Open Access Article
Open Access ArticleHarnessing magnetic fields for rare-earth complex crystallization–separations in aqueous solutions†
Amit Kumar ,
Han Geng
,
Han Geng and
Eric J. Schelter
and
Eric J. Schelter *
*
P. Roy and Diana T. Vagelos Laboratories, Department of Chemistry, University of Pennsylvania, 231 S. 34th St., Philadelphia, PA 19104, USA. E-mail: schelter@sas.upenn.edu
First published on 29th September 2022
Abstract
Magnetic field-directed crystallization separation of rare-earth (RE) metals is emerging as a new direction in the field of separation science, due to its simplicity, low energy input, and low cost of operation, as compared to traditional separation methods such as solvent extraction. Here, we report the use of Fe14Nd2B magnets for selective crystallization of paramagnetic Nd, Dy, Er, and Tm rare earth compounds from a mixture with diamagnetic La ones using the RE–DOTA complex system. All the separations were performed at milder temperatures of 3 °C to provide a thermal gradient, and the crystallizations were set up in aqueous solutions using the benign solvents water and acetone. A four-fold increase in the separation factor (41.4 ± 0.6) was observed for the Dy/La pair in the presence of a magnetic field as compared to the separation factor (10.5 ± 0.9) obtained without the application of the field. These results indicate that the use of the magnetic crystallization method for RE separations is effective in aqueous systems and can be a useful strategy for energy-efficient molecular separations of RE metals.
Introduction
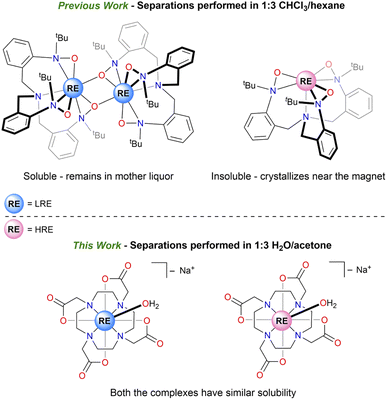
The prices of rare-earth (RE) elements (Sc, Y, and the lanthanides) have been volatile in the past decade after the “Rare-Earth Crisis” between 2009 and 2011, leading to supply shortages of these critical metals.1 Recycling RE elements from their mixtures can help mitigate this balance problem by adding recycled REs to the global supply chain.2 Historically and commercially, RE elements are separated using technologies such as solvent extraction, ion exchange chromatography, and selective precipitation, all of which use the difference in sizes of the RE elements.3–5 However, these processes require large amounts of solvents and energy, which contributes to the price of the elements and generates waste causing negative environmental footprints, especially near RE processing facilities.6,7Previous works in our group demonstrated various low-energy and environmentally friendly RE separation methods that used minimal solvents, targeted intrinsic properties of RE ions other than size, and were controlled by molecular speciation.8 A recent study aimed at targeting magnetic properties of RE3+ cations, due to their unfilled 4f orbitals, to achieve a magnetic separation step based on variable migration of paramagnetic cations in the presence of an applied magnetic field.9 This strategy was used in separating molecular RE-complexes of the [TriNOx]3− ligand using Fe14Nd2B permanent magnets (Chart 1).10 This low energy input separation method allowed for the enhancement of experimental separation factors11 by approximately 100% for the La/Dy pair. It was also suggested that the achieved separation depended on the free-ion angular momentum (J = 7.5 for Dy3+) of the trivalent RE cations in its electronic ground state as well as on the monomer–dimer equilibrium that was studied for this system. In this case, solubility differences between the more soluble dimeric [RE(TriNOx)]2 complex, formed by LRE (light rare earth), and the less soluble monomeric [RE(TriNOx)], formed by HRE (heavy rare earth), were primarily responsible for the enhancement in the separation factors (SF) (Chart 1). Due to the air-sensitive nature of [RE(TriNOx)] complexes, the separations were performed under an inert atmosphere and required the use of organic solvents including a chlorinated solvent, CHCl3.
For the current work, we were interested in investigating the effect of magnetic crystallization separation in environmentally friendly solvents, such as water, and using an established ligand framework that does not have monomer–dimer equilibrium. We hypothesized that the absence of this equilibrium would ensure that the effect of the magnetic field on the separations was only dependent on the identity of the RE ion.
For our work, we chose a common chelating ligand DOTA4− (1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetate), whose chemistry with RE cations is well-established, to prepare a series of complexes. We chose this system since, in addition to its well-established coordination chemistry and inertness towards exchange of the cations, magnetic studies have been performed on the RE–DOTA complexes, especially to understand properties such as their magnetic anisotropy and magnetic relaxation.12–14 Ideal magnetic crystallization separation ligand systems would include ligands that promote large magnetic anisotropy differences at the metal cations under aerobic conditions.
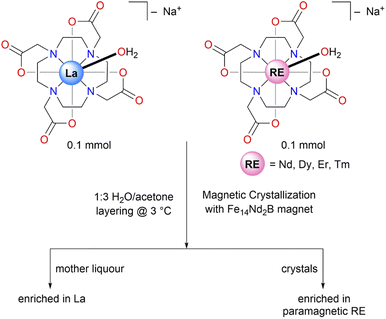
Herein, we report the separation of selected paramagnetic RE ions (Nd, Dy, Er, and Tm) from a diamagnetic RE ion (La) using a magnetic crystallization process. For these studies, we subjected the RE-DOTA complexes to magnetic and thermal-gradient crystallization using an external field provided by a Fe14Nd2B magnet. After performing ICP-OES (Inductively Coupled Plasma-Optical Emission Spectroscopy) to analyze metal content of the crystals and the mother liquor, we observed an enrichment of paramagnetic RE ions in the crystals and enrichment of diamagnetic La in the mother liquor, giving an upper bound of a four-fold increase in the separation factor for the Dy/La pair.
Results & discussion
For the current study, RE–DOTA (formulated as Na[RE(DOTA)(H2O)]·4H2O, RE = La, Nd, Dy, Er, Tm) complexes were synthesized using published procedures.12,15 Refluxing the RE-oxides RE2O3 with 0.5 equiv. H4DOTA (1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid) in the presence of 1 equivalent of NaOH in Milli-Q water for 3–4 days afforded the corresponding RE–DOTA complexes in 50–60% yields. The crude complexes were crystallized for the separations experiments by vapor diffusion of acetone into a concentrated solution of RE–DOTA complexes in water at room temperature. We performed unit cell checks on the single crystals of RE–DOTA complexes using X-ray diffraction (XRD) and confirmed the expected formulation of these complexes as Na[RE(DOTA)(H2O)]·4H2O, as previously reported.14 The presence of the RE-bound H2O molecule was of interest in our experiments, as the position of the hydrogen atoms on the bound water molecule dictates the easy axis orientation, and magnetic anisotropy therein, of the molecule in question.14 We envisioned that these magnetic properties of RE–DOTA complexes will result in enhanced separation of a paramagnetic RE ion from a diamagnetic RE ion in the presence of a magnetic field.With the RE–DOTA complexes in hand, we moved on to performing separations experiment using Fe14Nd2B permanent magnets (12.7 × 12.7 × 3.2 mm3) as was previously used in work by Evans,16 and also by our group in the recent work on separations using magnetic crystallization.10 Following similar experimental protocols from our previous work, we carried out our separations experiment on selected binary mixtures of RE–DOTA complexes where the concentration of individual RE-DOTA complexes in solution was kept constant at 0.025 M at 3 °C (Fig. 1). The binary mixtures were dissolved in 1 mL of water and layered with 3 mL of acetone to induce crystallization. The Fe14Nd2B permanent magnet in our group's previous work was attached to the vial by using an insulating tape or a rubber band. To avoid any changes to the experimental setup, we designed a sample holder using a 3D printer that contained a slot for holding a 4 mL vial and a slot for the magnet at a 2 mm distance from the vial (Fig. 2). The distance of 2 mm was chosen based on the reported magnetic flux density as a function of distance, measured by Berlinguette & coworkers using a Hall probe.17
After 72 h, crystals were observed near the magnet and the solid and filtrate portions were separated by decantation and analyzed by ICP-OES. As a control, we also performed the separations without the presence of a magnetic field. The results from the ICP-OES were used to calculate the enrichment factors (EF) and separation factors (SF) in both fractions (see ESI† for example calculation) (Table 1).
| Entry | Rare-earth pair | EF (crystals) | EF (mother liquor) | SF |
|---|---|---|---|---|
| 1 | La/Nd | 2.14 ± 0.18 | 1.48 ± 0.07 | 3.56 ± 0.36 |
| 2 | La/Dy | 2.95 ± 0.19 | 3.57 ± 0.54 | 10.5 ± 0.9 |
| 3 | La/Er | 2.61 ± 0.75 | 1.70 ± 0.36 | 4.60 ± 0.15 |
| 4 | La/Tm | 3.82 ± 0.76 | 1.20 ± 0.15 | 4.63 ± 1.49 |
| 5 | La/Nd | 2.48 ± 0.28 | 1.78 ± 0.04 | 3.86 ± 0.38 |
| 6 | La/Dy | 3.41 ± 0.06 | 12.1 ± 0.1 | 41.4 ± 0.6 |
| 7 | La/Er | 3.48 ± 0.39 | 1.78 ± 0.38 | 6.01 ± 0.26 |
| 8 | La/Tm | 4.08 ± 0.42 | 1.76 ± 0.17 | 7.15 ± 0.05 |
The observed separation factors with RE–DOTA complexes are relatively smaller as compared to that observed for the RE–TriNOx complexes (41.4 versus 494 for La/Dy pair). This difference is presumably due to the monomer–dimer equilibrium in the case of RE–TriNOx complexes, an equilibrium that is tied to the solubility differences for the two metal complex forms. Such a speciation equilibrium is not present in the case of the RE–DOTA complexes, however. Nonetheless, we see an increase in the SFs for all four pairs of binary mixtures with the application of the field as compared to SFs obtained without an applied field. Moreover, for La/Dy binary mixture, a four-fold increase in the SF in the presence of a magnet was observed as compared to separations performed without a magnet. This result could be attributable to the high J value of Dy3+ ion and also the presence of “missed” easy plane anisotropy in Dy–DOTA species.14 Notably, the four-fold increase in the SF observed for La/Dy pair for RE–DOTA complexes (from 10.5 to 41.4) is more than the two-fold increase in the SF observed for the RE–TriNOx case (from 258 to 494). On the other hand, the magnetic-field assisted enhancement in the separation factor (from 3.56 to 3.86) for the LRE (Nd) is significantly smaller than that observed (from 4.63 to 7.15 for Tm) for the HREs (Dy, Er, Tm). These could be attributed to both the differences in the ionic radii of the RE3+ ions and their total angular momentum values (J). For instance, the Nd3+ ion has the smallest J value (4.5) in our series and is closest in size to the La3+ ion (1.16 and 1.22 for C.N. of 10, respectively).18 We hypothesize that these collective properties could be responsible for the smaller enhancement in the separation factor observed for La/Nd pair as compared to La/HRE pairs. Notably, similar ionic radii of Er3+ and Tm3+ (1.06 and 1.05 for C.N. of 10, respectively) and the closeness in their J values (7.5 and 6.5, respectively) are reflected in their similar separation factors (6.01 and 7.15, respectively).
Unlike our group's previously published work in magnetic field-directed RE separations, for the current system we did not observe any meaningful trends in the enrichment and separation factors with the total angular momentum (J) for the free ion of the RE3+ cations (see ESI, Fig. S7 and S8†). Future work on magnetic crystallization separations will target systems that contain strong axial field ligands and weaker equatorial ligands to further increase the magnetic anisotropy at the metal cations and systems that include a solution equilibrium process. This strategy could possibly offer greater separation, especially in the case of a binary mixture containing a diamagnetic RE ion and a paramagnetic, oblate RE ion that are also similar in size.
Conclusions
We demonstrated that the magnetic crystallization method along with a concentration gradient controlled by temperature reduction can be used as a strategy for RE separations using a simple ligand system under aqueous and aerobic conditions. A four-fold increase in the separation factor was observed for the La/Dy pair indicating the importance of the total angular momentum (J) for the free ion of the paramagnetic RE3+ cation and magnetic anisotropy of the RE–DOTA complexes in the separation process. Notably, unlike our previous work, no clear trend was observed between the enrichment and separation factors and the J values for the free ion of the RE3+ cations. Moreover, the results described here indicate the presence of the monomer–dimer equilibrium for the previously reported RE(TriNOx) system is evidently an important design feature for achieving significant amplification of magnetic crystallization–separations performance. Future work will involve extending this method to other molecular systems that exhibit high magnetic anisotropy, and incorporation of metal complex equilibria.Author contributions
Amit Kumar synthesized the RE–DOTA complexes (RE = La, Nd, Dy, Er, and Tm), developed the separation procedure, and wrote/edited the manuscript and ESI. Han Geng assisted in the ICP-OES technique for determining the metal content after separation and contributed to the writing of the ESI. Eric J. Schelter provided guidance, support and funding for the experimental studies described within, contributed to the writing/editing of the manuscript and ESI, and managed all aspects of the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences Separations and Analysis program under Award Number DE-SC0017259 for financial support. The authors also thank the University of Pennsylvania. 3D printed sample holders printed courtesy of the University of Pennsylvania Libraries' Biotech Commons.Notes and references
- U.S. Department of Energy, Critical Materials Strategy, 2011, URL: https://www.energy.gov/sites/prod/files/DOE_CMS2011_FINAL_Full.pdf Search PubMed.
- K. Binnemans, P. T. Jones, B. Blanpain, T. Van Gerven, Y. Yang, A. Walton and M. Buchert, J. Cleaner Prod., 2013, 51, 1–22 CrossRef CAS
.
- B. Weaver, F. A. Kappelmann and A. C. Topp, J. Am. Chem. Soc., 1953, 75, 3943–3945 CrossRef CAS
.
- D. F. Peppard, G. W. Mason, J. L. Maier and W. J. Driscoll, J. Inorg. Nucl. Chem., 1957, 4, 334–343 CrossRef CAS
.
- D. Qi, Hydrometallurgy of Rare Earths, Elsevier Ltd, Burlington MA, USA, 2018 Search PubMed
.
- K. Li, T. Liang, L. Wang and S. Tian, Environ. Geochem. Health, 2018, 40, 2795–2805 CrossRef CAS PubMed
.
- Q. Liang, H. Yin, J. Li, L. Zhang, R. Hou and S. Wang, Medicine, 2018, 97, e12717 CrossRef CAS PubMed
.
-
(a) J. A. Bogart, C. A. Lippincott, P. J. Carroll and E. J. Schelter, Angew. Chem., 2015, 127, 8340–8343 CrossRef
; (b) J. A. Bogart, B. E. Cole, M. A. Boreen, C. A. Lippincott, B. C. Manor, P. J. Carroll and E. J. Schelter, Proc. Natl. Acad. Sci., 2016, 113, 14887–14892 CrossRef CAS PubMed
; (c) H. Fang, B. E. Cole, Y. Qiao, J. A. Bogart, T. Cheisson, B. C. Manor, P. J. Carroll and E. J. Schelter, Angew. Chem., 2017, 129, 13635–13639 CrossRef
; (d) B. E. Cole, I. B. Falcones, T. Cheisson, B. C. Manor, P. J. Carroll and E. J. Schelter, Chem. Commun., 2018, 54, 10276–10279 RSC
; (e) T. Cheisson, B. E. Cole, B. C. Manor, P. J. Carroll and E. J. Schelter, ACS Sustainable Chem. Eng., 2019, 7, 4993–5001 CrossRef CAS
; (f) B. E. Cole, T. Cheisson, R. F. Higgins, E. Nakamaru-Ogiso, B. C. Manor, P. J. Carroll and E. J. Schelter, Inorg. Chem., 2019, 59, 172–178 CrossRef
.
-
(a) B. Pulko, X. Yang, Z. Lei, S. Odenbach and K. Eckert, Appl. Phys. Lett., 2014, 105, 232407 CrossRef
; (b) A. Franczak, K. Binnemans and F. Jan, Phys. Chem. Chem. Phys., 2016, 18, 27342–27350 RSC
; (c) Z. Lei, B. Fritzsche and K. Eckert, J. Phys. Chem. C, 2017, 121, 24576–24587 CrossRef CAS
; (d) I. R. Rodrigues, L. Lukina, S. Dehaeck, P. Colinet, K. Binnemans and J. Fransaer, J. Phys. Chem. Lett., 2017, 8, 5301–5305 CrossRef CAS PubMed
; (e) I. R. Rodrigues, L. Lukina, S. Dehaeck, P. Colinet, K. Binnemans and J. Fransaer, J. Phys. Chem. C, 2018, 122, 23675–23682 CrossRef CAS
.
- R. F. Higgins, T. Cheisson, B. E. Cole, B. C. Manor, P. J. Carroll and E. J. Schelter, Angew. Chem., 2020, 132, 1867–1872 CrossRef
.
- We are defining separation factor (SF) and enrichment factor (EF) as: SFRE1:RE2 = EFcrystals × EFmotherliquour where EFcrystals = ηRE2/ηRE1 and EFmotherliquour = ηRE1/ηRE2 (η represents the molar ratios of RE1 and RE2 in either the crystals or the mother liquour portion). Each separation factor presented in this study is representative of the average of three measurements.
- P. E. Car, M. Perfetti, M. Mannini, A. Favre, A. Caneschi and R. Sessoli, Chem. Commun., 2011, 47, 3751–3753 RSC
.
- G. Cucinotta, M. Perfetti, J. Luzon, M. Etienne, P.-E. Car, A. Caneschi, G. Calvez, K. Bernot and R. Sessoli, Angew. Chem., Int. Ed., 2012, 51, 1606–1610 CrossRef CAS PubMed
.
- M. Briganti, E. Lucaccini, L. Chelazzi, S. Ciattini, L. Sorace, R. Sessoli, F. Totti and M. Perfetti, J. Am. Chem. Soc., 2021, 143, 8108–8115 CrossRef CAS PubMed
.
- M. E. Boulon, G. Cucinotta, J. Luzon, C. Degl'Innocenti, M. Perfetti, K. Bernot, G. Calvez, A. Caneschi and R. Sessoli, Angew. Chem., Int. Ed., 2013, 52, 350–354 CrossRef CAS PubMed
.
- K. R. Meihaus, J. F. Corbey, M. Fang, J. W. Ziller, J. R. Long and W. J. Evans, Inorg. Chem., 2014, 53, 3099–3107 CrossRef CAS PubMed
.
- C. Hunt, Z. Zhang, K. Ocean, R. P. Jansonius, M. Abbas, D. J. Dvorak, A. Kurimoto, E. W. Lees, S. Ghosh, A. Turkiewicz, F. A. Garcés Pineda, D. K. Fork and C. P. Berlinguette, J. Am. Chem. Soc., 2022, 144, 733–739 CrossRef CAS PubMed
.
-
(a) R. T. Shannon and C. T. Prewitt, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1969, 25, 925–946 CrossRef CAS
; (b) R. T. Shannon and C. T. Prewitt, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1970, 26, 1046–1048 CrossRef CAS
; (c) R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra04729b |
| This journal is © The Royal Society of Chemistry 2022 |