Open Access Article
Open Access ArticleA simple supramolecular complex of boronic acid-appended β-cyclodextrin and a fluorescent boronic acid-based probe with excellent selectivity for D-glucose in water†
Ko Sugita‡
a,
Yota Suzuki‡ a,
Yuji Tsuchido
a,
Yuji Tsuchido ab,
Shoji Fujiwara
ab,
Shoji Fujiwara ac,
Takeshi Hashimoto
ac,
Takeshi Hashimoto *a and
Takashi Hayashita
*a and
Takashi Hayashita *a
*a
aDepartment of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, 7-1, Kioi-cho, Chiyoda-ku, Tokyo 102-8554, Japan. E-mail: t-hasimo@sophia.ac.jp; ta-hayas@sophia.ac.jp
bDepartment of Life Science and Medical Bioscience, Graduate School of Advanced Science and Engineering, Waseda University (TWIns), Wakamatsu-cho, Shinjuku-ku, Tokyo 162-8480, Japan
cDepartment of Material and Life Chemistry, Faculty of Engineering, Kanagawa University, Rokkakubashi, Kanagawa-ku, Yokohama-shi, Kanagawa 221-8686, Japan
First published on 14th July 2022
Abstract
Various diboronic acid-based chemosensors for D-glucose have been developed for use in diabetes diagnostic systems. However, most of these chemosensors have limitations, such as poor water solubility, difficulties in synthesis, and inability to selectively detect D-glucose from among other saccharides. We report a simple chemosensor based on a supramolecular complex of fluorophenylboronic acid-appended β-cyclodextrin (FPB-βCyD) and an anthracene-based probe having a boronic acid moiety (1). Hydrophobic 1 is encapsulated in the cyclodextrin cavity of FPB-βCyD, making the supramolecular complex (1/FPB-βCyD) applicable in a water-rich solvent mixture (98% water). Interestingly, 1/FPB-βCyD showed a strong turn-on response to D-glucose with a 9.6-fold enhancement in fluorescence intensity, and no response to other saccharides. This study uncovers an innovative approach based on the supramolecular assembly of simple components for the development of a water-soluble D-glucose chemosensor with excellent selectivity.
Introduction
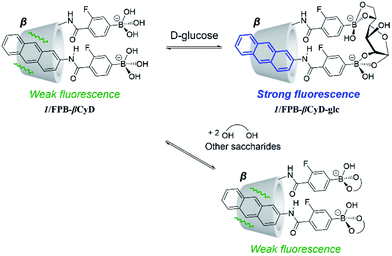
The modern diet based on high-calorie foods has increased the number of diabetic patients globally, and the total number is projected to exceed 300 million by 2030.1 There is a strong demand for an accurate blood glucose sensing system for the early diagnosis of diabetes because conventional enzyme-based D-glucose biosensors have low heat and pH stability and poor reproducibility.Recently, heat- and pH-stable diboronic acid sensors, which contain two boronic acid moieties that are precisely positioned to simultaneously react with the two 1,2-diol moieties of D-glucose, have emerged for D-glucose sensing.2,3 Nevertheless, the development of a truly D-glucose selective diboronic acid-based chemosensor has been continuously pursued as diboronic acids can also react with other saccharides possessing two 1,2-diol moieties (e.g., D-galactose). In addition, many diboronic acid compounds require complicated organic synthesis, and the presence of a highly hydrophobic fluorophore decreases their solubility in water. The aim of our research is to develop an ideal chemosensor with (1) high selectivity for D-glucose, (2) a simple structure, and (3) high water solubility to achieve D-glucose sensing.
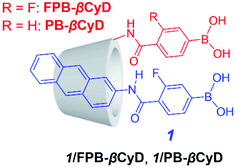
We present a supramolecular complex consisting of a m-fluorophenylboronic acid-based probe (1) and m-fluorophenylboronic acid-appended β-cyclodextrin (FPB-βCyD) as a fluorescent chemosensor for D-glucose that can be used in a water-rich solvent mixture of dimethyl sulfoxide (DMSO)/water (2/98 in v/v, Scheme 1). The structures of these components are so simple that the synthesis can be completed effortlessly. Cyclodextrin (CyD) is water-soluble and has a hydrophobic cavity that can encapsulate the hydrophobic probe; this allows the supramolecular complex to function as a sensor in an aqueous environment.4–7 To enhance the affinity of the boronic acid moiety for D-glucose, we introduced an electron-withdrawing fluorine group into the phenylboronic acid moiety to increase the Lewis acidity of the boron centre.5 We also elucidated the sensing mechanism of supramolecular complex 1/FPB-βCyD through comparison with a complex of 1 and phenylboronic acid-appended β-cyclodextrin (PB-βCyD).
Experimental
Reagents
FPB-βCyD, PB-βCyD, and 1 were synthesised according to our previous reports.5–7 Dimethyl sulfoxide (DMSO, Luminasol®, Dojindo Laboratories), sodium chloride (Fujifilm Wako Chemicals), disodium hydrogen phosphate (Fujifilm Wako Chemicals), sodium hydrogen carbonate (Fujifilm Wako Chemicals), sodium carbonate (Fujifilm Wako Chemicals), D-fructose (Fujifilm Wako Chemicals), D-glucose (Fujifilm Wako Chemicals), D-galactose (Fujifilm Wako Chemicals), D-mannose (Fujifilm Wako Chemicals), D-ribose (Fujifilm Wako Chemicals), D-xylose (Fujifilm Wako Chemicals), hydrogen chloride aq. (Fujifilm Wako Chemicals), 50% sodium hydroxide solution (super special grade, Fujifilm Wako Chemicals), and Milli-Q water were used for spectroscopic measurements.Instrumentation
The solution pH was measured by a HORIBA pH meter F-52 (Horiba, Japan). Fluorescence spectra were measured using a Hitachi F-7000 fluorescence spectrophotometer (Hitachi, Japan) equipped with a temperature controller (Hitachi, Japan) to keep the temperature constant at 25 °C. Induced circular dichroism (ICD) spectra were measured using a JASCO J-820 spectrophotometer (JASCO, Japan) equipped with a Peltier temperature controller (JASCO, Japan) and an EYELA Cool Ace CA-1111 (EYELA, Japan) to keep the temperature constant at 25 °C under a nitrogen atmosphere.Preparation of sample solutions
The ionic strength of sample solutions was adjusted to 0.10 M with sodium chloride. The pH of sample solutions was adjusted by adding hydrochloric acid aq. and 50% sodium hydroxide solution.Measurement of fluorescence spectra under various pH conditions
Sample solutions that contain DMSO, 0.2 mM of a cyclodextrin compound, 0.10 M of NaCl, 10 mM of phosphate buffer, a saccharide (0 or 30 mM), and 10 μM of 1 in a mixed solvent of DMSO/water (2/98 in v/v) were prepared. The solutions were acidified with a diluted hydrochloric acid aqueous solution until ca. pH 3. Fluorescence spectra for each of the sample solutions were measured under various pH conditions by the titration of NaOH aq. into the acidic sample solution. The solution pH was increased until ca. pH 11. Before every measurement, the sample solution was stirred for 25 minutes after each titration.Determination of acid dissociation constants
pH dependence of the fluorescence intensity at a specific wavelength was analysed using the KaleidaGraph program according to a theoretical sigmoidal curve derived from the acid dissociation model of a monobasic acid.Measurement of fluorescence spectra at various saccharide concentrations
A series of solutions that contains DMSO, NaCl, a cyclodextrin compound, carbonate buffer, and a saccharide with various concentrations (0–30 mM) was prepared individually. The pH of all the solutions was adjusted to 10. Before the measurement of fluorescence and ICD spectra, the solutions were stirred for 25 minutes after the addition of an appropriate amount of 1 DMSO solution to prepare solutions containing 10 μM of 1, 0.2 mM of a cyclodextrin compound, 0.10 M of NaCl, 10 mM of carbonate buffer, and a saccharide (0–30 mM) in a mixed solvent of DMSO/water (2/98 in v/v).Results and discussion
FPB-βCyD, PB-βCyD, and probe 1 were readily synthesised as described previously.5–7 Briefly, FPB-βCyD and PB-βCyD were obtained by the amide condensation reaction of 3-amino-β-cyclodextrin with the corresponding carboxyphenylboronic acid. Probe 1 was synthesised by the amide condensation reaction of 4-carboxy-3-fluorophenylboronic acid pinacol ester with 1-aminopyrene followed by the hydrolysis of the pinacol ester.Supramolecular complex 1/FPB-βCyD was prepared by dissolving FPB-βCyD (0.2 mM) and 1 (10 μM) in DMSO/water (2/98 in v/v). According to our previous studies, CyD compounds can encapsulate hydrophobic probes in their CyD cavities.4–7 Induced circular dichroism (ICD) spectra were measured to clarify the structure of 1/FPB-βCyD (Fig. S1†). In the absence and presence of saccharides, a weak positive Cotton effect was observed in the 270–290 nm region. In the presence of D-fructose, this Cotton effect was not observed because of the strong positive Cotton effect of D-fructose itself at 280 nm.8 We previously reported that bisignate Cotton effects are observed in the ICD spectra when two molecules of 1 are simultaneously held in the native γ-CyD cavity, and only one molecule of 1 is held in the native β-CyD.5 Therefore, 1 and FPB-βCyD form a supramolecular complex of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. The fluorescence spectrum of 1/FPB-βCyD showed a broad vibrational structure with peaks at 413 nm and 431 nm, with no band corresponding to anthracene dimer emission observable at around 520 nm (Fig. S2†).9 These features were identical to those of the 1
1 stoichiometry. The fluorescence spectrum of 1/FPB-βCyD showed a broad vibrational structure with peaks at 413 nm and 431 nm, with no band corresponding to anthracene dimer emission observable at around 520 nm (Fig. S2†).9 These features were identical to those of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex of 1 and native β-CyD, suggesting that 1/FPB-βCyD is a supramolecular complex of 1
1 stoichiometric complex of 1 and native β-CyD, suggesting that 1/FPB-βCyD is a supramolecular complex of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry.5 Anthracene compounds form photodimers that are non-emissive upon irradiation;10 however, the fluorescence spectra of 1/FPB-βCyD showed no change over time with excitation at 323 nm (Fig. S3†), suggesting that 1 molecules in solution are not positioned sufficiently close to each other to form photodimers, as these molecules are trapped by excess FPB-βCyD to form 1
1 stoichiometry.5 Anthracene compounds form photodimers that are non-emissive upon irradiation;10 however, the fluorescence spectra of 1/FPB-βCyD showed no change over time with excitation at 323 nm (Fig. S3†), suggesting that 1 molecules in solution are not positioned sufficiently close to each other to form photodimers, as these molecules are trapped by excess FPB-βCyD to form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexes.
1 stoichiometric complexes.
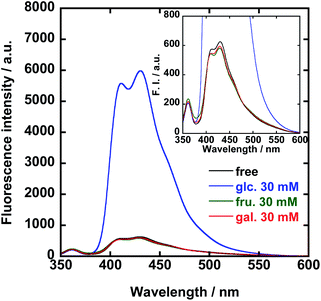
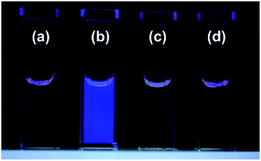
The fluorescence and UV-vis absorption spectra of 1/FPB-βCyD were measured at pH 10 in the absence and presence of D-glucose, D-fructose, and D-galactose, i.e., common saccharides in blood (Fig. 1 and S4†). Interestingly, whereas no spectral change was observed in the UV-vis absorption spectra, a 9.6-fold enhancement in the fluorescence intensity of 1/FPB-βCyD was observed at 431 nm upon the addition of 30 mM D-glucose. In contrast, the spectra of 1/FPB-βCyD showed little change when D-fructose and D-galactose were added. Whereas 1/FPB-βCyD solutions exhibited weak fluorescence that was not visible to the naked eye in the absence of saccharides as well as in the presence of D-fructose and D-galactose, strong blue fluorescence was observed in the presence of D-glucose under 365 nm UV irradiation using a commercial UV lamp (Fig. 2). The fluorescence spectra of 1/FPB-βCyD were measured under various pH conditions in the absence and presence of the saccharides (Fig. S5†). Fluorescence intensities at 413 nm and 431 nm increased with increasing pH. Given that FPB-βCyD is non-emissive in the entire pH region investigated, this pH dependence is likely attributable to the acid dissociation of 1, i.e., structural change in the boronic acid moiety from trigonal boronic acid (non-fluorescent) to tetrahedral boronate ion (weakly fluorescent) (Scheme S1a†). The fluorescence spectrum of 1/FPB-βCyD measured in the absence of saccharides at pH > 10 was almost identical to that measured in the presence of D-fructose or D-galactose. Interestingly, in the presence of D-glucose, 1/FPB-βCyD exhibited much stronger fluorescence than that observed in the absence of saccharides at pH > 10 (Fig. S5-5†). By applying non-linear least squares fitting, the apparent acid dissociation constants (pKappa1) were 9.14 ± 0.03 in the absence of saccharides, 8.54 ± 0.05 in the presence of D-glucose, 7.52 ± 0.04 in the presence of D-fructose, and 8.44 ± 0.03 in the presence of D-galactose (Fig. S6†). The decrease in pKappa1 values in the presence of saccharides is due to the formation of boronate ester ions through the reaction of a boronic acid and a saccharide (Scheme S1b†), suggesting that 1 reacts with saccharides to give boronate ester ions.11 It should be emphasised that D-fructose and D-galactose inherently react with 1 at pH 10, but do not form a fluorescent product with 1/FPB-βCyD. Therefore, 1/FPB-βCyD enables the highly selective detection of D-glucose in a water-rich solvent at pH > 10 with a turn-on response, emitting blue fluorescence visible to the naked eye.
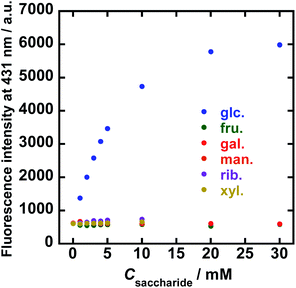
To evaluate the sensitivity of 1/FPB-βCyD for the saccharides, the fluorescence spectra of 1/FPB-βCyD were measured at various concentrations of common saccharides (D-glucose, D-fructose, and D-galactose) and less common saccharides (D-mannose, D-ribose, and D-xylose) at pH 10 (Fig. S7†). Spectral measurements were performed 25 minutes after mixing 1/FPB-βCyD with each saccharide (Fig. S8†) because the reaction took about 20 minutes to complete. Surprisingly, 1/FPB-βCyD showed extremely high selectivity for D-glucose, exhibiting a strong turn-on response with fluorescence enhancement at 431 nm (Fig. 3). On the other hand, 1/FPB-βCyD showed almost no fluorescence response to the other saccharides. The fluorescence intensity increased linearly in the D-glucose concentration range of 0–5 mM and reached a plateau at ca. 20 mM. Because D-glucose in blood is present at the mM level, 1/FPB-βCyD could potentially be used for monitoring glucose in actual blood samples.12 The conditional equilibrium constant K′ at pH 10 for the reaction of 1/FPB-βCyD with D-glucose was determined to be 139 ± 9 M−1 according to a theoretical equation derived from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model (eqn (S1) and Fig. S9†).
1 binding model (eqn (S1) and Fig. S9†).
The K′ values for the other reaction systems could not be determined because none of the other saccharides evaluated caused sufficient fluorescence enhancement. Using a 3σ approach and the data of reproducibility (n = 3) at the lower measurement points in Fig. 3, the limit of detection (LOD) was estimated to be 90 μM for D-glucose with the calibration curve of I = 562.66Cglu. + 575.57 (R2 = 0.983).
Competitive experiments indicated that the origin of fluorescence, i.e., the reaction product of 1/FPB-βCyD and D-glucose (1/FPB-βCyD-glc), was robust in the presence of other saccharides except D-fructose, which decreased the fluorescence intensity by only 6% (Fig. S10†). These results demonstrate that 1/FPB-βCyD selectively recognises D-glucose in the presence of other saccharides. When 0.1 mM of D-fructose was also present, the fluorescence intensity of 1/FPB-βCyD-glc decreased to 83%. This is due to the much higher affinity of D-fructose for monoboronic acids compared with D-glucose, thereby inhibiting the formation of 1/FPB-βCyD-glc.13 Nevertheless, as blood D-fructose concentrations are in the μM range, the presence of D-fructose is unlikely to interfere with D-glucose sensing by systems based on 1/FPB-βCyD in practical situations.14
To elucidate the D-glucose sensing mechanism of 1/FPB-βCyD, we examined the saccharide recognition characteristics of 1/PB-βCyD, a control supramolecular complex of probe 1 and PB-βCyD with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (Scheme 1), for comparison. The phenylboronic acid moiety of PB-βCyD has a lower affinity for saccharides than the m-fluorophenylboronic acid moiety of FPB-βCyD as the Lewis acidity of phenylboronic acid is lower than that of m-fluorophenylboronic acid.15 The fluorescence spectra of 1/PB-βCyD were measured under various pH conditions in the absence and presence of D-glucose, D-fructose, and D-galactose (Fig. S11†). When the pH of the solution was increased from 7 to around 10, an increase in fluorescence intensity was observed in the absence and presence of saccharides, as in the case of 1/FPB-βCyD; this was considered to be due to the deprotonation of the boronic acid moiety of 1 in 1/PB-βCyD. At pH ≤ 6, an emission band at 447 nm appeared, which possibly originated from exciplex formation between 1 and the phenylboronic acid moiety of PB-βCyD. The analysis of 1/PB-βCyD fluorescence intensity at 413 nm under various pH conditions revealed pKappa1 values of 9.25 ± 0.05 in the absence of saccharides, 8.62 ± 0.02 in the presence of D-glucose, 7.84 ± 0.06 in the presence of D-fructose, and 8.95 ± 0.05 in the presence of D-galactose (Fig. S12†). These pKappa1 values were larger than those of 1/FPB-βCyD under the corresponding conditions, suggesting that the acidity of 1 is slightly influenced by the structure of the boronic acid moiety appended to the β-CyD. 1/PB-βCyD exhibited a marked increase in fluorescence intensity in response to D-glucose at pH > 10, similar to 1/FPB-βCyD (Fig. S11-2†). It is notable that 1/FPB-βCyD displayed a much stronger turn-on fluorescence response to D-glucose than 1/PB-βCyD (9.6-fold vs. 2.8-fold enhancement in fluorescence intensity at 431 nm). This indicates that the m-fluorophenylboronic acid moiety of FPB-βCyD, which has a higher affinity for saccharides than the phenylboronic acid moiety of PB-βCyD, plays a key role in the fluorescence detection of D-glucose. On the basis of these findings, we propose the following mechanism for the sensing of saccharides by 1/FPB-βCyD (Scheme 2). The reaction of 1/FPB-βCyD with D-glucose produces fluorescent product 1/FPB-βCyD-glc through the simultaneous binding of the two boronic acid moieties of 1/FPB-βCyD to the two 1,2-diol moieties of D-glucose. This rigidifies the anthracene moiety of 1 in the cavity of FPB-βCyD, giving rise to enhanced fluorescence. As the affinity of FPB-βCyD for saccharides is higher than that of PB-βCyD, 1/FPB-βCyD forms a more stable product with D-glucose, showing a stronger (3.4-fold) turn-on fluorescence response to D-glucose than 1/PB-βCyD. In contrast, no such recognition through the two boronic acid moieties of 1/FPB-βCyD occurs with other saccharides; each boronic acid moiety reacts with a saccharide in 1
1 stoichiometry (Scheme 1), for comparison. The phenylboronic acid moiety of PB-βCyD has a lower affinity for saccharides than the m-fluorophenylboronic acid moiety of FPB-βCyD as the Lewis acidity of phenylboronic acid is lower than that of m-fluorophenylboronic acid.15 The fluorescence spectra of 1/PB-βCyD were measured under various pH conditions in the absence and presence of D-glucose, D-fructose, and D-galactose (Fig. S11†). When the pH of the solution was increased from 7 to around 10, an increase in fluorescence intensity was observed in the absence and presence of saccharides, as in the case of 1/FPB-βCyD; this was considered to be due to the deprotonation of the boronic acid moiety of 1 in 1/PB-βCyD. At pH ≤ 6, an emission band at 447 nm appeared, which possibly originated from exciplex formation between 1 and the phenylboronic acid moiety of PB-βCyD. The analysis of 1/PB-βCyD fluorescence intensity at 413 nm under various pH conditions revealed pKappa1 values of 9.25 ± 0.05 in the absence of saccharides, 8.62 ± 0.02 in the presence of D-glucose, 7.84 ± 0.06 in the presence of D-fructose, and 8.95 ± 0.05 in the presence of D-galactose (Fig. S12†). These pKappa1 values were larger than those of 1/FPB-βCyD under the corresponding conditions, suggesting that the acidity of 1 is slightly influenced by the structure of the boronic acid moiety appended to the β-CyD. 1/PB-βCyD exhibited a marked increase in fluorescence intensity in response to D-glucose at pH > 10, similar to 1/FPB-βCyD (Fig. S11-2†). It is notable that 1/FPB-βCyD displayed a much stronger turn-on fluorescence response to D-glucose than 1/PB-βCyD (9.6-fold vs. 2.8-fold enhancement in fluorescence intensity at 431 nm). This indicates that the m-fluorophenylboronic acid moiety of FPB-βCyD, which has a higher affinity for saccharides than the phenylboronic acid moiety of PB-βCyD, plays a key role in the fluorescence detection of D-glucose. On the basis of these findings, we propose the following mechanism for the sensing of saccharides by 1/FPB-βCyD (Scheme 2). The reaction of 1/FPB-βCyD with D-glucose produces fluorescent product 1/FPB-βCyD-glc through the simultaneous binding of the two boronic acid moieties of 1/FPB-βCyD to the two 1,2-diol moieties of D-glucose. This rigidifies the anthracene moiety of 1 in the cavity of FPB-βCyD, giving rise to enhanced fluorescence. As the affinity of FPB-βCyD for saccharides is higher than that of PB-βCyD, 1/FPB-βCyD forms a more stable product with D-glucose, showing a stronger (3.4-fold) turn-on fluorescence response to D-glucose than 1/PB-βCyD. In contrast, no such recognition through the two boronic acid moieties of 1/FPB-βCyD occurs with other saccharides; each boronic acid moiety reacts with a saccharide in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio, and thus, the structure of 1/FPB-βCyD is inherently unchanged, and the fluorescence spectrum is also unchanged. Remarkably, 1/FPB-βCyD exhibited no fluorescence enhancement in response to D-galactose, which also has two 1,2-diol moieties. The excellent selectivity of 1/FPB-βCyD for D-glucose originates from its two boronic acid moieties that are precisely positioned to match the distance between the two 1,2-diol moieties of D-glucose, which is shorter than that of D-galactose.16 Our results demonstrate that 1/FPB-βCyD selectively detects D-glucose via the formation of a stable supramolecular complex, 1/FPB-βCyD-glc, through the hydrophobic interaction of the anthracene moiety of 1 with the hydrophobic cavity of FPB-βCyD and the dynamic covalent binding of the two boronic acid moieties to the two 1,2-diol moieties of D-glucose.
1 stoichiometric ratio, and thus, the structure of 1/FPB-βCyD is inherently unchanged, and the fluorescence spectrum is also unchanged. Remarkably, 1/FPB-βCyD exhibited no fluorescence enhancement in response to D-galactose, which also has two 1,2-diol moieties. The excellent selectivity of 1/FPB-βCyD for D-glucose originates from its two boronic acid moieties that are precisely positioned to match the distance between the two 1,2-diol moieties of D-glucose, which is shorter than that of D-galactose.16 Our results demonstrate that 1/FPB-βCyD selectively detects D-glucose via the formation of a stable supramolecular complex, 1/FPB-βCyD-glc, through the hydrophobic interaction of the anthracene moiety of 1 with the hydrophobic cavity of FPB-βCyD and the dynamic covalent binding of the two boronic acid moieties to the two 1,2-diol moieties of D-glucose.
Conclusions
We have demonstrated that 1/FPB-βCyD, a water-soluble supramolecular complex composed of two simple components, recognises D-glucose with extremely high selectivity in a water-rich solvent (98% water). The two boronic acid moieties of 1/FPB-βCyD are precisely positioned to simultaneously react with the two 1,2-diol moieties of D-glucose. The reaction of 1/FPB-βCyD with D-glucose gives 1/FPB-βCyD-glc, which results in a 9.6-fold enhancement in fluorescence intensity that is visible to the naked eye, by the rigidification of 1 in the hydrophobic cavity of FPB-βCyD. In contrast, other saccharides including D-galactose are hardly recognised by the two reaction sites of 1/FPB-βCyD, and hence, no rigidification of 1 occurs. Consequently, the excellent selectivity of 1/FPB-βCyD for D-glucose is achieved. The fluorescence intensity of 1/FPB-βCyD linearly increased with increasing D-glucose concentration in the mM range, suggesting that 1/FPB-βCyD is a potential candidate for the basic structure of glucose sensing systems for diabetes diagnosis. Moreover, we have demonstrated that the novel approach that exploits the supramolecular assembly of boronic acid-appended CyD and boronic acid-based hydrophobic probe enables extremely selective detection of D-glucose in water. Furthermore, it requires neither difficult multi-step synthesis nor substantial amounts of organic solvents to dissolve the hydrophobic probe, unlike most of the conventional boronic acid-based chemosensors. The present approach represents a completely novel strategy and has the potential to become a breakthrough in the development of practical boronic acid-based chemosensors that are highly selective for D-glucose in water.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research Grant Numbers 20H02772 (T. Hay.), 18K05180 (T. Has.), 21K03452 (T. Has.), and 18K14255 (Y. T.), and a JSPS Research Fellowship for Young Scientists PD Grant Number 21J00709 (Y. S.).Notes and references
- S. Wild, G. Roglic, A. Green, R. Sicree and H. King, Diabetes Care, 2004, 27, 1047–1053, DOI:10.2337/diacare.27.5.1047.
- X. Sun and T. D. James, Chem. Rev., 2015, 115, 8001–8037, DOI:10.1021/cr500562m.
- J. Ramos-Soriano, S. J. Benitez-Benitez, A. P. Davis and M. C. Galan, Angew. Chem., Int. Ed., 2021, 60(31), 16880–16884, DOI:10.1002/anie.202103545.
- Y. Tsuchido, S. Fujiwara, T. Hashimoto and T. Hayashita, Chem. Pharm. Bull., 2017, 65, 318–325, DOI:10.1248/cpb.c16-00963.
- K. Sugita, Y. Tsuchido, C. Kasahara, M. A. Casulli, S. Fujiwara, T. Hashimoto and T. Hayashita, Front. Chem., 2019, 7, 806, DOI:10.3389/fchem.2019.00806.
- K. Aoki, R. Osako, J. Deng, T. Hayashita, T. Hashimoto and Y. Suzuki, RSC Adv., 2020, 10, 15299–15306, 10.1039/d0ra01455a.
- M. A. Casulli, I. Taurino, T. Hashimoto, S. Carrara and T. Hayashita, Small, 2020, 16, 2003359, DOI:10.1002/smll.202003359.
- A. Kimura, S. Chiba and M. Yoneyama, Carbohydr. Res., 1988, 175(1), 17–23, DOI:10.1016/0008-6215(88)80152-6.
- A. Das, A. Danao, S. Banerjee, A. M. Raj, G. Sharma, R. Prabhakar, V. Srinivasan, V. Ramamurthy and P. Sen, J. Am. Chem. Soc., 2021, 143, 2025–2036, DOI:10.1021/jacs.0c12169.
- H. Bouas-Laurent, A. Castellan, J. P. Desvergne and R. Lapouyade, Chem. Soc. Rev., 2000, 29, 43–55, 10.1039/A801821I.
- G. Springsteen and B. Wang, Tetrahedron, 2002, 58, 5291–5300, DOI:10.1016/S0040-4020(02)00489-1.
- The Emerging Risk Factor Collaboration, Lancet, 2010, 375, 2215–2222, DOI:10.1016/S0140-6736(10)60484-9.
- X. Wu, Z. Li, X.-X. Chen, J. S. Fossey, T. D. James and Y.-B. Jiang, Chem. Soc. Rev., 2013, 42, 8032–8048, 10.1039/C3CS60148J.
- T. Kawasaki, H. Akanuma and T. Yamanouchi, Diabetes Care, 2002, 25, 353–357, DOI:10.2337/diacare.25.2.353.
- J. Yan, G. Springsteen, S. Deeter and B. Wang, Tetrahedron, 2004, 60, 11205–11209, DOI:10.1016/j.tet.2004.08.051.
- M. D. Phillips and T. D. James, J. Fluoresc., 2004, 14, 549–559, DOI:10.1023/b:jofl.0000039342.10260.21.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, synthetic procedures, Fig. S1–S11 and Scheme S1. See https://doi.org/10.1039/d2ra03567g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |