani, Lett. Org. Chem., 2014, 11, 546–549 CrossRef CAS.
- D. Liu, W. Guo, W. Wu and H. Jiang, J. Org. Chem., 2017, 82, 13609–13616 CrossRef CAS PubMed.
-
(a) C.-K. Chan, C.-Y. Lai, W.-C. Lo, Y.-T. Cheng, M.-Y. Chang and C.-C. Wang, Org. Biomol. Chem., 2020, 18, 305–315 RSC;
(b) C.-K. Chan, C.-Y. Lai and C.-C. Wang, Org. Biomol. Chem., 2020, 18, 7201–7212 RSC;
(c) K. H. Asressu, C.-K. Chan and C.-C. Wang, RSC Adv., 2021, 11, 28061–28071 RSC;
(d) C.-K. Chan, C.-Y. Lai and C.-C. Wang, Synthesis, 2020, 18, 1779–1794 Search PubMed;
(e) C.-K. Chan, C.-Y. Lai and C.-C. Wang, Catalyst, 2021, 11, 877 CrossRef CAS;
(f) C.-K. Chan, Y.-H. Chung and C.-C. Wang, RSC Adv., 2022, 12, 8263–8273 RSC.
- D. Cantillo, O. de Frutos, J. A. Rincón, C. Mateos and C. O. Kappe, Org. Lett., 2014, 16, 896–899 CrossRef CAS PubMed.
-
(a) C. H. Low, J. N. Rosenberg, M. A. Lopez and T. Agapie, J. Am. Soc. Chem., 2018, 140, 11906–11910 CrossRef CAS;
(b) Y. Hiraga, R. Kuwahara and T. Hatta, Tetrahedron, 2021, 94, 132317 CrossRef CAS;
(c) R. Mondal and D. E. Herbert, Organometallics, 2020, 39, 1310–1317 CrossRef CAS;
(d) A. K. Bains and D. Adhikari, Catal. Sci. Technol., 2020, 10, 6309–6318 RSC;
(e) S. Forouzandehdel, M. Meskini and R. Rami, J. Mol. Struct., 2020, 1214, 128142 CrossRef CAS;
(f) Y. Yi, M.-N. Zhao, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Green Chem., 2017, 19, 1023–1027 RSC;
(g) Q. Yang, Y. Zhang, W. Zeng, Z.-C. Duan, X. Sang and D. Wang, Green Chem., 2019, 21, 5683–5690 RSC;
(h) J.-C. Xiang, M. Wang, Y. Cheng and A.-X. Wu, Org. Lett., 2016, 18, 24–27 CrossRef CAS PubMed.
- L. A. Lumangtad, E. Claeys, S. Hamal, A. Intasiri, C. Basrai, E. Yen-Pon, D. Beenfeldt, K. Vermeire and T. W. Bell, Bioorg. Med. Chem., 2020, 28, 115816 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterisation data, copies of 1H and 13C NMR spectra data. See https://doi.org/10.1039/d2ra04739j |
|
| This journal is © The Royal Society of Chemistry 2022 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *,
Yi-Hsiu Chung and
Cheng-Chung Wang
*,
Yi-Hsiu Chung and
Cheng-Chung Wang *
*







