Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Oxa-Michael-based divergent synthesis of artificial glutamate analogs†
Shuntaro Tsukamoto a,
Oriel Hlokoane
a,
Oriel Hlokoane ab,
Kei Miyako
ab,
Kei Miyako c,
Raku Irie
c,
Raku Irie a,
Ryuichi Sakai
a,
Ryuichi Sakai c and
Masato Oikawa
c and
Masato Oikawa *a
*a
aGraduate School of Nanobioscience, Yokohama City University, Seto 22-2, Kanazawa-ku, Yokohama 236-0027, Japan. E-mail: moikawa@yokohama-cu.ac.jp
bDepartment of Pharmacy, National University of Lesotho, P.O. Roma 180, Maseru, Lesotho
cFaculty of Fisheries Sciences, Hokkaido University, Hakodate 041-8611, Japan
First published on 10th August 2022
Abstract
Herein we report stereoselective generation of two skeletons, 1,3-dioxane and tetrahydropyranol, by oxa-Michael reaction as the key reaction from δ-hydroxyenone. The construction of the 1,3-dioxane skeleton, achieved through hemiacetal formation followed by oxa-Michael reaction from δ-hydroxyenone, was exploited to access structurally diverse heterotricyclic artificial glutamate analogs. On the other hand, formation of a novel tetrahydro-2H-pyranol skeleton was accomplished by the inverse reaction order: oxa-Michael reaction followed by hemiacetal formation. Thus, this study succeeded in showing that structural diversity in a compound collection can be acquired by interchanging the order of just two reactions. Among the skeletally diverse, heterotricyclic artificial glutamate analogs synthesized in this study, a neuronally active compound named TKM-50 was discovered in the mice in vivo assay.
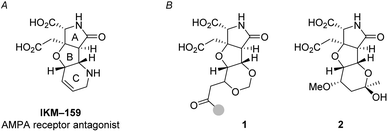
Ionotropic glutamate receptors (iGluRs) mediate the majority of the excitatory neurotransmissions such as learning, memory, and nociception in the mammalian central nervous system (CNS).1 To study and control the function of iGluRs, specific glutamate analogs have been developed in natural product chemistry2 and in medicinal chemistry.3,4 IKM-159 (Fig. 1A) is an artificial glutamate analog designed and developed based on dysiherbaine5,6 and kainic acid7 in our laboratories as an antagonist selective to (S)-2-amino-3-(3-hydroxy-5-methyl-4-isoxazolyl)propionic acid (AMPA)-type iGluR.8–12 The AMPA receptor consists of four subunits: GluA1, GluA2, GluA3, and GluA4.3 In vitro, IKM-159 selectively inhibits the GluA1/GluA2 heterodimer and GluA4 homodimer.10 In vivo, IKM-159 inhibits voluntary action of mice for 50 min to several hours upon intracerebroventricular injection. The potency and selectivity of IKM-159 are, however, not very satisfactory to selectively modulate the function of AMPA-type iGluR. As an attempt to improve the biological profiles of IKM-159, we have been studying its structural modification.
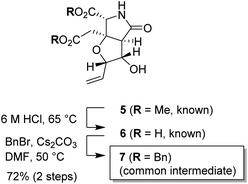
From the first-generation studies on structure–activity relationships (SARs) of IKM-159, it had been shown that the ring size and the heteroatom of the C-ring were important for neuroactivity of IKM-159.10,13 We then studied the second-generation SAR on the oxa analogs generated by a Prins-Ritter three-component coupling strategy, although all analogs were found to lose the original neuronal activity of IKM-159.14 Herein, we report our continuous effort along this line employing the homoallylic alcohol such as 5 and 7 (see Scheme 2) as the common intermediates.15
One of the strategies in this work is the thermodynamically controlled, stereoselective formation of 1,3-dioxane (1 in Fig. 1B) by hemiacetal formation followed by oxa-Michael reaction from δ-hydroxyenone derivative that we recently developed (Scheme 1).16 The other strategy is the novel stereoselective formation of tetrahydropyranol (2 in Fig. 1B) by the inverse reaction order; oxa-Michael reaction followed by hemiacetal formation (see Scheme 5). Thus, this study succeeded in showing that structural diversity in a compound collection can be acquired by interchanging the order of just two reactions; hemiacetal formation and oxa-Michael reaction. Among the skeletally diverse, heterotricyclic artificial glutamate analogs thus synthesized, a compound named TKM-50 (1ar) was discovered to be neuronally active in the mice in vivo assay.
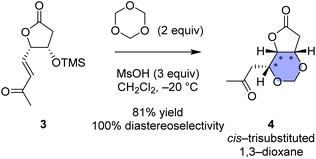 | ||
| Scheme 1 Our recent work regarding stereoselective 1,3-dioxane formation.16 For clarity and comparison, enantiomers of the reported compounds are shown in this scheme. | ||
The substrate used for the 1,3-dioxane formation was prepared from the known dimethyl ester 5 (Scheme 2).17 Exposure of dimethyl ester 5 to hydrochloric acid (6 M) at 65 °C provided dicarboxylic acid 6.17 Without purification, dicarboxylic acid 6 was treated with BnBr and Cs2CO3 to furnish the common intermediate 7 in 72% yield (2 steps).18
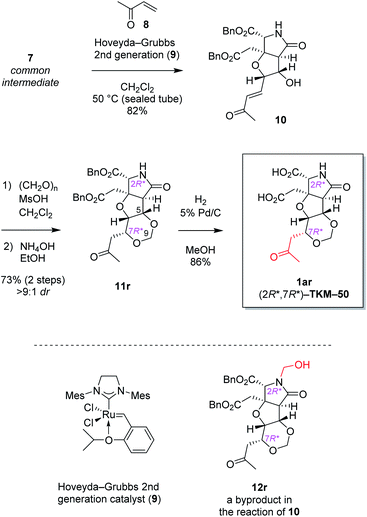
The alkene 7 was subjected to cross metathesis with methyl vinyl ketone (8) mediated by Hoveyda-Grubbs second generation catalyst (9)19 to provide enone 10 in 82% yield (Scheme 3). Upon exposure to paraformaldehyde as an equivalent of formaldehyde and 1,3,5-trioxane16 in the presence of MsOH, 1,3-dioxane ring formed smoothly by oxa-Michael reaction to give rise to desired (7R*)-heterotricycle 11r and the (7S*) epimer 11s (structure not shown) in the ratio of >9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
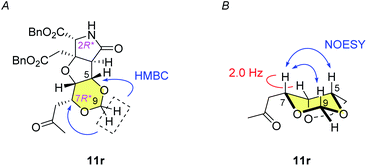
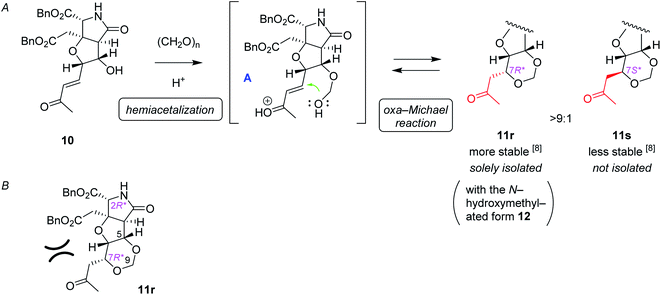
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as well as the N-hydroxymethylated product 12r (see Scheme 3) and the (7S*) epimer 12s (structure not shown). Since we had found that alkaline hydrolysis is of use to remove the N-hydroxymethyl group, the mixture of hemiaminals (12r/12s) and free amides (11r/11s) was treated with ammonium hydroxide20 to obtain free amide 11r in 73% isolated yield (2 steps), and free amide 11s in 10% yield (estimated by NMR, 2 steps). The formation of 1,3-dioxane ring of 11r was determined by the HMBC correlations (Fig. 2A), and the stereochemical configuration was established by a 3JH,H value and NOESY correlations denoted in Fig. 2B. Both configuration and conformation of 11r are identical to those we observed recently in the simple case (3 → 4, see Scheme 1),16 showing that the 1,3-dioxane formation in this study is also thermodynamically controlled (see below for the mechanism).
1, as well as the N-hydroxymethylated product 12r (see Scheme 3) and the (7S*) epimer 12s (structure not shown). Since we had found that alkaline hydrolysis is of use to remove the N-hydroxymethyl group, the mixture of hemiaminals (12r/12s) and free amides (11r/11s) was treated with ammonium hydroxide20 to obtain free amide 11r in 73% isolated yield (2 steps), and free amide 11s in 10% yield (estimated by NMR, 2 steps). The formation of 1,3-dioxane ring of 11r was determined by the HMBC correlations (Fig. 2A), and the stereochemical configuration was established by a 3JH,H value and NOESY correlations denoted in Fig. 2B. Both configuration and conformation of 11r are identical to those we observed recently in the simple case (3 → 4, see Scheme 1),16 showing that the 1,3-dioxane formation in this study is also thermodynamically controlled (see below for the mechanism).
 | ||
| Scheme 3 Stereoselective 1,3-dioxane formation leading to heterotricyclic artificial glutamate analog 1ar.a adr denotes the diastereoselectivity in the 1,3-dioxane formation. | ||
The proposed mechanism for the 1,3-dioxane formation is shown in Scheme 4A. Reaction of alcohol 10 and paraformaldehyde would form hemiacetal intermediate A under acidic conditions, which, then undergoes intramolecular oxa-Michael reaction to give 11r and 11s. Since the second conjugate addition is generally a thermodynamically controlled, reversible process, production of more stable (7R*) isomer 11r predominated over the (7S*) epimer 11s, as discussed also in our preliminary study.16 It should be also noted here that, in that preliminary study employing a simple substrate, the (7S) epimer had not been obtained.16 Generation of the less stable (7S*) epimer 11s in this study would be due to unfavorable steric interactions between the acetyl group and the benzyl ester on the near side in 11r (Scheme 4B), that make the energy difference between the two diastereomers (11r and 11s) smaller.
Then two benzyl groups of 11r were removed by hydrogenolysis21 to cleanly provide glutamate analog 1ar ((2R*,7R*)-TKM-50) in 86% yield (Scheme 3).
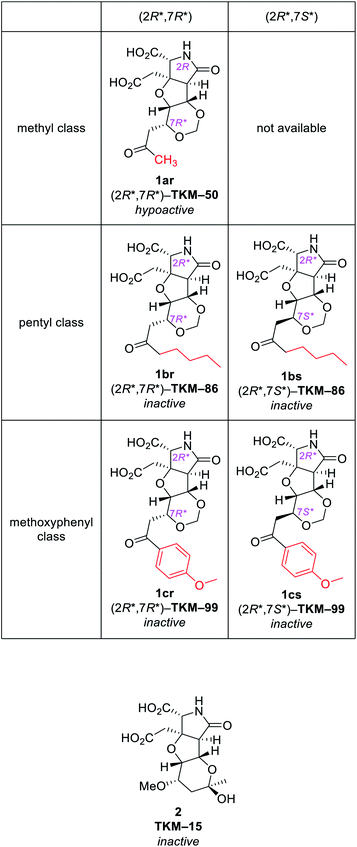
With the same reaction sequences for 1ar (Scheme 3), two more analogs 1br and 1cr were furthermore synthesized (Fig. 3). The marked decrease in diastereoselectivity in these oxa-Michael reactions (see Fig. 3) suggests that the steric repulsion between the pentyl/methoxyphenyl group and the benzyl ester on the near side is extremely large. The minor (7S*) diastereomers obtained in these oxa-Michael reactions were also isolated and deprotected to give 1bs and 1cs (see the ESI†), which were subjected to in vivo assay (see below).
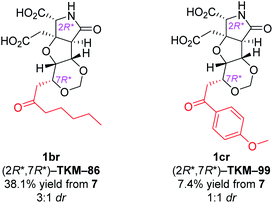 | ||
| Fig. 3 Other 1,3-dioxane analogs synthesized by the intramolecular oxa-Michael reaction.a adr denotes the diastereoselectivity in the 1,3-dioxane formation. | ||
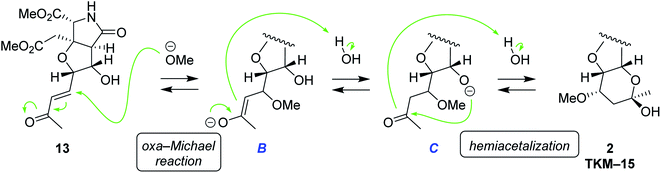
We also found that another skeleton can be constructed from δ-hydroxyenone being used for 1,3-dioxane formation, under alkaline hydrolytic conditions. Thus, as shown in Scheme 5, the δ-hydroxyenone 13 derived from homoallylic alcohol 5 by cross metathesis was selectively transformed into cyclic hemiacetal 2 in 53% yield (1 M LiOH in water, MeOH, rt). In this transformation, dimethyl ester and δ-hydroxyenone moiety independently suffer hydrolysis and cyclization, respectively, to generate glutamate analog 2 efficiently. The configuration of 2 was determined by combined analysis of NMR and DFT calculation (see the ESI†).22
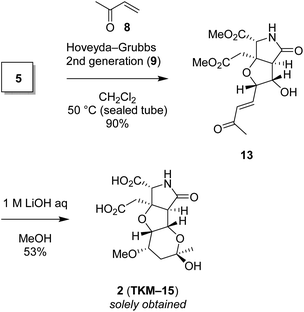 | ||
| Scheme 5 The heterotricyclic artificial glutamate analog 2, constructed by intermolecular oxa-Michael reaction of MeOH followed by acetalization. | ||
The plausible mechanism for the hemiacetal formation is shown in Scheme 6. In view of the fact that the hydroxy and carbonyl groups are located apart in 13, the six-membered-ring formation should take place after saturation of the trans-alkene. It is, therefore, supposed that oxa-Michael reaction of MeOH to enone 13 first generates saturated ketone C via enolate B.23 Under alkaline conditions, the alkoxide C intramolecularly attacks carbonyl group to give rise to hemiacetal 2. Considering the fact that oxa-Michael reaction and the acetalization are thermodynamically controlled, reversible processes, energetically favorable diastereomer 2 would have been obtained predominantly (see the ESI† for discussions on thermodynamic stability of 2). A related example had been reported in 1992 by Shing et al.24
Behavioral activities of all six compounds upon intracerebroventricular (i.c.v.) injection were evaluated in mice (Fig. 4).25 Injection of 1ar (TKM-50, 50 μg per mouse) resulted in loss of voluntary motor activity for 10 min after injection and then ataxia-like motions were recorded, thus annotated as hypoactive. The hypoactivity observed for 1ar (TKM-50) is thus somewhat weaker than IKM-159 which causes loss of mice spontaneous activity for up to 4 h.12 Other congeners, however, did not cause any noticeable behavioral changes at the same dose tested.
Conclusions
In this paper, we reported synthesis of skeletally diverse artificial glutamate analogs from a common precursor. Since we employed thermodynamically controlled, reversible process for the key cyclizations, most of the reactions proceeded stereoselectively. The cases that were less selective (1br and 1cr in Fig. 3) could even be reasonably explained, supporting the origin of the stereoselectivity we proposed in Scheme 4.16It is of interest to note that the formed skeleton changes significantly, just by interchanging the order of the oxa-Michael reaction and the hemiacetalization (see Schemes 4 and 6). Therefore, it is expected that our methodology is generally of use for discovery of biologically active small molecules.26 In fact, we succeeded in identifying neuroactive compound (1ar, TKM-50) in this study.
We are currently working on the construction of a larger compound library using this methodology and the development of alternative methodology for generation of other skeletons. The results will be reported in due course.
Author contributions
ST: investigation, writing the first draft and editing; OH: investigation and editing; KM: investigation; RI: formal analysis and editing; RS: funding acquisition, investigation and writing the first draft; MO: conceptualization, formal analysis, funding acquisition, project administration, supervision, writing the first draft and final editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the grant for Academic Research Promotion (No. SG2803) of Yokohama City University, Japan. The JSPS grant-in-aid for scientific research (15H04546, 18H0460009, 19H03040, 20H0475800) is also gratefully acknowledged. The financial support from the government of Japan through Japan International Cooperation Agency (JICA) is gratefully acknowledged by O.H.Notes and references
- G. Riedel, B. Platt and J. Micheau, Behav. Brain Res., 2003, 140, 1–47 CrossRef CAS.
- G. T. Swanson and R. Sakai, in Marine Toxins as Research Tools, ed. N. Fusetani and W. Kem, Springer Berlin Heidelberg, Berlin, Heidelberg, 2009, pp. 123–157 Search PubMed.
- P. Stawski, H. Janovjak and D. Trauner, Bioorg. Med. Chem., 2010, 18, 7759–7772 CrossRef CAS PubMed.
- S. K. Bagal, A. D. Brown, P. J. Cox, K. Omoto, R. M. Owen, D. C. Pryde, B. Sidders, S. E. Skerratt, E. B. Stevens, R. I. Storer and N. A. Swain, J. Med. Chem., 2013, 56, 593–624 CrossRef CAS PubMed.
- R. Sakai, H. Kamiya, M. Murata and K. Shimamoto, J. Am. Chem. Soc., 1997, 119, 4112–4116 CrossRef CAS.
- R. Sakai, T. Koike, M. Sasaki, K. Shimamoto, C. Oiwa, A. Yano, K. Suzuki, K. Tachibana and H. Kamiya, Org. Lett., 2001, 3, 1479–1482 CrossRef CAS PubMed.
- A. F. Parsons, Tetrahedron, 1996, 52, 4149–4174 CrossRef CAS.
- M. Ikoma, M. Oikawa, M. B. Gill, G. T. Swanson, R. Sakai, K. Shimamoto and M. Sasaki, Eur. J. Org. Chem., 2008, 2008, 5215–5220 CrossRef PubMed.
- M. Oikawa, M. Ikoma, M. Sasaki, M. B. Gill, G. T. Swanson, K. Shimamoto and R. Sakai, Eur. J. Org. Chem., 2009, 2009, 5531–5548 CrossRef PubMed.
- M. B. Gill, S. Frausto, M. Ikoma, M. Sasaki, M. Oikawa, R. Sakai and G. T. Swanson, Br. J. Pharmacol., 2010, 160, 1417–1429 CrossRef CAS PubMed.
- M. Oikawa, M. Ikoma, M. Sasaki, M. B. Gill, G. T. Swanson, K. Shimamoto and R. Sakai, Bioorg. Med. Chem., 2010, 18, 3795–3804 CrossRef CAS PubMed.
- L. Juknaite, Y. Sugamata, K. Tokiwa, Y. Ishikawa, S. Takamizawa, A. Eng, R. Sakai, D. S. Pickering, K. Frydenvang, G. T. Swanson, J. S. Kastrup and M. Oikawa, J. Med. Chem., 2013, 56, 2283–2293 CrossRef CAS PubMed.
- M. Oikawa, Y. Kasori, L. Katayama, E. Murakami, Y. Oikawa and Y. Ishikawa, Synthesis, 2013, 45, 3106–3117 CrossRef CAS.
- M. Chiba, Y. Ishikawa, R. Sakai and M. Oikawa, ACS Comb. Sci., 2016, 18, 399–404 CrossRef CAS PubMed.
- Racemate synthesis was studied in this study, however (2R)-enantiomer has been shown to be neuronally active in IKM-159. See ref. 12.
- O. Hlokoane, S. Tsukamoto, R. Irie and M. Oikawa, Chem. Lett., 2021, 50, 1464–1466 CrossRef CAS.
- M. Chiba, C. Fujimoto, R. Sakai and M. Oikawa, Bioorg. Med. Chem. Lett., 2015, 25, 1869–1871 CrossRef CAS.
- J. C. Lee, Y. S. Oh, S. H. Cho and J. D. Lee, Org. Prep. Proced. Int., 1996, 28, 480–483 CrossRef CAS.
- S. B. Garber, J. S. Kingsbury, B. L. Gray and A. H. Hoveyda, J. Am. Chem. Soc., 2000, 122, 8168–8179 CrossRef CAS.
- Z. Galla, E. Forró and F. Fülöp, Tetrahedron: Asymmetry, 2016, 27, 729–731 CrossRef CAS.
- W. H. Hartung and R. Simonoff, Org. React., 1953, 7, 263–326 Search PubMed.
- In our preliminary experiments, this transformation can be realized also in the presence of other alcohols such as EtOH or BnOH instead of MeOH..
- When monitoring the progress of the reaction 13 → 2 by LCMS and TLC, oxa-Michael product which is a protonated form for intermediated C (see Scheme 6) was detected as the reaction intermediate. Finally, the reaction product was converged to 2 via the oxa-Michael product, supporting the reaction is based on a stepwise mechanism we proposed in Scheme 6. This observation will be summarized and discussed in detail in a separate paper in the future..
- T. K. M. Shing, Z.-H. Zhou and T. C. W. Mak, J. Chem. Soc., Perkin Trans. 1, 1992, 1907–1910 RSC.
- H. Uchimasu, K. Matsumura, M. Tsuda, K. Kumagai, M. Akakabe, M. J. Fujita and R. Sakai, Tetrahedron, 2016, 72, 7185–7193 CrossRef CAS.
- C. J. Gerry and S. L. Schreiber, Nat. Rev. Drug Discovery, 2018, 17, 333–352 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03744k |
| This journal is © The Royal Society of Chemistry 2022 |