Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sustainable xanthophylls-containing poly(ε-caprolactone)s: synthesis, characterization, and use in green lubricants†
Eloy Rodríguez-deLeón *a,
Moustapha Bah
*a,
Moustapha Bah a,
José E. Báez
a,
José E. Báez *b,
María T. Hernández-Sierra
*b,
María T. Hernández-Sierra c,
Karla J. Moreno
c,
Karla J. Moreno c,
Alejandro Nuñez-Vilchis
c,
Alejandro Nuñez-Vilchis a,
José Bonilla-Cruz
a,
José Bonilla-Cruz d and
Kenneth J. Shea
d and
Kenneth J. Shea e
e
aPosgrado en Ciencias Químico Biológicas, Faculty of Chemistry, Autonomous University of Queretaro (UAQ), Cerro de Las Campanas, Querétaro, 76010, Mexico. E-mail: eloy.rodriguez@uaq.mx
bDepartment of Chemistry, Division of Natural and Exact Sciences, University of Guanajuato (UG), Campus Guanajuato, Noria Alta S/N, Guanajuato 36050, Mexico. E-mail: jebaez@ugto.mx
cDepartment of Mechanical Engineering, National Technology Institute of Mexico at Celaya, Celaya 38010, Guanajuato, Mexico
dCentro de Investigación en Materiales Avanzados S.C. (CIMAV-Monterrey), Av. Alianza Norte 202, PIIT, Autopista Monterrey-Aeropuerto Km 10, Apodaca 66628, N.L., Mexico
eDeparment of Chemistry, University of California, Irvine, (UCI), Irvine 92697-2025, California, USA
First published on 27th October 2022
Abstract
Three xanthophylls [(3R,3′R,6′R)-lutein (1), (3R,3′S)-zeaxanthin (2), and (3R,3′S)-astaxanthin (3)] were used for the first time as initiators in the ring-opening polymerization (ROP) of ε-caprolactone (CL) catalyzed by tin(II) 2-ethylhexanoate [Sn(Oct)2] for the synthesis of novel sustainable xanthophyll-containing poly(ε-caprolactone)s (xanthophylls-PCL). The obtained polyesters were characterized by 1H and 13C NMR, FT-IR, DSC, SEC, and MALDI-TOF MS, and their use as additives in green lubricants was evaluated using a sliding friction test under boundary conditions. Xanthophylls-PCL were obtained with good conversions and with molecular weights determined by SEC to be between 2500 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 Da. The thermal properties of xanthophyll-polyesters showed a crystalline domain, detected by DSC. Lastly, the green lubricant activity of these polymers was evaluated and the results showed that xanthophylls-PCL could be employed as additives for biodegradable lubricant applications since they have better tribological behavior than current additives, which demonstrates their potential as future commercial materials with interesting eco-friendly properties for diverse applications.
500 Da. The thermal properties of xanthophyll-polyesters showed a crystalline domain, detected by DSC. Lastly, the green lubricant activity of these polymers was evaluated and the results showed that xanthophylls-PCL could be employed as additives for biodegradable lubricant applications since they have better tribological behavior than current additives, which demonstrates their potential as future commercial materials with interesting eco-friendly properties for diverse applications.
Introduction
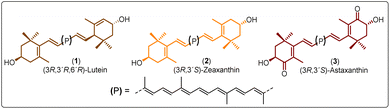
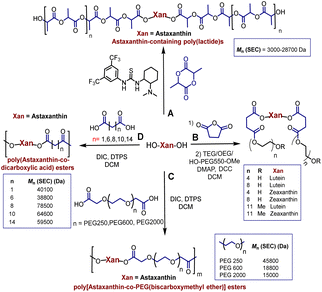
Polyesters derived from renewable resources are nowadays an expanding area with growing scientific activity,1–3 due to the growing need for polymers that are currently obtained from petroleum-based resources.4–6 Moreover, sustainability is a relevant topic today and for future generations.7 In this sense, at a global level, petroleum as a starting material for plastics production has limited availability.4 For this reason, natural products represent an alternative to produce biodegradable and eco-friendly materials.8,9 Among all the widespread compounds present in nature, carotenoids stand out as they perform important and crucial roles in the organisms in which they are present. For instance, carotenoids are responsible for yellow, orange, and red colors of birds'plumage and scales of some fish.10,11 In these animals, the intensity and brightness of their colors seem to be an indicator of good health.12 Also, carotenoids are selectively accumulated in the human macula lutea, in the center of the retina, where they comply with eye protection functions.13,14 Different studies have corroborated that xanthophylls such as lutein (1) and zeaxanthin (2) are efficient to prevent age-related macular degeneration (AMD).15–17 Carotenoids are a family of compounds that are composed of two classes: carotenes and xanthophylls. Chemically, carotenes are those formed only by carbon and hydrogen. Examples of these are α- and β-carotenes, and lycopene. Xanthophylls, on the other hand, are oxygenated carotenes with epoxy, hydroxy, methoxy or carbonyl groups. Examples of these are: lutein (1), zeaxanthin (2), and astaxanthin (3) (Fig. 1).18 One important function of the carotenoids in organisms is their protective action against reactive oxygen species (ROS).10,19 This feature can take advantage to create new materials that own the intrinsic characteristics of xanthophylls such as their antioxidant properties. Previously, our group reported the use of compounds 1, 2, and 3 as chain extender agents in poly(ester-urethane)s synthesis.20 Likewise, there are various studies in which carotenoids are used as functional diols to obtain diverse polymeric esters (Scheme 1).As illustred in Scheme 1, astaxanthin was used as initiator in the ring opening polymerization (ROP) of D,L-lactide in presence of a bifunctional organocatalyst (thiourea/tertiary amine) to obtain astaxanthin-containing poly(lactide)s (route A).21 Route B shows the procedure leading to the synthesis of poly(ethylene glycols) (PEGs) from lutein or zeaxanthin disuccinate conjugated with tetra, octa or poly(ethylene glycol).22 Route C represents a copolymerization of astaxanthin with various polyethyleneglycol-[biscarboxymethyl ether]s, while in route D, compound 3 was used with different diacids resulting in “polyactive” polyesters (antimicrobial agents against Staphylococcus aureus MRSA252 and MSSA476).23–25 It is worth highlighting that the stereochemistry of the astaxanthin used in routes A, C and D was not defined, while the stereochemistries of lutein and zeaxanthin used in route B were (3R,3′R,6′R) and (3R,3′R), respectively.
Xanthophylls such as 1, 2, and 3 have never been used before as initiators to produce poly(ε-caprolactone)s. In the present contribution, we synthesized novel xanthophylls-containing poly(ε-caprolactone)s with important characteristics due to the fact that they are biocompatible and biodegradable materials,26–29 and can be used as additives in green lubrication.
Experimental section
Materials
(3R,3′R,6′R)-lutein (1) was extracted from marigold oleoresin, which was purchased from Alcosa Biotech (Alcosa Biotech, Apaseo el Grande, Guanajuato, México). (3R,3′S)-zeaxanthin (2) and (3R,3′S)-astaxanthin (3) were obtained by partial synthesis from compound 1 (as we have described previously30). ε-caprolactone, 1,8-octanediol, tin(II) 2-ethylhexanoate, and deuterated chloroform (CDCl3) were acquired from Sigma Aldrich Co (St Louis, Mo, USA) and used as received.Instruments
Fourier transform-infrared (FTIR) spectra of all polyesters and xanthophylls were obtained with the attenuated total reflectance spectroscopy (ATR) technique in a PerkinElmer Spectrum 100 spectrometer. Nuclear magnetic resonance (NMR) spectra of 1H and 13C were recorded on a 500 MHz Bruker Avance III HD instrument and CDCl3 as solvent. Spectra were referenced to the chemical shift of the residual solvent protons at 7.26 ppm in the 1H NMR and the residual solvent carbons at 77.16 ppm in the 13C NMR spectrum.Size exclusion chromatograph 1260 Infinity Agilent Technologies coupled to a refractive index detector (1260 RID) was used to determine the number average molecular weight (Mn) and dispersity (ĐM) of the synthesized polyesters. These analyses were recorded using as stationary phase a column PLgel 5 mm MIXED-D and THF as mobile phase, at a flow rate of 1.0 mL min−1. All chromatograms were acquired at 37 °C and the results reported relative to polystyrene standards.
Differential Scanning Calorimetry (DSC) thermograms were performed on a TA Q200 instrument. Samples were sealed in aluminum pans. The thermal analysis was obtained using the next method: three scans were performed with two heating cycles (from 0 to 100 °C, 100 to −30 °C, and −30 to 100 °C). The flow rate of cooling/heating was 10 °C min−1 under nitrogen purge. All data presented are taken from the second heating cycle and the melting points (Tm) are given as the minimum of the endothermic transition.
Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) spectra were recorded in linear mode by using an AB SCIEX TOF/TOF 5800 SYSTEM equipped with a nitrogen laser emitting at 349 nm, an input bandwith = 1000 MHz with a 3 ns pulse width and working in positive mode. 2,5-Dihydroxybenzoyc acid was used as matrix in a concentration of 10 mg mL−1 in THF as solvent. Polyester samples were dissolved in THF (3 mg mL−1), then 10 μL of sample solution was mixed with 10 μL of matrix solution. Different aliquots were placed on a stainless-steel plate and the solvent was evaporated before starting the acquisition.
Synthesis of xanthophylls-containing poly(ε-caprolactone)s (HOPCLOH)
Polymerization reaction was performed in bulk into a dry vial, in which ε-caprolactone (50 mmol, 5.707 g), tin(II) 2-ethylhexanoate (two drops, 24 mg), and 2.5 mmol of the corresponding xanthophyll (compound 1, 2, or 3) were placed. The mixture was stirred and heated at 120 °C for different periods of time (20 hours to 48 hours). The polyesters obtained were analyzed and used without purification. Three different macrodiols derived from each xanthophyll and CL were obtained by ROP. 1H NMR data of the polyester derived from zeaxanthin (zeaxanthin-PCL) (Fig. S6,† 500 MHz, CDCl3, ppm): δ 6.47 [m, 4H, (11,11′ & 15,15′, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), zeaxanthin], 6.20 [m, 2H, (12,12′, –C
CH–), zeaxanthin], 6.20 [m, 2H, (12,12′, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), zeaxanthin], 6.17 [m, 4H, (14,14′ & 10,10′, –C
CH–), zeaxanthin], 6.17 [m, 4H, (14,14′ & 10,10′, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), zeaxanthin], 6.09 [m, 2H, (8,8′, –C
CH–), zeaxanthin], 6.09 [m, 2H, (8,8′, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), zeaxanthin], 5.97 [m, 2H, (7,7′, –C
CH–), zeaxanthin], 5.97 [m, 2H, (7,7′, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), zeaxanthin], 5.00 [m, 2H, (3,3′, –C
CH–), zeaxanthin], 5.00 [m, 2H, (3,3′, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –O–), zeaxanthin], 4.08 [t, 2H, (f, –CH2–O–), PCL], 3.58 [t, 2H, (f′, –CH2–OH), PCL], 2.49 [t, 2H, (b, –CH2–CO–), PCL], 1.90 [s, 12H, (19,19′ & 20,20′, –CH3), zeaxanthin], 1.62 [m, 4H, (c & e, –CH2–), PCL], 1.10 [m, 2H, (d, –CH2–), PCL], 0.92 [s, 6H, (16,17, –CH3), zeaxanthin]. Mn(SEC) = 8023, ĐM = 1.48. FT-IR (cm−1; Fig. S15†): 3438 (v O–H), 2944 (vas CH2), 2865 (vs CH2), 1721 (v C
–O–), zeaxanthin], 4.08 [t, 2H, (f, –CH2–O–), PCL], 3.58 [t, 2H, (f′, –CH2–OH), PCL], 2.49 [t, 2H, (b, –CH2–CO–), PCL], 1.90 [s, 12H, (19,19′ & 20,20′, –CH3), zeaxanthin], 1.62 [m, 4H, (c & e, –CH2–), PCL], 1.10 [m, 2H, (d, –CH2–), PCL], 0.92 [s, 6H, (16,17, –CH3), zeaxanthin]. Mn(SEC) = 8023, ĐM = 1.48. FT-IR (cm−1; Fig. S15†): 3438 (v O–H), 2944 (vas CH2), 2865 (vs CH2), 1721 (v C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1176 [vas C–(C
O), 1176 [vas C–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O], 1045 (v, C–O), 960 (ω, C
O)–O], 1045 (v, C–O), 960 (ω, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H), 731 (ρ, CH2). DSC data (Table 2): 51.7 and 53.5 °C, ΔHm = 77.97 J g−1, χi = 58%.
C–H), 731 (ρ, CH2). DSC data (Table 2): 51.7 and 53.5 °C, ΔHm = 77.97 J g−1, χi = 58%.
Evaluation of xanthophylls-PCL as additives for biodegradable lubricants
The polyesters derived from lutein, zeaxanthin, and astaxanthin with DP = 20 were selected as representative additive samples. Castor oil (CastO) was selected as the lubricant base, because it is one of the most representative vegetable oils for the manufacturing of bio-lubricant products.31 Each polymer (0.5% weight) was diluted in castor oil at 70 °C. This mixture was stirred during 5 minutes until completely homogeneous lubricating solutions were obtained. The physical properties of bio-lubricant mixtures such as density, viscosity, and viscosity index were determined by the ASTM D891, ASTM D445, and ASTM D341 standards methods, respectively. Likewise, their friction and wear performance were evaluated by pin-on-disk tests based on the ASTM G 99 procedure. The performance of the polyester derived from 1,8-octanediol and CL (1,8-octanediol-PCL) with DP = 20 was also studied for comparison purposes. During experiments, friction coefficient was recorded by the equipment, while wear was evaluated at the end of the tests by calculating the wear rate.32Results and discussion
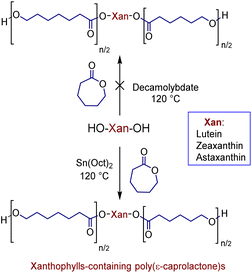
In previous studies, we have shown that the ammonium decamolybdate, (NH4)8[Mo10O34], is an excellent catalyst for ring-opening polymerization (ROP) of ε-caprolactone (CL).33–37 However, this catalyst is not efficient for the ROP when the xanthophylls are used as initiators, since the reaction did not occur (Scheme 2). This inefficiency is probably caused by the formation of other products. To confirm this assumption, the reaction between lutein and the ammonium decamolybdate was carried out in DCE as solvent at 80 °C for 24 hours (ES1). The reaction was monitored through reverse phase HPLC and the results indicated the presence of less polar products [probably dehydration products (anhydroluteins)] in the chromatogram (Fig. S2†). For this reason, we used the industrially relevant and accepted by the U.S. FDA38,39 catalyst, Sn(Oct)2, to obtain all xanthophylls-containing poly(ε-caprolactone)s (Scheme 2).With the use of this catalyst, a solvent-free polymerization and at 120 °C, we synthesized three different macrodiols derived from each xanthophyll (1, 2, and 3) and CL with degrees of polymerization (DP) of 10, 20, and 40. For comparison, three different macrodiols were synthesized using 1,8-octanediol (Oct) as initiator, CL, and (NH4)8[Mo10O34] as catalyst with the same DPs (10, 20, & 40) [1,8-octanediol-PCL].
The polyesters were obtained with high conversions (from 94 to 99%). The time of reactions for these xanthophyll-PCLs varied from 20 to 48 hours, except those macrodiols synthesized using (NH4)8[Mo10O34] as catalyst (Table 1). The molecular weights for these macrodiols were determined by size-exclusion chromatography (SEC) analysis (Fig. 2) to be between 2500 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 Da (Table 1), where an unimodal distribution was observed.
500 Da (Table 1), where an unimodal distribution was observed.
| [M]/[I]a | [I]b | DPc | Mnd | ĐMd,e | Timef (hours) | Conversionc (%) |
|---|---|---|---|---|---|---|
a [Monomer]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [initiator] ratio.b [I]: initiator.c Determined by 1H NMR.d Determined by SEC analysis.e ĐM: dispersity.f Time of reaction.g Synthesized using ammonium decamolybdate as catalyst. [initiator] ratio.b [I]: initiator.c Determined by 1H NMR.d Determined by SEC analysis.e ĐM: dispersity.f Time of reaction.g Synthesized using ammonium decamolybdate as catalyst. |
||||||
| 10 | 1 | 11.0 | 3803 | 1.85 | 44.00 | 96 |
| 20 | 19.6 | 5361 | 1.67 | 48.00 | 94 | |
| 40 | 40.0 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 365 |
1.58 | 48.05 | 94 | |
| 10 | 2 | 10.0 | 4716 | 1.28 | 24.10 | 97 |
| 20 | 19.8 | 5000 | 1.24 | 24.00 | 97 | |
| 40 | 40.0 | 8023 | 1.48 | 24.00 | 99 | |
| 10 | 3 | 10.0 | 4927 | 1.38 | 20.00 | 99 |
| 20 | 20.0 | 5427 | 1.39 | 20.50 | 99 | |
| 40 | 41.2 | 8466 | 1.46 | 20.05 | 99 | |
| 10g | Oct | 9.9 | 2767 | 1.65 | 2.08 | 99 |
| 20g | 19.4 | 4978 | 1.41 | 2.14 | 97 | |
| 40g | 40.0 | 9134 | 1.40 | 2.05 | 98 | |
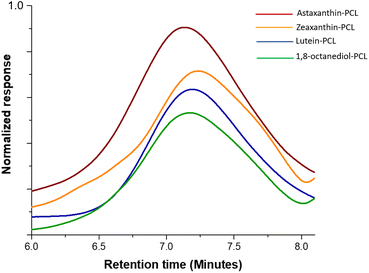 | ||
| Fig. 2 Refractive index-detected SEC chromatograms of the xanthophylls-PCL and 1,8-octanediol-PCL with DP = 20. | ||
The xanthophyll-polyesters showed major dispersity (ĐM) between 1.24 & 1.85, compared to polymers derived from 1,8-octanediol, which are on average of ĐM = 1.48 (Table 1). Therefore, the effect of the primary versus secondary alcohols (1,8-octanediol and xanthophylls, respectively) on the ĐM was negligible. On the other hand, with regard to the reactivity, we confirmed that, among the three xanthophylls, astaxanthin is the most reactive for ROP, followed by zeaxanthin and finally lutein, based on their percentages of conversion obtained by 1H NMR and the time of reaction. This behavior in the reactivity of compound 1 is probably due to the less reactive allylic alcohol in the 3′ position, compared with the secondary alcohols in the zeaxanthin and astaxanthin.
Analysis by 1H and 13C NMR confirmed the presence of the xanthophylls in the structure of the polyesters. Fig. 3a shows the 1H NMR spectrum of the polyester derived from compound 1, lutein-poly(ε-caprolactone) diol (lutein-PCL), which indicates the characteristic signals for the oxygenated methylene (–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O–) in the repetitive unit (f, δ 3.99) and the terminal hydroxymethylene (f', δ 3.58, –C
2–O–) in the repetitive unit (f, δ 3.99) and the terminal hydroxymethylene (f', δ 3.58, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH) of the PCL. The ratio of peak f area to that of peak f' is 9.8
2–OH) of the PCL. The ratio of peak f area to that of peak f' is 9.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which confirms that the main reaction product is HOPCLOH. This ratio multiplied by two indicates the degree of polymerization (DP), in this case, 19.6. The most intense signals were assigned to PCL: 2.24 ppm (b, –C
1, which confirms that the main reaction product is HOPCLOH. This ratio multiplied by two indicates the degree of polymerization (DP), in this case, 19.6. The most intense signals were assigned to PCL: 2.24 ppm (b, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CO–O–), 1.58 ppm (c & e, –CH2–), and 1.30 ppm (d, –CH2–). This spectrum also revealed a shift of resonances corresponding to the lutein protons upon poly(ε-caprolactone) chain growth, most notably in the chemical shift attributed to the protons attached to the 3 & 3′ carbons from 4.01 and 4.25 (Fig. S3†) to 5.00 and 5.26 ppm upon polymerization, respectively. The same effect was observed in the macrodiols derived from compounds 2 and 3 (Table S2†). The protons 3 & 3′ in the 1H spectrum of zeaxanthin appears from 4.01 ppm (Fig. S4†) to 5.00 ppm (Fig. S6†) upon polymerization. While in the 1H spectrum of astaxanthin-poly(ε-caprolactone) homopolymer (astaxanthin-PCL) was observed from 4.33 ppm (free astaxanthin, Fig. S5†) to 5.34 ppm (Fig. S7†). Finally, in the 1H NMR spectrum of lutein-PCL, some signals, such as vinylic protons between 6.57 and 6.03 ppm and those singlets of the methyl groups at 1.97 ppm (19, 19′, 20, & 20′) and 1.07 ppm (16, 16′, 17, & 17′) corresponding to the xanthophyll moiety were assigned.
2–CO–O–), 1.58 ppm (c & e, –CH2–), and 1.30 ppm (d, –CH2–). This spectrum also revealed a shift of resonances corresponding to the lutein protons upon poly(ε-caprolactone) chain growth, most notably in the chemical shift attributed to the protons attached to the 3 & 3′ carbons from 4.01 and 4.25 (Fig. S3†) to 5.00 and 5.26 ppm upon polymerization, respectively. The same effect was observed in the macrodiols derived from compounds 2 and 3 (Table S2†). The protons 3 & 3′ in the 1H spectrum of zeaxanthin appears from 4.01 ppm (Fig. S4†) to 5.00 ppm (Fig. S6†) upon polymerization. While in the 1H spectrum of astaxanthin-poly(ε-caprolactone) homopolymer (astaxanthin-PCL) was observed from 4.33 ppm (free astaxanthin, Fig. S5†) to 5.34 ppm (Fig. S7†). Finally, in the 1H NMR spectrum of lutein-PCL, some signals, such as vinylic protons between 6.57 and 6.03 ppm and those singlets of the methyl groups at 1.97 ppm (19, 19′, 20, & 20′) and 1.07 ppm (16, 16′, 17, & 17′) corresponding to the xanthophyll moiety were assigned.
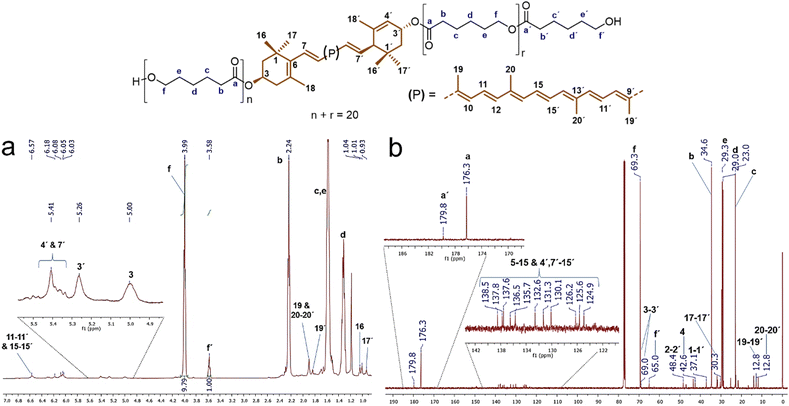 | ||
| Fig. 3 (a) 1H NMR (CDCl3, 500 MHz) and (b) 13C NMR (CDCl3, 125 MHz) spectra of polyester (DP = 19.6) derived from lutein (1). | ||
In the same way, the 13C spectrum also shows evidence for the presence of xanthophyll in the structure of the polymer. Fig. 3b shows the 13C spectrum of the macrodiol derived from compound 1 with DP = 20. The signals at 176.3 and 179.8 ppm correspond to the ester carbonyl group of the main chain (a) and the ester carbonyl of the end group (a'), respectively. Signals at 138.5 to 124.9 ppm correspond to vinylic carbons of the xanthophyll moiety (5–15 & 4′, 7′–15′) of the PCL. Meanwhile the signal at δ 69.3 (f, –![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O–CO–) was assigned to the methylene attached to the sp3 oxygen of the ester group and the signal at δ 34.6 (b, –
2–O–CO–) was assigned to the methylene attached to the sp3 oxygen of the ester group and the signal at δ 34.6 (b, –![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CO–O–) is ascribed to the methylene bound to the ester group. The other most intense peaks at 29.3 ppm (e, –CH2–), 29.0 ppm (d, –CH2–), and 23.0 ppm (c, –CH2–) observed in the spectrum were assigned to the other methylenes of PCL. In addition, the signals corresponding to the lutein moiety, such as those of the methylenes at 48.4 and 42.6 ppm corresponding to carbons 2, 2′ and 4, respectively, as well as methyl groups at δ 30.3 (17,17′) and 12.8 (19,19′ & 20,20′), and that methines 3 and 3′ at δ 69.0 and 69.3 were identified, respectively.
2–CO–O–) is ascribed to the methylene bound to the ester group. The other most intense peaks at 29.3 ppm (e, –CH2–), 29.0 ppm (d, –CH2–), and 23.0 ppm (c, –CH2–) observed in the spectrum were assigned to the other methylenes of PCL. In addition, the signals corresponding to the lutein moiety, such as those of the methylenes at 48.4 and 42.6 ppm corresponding to carbons 2, 2′ and 4, respectively, as well as methyl groups at δ 30.3 (17,17′) and 12.8 (19,19′ & 20,20′), and that methines 3 and 3′ at δ 69.0 and 69.3 were identified, respectively.
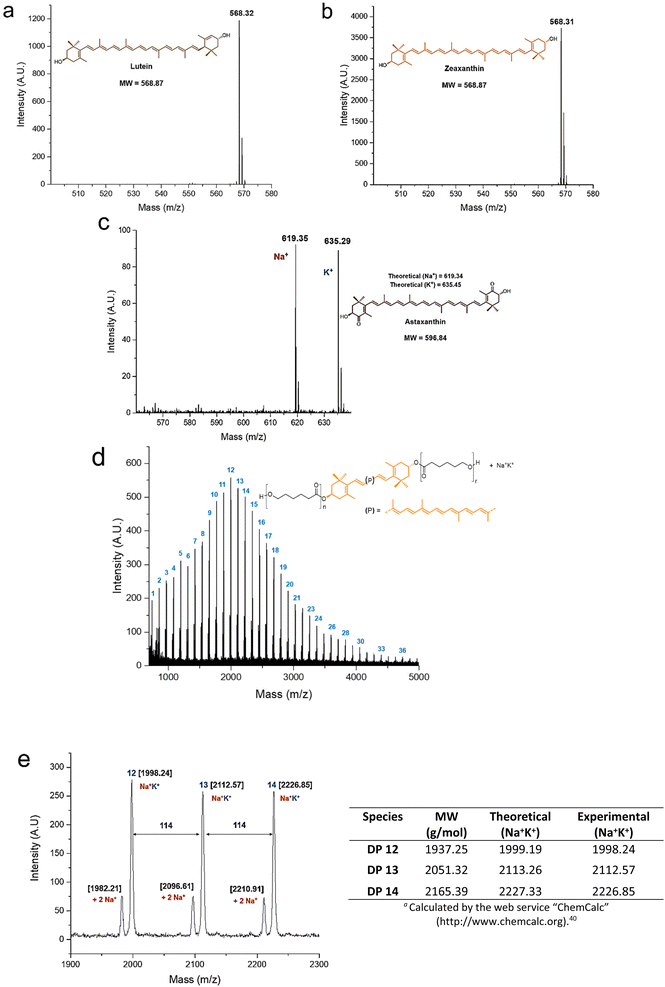
The MALDI-TOF analysis provided useful information for the characterization of the polyesters and their initiators (Fig. 4). The xanthophylls 1 and 2 were shown as molecular ion species (Fig. 4a and b), while compound 3 is observed doped with sodium and potassium ions (Fig. 4c). In the MALDI-TOF mass spectrum for zeaxanthin-poly(ε-caprolactone) diol with degree of polymerization equal to 10 (Fig. 4d) it was clearly observed the characteristic pattern of the curve profile with a unimodal molecular weight distribution of a PCL oligoester with a specific DP. Notably, the major repeat unit is 114 Da corresponding to CL unit. In an expanded view of the MALDI-TOF mass spectrum of zeaxanthin-PCL with DP = 10 (Fig. 4e), fragments between 1900 and 2300 Da are observed; in this zoom, the oligomers with 12–14 CL repeat units are visualized. The most intense peaks are doped with sodium and potassium ions (Na+K+), while low-intensity peaks are doped with two sodium ions (2Na+).
This pattern also was observed in other samples (Fig. S11†).40 On the other hand, a previous report about “retinol initiated poly(lactide)s” has described the presence of epoxide groups as a consequence of oxidation in the structure of retinoid.41 This might occur in the xanthophylls-PCL. However, the MALDI–TOF analysis of our samples did not reveal the presence of a mixture of natural and epoxidized xanthophylls (Fig. S14†), as it was described in the poly(lactide)s derived from retinol. Finally, it is worthy to mention that the presence of two sodiums or sodium-potassium adducts was not observed in the polyesters derived from 1,8-octanediol (Fig. S13†). Additionally, mass signals for macrocyclic species are not visualized, which indicates that intramolecular transesterification reactions neither occur. Take into consideration that xanthophylls are susceptible to epoxidations, this particularity can be used to increase the functionality of these polymers, which eventually we will describe in a future contribution (Scheme S1†).
In the same way, FTIR-ATR spectroscopy was useful for the characterization of the macrodiols. Fig. 5b shows the FT-IR spectrum of astaxanthin-PCL, where it can be observed the out-of-plane C–H bending vibration band at 961 cm−1 [(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H)] (ω), characteristic of trans-disubstituted alkenes, which corroborated the xanthophyll insertion. Likewise, the typical stretching vibrations of –CH2– (νas and νs) were shown at 2943 and 2865 cm−1, respectively. Other signals at 1175 and 731 cm−1 were assigned to stretching vibration of [–C–(C
C–H)] (ω), characteristic of trans-disubstituted alkenes, which corroborated the xanthophyll insertion. Likewise, the typical stretching vibrations of –CH2– (νas and νs) were shown at 2943 and 2865 cm−1, respectively. Other signals at 1175 and 731 cm−1 were assigned to stretching vibration of [–C–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O–] and bending vibration of (–CH2–) (rocking, ρ). With regard to the xanthophyll 3 used as initiator (Fig. 5a), a broad band at 3452 cm−1 was attributed to the hydroxyl groups and another thin band at 3020 cm−1 to the C–H stretching of the vinylic group. Additionally, the ketone carbonyl band at 1656 cm−1 and the stretching vibrations of methylene groups (–CH2–) were observed at 2859 (νs) and 2921 (νas) cm−1. These data match those published by others.42,43
O)–O–] and bending vibration of (–CH2–) (rocking, ρ). With regard to the xanthophyll 3 used as initiator (Fig. 5a), a broad band at 3452 cm−1 was attributed to the hydroxyl groups and another thin band at 3020 cm−1 to the C–H stretching of the vinylic group. Additionally, the ketone carbonyl band at 1656 cm−1 and the stretching vibrations of methylene groups (–CH2–) were observed at 2859 (νs) and 2921 (νas) cm−1. These data match those published by others.42,43
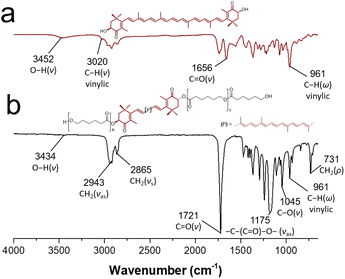 | ||
| Fig. 5 FT-IR spectrum of (a) free astaxanthin and (b) astaxanthin-poly(ε-caprolactone) homopolymer (DP = 20). | ||
Moreover, the thermal properties, such as melting temperature (Tm) and crystallinity (χi), of all polyesters were examined by differential scanning calorimetry (DSC). Table 2 summarizes the melting temperatures of all polymers which were observed between 36.1 and 53.5 °C. There is a clear correlation in the increase of the Tm as DP increases. The degree of crystallinity for all polymers was calculated from the endothermic peak area ΔHi by the equation χi = ΔHi/ΔH°i, where ΔH°i is the heat of fusion (135.3 J g−1, perfect PCL crystal).44
| Initiator | DPa | Tmb (°C) | ΔHmb (J g−1) | χic (%) |
|---|---|---|---|---|
| a Determined by 1H NMR.b Obtained by DSC, second heating cycle.c Obtained by the equation χi = (ΔHi/ΔH°i) (100). | ||||
| Lutein | 11.0 | 40.0, 44.7 | 56.77 | 42 |
| 19.6 | 47.1, 49.7 | 65.57 | 48 | |
| 40.0 | 51.3, 53.0 | 74.77 | 55 | |
| Zeaxanthin | 10.0 | 43.5, 47.9 | 70.23 | 52 |
| 19.8 | 48.0, 50.2 | 74.82 | 55 | |
| 40.0 | 51.7, 53.5 | 77.97 | 58 | |
| Astaxanthin | 10.0 | — | — | — |
| 20.0 | 46.77 | 56.39 | 42 | |
| 41.2 | 50.01 | 66.94 | 49 | |
| 1,8-Octanediol | 9.9 | 36.1, 41.0 | 84.62 | 62 |
| 19.4 | 44.3, 46.8 | 75.87 | 56 | |
| 40.0 | 50.1, 52.3 | 77.73 | 57 | |
Fig. 6 shows the DSC thermograms of the macrodiols derived from the zeaxanthin and those derived from 1,8-octanediol with DP = 10, 20, and 40.
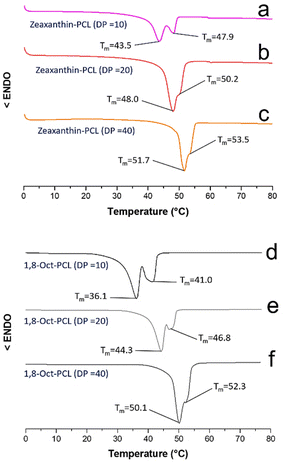 | ||
| Fig. 6 DSC thermograms of the three different macrodiols derived from zeaxanthin (a–c) and the macrodiols derived from 1,8-octanediol (d–f). Conditions: 10 °C min−1 (endo down); second heating cycle. | ||
The melting temperatures of the polyesters derived from compound 2 were detected between 43.5 and 53.5 °C (Fig. 6a–c). In the thermogram of zeaxanthin-PCL with DP = 10 (Fig. 6a) two endothermic peaks (Tm = 43.5 and 47.9 °C) were observed. The two signals may be explained in terms of two different sizes of crystallites that arise from two different environments, for example, crystallites immersed in an amorphous phase (43.5 °C) and in zones that are less amorphous in the PCL (47.9 °C). Similar behaviour in the 1,8-Oct-PCL with DP = 10 (Fig. 6d) was observed.
While the Tm of the 1,8-octanediol-PCL was observed between 36.1 and 52.3 °C (Fig. 6d–f). Generally, the polymers derived from 1,8-octanediol have a slightly lower Tm than xanthophylls-PCL. These results may be attributed to the significant crystalline domains in the xanthophylls, acting as nucleation agents (Fig S15†). The Tm of xanthophylls-PCL are near 45 °C, which is consistent with PCL oligoesteres previously reported.45 A single endothermic peak (Tm) was observed for astaxanthin-PCL samples (Fig. S16†). All the polyesters show a tendency towards increased crystallinity with increasing DP.
Table 3 summarizes the physical properties, as well as the friction and wear performance of bio-lubricants. The density of the bio-lubricants was not significantly affected with the addition of the polyesters. However, the viscosity and viscosity index were influenced by the type of initiator. In comparison with 1,8-octanediol-PCL, lutein-PCL allowed to increase the viscosity and the viscosity index of bio-lubricants up to 7% and 15%, respectively. Zeaxanthin-PCL decreased the viscosity by 12%, but increased the viscosity index by 26%. However, astaxanthin-PCL decreased both the viscosity and viscosity index to 5% and 26%, correspondingly. Regarding the effect these additives produced on the friction and wear performance, it can be observed that the lowest friction coefficient value was achieved with the additive containing lutein-PCL. With this component, the friction coefficient was 50% lower than that obtained with 1,8-octanediol-PCL. Nevertheless, an increase in friction of 28% was observed with astaxanthin-PCL. On the other hand, the wear rate was notably improved by all the xanthophylls-PCL. The higher contribution was observed with zeaxanthin-PCL, whose wear rate value was up to 39% lower than that acquired with 1,8-octanediol-PCL.
| Bio-lubricant | Densitya (Kg m−3) | Kinematic viscositya (mm2 s−1) | Viscosity index | FCb | Wear rateb (mm3 Nm−1) |
|---|---|---|---|---|---|
| a Properties at 40 °C.b Friction and wear test employing tungsten carbide pins of 3 mm, steel disks of 25.4 mm, 60 mL of bio-lubricant at 100 °C, normal load of 1 N, sliding speed of 0.025 m s−1, and total sliding distance of 377 m. | |||||
| CastO – 0.5% wt lut-PCL | 943 | 286 | 153 | 0.07 | 1.45 × 10−5 |
| CastO – 0.5% wt zea-PCL | 944 | 236 | 167 | 0.12 | 1.24 × 10−5 |
| CastO – 0.5% wt ast-PCL | 951 | 254 | 98 | 0.18 | 1.71 × 10−5 |
| CastO – 0.5% wt 1,8-PCL | 942 | 267 | 133 | 0.14 | 2.02 × 10−5 |
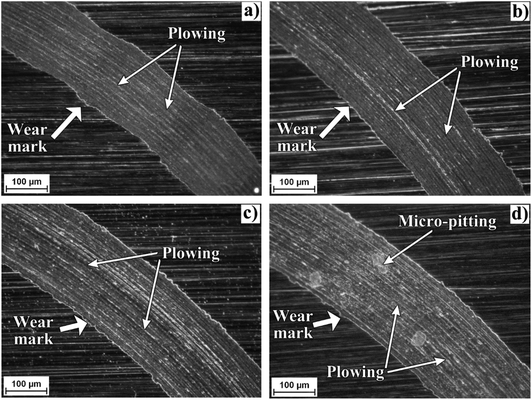
Fig. 7 shows an optical micrograph of a region of the disk surfaces tested with the studied bio-lubricants. Within the wear track marks, it can be observed that wear damage was different depending on the bio-lubricant employed. Worn surfaces lubricated with castor oil and xanthophylls-PCL exhibited only plowing wear which is characterized by the formation of grooves and furrows. Meanwhile, the surface tested with the bio-lubricant with 1,8-octanediol-PCL, besides showing wider and deeper grooves, exhibited pitting marks which are a form of localized corrosion that leads to the creation of small holes in the metal. In this sense, the intrinsic antioxidant activity of carotenoids into the xanthophylls-PCL could be contributed to the diminishing corrosion process. In a previous contribution, we demonstrated that xanthophylls 1, 2, and 3 are excellent additives for totally biodegradable lubricants, because they improve the tribological and antioxidant properties.32 In this context, the xanthophylls-PCL could be also employed with this purpose. The best results obtained in this work are the samples that contain lutein and zeaxanthin homopolymers may be due to the improvement in viscosity and viscosity index, which is a scale used to measure the change of the resistance to flow of a lubricant with the increase of its temperature. Furthermore, the greater the viscosity, the greater the layer that separates the mechanical elements, thus reducing friction and wear between them.
Finally, different studies have reported the use of carotenoids as stabilizers for both polymers and biopolymers to improve the resistance to the factors that promote their deterioration such as UV light, oxygen, heat, among others.46 Eventually, our group will report in a future contribution the properties of these xanthophylls-PCL and the free carotenoids as additives (compatibilizers, plasticizers, and antioxidants) in polymeric blends for the development of food packaging with antioxidant properties.
Conclusions
Xanthophylls-containing poly(ε-caprolactone)s were successfully synthesized by solvent-free ring-opening polymerization (ROP) of ε-caprolactone (CL), tin(II) octoate Sn(Oct)2 as catalyst, and using three different xanthophylls, (3R,3′R,6′R)-lutein (1) extracted from a renewable source (marigold oleoresin, Tagetes erecta L.), (3R,3′S)-zeaxanthin (2) and (3R,3′S)-astaxanthin (3) obtained by semisynthesis from 1, as initiators. Xanthophylls incorporation into the structure of the polyesters were confirmed by 1H and 13C NMR, MALDI-TOF mass spectrometry, and FT-IR spectroscopy analysis. Thermal properties of xanthophyll-polyesters showed a crystalline domains and melting temperatures between 36.1 and 53.5 °C, characteristic of classical PCL homopolymer, detected by DSC.All xanthophylls-PCL showed a better tribological behavior than current additives, which demonstrates their potential as future commercial materials with antioxidant and eco-friendly properties for diverse applications ranging from additives in green lubricants, antioxidant coatings in metallic surfaces for corrosion prevention, as additives for manufacturing of food packaging materials with antioxidant capacity, among others. Finally, this work represents the first report on the use of xanthophylls as initiators in the ring-opening polymerization of ε-caprolactone.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Eloy Rodríguez-deLeón highly thanks the Autonomous University of Queretaro (UAQ) for the current opportunity to work as an assistant professor, the Sistema Nacional de Investigadores (SNI) of CONACyT and the Mexican government (4T) for the distinction as researcher level I. Eloy Rodríguez-deLeón and J. E. Báez are thankful to Laboratorio Nacional de Caracterización de Propiedades Fisicoquímicas y Estructura Molecular (LACAPFEM UG-UAA-CONACYT) of the University of Guanajuato for the NMR spectra recording, and Patricia Cerda Hurtado (CIMAV-Unidad Monterrey) for the acquisition of the SEC chromatograms. Lastly, J. E. Báez thanks CONACYT Ciencia Básica for the grant “Proyecto SEP/284893” and CONACYT-SENER/177007 for the purchase of the DSC instrument.Notes and references
- S. A. Miller, ACS Macro Lett., 2013, 2, 550–554 CrossRef CAS PubMed.
- C. Vilela, A. S. Sousa, A. C. Fonseca, A. C. Serra, J. F. J. Coelho, C. S. R. Freire and A. J. D. Silvestre, Polym. Chem., 2014, 5, 3119–3141 RSC.
- F. Della Monica and A. W. Kleij, Polym. Chem., 2020, 11, 5109–5127 RSC.
- P. A. Wilbon, F. Chu and C. Tang, Macromol. Rapid Commun., 2013, 34, 8–37 CrossRef CAS PubMed.
- A. C. Weems, K. R. Delle Chiaie, J. C. Worch, C. J. Stubbs and A. P. Dove, Polym. Chem., 2019, 10, 5959–5966 RSC.
- S. L. Kristufek, K. T. Wacker, Y.-Y. T. Tsao, L. Su and K. L. Wooley, Nat. Prod. Rep., 2017, 34, 433–459 RSC.
- R. Beddoe, R. Costanza, J. Farley, E. Garza, J. Kent, I. Kubiszewski, L. Martinez, T. McCowen, K. Murphy, N. Myers, Z. Ogden, K. Stapleton and J. Woodward, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 2486–2489 Search PubMed.
- A. Gandhini, Green Chem., 2011, 13, 1061–1083 RSC.
- K. T. Wacker, A. C. Weems, S.-M. Lim, S. Khan, S. E. Felder, A. P. Dove and K. L. Wooley, Biomacromolecules, 2019, 20, 109–117 CrossRef CAS PubMed.
- R. Álvarez, B. Vaz, H. Gronemeyer and A. R. de Lera, Chem. Rev., 2014, 114, 1–125 CrossRef PubMed.
- A. M. LaFountain, S. Kaligotla, S. Cawley, K. M. Riedl, S. J. Schwartz, H. A. Frank and R. O. Prum, Arch. Biochem. Biophys., 2010, 504, 142–153 CrossRef CAS PubMed.
- J. Ng, A. L. Kelly, D. J. Macguigan and R. E. Glor, J. Hered., 2013, 104, 862–873 Search PubMed.
- G. Britton, S. Liaaen-Jensen and H. Pfander, Carotenoids volume 5: Nutrition and Health, Birkhäuser Verlag, Basel, 1st edn, 2009, pp. 7–8 Search PubMed.
- M. A. K. Widjaia-Adhi, S. Ramkumar and J. von Lintig, FASEB J., 2018, 32, 6305–6315 CrossRef PubMed.
- M. P. Horvath, E. George, Q. T. Tran, K. Baumgardner, G. Zharov, S. Lee, H. Sharifzadeh, T. Mattinson, B. Li and P. S. Bernstein, Acta Crystallogr., Sect. F: Struct. Biol. Commun., 2016, 72, 609–618 CrossRef CAS PubMed.
- A. Kijlstra, Y. Tian, E. R. Kelly and T. T. J. M. Berendschot, Prog. Retinal Eye Res., 2012, 31, 303–315 CrossRef CAS PubMed.
- S. Zampatti, F. Ricci, A. Cusumano, L. Tonino, G. Novelli and E. Giardina, Nutr. Res., 2014, 34, 95–105 CrossRef CAS PubMed.
- IUPAC Commission of Nomenclature of Organic Chemistry, IUPAC-IUB Commission on Biochemical Nomenclature, Pure Appl. Chem., 1975, 41, 405–431 CrossRef.
- C. I. Cazzonelli, Funct. Plant Biol., 2011, 38, 833–847 CrossRef CAS PubMed.
- E. Rodríguez-deLeón, M. Bah, J. O. C. Jiménez-Halla, J. Bonilla-Cruz, M. Estévez and J. E. Báez, Polym. Chem., 2019, 10, 6580–6587 RSC.
- H. Middleton, S. Tempelaar, D. M. Haddleton and A. Dove, Polym. Chem., 2011, 2, 595–600 RSC.
- M. Háda, D. Petrovics, V. Nagy, K. Böddi, J. Deli and A. Agócs, Tetrahedron Lett., 2011, 52, 3195–3197 CrossRef.
- S. Weintraub, T. Shpigel, L. G. Harris, R. Shuster, E. C. Lewis and D. Y. Lewitus, Polym. Chem., 2017, 8, 4182–4189 RSC.
- S. Weintraub, L. G. Harris, K. Thevissen and D. Y. Lewitus, Materialia, 2018, 3, 15–20 CrossRef CAS.
- V. Naggy, J. Deli, and A. Agócs, Chemical synthesis of carotenoid esters from the book Carotenoid Esters in Food Chemistry: physical, chemical and biological properties, The Royal Society of Chemistry, London, 1st edn, 2019, pp. 77–87 Search PubMed.
- D. Kai, K. Zhang, L. Jiang, H. Z. Wong, Z. Li, Z. Zhang and X. J. Loh, ACS Sustainable Chem. Eng., 2017, 5, 6016–6025 CrossRef CAS.
- M. Bartnikowski, T. R. Dargaville, S. Ivanovski and D. W. Hutmahcer, Prog. Polym. Sci., 2019, 96, 1–20 CrossRef CAS.
- R. Liang, J. Zhao, B. Li, P. Cai, X. J. Loh, C. Xu, P. Cheng, D. Kai and L. Zheng, Biomaterials, 2020, 230, 119601 CrossRef CAS PubMed.
- A. C. Weems, M. M. Pérez-Madrigal, M. C. Arno and A. P. Dove, Biomacromolecules, 2020, 21, 1037–1059 CrossRef CAS PubMed.
- E. Rodríguez-deLeón, J. E. Báez, J. O. C. Jímenez-Halla and M. M. Bah, Molecules, 2019, 24(7), 1–13 CrossRef PubMed.
- P. Ghosh, M. Hoque and G. Karmakar, Polym. Bull., 2018, 75, 501–514 CrossRef CAS.
- K. J. Moreno, M. T. Hernández-Sierra, J. E. Báez, E. Rodríguez-deLeón, L. D. Aguilera-Camacho and J. S. García-Miranda, Materials, 2021, 14, 1–17 Search PubMed.
- J. E. Báez, M. Martinez-Rosales and A. Martinez-Richa, Polymer, 2003, 44, 6767–6772 CrossRef.
- J. E. Báez and A. Martínez-Richa, Macromolecules, 2005, 38, 1599–1608 CrossRef.
- J. E. Báez and A. Martínez-Richa, Polymer, 2005, 46, 12118–12129 CrossRef.
- J. E. Báez, A. Marcos-Fernández, R. Lebrón-Aguilar and A. Martinez-Richa, Polymer, 2006, 47, 8420–8429 CrossRef.
- J. E. Báez and A. Marcos-Fernández, Int. J. Polym. Anal. Charact., 2011, 16, 269–276 CrossRef.
- M. Hoffman, C. Alberti, F. Scheliga, R. R. R. Meiβner and S. Enthaler, Polym. Chem., 2020, 11, 2625–2629 RSC.
- U.S. Food & Drug Administration. https://www.fda.gov/, accessed December 2021.
- L. Patiny and A. Borel, J. Chem. Inf. Model., 2013, 53, 1223–1228 CrossRef CAS PubMed.
- I. Yildirim, T. Yildirim, D. Kalden, G. Festag, N. Fritz, C. Weber, S. Schubert, M. Westerhausen and U. S. Schubert, Polym. Chem., 2017, 8, 4378–4387 RSC.
- B. Subramanian, M.-H. Thibault, Y. Djaoued, C. Pelletier, M. Touaibia and N. Tchoukanova, Analyst, 2015, 140, 7423–7433 RSC.
- C. Yuan, Z. Jin and X. Xu, Carbohydr. Polym., 2012, 89, 492–496 CrossRef CAS PubMed.
- V. Crescenzi, G. Manzini, G. Calzolari and C. Borri, Eur. Polym. J., 1972, 8, 449–463 CrossRef CAS.
- J. E. Báez, K. J. Shea, P. R. Dennison, A. Obregón-Herrera and J. Bonilla-Cruz, Polym. Chem., 2020, 11, 4228–4236 RSC.
- N. T. Dintcheva and F. D′Anna, ACS Sustainable Chem. Eng., 2019, 7, 12656–12670 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra04502h |
| This journal is © The Royal Society of Chemistry 2022 |