Open Access Article
Open Access ArticleDry gel synthesis of hierarchical ZSM-5 zeolite using hydroxyl propyl methyl cellulose (HPMC) as a mesoporogen and its catalytic activity in alkylation reactions†
Phan Huy Hoang * and
Nguyen Thi Thu Thao
* and
Nguyen Thi Thu Thao
School of Chemical Engineering, Hanoi University of Science & Technology, No. 1, Dai Co Viet Street, Hanoi, Vietnam. E-mail: hoang.phanhuy@hust.edu.vn
First published on 30th August 2022
Abstract
In this work, a “green” and facile method for synthesis of hierarchical ZSM-5 zeolite was presented by combination of a bio-mesoporogen and a dry gel conversion (DGC) process. ZSM-5 zeolite with high hierarchy factors and excellent mesoporosity was synthesized by adding hydroxypropylmethylcellulose (HPMC), which originated from cellulose biomass, to a zeolitic synthetic gel. The obtained zeolite samples were analyzed by X-ray diffraction (XRD), nitrogen adsorption–desorption (BET) and scanning electron microscopy (SEM) to determine their properties. The influence of crystallization time on the crystallinity of ZSM-5 zeolite was investigated. Moreover, the results showed that HPMC as a pore directing agent is an important factor for the formation of hierarchical zeolite with high mesoporosity. The as-prepared ZSM-5 sample with high pore volume, large surface area and abundant accessible acid sites, which seriously improves diffusion efficiency and catalytic activity, exhibited high catalytic performance in the benzylation reaction.
Introduction
ZSM-5 zeolite – a type of zeolite with a high Si content, is one of the most important crystalline microporous materials with a rather large specific surface.1–3 ZSM-5 zeolite has been widely used as a catalyst in industrial chemical, ion exchange and adsorption fields due to its unique pore structure and good thermal stability.4,5 It has selective adsorption and catalytic ability due to the uniform pore size of zeolite that only allows molecules of suitable shape and size to pass through. However, the microporous structure of the ZSM-5 zeolite is a drawback when applied as a catalyst in chemical reactions. These micropores hinder the diffusion of materials with large size in/out the catalyst matrix, leading to not high efficiency in catalytic reactions. Hence, this would limit the applications of zeolite in the chemical industry.Therefore, many efforts have been focusing on solving this issue by synthesis and application of mesoporous ZSM-5 zeolite in catalysis and adsorption. Generally, ZSM-5 zeolite was hydrothermally synthesized in the presence of organic mesoporogens,4,6–8 a three-dimension ordered mesoporous (3DOM) carbon compound,9 and amphiphilic organosilane surfactants.10 However, popular mesoporogen agents used in the synthesis of zeolite are mostly synthetic chemicals, expensive, toxic and may negatively influence the environment which limit the massive industrial production of zeolites.4,6 It is recognized that carbohydrate materials have been used successfully as pore-directing agents to synthesize hierarchical ZSM-5 zeolite with greater pore sizes and specific area.4,11–13 Its lifetime and catalytic activity are, thus, obviously improved. Therefore, cellulose derivatives, which are originated from carbohydrate biomass, biodegradable, and environmentally friendly, could be considered as promising alternative pore-directing agents for synthesis of hierarchical ZSM-5 zeolite.
Furthermore, in most cases, synthesis of ZSM-5 zeolite is mostly conducted followed the traditional hydrothermal method, which uses large amounts of water, alcohols, or ammonium hydroxide solution as solvent under autogenous pressure at high temperatures.5,6,14 This method has its own shortcomings as high autogenous pressure, generation of a large amount of waste solvents and low efficiency.5,15 In addition, the zeolite crystallization in solvent using the conventional hydrothermal routes is difficult to control, which is not profitable to the regulation of zeolite properties, especially in the case of achievement of smaller ZSM-5 zeolite particles in bulk synthesis. It is known that smaller particles of ZSM-5 zeolite are much more effective than large particles.16,17 The smaller size ZSM-5 zeolite possesses the important advantages of shorter diffusion paths, higher resistance to coke poisoning, and more active sites in catalytic process. It is worth noting that, the important advantages of the dry gel conversion (DGC) method over traditional hydrothermal method are higher zeolite product yield, fast crystallization, less reactor volume requirement, less waste generation, lowering of the consumptions of expensive templates and possible continuous production.17–20 Due to the higher nucleation density for ZSM-5 crystals in the dry gel conversion leading to the formation of small zeolite crystals.
We were, therefore, motivated that the efficient and green synthesis method could be made by taking fully the advantages that are offered by both cellulose derivatives mesoporogen and dry gel conversion (DGC) synthesis method. Such a synthesis could open a new route for “green”, fast, and versatile synthesis of hierarchical ZSM-5 zeolite to meet the increasingly stringent environmental demands.
Herein, we report a “green” and new route for synthesis of hierarchical ZSM-5 zeolite by combination of bio-pore directing agent – hydroxypropylmethylcellulose (HPMC), which is originated from biomass, and dry gel conversion (DGC) method. Hydroxypropylmethylcellulose (HPMC) is a derivative of natural cellulose, which are harmless, biodegradable, cheap, stable, and have been used in various fields. Texture properties of the as-obtained zeolite product from the synthesis procedures mentioned above such as the mesopores volume, pore size, and surface area were obviously improved. Indeed, this mesoporous zeolite catalyst shows advantages in reactions dealing with great molecular size bulky reactants when applied as catalyst for benzylation of aromatic compounds showing high conversion yield of desired product.
Experimental
Materials
NaAlO2, TPAOH 25%, tetraethyl orthosilicate (TEOS), KOH, hydroxypropyl methylcellulose (HPMC), benzene and benzyl alcohol were purchased from Sigma and all chemicals were used as received.Zeolite synthesis
An alumina precursor solution was prepared by adding 0.082 g sodium aluminate (NaAlO2) to a solution containing of 6.184 g tetrapropylammonium hydroxide (TPAOH) and 0.284 g potassium hydroxide (KOH). This solution was stirred until completely dissolved. Then different amount of HPMC pore-directing agent (0.18–0.24–0.3 g) was added to the above alumina precursor solution and stirred until completely dissolved. Finally, 10 g tetraethyl orthosilicate (TEOS) was added to the above solution to prepare a synthetic solution. This mixture is stirred with a magnetic stirrer and heated at 80 °C for 12 hours. After grinding, the dry powder was transformed to an autoclave containing a Teflon tube. Water was added at the bottom of autoclave in such a manner that dry gel and water were not in direct contact with each other. The autoclave was kept in the oven at 170 °C for 5, 10, 15 and 20 h to study the effect of crystallization time. At the end of the process, reaction mixture was washed with DI water and centrifuged with 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to separate the solid product. Zeolite particles were then dried at 100 °C and calcined at 550 °C for 5 h.
000 rpm to separate the solid product. Zeolite particles were then dried at 100 °C and calcined at 550 °C for 5 h.
Alkylation reaction
The benzylation of benzyl alcohol and benzene was performed at 80 °C in several reaction time with the mixture of 0.50 g ZSM-5 zeolite catalyst, 2.0 mL benzyl alcohol and 136 mL benzene. The reaction mixture was characterized to determine the conversion yield.Characterization
X-ray diffraction (XRD) was performed on a Rigaku D/max lllC (3 kW) with a h h−1 goniometer equipped using a Cu Kα radiation generator. Nitrogen adsorption–desorption isotherms were measured at 77 K on a Micromeritics (ASAP 2010, USA) using the Brunauer–Emmett–Teller (BET) method. Prior to analysis, all the samples were degassed at 300 °C for 10 h. The t-plot method was employed to estimate the micropore volume, and micropore surface area and external surface area. The Si/Al molar ratio was measured by the method of inductively coupled plasma (ICP) on a Varian 720 instrument. Scanning electron microscopy (SEM) was performed using a JSM-7000F, JEOL, Japan. The catalyst reaction products were analyzed with GC (Shimadzu instrument with FID detection, TR-WAX-TR1MS column of 60 m length and 0.32 mm inner) and characterized by GC-MS (TERMO Electron Corporation instrument).Results and Discussions
Hierarchical ZSM-5 zeolite synthesis
It is known that in the process of dry gel conversion, the fast crystallization is occurred due to a large number of nuclei formed in a short time.5,17 In order to investigate the effect of the crystallization time on the DGC synthesis process, experiments were conducted at different synthetic time (5, 10, 15 and 20 h) using 0.24 g hydroxypropyl methylcellulose (HPMC) pore-directing agent. XRD patterns of the samples crystallized at different times were shown in Fig. 1. The XRD patterns of the ZSM-5 zeolite showed that the crystallization time significantly influenced the crystallinity of the products. The XRD pattern of zeolite product obtained after 5 h crystallization time presented the characteristic diffraction peaks of MFI-type structure at 7–9°, 22–25° and 29–30°.21–23 Even the intensity of peak and crystallinity was not high however it implied that well-crystallized ZSM-5 zeolite was successfully synthesized by DGC method using cellulose-derivative pore-expanding agent. The result steadily suggested that nuclei of zeolite crystal have been formed in the early stage of crystallization.5 It is a very short crystallization time of ZSM-5 zeolite compared to the conventional hydrothermal method. Moreover, the result also strongly indicated that cellulose-derivative (HPMC) can be acted as suitable bio-mesoporogen, an alternate of conventional pore-directing agent for synthesis of mesoporous ZSM-5 zeolite.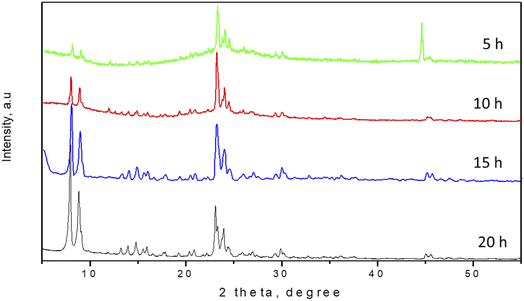 | ||
| Fig. 1 XRD patterns of hierarchical ZSM-5 zeolite synthesized by the DGC method using HPMC mesoporogen with different crystallization times. | ||
Increase the heating time the gel led to continuing increase in the intensity of the peaks of XRD pattern and increase the crystallinity of ZSM-5 crystals. When the crystallization time increased from 5 h to 15 h, there is almost no significant change on the XRD patterns but the intensities of the XRD peaks gradually improved. When the reaction time further increased to 20 h, the crystallinity of the sample did not increase further, and there was no impurity appeared indicating that the crystallization has been completed after 15 h reaction time.
In this current work HPMC, a cellulose derivative was applied as pore-directing agents for the synthesis of hierarchical ZSM-5 zeolite to improve the texture properties. To investigate the effect of pore-expanding substance to the texture properties of ZSM-5 zeolite, the amount of HPMC was changed from 0.18 to 0.3 g. The as-obtained products were denoted corresponding to the amount of HPMC used as Z-HPMC-0.18; Z-HPMC-0.24 and Z-HPMC-0.30. It is clearly seen from Fig. 2 that all three samples showed well-resolved peaks in the range of 5–55°, which can be indexed to the characteristic diffractions of MFI-type zeolite framework without any impurity phases. There was no amorphous background indicated the high crystallinity of ZSM-5 crystal. It can be also found that amount of pore-extended agent used in the synthetic process slightly affected on the crystallization of ZSM-5 zeolite. In particular, the amorphous nature of the material increased with the increase of HPMC mesoporogen amount.
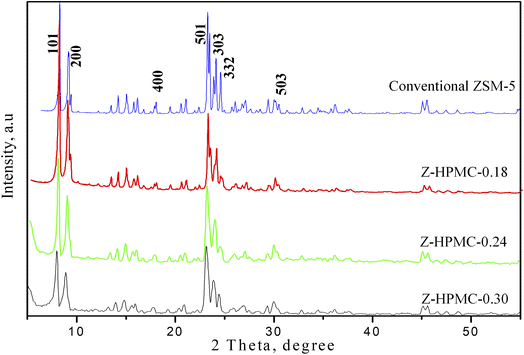 | ||
| Fig. 2 XRD patterns of hierarchical ZSM-5 zeolite synthesized by the DGC method using different amount of HPMC mesoporogen after 15 h crystallization time and conventional ZSM-5. | ||
The sharp peaks of XRD pattern were observed from sample of lower amount of HPMC. It can be explained that at a higher dosage of mesoporogen the formation of semi-crystalline ZSM-5 material containing a larger amount of amorphous material has been occurred and the presence of this amorphous material would lead to form of meso and macropores.4,11
Fig. 3 shows the nitrogen adsorption isotherms of the as-obtained ZSM-5 samples with different amount of HPMC pore-expanding agent. As seen from Fig. 3, conventional ZSM-5 exhibited a representative type I isotherm, which reflected its microporous structure without mesoporosity. While the samples showed the type IV isotherm with a hysteresis loop, which suggests the presence of large mesopores. The sharp uptake in nitrogen adsorption at relative pressures of 0.5–0.9 P/P0 revealed the capillary condensation of the gas inside the mesopores. Compared to conventional ZSM-5 zeolite, the hierarchical ZSM-5 samples showed a broader hysteresis loop from P/P0 = 0.5 to P/P0 = 1 due to the co-existence of both micropores and mesopores. Moreover, the concentration of HPMC pore mesoporogen in the initial synthetic gel was observed to strongly influence the porosity of the hierarchical ZSM-5 samples.
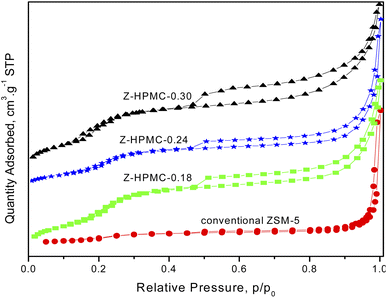 | ||
| Fig. 3 N2 adsorption/desorption isotherms of as-obtained hierarchical ZSM-5 zeolite samples with different dosage of HPMC pore-expanding agent. | ||
The porosity of samples significantly increased (relative pressure range P/P0 of 0.6–0.9) with the increase of mesoporogen dosage. This finding of improving porosity with increasing HPMC dosage is also consistent with the XRD data, where the increase in amount of HPMC was observed to enhance the amorphous phase of material.
Table 1 summarizes the textural properties of the of as-synthesized hierarchical ZSM-5 zeolites. It is obviously seen that all three zeolite samples using HPMC pore-expanding agent showed high pore volume and high surface area which could be attributed to the presence of mesopores in the zeolite structures. The BET surface area and pore volume of as-obtained zeolite samples were much higher than that of traditional ZSM-5 zeolite and the surface area and pore volume of Z-HPMC-0.30 was highest in all samples. It can be also found that the surface area, pore volume and pore diameters of samples improved with the increase in HPMC agent. This further indicated that an increase of pore-expanding agent led to form the zeolite materials with higher porosity. Overall, the influence of mesoporogen on mesoporosity clearly demonstrated the mesoporous expanding role of HPMC- a cellulose derivative. It is noted that HPMC is soluble macromolecular derivative of cellulose containing many hydroxyl groups, which endow it the ability to improve the interface interaction.4,24 Thus, HPMC could be well dispersed in silica due to satisfactory affinity to silica. In the synthesis process, HPMC macromolecules were integrated into the zeolite structure through the hydrogen bond interaction between Si–OH and the hydroxyl groups during the crystallization. After calcination, the pore directing agent was removed and the hierarchical ZSM-5 zeolite was achieved.12,25 HPMC mesoporogens was hence responsible for the formation of mesopores in the zeolite.
| Sample | SBET m2 g−1 | Smicro m2 g−1 | Vtotal m3 g−1 | Vmicro | dpore nm | Si/Al |
|---|---|---|---|---|---|---|
| ZSM-5 | 300 | 218 | 0.17 | 0.11 | 2.2 | 48 |
| Z-HPMC-0.18 | 415 | 192 | 0.25 | 0.09 | 3.6 | 51 |
| Z-HPMC-0.24 | 442 | 190 | 0.29 | 0.08 | 5.3 | 52 |
| Z-HPMC-0.30 | 481 | 198 | 0.31 | 0.06 | 5.8 | 52 |
The Si-to-Al ratios of ZSM-5, Z-HPMC-0.18, Z-HPMC-0.24 and Z-HPMC-0.30 zeolite were 48, 51, 52 and 52, respectively. These results are relatively similar and indicated that the content of framework aluminum in the zeolites was almost unchanged in both cases of using and non-using HPMC pore-directing agent.
Fig. 4 exhibited the SEM images of three zeolite samples in comparison with that of conventional ZSM-5 zeolite. SEM image (Fig. 4A) showed representative conventional zeolite particles possessing an orthorhombic morphology with smooth surface and relatively uniform diameter of around 500 nm. While the surfaces of the hierarchical zeolite particles showed much rougher than that of conventional zeolite. As seen from Fig. 4B–D that non-smooth surface of hierarchical zeolite particles was composed of nano-particle aggregates without observable amorphous structure. Noticeably, the aggregation of ZSM-5 zeolite crystals into particles led to the formation of some inter-crystalline mesopores.4,26 It is interesting that the morphology of ZSM-5 particles depends on the mesoporosity (dosage of mesoporogen). Z-HPMC-0.18 sample exhibited the similar shape with rough surface and particle size of about 300 nm, which are smaller conventionally ZSM-5 crystals. Whereas the Z-HPMC-024 and HPMC-0.30 samples exhibited the different shape with voids of internanoparticles and grainy surface. The smaller size of hierarchical zeolite could be attributed to the vapor assisted crystallization of dry gel conversion (DGC) method.
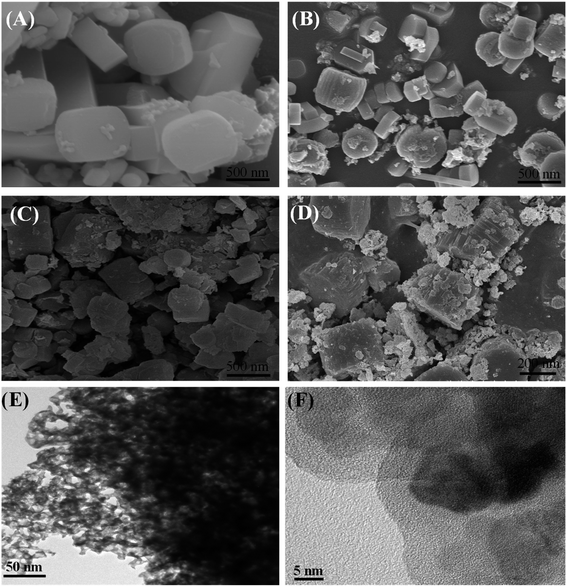 | ||
| Fig. 4 SEM images of various zeolite samples: (A) conventional ZSM-5 zeolite, (B) Z-HPMC-0.18, (C) Z-HPMC-0.24 and (D) Z-HPMC-0.30 samples; (E) and (F) TEM images of Z-HPMC-0.30 samples. | ||
It is seen from the TEM image of Z-HPMC-0.30 samples shown in Fig. 4E that there were many brighter areas corresponding to the mesopores created by the removal of porogens distributed homogenously in the crystals. This reflected the development of mesoporosity in the as-synthesized hierarchical ZSM-5 zeolite. Moreover, the high-resolution TEM images of mesoporous ZSM-5 (Fig. 4F) showed clear lattice fringes indicating high crystallinity of mesoporous ZSM-5, which is consistent with XRD patterns.
Catalytic activity test
The presence of a larger amount of mesoporosity in hierarchical zeolite should lead to faster diffusion of reactant and higher conversion yield. Therefore, the alkylation between benzene and benzyl alcohol (as shown in scheme of Fig. 5) was chosen to test the improvement of catalytic activity of the hierarchical ZSM-5 catalyst synthesized by using HPMC pore expanding agent.Table 2 summarized the results obtained benzylation reaction at different conditions (also see Table S1 in ESI†). It is found that the conversion of benzyl alcohol on hierarchical ZSM-5 zeolite was increased with the increase of mesoporosity of zeolite catalysts, and higher than that on traditional ZSM-5 zeolite.
| Catalyst samples | Dosage of catalyst, wt% over benzyl alcohol | Conversion of benzyl alcohol, % |
|---|---|---|
| a Reactions conducted after 6 h reaction time at 80 °C. | ||
| ZSM-5 | 30 | 14.7 ± 0.5 |
| Z-HPMC-0.18 | 30 | 50.6 ± 0.5 |
| Z-HPMC-0.24 | 30 | 61.5 ± 0.5 |
| Z-HEMC-0.30 | 30 | 73.8 ± 0.5 |
| Z-HEMC-0.30 | 20 | 64.1 ± 0.5 |
| Z-HEMC-0.30 | 40 | 80.2 ± 0.5 |
The Z-HPMC-0.18, Z-HPMC-0.24 and Z-HPMC-0.30 samples showed 50.6 ± 0.5, 61.5 ± 0.5% and 73.8% conversion yields of benzyl alcohol, respectively, which are significantly higher than 14.7 ± 0.5% yielded by the conventional ZSM-5. The Z-HPMC-0.30 sample with the highest mesoporosity gave the highest conversion of benzyl alcohol. The highly improved catalytic performance of the as-obtained hierarchical zeolite catalyst could be attributed to the large number of active sites on the high surface area as well as the presence of mesopore that is an important factor in the alkylation reaction with large size reactant molecules. Moreover, the uniform and small crystal size of ZSM-5 catalyst could reduce the diffusion path and increase the total numbers of acid sites which lead to high conversion yield of benzylation.17 The conversion of benzyl alcohol also increased with increasing amount of catalyst until the maximum conversions of 80.2 ± 0.5% were achieved at a catalyst dosage of 40% (wt% over benzyl alcohol).
The recycling of the catalyst is very important for chemical process. Thus, the reusability and recyclable efficiency of the catalyst was investigated (see Table S2, ESI†). After reaction, the Z-HPMC-0.30 catalyst was recovered and reused for another reaction cycle under the same conditions. During the five runs investigated, the conversion decreased from 73.8% to 68.4%, revealing that there was only little decrease in conversion. The obtained results showed that the hierarchical zeolite catalyst could be recycled and reused efficiently for at least five times with only modest reduction in the conversion of benzyl alcohol. This indicated that as-prepared hierarchical ZSM-5 is a promising catalyst for the alkylation between benzene and benzyl alcohol with high activity and stability.
Conclusion
In summary, a “green” and facile route for synthesis of hierarchical ZSM-5 zeolite has been presented by combination of bio-pore directing agent – hydroxypropylmethylcellulose (HPMC) and dry gel conversion (DGC) method. The highly crystallized mesostructured ZSM-5 zeolite was synthesized by adding different amount of pore expanding agent. It is found that the mesoporosity of zeolite samples increased with the increase of dosage of HPMC pore extended agent. The as-obtained hierarchical ZSM-5 zeolite with excellent mesoporosity was applied as catalyst for alkylation between benzene and benzyl alcohol. The hierarchical zeolite had a high mesopore volume and large external surface area, providing decreased reactant and product diffusion path length and extra mesopore channel for organic reaction. In particular, the zeolite catalyst showed the high catalytic activity for benzylation reaction with high conversion. Moreover, the catalyst could be reused and recycled for five times without decrease of catalytic activity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.01-2020.14.References
- M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki and R. Ryoo, Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts, Nature, 2009, 461(7261), 246–249 CrossRef CAS PubMed.
- J. Zhou, Z. Hua, Z. Liu, W. Wu, Y. Zhu and J. Shi, Direct synthetic strategy of mesoporous ZSM-5 zeolites by using conventional block copolymer templates and the improved catalytic properties, ACS Catal., 2011, 1(4), 287–291 CrossRef CAS.
- P. H. Hoang, H. S. Park and D. P. Kim, Ultrafast and continuous synthesis of unaccommodating inorganic nanomaterials in droplet- and ionic liquid-assisted microfluidic system, J. Am. Chem. Soc., 2011, 133(37), 14765–14770 CrossRef CAS PubMed.
- P. H. Hoang and N. M. Dat, Study on using cellulose derivatives as pore directing agent for preparation of hierarchical ZSM-5 zeolite catalyst, Adv. Powder Technol., 2021, 32(10), 3927–3933 CrossRef CAS.
- X. Chen, R. Jiang, Y. Gao, Z. Zhou and X. Wang, Synthesis of nano-ZSM-5 zeoliteviaa dry gel conversion crystallization process and its application in MTO reaction, CrystEngComm, 2021, 23(15), 2793–2800 RSC.
- Y. H. Chen, D. M. Han, H. X. Cui and Q. Zhang, Synthesis of ZSM-5 via organotemplate-free and dry gel conversion method: Investigating the effects of experimental parameters, J. Solid State Chem., 2019, 279, 120969 CrossRef CAS.
- Q. Wu, X. Wang, G. Qi, Q. Guo, S. Pan, X. Meng, J. Xu, F. Deng, F. Fan, Z. Feng, C. Li, S. Maurer, U. Müller and F. S. Xiao, Sustainable synthesis of zeolites without addition of both organotemplates and solvents, J. Am. Chem. Soc., 2014, 136(10), 4019–4025 CrossRef CAS PubMed.
- J. A. Rabo and M. W. Schoonover, Early discoveries in zeolite chemistry and catalysis at Union Carbide, and follow-up in industrial catalysis, Appl. Catal., A, 2001, 222(1–2), 261–275 CrossRef CAS.
- A. Boisen, I. Schmidt, A. Carlsson, S. Dahl, M. Brorson and C. J. H. Jacobsen, TEM stereo-imaging of mesoporous zeolite single crystals, Chem. Commun., 2003, 3(8), 958–959 RSC.
- Y. Fang and H. Hu, An ordered mesoporous aluminosilicate with completely crystalline zeolite wall structure, J. Am. Chem. Soc., 2006, 128(33), 10636–10637 CrossRef CAS PubMed.
- D. Nandan, S. K. Saxena and N. Viswanadham, Synthesis of hierarchical ZSM-5 using glucose as a templating precursor, J. Mater. Chem. A, 2014, 2(4), 1054–1059 RSC.
- M. Kustova, K. Egeblad, K. Zhu and C. H. Christensen, Versatile route to zeolite single crystals with controlled mesoporosity: In situ sugar decomposition for templating of hierarchical zeolites, Chem. Mater., 2007, 19(12), 2915–2917 CrossRef CAS.
- G. V. Brião, S. L. Jahn, E. L. Foletto and G. L. Dotto, Adsorption of crystal violet dye onto a mesoporous ZSM-5 zeolite synthetized using chitin as template, J. Colloid Interface Sci., 2017, 508, 313–322 CrossRef PubMed.
- K. Shen, N. Wang, W. Qian, Y. Cui and F. Wei, Atmospheric pressure synthesis of nanosized ZSM-5 with enhanced catalytic performance for methanol to aromatics reaction, Catal. Sci. Technol., 2014, 4(11), 3840–3844 RSC.
- L. Ren, Q. Wu, C. Yang, L. Zhu and C. Li, Solvent-free Synthesis of Zeolites from Solid Raw Materials, J. Am. Chem. Soc., 2012, 134, 15173–15176 CrossRef CAS PubMed.
- F. Mohammadparast, R. Halladj and S. Askari, The Crystal Size Effect of Nano-Sized ZSM-5 in the Catalytic Performance of Petrochemical Processes: A Review, Chem. Eng. Commun., 2015, 202(4), 542–556 CrossRef CAS.
- F. Mohammadparast, R. Halladj and S. Askari, The synthesis of nano-sized zsm-5 zeolite by dry gel conversion method and investigating the effects of experimental parameters by Taguchi experimental design, J. Exp. Nanosci., 2018, 13(1), 160–173 CrossRef CAS.
- P. S. Niphadkar, N. P. Tangale, P. N. Joshi and S. V. Awate, Crystallization kinetics of Sn-MFI molecular sieve formation by dry gel conversion method, Microporous Mesoporous Mater., 2013, 182, 73–80 CrossRef CAS.
- Y. Yan, R. Cai, Y. Liu and S. Gu, Ambient pressure dry-gel conversion method for zeolite MFI synthesis using ionic liquid and microwave heating, J. Am. Chem. Soc., 2010, 132(37), 12776–12777 CrossRef PubMed.
- Y. Wang, J. Song, N. C. Baxter, G. T. Kuo and S. Wang, Synthesis of hierarchical ZSM-5 zeolites by solid-state crystallization and their catalytic properties, J. Catal., 2017, 349, 53–65 CrossRef CAS.
- X. Li, X. Li, W. Qi, J. Shi, J. Zhang, Y. Xu and J. Pang, Preparation of Magnetic Biomass-based Solid Acid Catalyst and Effective Catalytic Conversion of Cellulose into High Yields of Reducing Sugar, BioResources, 2015, 10(4), 6720–6729 CAS.
- P. H. Hoang and T. D. Cuong, Simultaneous Direct Production of 5-Hydroxymethylfurfural (HMF) and Furfural from Corncob Biomass Using Porous HSO3-ZSM-5 Zeolite Catalyst, Energy Fuels, 2021, 35(1), 546–551 CrossRef CAS.
- B. Liu, C. Li, Y. Ren, Y. Tan, H. Xi and Y. Qian, Direct synthesis of mesoporous ZSM-5 zeolite by a dual-functional surfactant approach, Chem. Eng. J., 2012, 210, 96–102 CrossRef CAS.
- H. Tao, C. Li, J. Ren, Y. Wang and G. Lu, Synthesis of mesoporous zeolite single crystals with cheap porogens, J. Solid State Chem., 2011, 184(7), 1820–1827 CrossRef CAS.
- H. Zhu, Z. Liu, D. Kong, Y. Wang, X. Yuan and Z. Xie, Synthesis of ZSM-5 with intracrystal or intercrystal mesopores by polyvinyl butyral templating method, J. Colloid Interface Sci., 2009, 331(2), 432–438 CrossRef CAS PubMed.
- Q. Wang, S. Xu, J. Chen, Y. Wei, J. Li, D. Fan, Z. Yu, Y. Qi, Y. He, S. Xu, C. Yuan, Y. Zhou, J. Wang, M. Zhang, B. Su and Z. Liu, Synthesis of mesoporous ZSM-5 catalysts using different mesogenous templates and their application in methanol conversion for enhanced catalyst lifespan, RSC Adv., 2014, 4(41), 21479–21491 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra04325d |
| This journal is © The Royal Society of Chemistry 2022 |