Open Access Article
Open Access ArticlePreparation of different morphology Cu/GO nanocomposites and their catalytic performance for thermal decomposition of ammonium perchlorate†
Shengnan Lia,
Ziteng Niua,
Yuke Jiaoa,
Peng Jina,
Desheng Yanga,
Chaofei Baia,
Jiaran Liua,
Guoping Li *ab and
Yunjun Luo
*ab and
Yunjun Luo ab
ab
aSchool of Materials Science and Engineering, Beijing Institute of Technology, Beijing, 100081, China
bKey Laboratory for Ministry of Education of High Energy Density Materials, Beijing Institute of Technology, Beijing, 100081, China
First published on 15th August 2022
Abstract
Cu nanoparticles are more active catalytically than CuO nanoparticles, which have been widely studied as catalysts for organic synthesis, electrochemistry, and optics. However, Cu nanoparticles are easily agglomerated and oxidized in air. In this research, columnar, flower-like, bubble-like and teardrop-shaped Cu/GO nanocomposites were fabricated via a water-solvent thermal method and high temperature calcination technique using deionized water (H2O), methanol (CH3OH), ethanol (CH3CH2OH) and ethylene glycol (EG) as the solvent, respectively. The structures, the morphology and the catalytic performance and catalytic mechanism for thermal decomposition of ammonium perchlorate (AP) of the Cu/GO nanocomposites have been studied by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), scanning electron microscopy (SEM), nitrogen adsorption tests (BET), simultaneous thermogravimetry-differential scanning calorimetry (TGA/DSC) and thermogravimetric couplet with Fourier transform infrared spectroscopy (TGA–FTIR), respectively. The experimental results show that the morphology of the Cu/GO nanocomposites has a significant effect on the surface area and the teardrop-shaped Cu/GO nanocomposites have the largest specific surface area and the best catalytic performance among them. When 5 wt% of the Cu/GO nanocomposites was added, the decomposition temperature of AP decreased from 426.3 °C to 345.5 °C and the exothermic heat released from the decomposition of AP increased from 410.4 J g−1 to 4159.4 J g−1. In addition, the four morphological Cu/GO nanocomposites exhibited good stability, their catalytic performance for thermal decomposition of AP remained stable after 1 month in air. Excellent catalytic performance and stability were attributed to the strong catalytic activity of pure metal nanoparticles, and GO can accelerate electron movement and inhibit the agglomeration of nanoparticles, as well as the multiple effects of inhibiting the oxidation of Cu nanoparticles in air. Therefore, it has important application potential in high-energy solid propellant.
1 Introduction
With the rapid development of aerospace and weaponry technology, propulsion vehicles play a pivotal role.1,2 For example, launch vehicles that send satellites, space stations, spacecraft, and probes into space, as well as torpedoes and cruise missiles that achieve ultra-long-range precision strikes all require propulsion vehicles to achieve this. As the power source of propulsion vehicle engines, solid propellant is greatly significant. Ammonium perchlorate (AP) is the main component of the composite solid propellant, and its thermal decomposition characteristics directly affect the combustion performance of the propellant.3,4 However, at present, the decomposition temperature of AP is relatively high, the heat release is relatively low, and the temperature span between the high temperature decomposition peak and the low temperature decomposition peak is too large, resulting in a lack of concentration of the heat release, which is not conducive to the combustion of the propellant. Improving the thermal decomposition performance of AP is of great significance for improving the combustion performance of the propellant.5–7 The introduction of catalyst can effectively reduce the high temperature decomposition temperature of AP, and lower high temperature decomposition temperature and more heat release can lead to higher combustion rate. Therefore, it is of great significance to develop AP catalyst with excellent performance and change the thermal decomposition process of AP for further development of propellants.Transition metal oxide nanoparticles (Fe2O3, CuO, TiO2, Mn3O4, and Cr2O3) have been shown to be effective catalytic performance for thermal decomposition of AP.8–13 However, compared with transition metal oxide nanoparticles, transition metal nanoparticles (Ni, Al, etc.) are more active in the thermal decomposition of AP than commonly used transition metal oxide nanoparticles.14–17 Nevertheless, due to their small size, high surface activity, and easy agglomeration, transition metal oxide nanoparticles and transition metal nanoparticles cannot fully contact the medium and reduce the catalytic activity, which is not conducive to the combustion of the propellant, and many excellent properties of nanomaterials are lost.18,19 Loading them on 2D materials is an effective means to suppress nanoparticle agglomeration.20–23 Graphene oxide (GO) as a classical 2D material, has many excellent properties, such as large specific surface area, strong mechanical properties (strength of 130 GPa and Young's modulus of 1.0 TPa), good electrical conductivity (carrier mobility of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 (V−1 s−1)), high thermal conductivity (thermal conductivity of 5300 W (m−1 K−1)) and abundant surface oxygen-containing functional groups.24 The presence of these oxygen-containing functional groups allows GO to have a large number of active sites, which can attract polar molecules and can be compounded with a variety of nanoparticles to prepare nanocomposites.25–27 CuO nanoparticles with different morphologies have been prepared, which have good catalytic effect on the thermal decomposition of AP, and the morphology of nanoparticles has a certain influence on the catalytic effect.28,29 However, the study of the catalytic effect of Cu nanoparticles with higher activity on the thermal decomposition of AP is basically in a blank, so the preparation of Cu nanoparticles with different morphologies is of great significance for the propulsion system.
000 cm2 (V−1 s−1)), high thermal conductivity (thermal conductivity of 5300 W (m−1 K−1)) and abundant surface oxygen-containing functional groups.24 The presence of these oxygen-containing functional groups allows GO to have a large number of active sites, which can attract polar molecules and can be compounded with a variety of nanoparticles to prepare nanocomposites.25–27 CuO nanoparticles with different morphologies have been prepared, which have good catalytic effect on the thermal decomposition of AP, and the morphology of nanoparticles has a certain influence on the catalytic effect.28,29 However, the study of the catalytic effect of Cu nanoparticles with higher activity on the thermal decomposition of AP is basically in a blank, so the preparation of Cu nanoparticles with different morphologies is of great significance for the propulsion system.
In this research, four morphologies (columnar, flower-like, bubble-like and teardrop-shaped) Cu/GO nanocomposites were prepared by a water-solvent thermal method using graphene as a carrier under four solvent (H2O, CH3OH, CH3CH2OH and EG) conditions. Cu nanoparticles was loaded on GO, which effectively hindered the agglomeration of Cu nanoparticles and enhanced the stability of Cu nanoparticles in air. The structures, the morphology and their catalytic performance and catalytic mechanism for thermal decomposition of AP of the Cu/GO nanocomposites have been studied by XRD, XPS, TEM, SEM, BET, TGA/DSC and TGA–FTIR, respectively. It has significant potential for application in high-energy solid propellants.
2 Experimental section
2.1 Materials
Graphene oxide (GO) (>95%, 5–10 μm) was purchased from Suzhou Hengqi Graphene Technology Co. Ltd (China). Nanometer copper oxide (CuO) (99.5% metals basis, 150–250 nm) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd (China). Deionized water (H2O) was prepared in laboratory. Copper trihydrate nitrate (Cu(NO3)2·3H2O), urea, ethanol (CH3CH2OH), methanol (CH3OH) and ethylene glycol (EG) were supplied by Beijing Chemical Reagents Co. (China) and were used as received. Ammonium perchlorate (AP) was purchased from Dalian North Potassium Chlorate Co. Ltd (China) and were dried at 60 °C for 10 h before use.2.2 Synthesis of Cu/GO nanocomposites
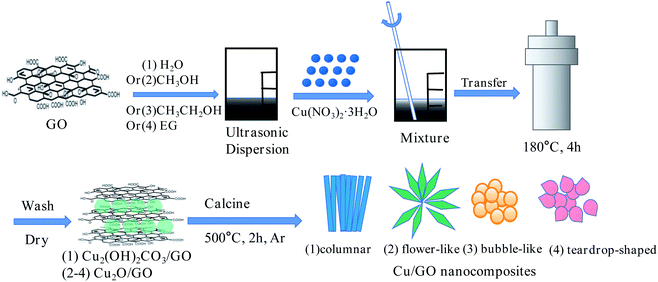
Synthesis of columnar Cu/GO nanocomposites: GO (112.5 mg) and deionized water (15 mL) were added to a 50 mL centrifuge tube, and ultrasonic was performed to uniform dispersion (ultrasonic power 95%, time 60 min) to prepare GO dispersion solution (7.5 mg mL−1). 1.45 g Cu(NO3)2·3H2O and 0.961 g urea and 30 mL deionized water were added in a 100 mL centrifuge tube. Then they were stirred manually for 30 min until completely dissolved. 10 mL GO dispersion was added to the fully dissolved solution. The solution was ultrasound for 30 minutes to prepare the precursor solution. Then the precursor solution was transferred to a 100 mL stainless steel autoclave. The synthesis was conducted at 180 °C for 4 h in a oven. The resulting powder was washed three times with ethanol and deionized water. After that, the powder was frozen at −80 °C for 5 hours to obtain the primary product of the nanocomposites. The primary product of the nanocomposites was calcined at 500 °C in a tube furnace (heating rate: 10 °C min−1) for 120 min in Ar. Finally, the columnar Cu/GO nanocomposites be obtained.Synthesis of flower-like, bubble-like and teardrop-shaped Cu/GO nanocomposites: under the same preparation conditions, different solvents (CH3OH, CH3CH2OH, and EG) were used to obtain flower-like, bubble-like and teardrop-shaped Cu/GO nanocomposites, respectively.
2.3 Preparation of catalytic samples (the Cu/GO-AP mixtures)
A certain amount of AP and Cu/GO nanocomposites were placed into a mortar (the mass ratio of the Cu/GO nanocomposites to AP was 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95), and thoroughly ground until it was uniform, that is, Cu/GO-AP mixtures were obtained.
95), and thoroughly ground until it was uniform, that is, Cu/GO-AP mixtures were obtained.
2.4 Characterization
The structures and the morphology of the Cu/GO nanocomposites were investigated by scanning electron microscope (SEM, ZEISS GeminiSEM 300), transmission electron microscope (TEM, Tecnai G2 F30), high-resolution transmission electron microscopy (HRTEM) and energy-dispersive X-ray spectroscopy (EDS). The X-ray diffraction (XRD) patterns of the samples were measured by Ultima IV X-ray diffraction instrument with Cu Kα source. The elemental composition and oxidation state information of teardrop-shaped Cu/GO nanocomposites and GO were carried out by X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha). Nitrogen adsorption and desorption isotherms of the Cu/GO nanocomposites were tested by Micromeritics ASAP2460 porosimetry analyzer. Pore size and surface areas were obtained by using the Barrett–Joyner–Halenda (BJH) and Brunauer–Emmett–Teller (BET) method respectively. The catalytic performance was detected by simultaneous thermogravimetry-differential scanning calorimetry (TGA/DSC1, MettlerToledo) at a heating rate of 10 °C min−1 from 30 °C to 500 °C in nitrogen. The catalytic kinetic of the Cu/GO nanocomposites for thermal decomposition of AP was performed on TGA/DSC1 at different heating rate (5 °C min−1, 10 °C min−1, 15 °C min−1, 20 °C min−1) from 30 °C to 500 °C in nitrogen. The catalytic mechanism of the Cu/GO nanocomposites for thermal decomposition of AP was carried out with thermogravimetric couplet with Fourier transform infrared spectroscopy (TGA/DSC3 MettlerToledo, FTIR Nicolet iS50) at a heating rate of 10 °C min−1 from 30 °C to 500 °C in nitrogen.3 Results and discussion
Different morphology Cu/GO nanocomposites were synthesized by a two-step method, as shown in Fig. 1. Firstly, GO was used as the carrier, deionized water (H2O), methanol (CH3OH), ethanol (CH3CH2OH) and ethylene glycol (EG) were used as solvent respectively and Cu(NO3)2·3H2O was used as the precursor. The initial products of Cu2(OH)2CO3/GO, Cu2O/GO, Cu2O/GO and Cu2O/GO nanocomposites were respectively synthesized under high temperature and high pressure. The XRD diffraction images of initial products showed in Fig. S1.† Then, the initial product of the nanocomposites was placed in a tubular furnace quartz tube, and calcined at high temperature under argon atmosphere (500 °C, 2 h). The GO was partially reduced to G. The C elements on GO transform the amorphous Cu2(OH)2CO3 and Cu2O into crystalline Cu at high temperature. Finally, columnar, flower-like, bubble-like and teardrop-shaped Cu/GO nanocomposites were prepared. The layered structure of GO inhibits ion agglomeration, while GO contains a large number of electronegative groups (carboxyl and hydroxyl groups) that interact electrostatically with ions to load Cu nanoparticles onto GO.3.1 The structures analysis of the Cu/GO nanocomposites
The structures of Cu/GO nanocomposites prepared with methanol, deionized water, ethanol and ethylene glycol as solvents were determined by X-ray diffraction (XRD), and the XRD patterns are shown in Fig. 2. The diffraction peaks at 2θ = 43.32°, 50.45° and 74.12° correspond to the (1,1,1), (2,0,0), (2,2,0) crystal surfaces of Cu. The relative intensity and positions of diffraction peaks are consistent with the standard card (PDF #85-1326). However, the diffraction peaks of Cu/GO which prepared in CH3OH have a slight deviation to the right. That probably because the oxidation of sample, O have smaller atomic radius compared to Cu, when oxidation happen, smaller oxygen atoms replaced copper atoms, caused the lattice contraction. No other impurity peaks appear, which indicated that Cu/GO nanocomposites are high crystallinity.In order to further characterize the elemental composition and oxidation state information of the Cu/GO nanocomposites, the valence electron states and bonding modes of GO and the teardrop-shaped Cu/GO nanocomposites prepared with ethylene glycol were characterized by XPS. XPS full spectra of GO and the teardrop-shaped GO/Cu nanocomposites (a), high-resolution Cu 2p XPS spectra of the teardrop-shaped GO/Cu nanocomposites (b), high-resolution C 1s XPS spectra of GO (c) and the teardrop-shaped GO/Cu nanocomposites (d) were shown in Fig. 3. As can be seen from Fig. 3(a), the characteristic peaks of C 1s and O 1s appear of both GO and Cu/GO nanocomposites in the full spectrum. However, the relative strength of the O 1s in the Cu/GO nanocomposites is relatively weak. This is caused by the elimination of part GO oxygen groups during calcination at high temperature. We observed that two intense peaks at 933.1 eV and 952.5 eV correspond to the Cu 2p3/2 and Cu 2p1/2, and two accompanying peaks with binding energy of 943.1 eV and 962.9 eV in the high-resolution Cu 2p XPS spectrum of the GO/Cu nanocomposites in Fig. 3(b), which shows that the amorphous copper oxide changes into the copper with high crystallinity after high calcination. Meanwhile, three binding energy peak C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.6 eV), C–OH/C–O–C (286.5 eV) and C
C (284.6 eV), C–OH/C–O–C (286.5 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (287.8 eV)30 all appear in the high-resolution C 1s XPS spectra of GO and the teardrop-shaped GO/Cu nanocomposites in Fig. 3(c) and (d). But the relative strength of the C–OH/C–O–C in the GO/Cu nanocomposites is significantly lower than the peak strength in the GO. It further shows that most oxygen groups of GO were eliminated during high temperature calcination. In order to further characterize the valence state of Cu, the Auger electron spectrum was characterized, the result was shown in Fig. 3(e). Only one characteristic peak can be seen at 567.8 eV, which correspond to Cu.
O (287.8 eV)30 all appear in the high-resolution C 1s XPS spectra of GO and the teardrop-shaped GO/Cu nanocomposites in Fig. 3(c) and (d). But the relative strength of the C–OH/C–O–C in the GO/Cu nanocomposites is significantly lower than the peak strength in the GO. It further shows that most oxygen groups of GO were eliminated during high temperature calcination. In order to further characterize the valence state of Cu, the Auger electron spectrum was characterized, the result was shown in Fig. 3(e). Only one characteristic peak can be seen at 567.8 eV, which correspond to Cu.
The micromorphology of Cu/GO nanocomposites is crucial for the catalytic effect of the thermal decomposition of AP. Fig. 4 shows SEM images of Cu/GO nanocomposites prepared by methanol (a), deionized water (b), ethanol (c) and ethylene glycol (d) as solvents, STEM-EDX (e), HR-TEM micrograph (f) and EDX image (g) of the teardrop-shaped GO/Cu nanocomposites. As can be seen from Fig. 4(a–d), with the increase of solvent viscosity (methanol ∼0.6 mPa s−1, deionized water ∼0.7972 mPa s−1, ethanol ∼0.983 mPa s−1, ethylene glycol ∼16.1 mPa s−1), the growth rate of the catalyst crystals was gradually decreased,31,32 the formation process is shown in Fig. 5. At high growth rates, the nanocrystals grow along both [0 0 1] and [0 1 0] directions,33,34 forming regular lamellar structures, and with increasing reaction time, the lamellar crystals form flower-like structures through self-assembly, as shown in Fig. 4(a). With the increase of viscosity, the growth of the nanocrystals along the [0 0 1] direction was significantly hindered, mainly along the [0 1 0] direction, and eventually formed a columnar structure, as shown in Fig. 4(b). With further increase of viscosity, the growth rate decreases in both directions and smaller lamellar crystals are formed, and regular self-assembly cannot be performed, and nanoparticles of similar size are randomly stacked together to form a bubble-like structure, as shown in Fig. 4(c). When the viscosity of the solvent increases to a certain extent, the movement of the nanoparticles during assembly is hindered, and in contrast to (c), a tightly arranged teardrop-like structure is formed as shown in Fig. 4(d). The microstructure of the Cu/GO nanocomposites, especially the uniform dispersion of the Cu nanoparticles in the GO sheet, has important implications for the catalytic effect of AP thermal decomposition. We used scanning transmission electron microscopy-energy dispersion X-ray spectroscopy (STEM-EDX) tests to detect the element distribution of the teardrop-shaped GO/Cu nanocomposites. As can be seen from Fig. 4(e), the Cu elements are uniformly dispersed on the GO nanosheets, and no agglomeration phenomenon is observed. This is due to the fact that the GO surface contains a lot of oxygen-containing functional groups such as hydroxyl and carboxyl groups. Those groups provide more attachment sites for nanoparticles, effectively inhibiting the agglomeration of nanoparticles and uniformly dispersing Cu/GO nanocomposites over the lamella of graphene. Due to the large number of oxygen-containing functional groups on the GO surface, the Cu/GO nanocomposites have a certain amount of oxygen. Besides, the oxygen-containing groups eliminated in the process of calcination, the free oxygen can adsorb with copper, result in oxygen gathered in where copper located. More importantly, the sampling depth of EDX is about 1 μm, after placed some time in air, the oxidation of the surface of copper also made the content of oxygen higher. That is also observed in XRD diffraction image of flower-like Cu/GO nanocomposites (Fig. 2). The lattice stripe of Cu can be clearly observed from Fig. 4(f). The crystal surface spacing of the nanoparticles calculated by Nanoscope Analysis software is 0.208 nm, corresponding to the (1,1,1) crystal surface of Cu,35 which is consistent with the results of XRD and XPS, indicated that Cu/GO nanocomposites was successfully prepared.
The morphology of nanoparticles has a significant effect on the specific surface area, and in general, the larger the specific surface area, the better the catalytic performance.28 As a fuel speed catalyst for solid propellant, the large specific surface area of the Cu/GO nanocomposites expose more active sites, thus improving the catalytic activity. Therefore, the specific surface area and porous structure characteristics of different morphology Cu/GO nanocomposites were studied by N2 adsorption–desorption isotherm method. The N2 adsorption detachment curvature and aperture distribution curves are shown in Fig. 6. As can be seen from Fig. 6, the nitrogen adsorption–desorption curve of the Cu/GO nanocomposites presented the H3 characteristic hysteresis ring-type IV isotherm. The pore size distribution ranges from 2 to 50 nm, indicating that it has layered mesopore structure characteristics and more small aperture sizes. As shown in Table 1, the specific surface area of Cu/GO was calculated by BET method. All four Cu/GO nanocomposites have large surface area. And the specific surface area of teardrop-shaped Cu/GO nanocomposites prepared with ethylene glycol as solvent can reach 39.77 m2 g−1.
 | ||
| Fig. 6 N2 adsorption/desorption isotherms and pore size distribution of Cu/GO nanocomposites with different morphologies. | ||
| Samples | Specific surface area/m2 g−1 |
|---|---|
| Bubble-like | 13.28 |
| Columnar | 19.30 |
| Flower-like | 22.48 |
| Teardrop-shaped | 39.77 |
The conductivity of catalyst is important in electron transfer. Higher conductivity means higher catalytic activity. The conductivity of teardrop-shaped Cu/GO nanocomposites and commercial copper oxide were characterized. As shown in Fig. 7, the conductivity of Cu/GO nanocomposites is much better than commercial CuO (150–250 nm), which is widely used as catalyst. With high conductivity, Cu/GO nanocomposites can accelerate the rate of electron transfer and speed of O2 to O2− conversion.
3.2 The catalytic performance of the Cu/GO nanocomposites for thermal decomposition of AP
It is interesting that four morphology Cu/GO nanocomposites were placed in the air after 1 month, the total heat release of Cu/GO/AP is not reduced. As shown in Fig. 8(d), four morphologies of Cu/GO nanocomposites still have good catalytic effect on AP. This is attributed to the strong catalytic activity of pure metal nanoparticles, GO accelerated electron movement of lamellar structure, and GO inhibition of nanoparticle agglomeration while also inhibiting the multiple effects of nanoparticles in oxidation in air.
 | ||
| Fig. 8 TG curves (a), DTG curves (b), DSC curves (c) and exothermic histograms (d) of 5 wt% the Cu/GO nanocomposites and AP blends. | ||
The comparison of the decomposition temperature and the heat release of different catalysts on AP thermal decomposition was shown in Table 2. It can be seen that, four Cu/GO nanocomposites with different morphologies have similar decomposition temperature with different types of CuO, PbO, CuFe2O4, [Cu(vimi)4]DCA2. More importantly, the heat release of four Cu/GO nanocomposites with different morphologies is much higher than other normal catalysts.
| Sample | Decomposition temperature (°C) | Heat release (J g−1) | Ref. |
|---|---|---|---|
| Flower-like Cu/GO | 353.7 | 2985.2 | This work |
| Columnar Cu/GO | 343.7 | 3096 | This work |
| Bubble-like Cu/GO | 349.6 | 3690.8 | This work |
| Teardrop-shaped Cu/GO | 345.5 | 4159.4 | This work |
| CuO | 353.8 | 1028 | 28 |
| Flower-like CuO | 353.17 | 1211 | 40 |
| CuO/GO | 337 | 1543.5 | 41 |
| Nano PbO | 400.9 | 1587 | 8 |
| Hollow flower-like nickel particles (40 nm) | 332 | 1163 | 10 |
| Hollow flower-like nickel particles (200 nm) | 374 | 697.2 | 10 |
| [Cu(vimi)4] DCA2 (3%) | 327 | 1599.2 | 12 |
| CuFe2O4/g-C3N4 (2.5%) | 318.6 | 1602 | 13 |
| CuFe2O4/GO | 331.4 | 1450 | 13 |
| CuFe2O4 | 331.8 | 1115 | 13 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95), and performed TG-DSC test at different heating rates (5 K min−1, 10 K min−1, 15 K min−1, 20 K min−1). According to the relationship between the peak exothermic heat and the heating rate, the kinetic parameters (activation energy and the leading factor) of AP under Cu/GO nanocomposites catalysis can be obtained. The relationship between thermal decomposition and heating rate of AP can be described by Kissinger's equation:
95), and performed TG-DSC test at different heating rates (5 K min−1, 10 K min−1, 15 K min−1, 20 K min−1). According to the relationship between the peak exothermic heat and the heating rate, the kinetic parameters (activation energy and the leading factor) of AP under Cu/GO nanocomposites catalysis can be obtained. The relationship between thermal decomposition and heating rate of AP can be described by Kissinger's equation:| ln(β + Tp2) = ln(AR/Ea) − Ea/RTp |
In this equation, β is the heating rate, Tp is the peak temperature of the AP high-temperature decomposition peak, Ea is the activation energy, R is the ideal gas constant, and A is the leading factor. As can be seen from the Kissinger equation, ln(β/Tp2) and (1/Tp) are linear relations. The line is fitted with ln(β/Tp2) as the ordinate and (1/Tp) as the abscissa. The activation energy and leading factor of AP thermal decomposition can be obtained from the slope and intercept of the straight line.
Fig. S3† shows the TG curves, DTG curves, and DSC curves of pure AP and AP in the presence of different morphology Cu/GO nanocomposites. Taking the thermal decomposition curve of AP as an example, as the heating rate increases, the TG, DTG and DSC curves move to the high temperature direction. This is due to the rapid heating rate of the temperature lead to the increase of gradient inside the system, which made the inhomogeneous heating of AP, caused the decomposition of AP at a higher temperature. Through the Kissinger equation, the liner fitting results of pure AP and AP in the presence of different morphology of Cu/GO were fitted. The fitting results were shown in Fig. 9. The corresponding kinetic parameters was shown in Table 3. The results showed that all four nanocomposites can reduce the activation energy of AP thermal decomposition, indicating that the existence of composites make the decomposition of AP proceed at lower temperature. In addition, the catalytic activities of Cu/GO nanocomposites with different morphologies in this work were compared with different catalysts. The Table 4 showed the comparison of the activation energy Ea of AP thermal decomposition with different catalysts given in recent literature. According to those results, Cu/GO nanocomposites have excellent catalytic action and most heat release. Among four Cu/GO nanocomposites, the teardrop-shaped Cu/GO nanocomposites is the most desirable catalyst.
| Sample | Ea (kJ mol−1) | A (min−1) | r |
|---|---|---|---|
| AP | 156.89 | 1.5 × 1016 | 0.994 |
| Bubble-like Cu/GO-AP mixtures | 73.41 | 7.4 × 107 | 0.995 |
| Flower-like Cu/GO-AP mixtures | 86.22 | 1.5 × 1010 | 0.992 |
| Columnar Cu/GO-AP mixtures | 65.7 | 1.1 × 107 | 0.967 |
| Teardrop-shaped Cu/GO-AP mixtures | 61.19 | 1.1 × 106 | 0.994 |
| Catalyst | Ea (kJ mol−1) | Ref. | |
|---|---|---|---|
| Pure AP | AP + catalyst | ||
| Bubble-like Cu/GO | 156.89 | 73.41 | This work |
| Flower-like Cu/GO | 156.89 | 86.22 | This work |
| Columnar Cu/GO | 156.89 | 65.7 | This work |
| Teardrop-shaped Cu/GO | 156.89 | 61.19 | This work |
| CuO | 213.4 | 86.7 | 42 |
| Linear nano-MnO2 | 203.87 | 195 | 43 |
| Sea-urchin nano-MnO2 | 203.87 | 150 | 43 |
| G/CuO | 129 | 71.47 | 44 |
| Al/G/CuO | 129 | 56.18 | 44 |
| 2% N–CuO/PbO | 156.7 | 102.3 | 8 |
| 0.5% CuFe2O4/g-C3N4 | 161 | 116.4 | 13 |
The catalytic process of Cu/GO-AP mixtures is shown in Fig. 11. In the low temperature decomposition stage, AP partially decomposes to produce NH3 and HClO4. Then REDOX reaction occurs in the gas phase, releasing a small amount of heat. In the high temperature decomposition stage, AP is completely decomposed, which is the main decomposition process of AP thermal decomposition. In this stage, a large quantity of heat was released.
| NH4+ + ClO4− = NH3(a) + HClO4(a) = NH3(g) + HClO4(g) |
The main process of AP decomposition is caused by the REDOX reaction of decomposition products of HClO4 with NH3 in the gas phase. If the gas molecule diffuses and escapes, then the decomposition product of HClO4 cannot fully react with NH3. Cu/GO nanocomposites as carriers for AP thermal decomposition reactions have large specific surface area, which gives the ability to adsorb gaseous molecules. The folds of the GO surface can also adsorb large amounts of gas molecules, providing a large number of active sites. Thus, in the second exothermic decomposition step of AP, Cu/GO nanocomposites adsorb gas-reactive molecules on their surface to react on the condensed phase. Meanwhile, there are many defects on the Cu nanoparticle lattice, and nitrogen oxides can easily react with Cu to form copper oxide.45,46 AP decomposition is accelerated by catalyzing the decomposition of nitrogen oxides.
| Cu + N2O = CuO + N2 |
| 4Cu + 2NO2 = 4CuO + N2 |
Moreover, due to the high thermal conductivity and high electrical conductivity, the rate of electron transfer and speed of O2 to O2− conversion accelerated. Finally, the AP decomposition heat increased, and the thermal decomposition rate increased.14
4. Conclusions
In summary, four novel morphologies (columnar, flower-like, bubble-like and teardrop-shaped) Cu/GO nanocomposites were prepared and characterized. It was confirmed that the four morphological Cu/GO nanocomposites have large specific surface area and excellent catalytic performance for thermal decomposition of AP. Among them, the teardrop-shaped Cu/GO nanocomposites has the largest specific surface area (39.77 m2 g−1) and the best catalytic performance. When 5 wt% of the Cu/GO nanocomposites was added, the decomposition temperature of AP decreased from 426.3 °C to 345.5 °C, the exothermic heat released from the decomposition of AP increased from 410.4 J g−1 to 4159.4 J g−1. Through the studies on pyrolysis volatiles of the Cu/GO-AP mixtures, it can be concluded that the catalytic mechanism of the Cu/GO nanocomposites for thermal decomposition of AP. Moreover, due to the presence of the layered structure GO, both the agglomeration of Cu nanoparticles and the oxidation of Cu nanoparticles in air are inhibited. All the results indicated that the Cu/GO nanocomposites had great potential applications in the future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Special Planning Projects (70902010115).Notes and references
- P. Ren, H. Wang, G. Zhou, J. Li, Q. Cai, J. Yu and Y. Yuan, Chin. J. Aeronaut., 2021, 34, 208–224 CrossRef.
- S. Jain, S. Chakraborty and L. Qiao, Combust. Flame, 2019, 206, 282–291 CrossRef CAS.
- I. P. S. Kapoor, P. Srivastava and G. Singh, Propellants, Explos., Pyrotech., 2009, 34, 351–356 CrossRef CAS.
- J. Wang, W. Zhang, Z. Zheng, Y. Gao, K. Ma, J. Ye and Y. Yang, J. Alloys Compd., 2017, 724, 720–727 CrossRef CAS.
- J. M. Gao, L. Wang, H. J. Yu, A. G. Xiao and W. B. Ding, Propellants, Explos., Pyrotech., 2011, 36, 404–409 CrossRef CAS.
- J. N. Modanlou and A. Eslami, J. Therm. Anal. Calorim., 2019, 136, 913–923 CrossRef.
- G. Marothiya, C. Vijay, K. Ishitha and P. A. Ramakrishna, Combust. Flame, 2017, 182, 114–121 CrossRef CAS.
- G. Zhang, B. Gou, Y. Yang, M. Liu, X. Li, L. Xiao, G. Hao, F. Zhao and W. Jiang, ACS Omega, 2020, 5, 32667–32676 CrossRef CAS PubMed.
- X. Zhou, C. Hu, X. Liu, W. Chen, Q. Tang and Y. Li, J. Rare Earths, 2020, 38, 108–112 CrossRef CAS.
- T. Zhang, H. Shi, Y. Zhang, Q. Liu, W. Fei and T. Wang, Appl. Surf. Sci., 2021, 552, 149506 CrossRef CAS.
- D. N. Ma, X. M. Li, X. Q. Wang and Y. J. Luo, Eur. J. Inorg. Chem., 2021, 10, 982–988 CrossRef.
- G. Lei, Y. zhong, Y. xu, F. yang, J. Bai, Z. Li, J. Zhang and T. Zhang, Chin. J. Chem., 2021, 39, 1193–1198 CrossRef CAS.
- C. Wan, J. Li, S. Chen, W. Wang and K. Xu, J. Anal. Appl. Pyrolysis, 2021, 160, 105372 CrossRef CAS.
- S. Wang, B. Ye, C. An, J. Wang and Q. Li, J. Mater. Sci., 2019, 54, 4928–4941 CrossRef CAS.
- L. Liu, F. Li, L. Tan, L. Ming and Y. Yi, Propellants, Explos., Pyrotech., 2004, 29, 34–38 CrossRef CAS.
- J. Wang, X. Lian, Q. Yan, D. Gao, F. Zhao and K. Xu, ACS Appl. Mater. Interfaces, 2020, 12, 28496–28509 CrossRef CAS PubMed.
- C. Sang, S. Jin, G. Li and Y. Luo, J. Sol-Gel Sci. Technol., 2021, 98, 559–567 CrossRef CAS.
- S. H. Ba, S. Y. Cai, F. Lu and J. Chin, Energy Mater., 2021, 29, 460–470 CAS.
- M. Nekoeinia, F. Salehriahi, O. Moradlou, H. Kazemi and S. Yousefinejad, New J. Chem., 2018, 42, 9209–9220 RSC.
- S. Isert, L. Xin, J. Xie and S. F. Son, Combust. Flame, 2017, 183, 322–329 CrossRef CAS.
- P. Chandrababu, J. Thankarajan, V. S. Nair and R. Raghavan, Thermochim. Acta, 2020, 691, 178720 CrossRef CAS.
- S. Reshmi, K. B. Catherine and C. P. R. Nair, Int. J. Nanotechnol., 2011, 8, 979–987 CrossRef CAS.
- X. Cui, P. Ren, D. Deng, J. Deng and X. Bao, Energy Environ. Sci., 2016, 9, 123–129 RSC.
- M. Li, L. Zhang, Q. Xu, J. Niu and Z. Xia, J. Catal., 2014, 314, 66–72 CrossRef CAS.
- M. Zhang, F. Zhao, Y. Yang, J. Zhang, N. Li and H. Gao, CrystEngComm, 2018, 20, 7010–7019 RSC.
- W. Wang, S. Guo, D. Zhang and Z. Yang, J. Saudi Chem. Soc., 2019, 23, 133–140 CrossRef CAS.
- W. Wang, B. Liu, K. Xu, Y. Zu, J. Song and F. Zhao, Ceram. Int., 2018, 44, 19016–19020 CrossRef CAS.
- Y. Hu, S. Yang, B. Tao, X. Liu, K. Lin, Y. Yang, R. Fan, D. Xia and D. Hao, Vacuum, 2019, 159, 105–111 CrossRef CAS.
- L. Chen, L. Li and G. Li, J. Alloys Compd., 2008, 464, 532–536 CrossRef CAS.
- J. Zhi, Z. Feng-Qi and J. Propul, Technol, 2002, 23, 258–261 Search PubMed.
- S. Chaturvedi and P. N. Dave, J. Saudi Chem. Soc., 2013, 17, 135–149 CrossRef CAS.
- Y. Yuan, W. Jiang, Y. Wang, P. Shen, F. Li, P. Li, F. Zhao and H. Gao, Appl. Surf. Sci., 2014, 303, 354–359 CrossRef CAS.
- M. A. Dar, Q. Ahsanulhaq, Y. S. Kim, J. M. Sohn, W. B. Kim and H. S. Shin, Appl. Surf. Sci., 2009, 255, 6279–6284 CrossRef CAS.
- Q. Zhang, K. Zhang, D. Xu, G. Yang, H. Huang, F. Nie, C. Liu and S. Yang, Prog. Mater. Sci., 2014, 60, 208–337 CrossRef CAS.
- Y. Xie, B. Liu, Y. Li, Z. Chen, Y. Cao and D. Jia, New J. Chem., 2019, 43, 12118–12125 RSC.
- K. Kishore and K. Sridhara, Solid propellant chemistry: condensed phase behaviour of ammonium perchlorate-based solid propellants, Defence Research & Development Organisation, Ministry of Defence, 1999 Search PubMed.
- F. Solymosi and K. Fanagy, Symp. (Int.) Combust., [Proc.], 1967, 11, 429–437 CrossRef CAS.
- W. A. Rosser and S. H. Inami, Combust. Flame, 1968, 12, 427 CrossRef CAS.
- P. W. M. Jacobsete, Combust. Flame, 1969, 13, 419 CrossRef.
- Y. Xu, D. Chen, X. Jiao and K. Xue, Mater. Res. Bull., 2007, 42, 1723–1731 CrossRef CAS.
- H. Akbi, S. Rafai, A. Mekki, S. Touidjine, M. A. Fertassi and D. E. Kadri, J. Organomet. Chem., 2022, 957, 122159 CrossRef CAS.
- Y. Zhang, K. Li, J. Liao, X. Wei and L. Zhang, Appl. Surf. Sci., 2020, 499, 143875 CrossRef CAS.
- Y. Chen, K. Ma, J. Wang, Y. Gao, X. Zhu and W. Zhang, Mater. Res. Bull., 2018, 101, 56–60 CrossRef CAS.
- M. A. Fertassi, K. T. Alali, Q. Liu, R. Li, P. Liu, J. Liu, L. Liu and J. Wang, RSC Adv., 2016, 6, 74155–74161 RSC.
- W. Sun, X. Ling, W. Wei, H. Hu, Z. Jiang, Z. Yan and J. Xie, Appl. Surf. Sci., 2019, 493, 710–718 CrossRef CAS.
- A. T. Aqueel Ahmed, B. Hou, H. S. Chavan, Y. Jo, S. Cho, J. Kim, S. M. Pawar, S. N. Cha, A. I. Inamdar, H. Kim and H. Im, Small, 2018, 14, 1800742 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available See https://doi.org/10.1039/d2ra03772f |
| This journal is © The Royal Society of Chemistry 2022 |