Open Access Article
Open Access ArticleHydroxyapatite coating on an aluminum/bioplastic scaffold for bone tissue engineering
Oratai Jongprateep *ab,
Nonthaporn Jitanukula,
Khotamy Saphongxayab,
Benjamon Petchareanmongkola,
Ampika Bansiddhia,
Apirat Laobuthee
*ab,
Nonthaporn Jitanukula,
Khotamy Saphongxayab,
Benjamon Petchareanmongkola,
Ampika Bansiddhia,
Apirat Laobuthee a,
Amornrat Lertworasirikula and
Ratchatee Techapiesancharoenkijab
a,
Amornrat Lertworasirikula and
Ratchatee Techapiesancharoenkijab
aDepartment of Materials Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, Thailand. E-mail: fengotj@ku.ac.th
bICE-Matter Consortium, ASEAN University Network/Southeast Asia Engineering Education Development Network (AUN/SEED Net), Bangkok, Thailand
First published on 21st September 2022
Abstract
Three-dimensional printing can produce scaffolds with shapes and dimensions tailored for practical clinical applications. Enhanced osteoconductivity of such scaffolds is generally desired. Hydroxyapatite (HA) is an inorganic ceramic that can be used to coat such scaffolds and to accelerate healing during the bone restoration process. In this study, HA-coated aluminum/bioplastic scaffolds were fabricated, and their structural characteristics and osteoconductivity were evaluated. Aluminum/bioplastic scaffolds were fabricated by three-dimensional printing, and HA slurries with solids loadings of 10–20 vol% were used for coating. As solids loadings increased, the thickness of the coating layers slightly increased, whereas pore sizes decreased. The average compressive strength was comparable to that of cancellous bone. Potential osteoconductivity was tested by simulated body fluid immersion for 28 days, and the formation of the HA phase on the surface along with a weight increase indicates the potential bioactivity of the samples.
1 Introduction
Bones are an important part of the human body: they support the rest of the body, are connected to muscles, protect organs and bone marrow, and store minerals such as calcium and phosphorus. Various bone diseases can occur, including spinal disc degeneration, scoliosis, osteoporosis, bone infection, tumors, congenital defects, and osteoarthritis.1 Apart from diseases, bones can fracture because of high impact forces or stress. The self-healing abilities of bones may not be sufficient for severe fractures. Several techniques have been employed to heal bone fractures or promote bone regeneration. One such technique is bone tissue engineering (BTE), which uses bone scaffolds.2 The fundamental requirements for the practical application of bone scaffolds include high porosity, nontoxicity, corrosion resistance, and high durability. In addition, the bone scaffold materials must be biocompatible so that they are not rejected and do not cause any undesirable effects when implanted. The materials should also have mechanical properties and strength similar to that of real human bones.2–8Various materials have been employed for bone scaffolds and implants. Metals such as magnesium, zirconium, titanium, and stainless steel are commonly used in implants because of their high strength, ease of forming, and low toxicity.3,8 Titanium alloy is a well-known biomaterial that is widely used as a bone replacement, to which magnesium, nickel, cobalt, and/or chromium are added to enhance strength, flexibility, and malleability.12–16 Moghaddam et al.13 reported that adding vanadium and aluminum to titanium can also improve strength and corrosion resistance. The Ti–6Al–4V alloy is one of the most commonly used materials in medical devices owing to its good mechanical properties, corrosion resistance, and low cost.17,18 In addition to being a component of robust titanium alloys, aluminum is extensively used as a major component in medical devices due to its strength, durability, flexibility, lightweight, corrosion resistance, recyclability, and abundance.19 To achieve low potential for severe toxicity and to attain good biocompatibility with the human body, surface-treated aluminium alloy is used in biomedical applications.20–22
Processing the material to obtain the required shape is also important. Numerous material processing techniques such as casting and powder metallurgy are available. However, these techniques require the utilization of molds, which have high tool costs. Three-dimensional (3D) printing is capable of fabricating near-net-shape samples without a mold as well as unique and customized parts. This technique can also produce small parts or specific details, so it is suitable for producing bone scaffolds.23,24
Despite the desirable mechanical properties of metals or alloys, their application in implants has several drawbacks. Metal implants have low osteoconductivity, which slows the healing process. Numerous studies have reported the corrosion of implants over time.3,9–11 Metals are also toxic in high doses.25 Metal ion leaching may cause harmful conditions to human body. For example, excessively high content of aluminium in human blood can induce problems in internal organ such as kidney, lung, liver, and bone.26–28 It has been reported that the majority of aluminium intake from food, medicine, and medical implants is eliminated through urine excretion. The high level of remaining aluminium ions, however, can result in chronic renal disease,28 inflammation of lung, intestine, heart, and testis, as well as weakened immunity system.29–32 High content of aluminium ions also induces the risk of triggering neurodegenerative diseases, including Alzheimer's disease and Parkinson's disease, and multiple sclerosis.33–35
While aluminium toxicity is not coverable, many of the above drawbacks can be prevented by coating the implant with biocompatible materials. Hydroxyapatite (HA) (Ca10(OH)2(PO4)6) is an inorganic ceramic that is used in BTE owing to its chemical composition similar to that of bones, high osteoinductivity and osteoconductivity, and excellent biocompatibility.36–42 However, its relatively low strength has limited the application of HA as a bone substitute.43,44 An effective option for producing a high-strength bone restoration material with enhanced osteoconductivity is to coat a metal or alloy implant with HA. Coating a metal implant with HA promotes bone growth, strengthens the bond between the metal and bone tissue, accelerates healing and bone restoration,45 and improves osteoconductivity and corrosion resistance.46,47 The ion reaction between HA and body fluid can result in bone regeneration.48,49 HA coating can be achieved by various techniques, including the sol–gel method, electrophoresis, thermal spray technique, chemical vapor deposition, and ion beam sputtering. The hydrothermal technique offers advantages such as low processing temperature and short processing time.50
In this study, simple and robust processing techniques were combined to fabricate bone scaffolds and coat them with HA. The study had three main parts: 3D printing of aluminum/bioplastic scaffolds, synthesis of HA powder by solution combustion, and coating the scaffolds with HA by the hydrothermal technique. The chemical composition and microstructure of the synthesized HA powder were examined, and the properties of the coated scaffolds were evaluated in terms of strength and potential bioactivity.
2 Experimental
2.1 Sample preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P molar ratio of 2.3
P molar ratio of 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was prepared. Glycine, which acted as the combustion fuel, was added to the prepared solution to obtain an aqueous solution with a Ca
1 was prepared. Glycine, which acted as the combustion fuel, was added to the prepared solution to obtain an aqueous solution with a Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycine molar ratio of 2.3
glycine molar ratio of 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9. Combustion reaction was initiated when the prepared solution was heated at 400 °C for approximately 1 h. Upon the completion of the combustion reaction, the synthesized powder was collected and calcined at 600 °C for 3 h.
1.9. Combustion reaction was initiated when the prepared solution was heated at 400 °C for approximately 1 h. Upon the completion of the combustion reaction, the synthesized powder was collected and calcined at 600 °C for 3 h.
2.2 Coating procedure
The hydrothermal process was used to coat the scaffolds with HA. The synthesized HA powder, polyvinyl alcohol ((–CH2CH(OH)–)n, 1500, Daejung), and dispersant (Darvan 821/D821A6) were mixed with deionized water to prepare slurries with solids loadings of 10, 15, and 20 vol% HA. The scaffolds were then immersed into the HA slurry, which was heated at 160 °C for 5 h. Then, the scaffolds were calcined at 600 °C for 3 h.2.3 Characterization
3 Results and discussion
3.1 Characteristics of uncoated scaffolds
Table 1 presents the EDS results for the scaffold composition. The scaffolds mainly consisted of aluminum and had a composition of 38.9 at% aluminum, 28.2 at% oxygen, and 32.9 at% carbon. The high carbon content was attributed to the high polymeric binder content in the composite filament.
| Element | Composition (at%) |
|---|---|
| Al | 38.86 |
| O | 28.17 |
| C | 32.97 |
Because the scaffold was fabricated from a commercially available composite filament consisting of aluminum and polymeric material, the polymeric binder needed to be identified. Fig. 7 shows the FTIR spectrum of the scaffold. The characteristic absorption bands of the peaks observed at 3440, 2956, 1733, 1440, and 1148 cm−1 correspond to the –OH(COOH), –CH3(S), –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –CH3(B), and –C–O(COOH) functional groups, which are commonly observed in polylactic acid (PLA).52,53
O, –CH3(B), and –C–O(COOH) functional groups, which are commonly observed in polylactic acid (PLA).52,53
Thermogravimetric analysis (TGA) was employed to determine the volatile components, specifically the polymeric binder in the filament, according to the change in weight. As the sample was heated from room temperature to 500 °C, a noticeable loss in mass occurred at 225–400 °C, as shown in Fig. 8(a). The extrapolated onset temperature corresponding to the degradation of the polymeric binder was 318.4 °C. This is close to the degradation temperature for PLA, which has been reported to be 300–372 °C. The TGA results agree with the study which indicates that the polymeric binder in the filament mainly comprised PLA.54–56
DSC measures the temperatures and heat flows associated with transitions in materials, and it was employed for thermal analysis of the scaffolds. The DSC curves exhibited prominent endothermic signals close to 58 °C and 170 °C, as shown in Fig. 8(b). These signals may represent the glass transition temperature (Tg) and melting temperature (Tm), respectively, of the polymeric component. An exothermic signal representing the crystallization temperature (Tc) was clearly observed close to 83 °C. The DSC results agreed with the FTIR and TGA results.
PLA is a biocompatible polymer extensively used in medical applications, including implants or bone scaffolds, owing to its biodegradability, nontoxicity, and environmental friendliness.57–60 Da Silva et al.61 noted that PLA can degrade in the human body and can be excreted in the urine and during breathing.
3.2 Characteristics of the coating powder
 | ||
| Fig. 10 (a) and (b) Scanning electron micrographs showing the morphology of HA particle. (c) Scanning electron micrographs showing agglomeration of HA particles into clusters. | ||
The coating particle size can affect the characteristics of the scaffold. For biomedical applications, finer HA particles are preferred because of the high surface area. That could enhance interaction between HA and scaffold. According to Hu et al. and Christian et al., bioactivities of the calcium phosphate are associated with size of the particles. Fine particle sizes provide large active surface area and nucleation sites for formation of apatite layer, promoting cell proliferation and osteoconductivity.62–66
3.3 Characteristics of the coated scaffolds
 | ||
| Fig. 12 Scanning electron micrographs showing the microstructure of scaffolds coated by (a) 10, (b) 15, and (c) 20 vol% HA. | ||
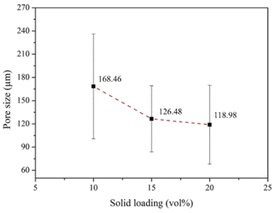
To enhance osteoconductivity, a proper pore structure for the HA coating is required. A pore size of 50–500 μm is considered appropriate for practical applications.68,69 Fig. 13 shows the scanning electron micrographs of the pore structures of the coated scaffolds. ImageJ was employed to analyze the pore size. The results indicated that increasing the solids loadings of HA reduced the pore size. Fig. 14 shows that scaffolds coated by 10, 15, and 20 vol% HA had average pore sizes of 168.46 ± 67.60, 126.49 ± 42.67, and 118.98 ± 50.85 μm, respectively. According to Won et al.,70 scaffolds with pore sizes of 100–325 μm are optimal for BTE.
 | ||
| Fig. 13 Scanning electron micrographs showing the pore structure of scaffolds coated by (a) 10, (b) 15, and (c) 20 vol% HA. | ||
In addition to the pore size, porosity affects the development of tissue and blood vessels as well as compressive strength.71 ImageJ and Origin Pro8.5 were employed to analyze the porosity of the coated scaffolds. Fig. 15 shows that the average porosities of the scaffolds coated by 10, 15, and 20 vol% HA were 69.48% ± 2.00%, 66.73% ± 4.61%, and 64.40% ± 2.97%, respectively. These values are comparable to those reported by other researchers and are suitable for practical application. According to Kim et al.,72 bone scaffolds with a structure similar to cancellous bone should have a porosity of 50%–90%.
Fig. 16 shows the chemical compositions of the coated scaffolds according to EDS. Elemental mapping was conducted to examine the distribution of the elements on the scaffold surface. All elements had a fairly uniform distribution. Table 2 presents the quantitative analysis of the key elements: Ca, P, Al, and O. The Ca/Al ratio differed significantly according to solids loadings: with the lowest and the highest in the samples coated with 10 and 20 vol% HA, respectively. It is generally accepted that the elemental composition values obtained from the EDS analysis rely on interactions of X-ray and the sample. Therefore, if the HA coating layer is uniform, non-porous and thick, aluminium signal may not be detected. Nevertheless, since the HA coating layers in this study demonstrated highly porous structure, X-ray could penetrate to aluminium surface through pores.
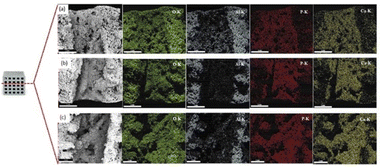 | ||
| Fig. 16 Scanning electron micrographs, EDS spectrum, and elemental mapping of the scaffolds coated by (a) 10, (b) 15, and (c) 20 vol% HA. | ||
| Element | Chemical composition (at%) | ||
|---|---|---|---|
| 10 vol% HA | 15 vol% HA | 20 vol% HA | |
| Ca | 17.605 ± 0.70 | 23.59 ± 1.34 | 26.29 ± 2.57 |
| P | 6.25 ± 0.32 | 9.09 ± 0.56 | 9.18 ± 0.15 |
| O | 59.20 ± 0.27 | 55.47 ± 0.31 | 56.07 ± 1.09 |
| Al | 18.29 ± 0.61 | 11.84 ± 1.59 | 8.45 ± 1.32 |
| Ca/Al | 0.96 ± 0.005 | 2.01 ± 0.38 | 3.17 ± 0.80 |
For the samples coated with 10 vol% HA, the largest pore sizes, the highest porosity and thinner HA coating layer were evident. On the contrary, thicker coating layer with finer pores and reduced porosity was observed in the samples coated with 20 vol% HA. For the coated scaffold with HA at higher solids loading, finer pore size and lower porosity suppress aluminum signal in the EDS spectrum, resulting in the enhanced Ca/Al ratio.
 | ||
| Fig. 17 Scanning electron micrographs showing thickness of the (a) uncoated sample, coated with (b) 10 vol% solids loading, (c) 15 vol% solids loading, and (d) 20 vol% solids loading. | ||
| Sample no. | Compressive strength (MPa) | |||
|---|---|---|---|---|
| Uncoated | Coated with 10 vol% HA | Coated with 15 vol% HA | Coated with 20 vol% HA | |
| 1 | 0.15 | 2.26 | 1.50 | 0.23 |
| 2 | 0.18 | 2.57 | 1.83 | 0.37 |
| 3 | 0.19 | 2.91 | 1.91 | 0.73 |
| 4 | 0.20 | 2.99 | 2.04 | 0.75 |
| 5 | 0.23 | 3.12 | 2.15 | 0.77 |
| 6 | 0.24 | 3.22 | 2.23 | 1.13 |
| 7 | 0.25 | 3.30 | 2.35 | 1.31 |
| 8 | 0.35 | 3.48 | 3.01 | 1.41 |
| Average | 0.22 | 2.98 | 2.13 | 0.84 |
| Standard deviation | 0.06 | 0.40 | 0.44 | 0.42 |
The low average compressive strength in the samples the high solids loading may be attributed to the nonuniformity and excessive thickness of the coating. As shown in Fig. 19, the samples coated with 20 vol% HA revealed the greatest standard deviation compared to its mean compressive strength. The coefficient of variation (the ratio between standard deviation of dataset and the mean of dataset) of the samples is 0.5, indicating that the compressive strength values of the samples are relatively scattered. The dispersion of the strength values might be associated with nonuniform coating. As shown in Fig. 20, the scanning electron micrographs of the samples coated with 10 vol% HA reveal thinner coating layer with relatively uniform thickness. On the contrary, thicker but nonuniform coating layers were observed in the sample coated the 15 and 20 vol% HA.
 | ||
| Fig. 20 Scanning electron micrographs showing the sample coated with (a) 10 vol% solids loading, (b) 15 vol% solids loading, and (c) 20 vol% solids loading. | ||
Schmidmaier et al.74 reported that a thick coating layer may be at a higher risk of detachment from the implant. Thus, a high solids loading may result in a nonuniform coating with poor adhesion and compressive strength.76
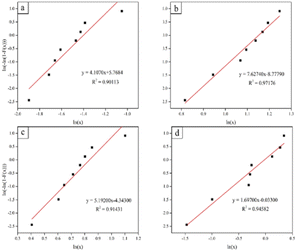 | ||
| Fig. 21 Weibull plots for the compressive strength of scaffolds: (a) control, (b) coated with 10 vol% HA, (c) coated with 15 vol% HA, and (d) coated with 20 vol% HA. | ||
| Compressive strength (MPa) | Reliability | |||
|---|---|---|---|---|
| Uncoated | Coated with 10 vol% HA | Coated with 15 vol% HA | Coated with 20 vol% HA | |
| 0.50 | 0.00 | 1.00 | 1.00 | 0.74 |
| 1.00 | 0.00 | 1.00 | 0.99 | 0.38 |
| 1.50 | 0.00 | 1.00 | 0.90 | 0.15 |
| 2.00 | 0.00 | 0.97 | 0.62 | 0.04 |
| 2.50 | 0.00 | 0.85 | 0.22 | 0.01 |
| 3.00 | 0.00 | 0.51 | 0.02 | 0.00 |
| 3.50 | 0.00 | 0.11 | 0.00 | 0.00 |
| 4.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 4.50 | 0.00 | 0.00 | 0.00 | 0.00 |
| 5.00 | 0.00 | 0.00 | 0.00 | 0.00 |
The bioactivity of the coated scaffolds can be evaluated as a result of HA immersion into SBF for 28 days. During the course of SBF immersion, HA dissolves in SBF and release Ca2+ and PO43−, which increased ion concentrations around the HA and provided favorable sites for apatite nucleation and crystallization. Through electrostatic attraction, negatively charged OH− and PO43− ions interact with positively charged Ca2+ ions, resulting in chemical bonding and formation of HA layer on the surface of the scaffold.48,78–80
Fig. 22 shows scanning electron micrographs of the scaffolds coated by HA slurries before and after SBF immersion. The micrographs revealed porous morphology similar to that of HA, indicating potential bone growth with the HA coating. To ensure that the HA-like morphology did not belong to the existing HA coating layer, changes in weight after SBF immersion were used as indicators of bone-bonding ability. Table 5 demonstrates that the coated scaffolds increased in mass by more than 10% after immersion. This is similar to the observations of Chandra et al.,81 who reported a mass of 5.93–6.79% after Fe-doped HA samples were immersed in SBF for 4 weeks.
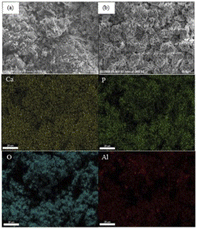 | ||
| Fig. 22 Scanning electron micrographs and elemental mapping of the scaffolds coated by hydroxyapatite slurries with 10 vol% (a) before immersion in SBF, and (b) after immersion in SBF 28 days. | ||
| HA (vol%) | Weight before SBF immersion (g) | Weight after SBF immersion (g) | Weight increase (%) |
|---|---|---|---|
| 10 | 2.9065 ± 0.05 | 3.2849 ± 0.05 | 11.78 |
| 15 | 2.9942 ± 0.05 | 3.4032 ± 0.05 | 13.65 |
| 20 | 2.077 ± 0.05 | 2.3016 ± 0.05 | 10.81 |
The presence of HA was also confirmed by EDS. Elemental mapping of the coated scaffolds, as shown in Fig. 22 reveals a uniform distribution of elements on the surfaces. Table 6 presents a quantitative analysis of the key elements: Ca, P, Al, and O. The Ca/P ratios of 2.81 ± 0.21, 2.53 ± 0.09 and 2.90 ± 0.08 were evident in the samples coated by 10, 15, and 20 vol% HA, respectively. Elemental analysis of the samples prior to SBF immersion was previously shown in Table 2. The Ca/P ratios of 2.81 ± 0.03, 2.53 ± 0.01, and 2.86 ± 0.32 were observed in the samples coated by 10, 15, and 20 vol% HA, respectively. The Ca/P values of the HA coating after SBF immersion is close to that obtained prior to SBF immersion, indicating presence of HA after SBF immersion.
| Element | Chemical composition (at%) | ||
|---|---|---|---|
| 10 vol% HA | 15 vol% HA | 20 vol% HA | |
| Ca | 8.195 ± 2.22 | 14.61 ± 0.20 | 20.41 ± 14.86 |
| P | 2.88 ± 0.57 | 5.78 ± 029 | 7.10 ± 5.32 |
| O | 69.65 ± 0.43 | 65.45 ± 0.70 | 64.48 ± 13.22 |
| Al | 19.26 ± 2.36 | 14.14 ± 1.2 | 7.69 ± 6.53 |
| Ca/P | 2.81 ± 0.21 | 2.53 ± 0.09 | 2.90 ± 0.08 |
It should be addressed that non-stoichiometric apatite with the high value of Ca/P was obtained in this study. Stoichiometric HA has the Ca/P ratio of 1.67. Substantial variation of Ca/P ratio from the stoichiometric value can occur as a result of ion substitution. The loss of negative charges, which leads to the increase of Ca/P ratio, may occur when trivalent PO43+ ions in HA structure are substituted by bivalent ions such as carbonate. Carbonate substitution in HA can be represented by the following formula:
| Ca10−x+u(PO4)6−x(CO3)x(OH)2−x+2u with x ≤ 2 and u ≤ x/2 |
It is noted that the hydroxyapatite powder used for coating was synthesized by the solution combustion technique, where carbon containing compound (glycine) was used as combustion fuel. Therefore, carbonate substitution can be possible. Average Ca/P ratios of the synthesized powder, coated layers (both before and after SBF immersion) were in a comparable range.
Potential enhancement of bioactivity in ion-substituted HA may be associated with increase solubility. It is commonly accepted that HA is hardly soluble. Ion substitution potentially creates ionic vacancies, strains, and heterogeneity of composition, which destruct crystal cohesion and result in increased solubility. It has been reported that when the carbonate substitution increases, higher solubility product constant (Ksp) increases. Nucleation ability of HA is also related to supersaturation ratio which generally associates with high solubility.82
Another possible explanation for high Ca/P ratio involves formation of a hydrated surface layer. The existence of a hydrated layer, mainly containing bivalent ions such as Ca2+, promotes spontaneous ion release and ion exchange. In addition, interfacial energy is potentially decreased, which accommodate the nucleation of HA.66
From the aforementioned discussion related to non-stoichiometric HA, it can be possibly deduced that high Ca/P ratio may be advantageous in terms of enhancement of bioactivity. A similar observation has been reported by Yang et al., who states that the coating prepared by plasma electrolytic oxidation at different Ca/P ratios yields different bioactivity. Improvement of the bioactivity of the coated implant can be achieved with higher the Ca/P ratio.83
4 Conclusions
Aluminum/PLA scaffolds were successfully fabricated and coated with HA. The scaffold was 3D-printed, whereas a hydrothermal method was used for the HA coating. HA powder with an average particle size of <200 nm was synthesized by solution combustion and used for the coating process. The uncoated scaffolds showed a rough surface morphology with segregated grains of aluminum. By contrast, the coated scaffolds revealed a flower-like morphology. The pore size and thickness of the HA coating were affected by the solids loading of the HA slurry. An average pore size of 118.98–168.46 μm and coating thickness of 38.21–46.53 μm were observed for scaffolds coated with 10, 15, and 20 vol% HA. An average compressive strength value of 0.84–2.98 MPa was achieved. The optimal coating properties were obtained at 10 vol% HA. The coated scaffolds were then tested for reliability and potential bioactivity. The scaffolds coated with 10 vol% HA achieved 100% reliability when subjected to a compressive stress of 1.5 MPa. The mass of the coated scaffolds increased by more than 10% after immersion in SBF for 28 days, which suggest their potential bioactivity.Author contributions
Research design: O. J. (leader), N. J. (contributor), K. S. (contributor), B. P. (contributor), A. B. (contributor), A. L. (contributor), A. L. (contributor), and R. L. (contributor); acquisition, analysis, or interpretation of data: O. J. (leader), N. J. (contributor), K. S. (contributor), B. P. (contributor); drafting the paper or revising it critically: O. J. (leader), N. J. (contributor), K. S. (contributor), B. P. (contributor); approval of the submitted and final versions: O. J. (leader).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the Administrative and Management Unit for Enhancing the Competitiveness of the Country. Equipment support was provided by the Department of Materials Engineering, Faculty of Engineering, Kasetsart University. The authors thank Ms. Siraprapa Pitiphattharabun and Mr Krittin Auewattanapun for the valuable discussions and assistance.Notes and references
- T. Tariverdian, F. Sefat, M. Gelinsky and M. Mozafari, Handbook of Tissue Engineering Scaffolds: Volume One, Elsevier, 2019, pp. 189–209 Search PubMed.
- T. Ghassemi, A. Shahroodi, M. H. Ebrahimzadeh, A. Mousavian, J. Movaffagh and A. Moradi, Arch. Bone Jt. Surg., 2018, 6, 90–99 Search PubMed.
- T. M. De Witte, L. E. Fratila-Apachitei, A. A. Zadpoor and N. A. Peppas, Regener. Biomater., 2018, 5, 197–211 CrossRef CAS PubMed.
- B. N. Brown, J. E. Valentin, A. M. Stewart-Akers, G. P. McCabe and S. F. Badylak, Biomaterials, 2009, 30, 1482–1491 CrossRef CAS PubMed.
- D. W. Hutmacher, Biomaterials, 2000, 21, 2529–2543 CrossRef CAS PubMed.
- J. R. Porter, T. T. Ruckh and K. C. Popat, Biotechnol. Prog., 2009, 25, 1539–1560 CAS.
- T. Kokubo, H.-M. Kim and M. Kawashita, Biomaterials, 2003, 24, 2161–2175 CrossRef CAS PubMed.
- B. I. Oladapo, S. A. Zahedi and A. O. M. Adeoye, Composites, Part B, 2019, 158, 428–436 CrossRef CAS.
- J. Kruger, Int. Mater. Rev., 1988, 33, 113–130 CrossRef CAS.
- S. P. Patterson, R. H. Daffner and R. A. Gallo, Am. J. Roentgenol., 2005, 184, 1219–1222 CrossRef PubMed.
- C. Liu, P. Wan, L. L. Tan, K. Wang and K. Yang, J. Orthop. Translat., 2014, 2, 139–148 CrossRef.
- R. Saidi, M. H. Fathi and H. Salimijazi, Bull. Mater. Sci., 2015, 38, 1367–1374 CrossRef CAS.
- N. Shayesteh Moghaddam, M. Taheri Andani, A. Amerinatanzi, C. Haberland, S. Huff, M. Miller, M. Elahinia and D. Dean, Biomanuf. Rev., 2016, 1, 1–16 CrossRef.
- G. Eddy Jai Poinern, S. Brundavanam and D. Fawcett, Am. J. Biomed. Eng., 2013, 2, 218–240 CrossRef.
- M. T. Andani, N. Shayesteh Moghaddam, C. Haberland, D. Dean, M. J. Miller and M. Elahinia, Acta Biomater., 2014, 10, 4058–4070 CrossRef CAS PubMed.
- J. A. McGeough, The Engineering of Human Joint Replacements: McGeough/The Engineering, John Wiley & Sons Ltd, Chichester, UK, 2013 Search PubMed.
- M. C. Bottino, P. G. Coelho, V. A. R. Henriques, O. Z. Higa, A. H. A. Bressiani and J. C. Bressiani, J. Biomed. Mater. Res., Part A, 2009, 88, 689–696 CrossRef PubMed.
- S. Sista, C. Wen, P. D. Hodgson and G. Pande, Mater. Sci. Eng., C, 2013, 33, 1573–1582 CrossRef CAS PubMed.
- M. Niinomi, M. Nakai and J. Hieda, Acta Biomater., 2012, 8, 3888–3903 CrossRef CAS.
- D. D. Kiradzhiyska, Y. N. Feodorova, M. M. Draganov, Ch. A. Girginov, A. P. Viraneva, T. A. Yovcheva and R. D. Mantcheva, AIP Conference Proceedings, AIP Publishing LLC, Istanbul, Turkey, 2016 Search PubMed.
- K. Kisters, B. Winterberg, M. Barenbrock, M. Hausberg and K. H. Rahn, Clin. Nephrol., 1999, 5, 191–193 Search PubMed.
- T. V. Kolekar, N. D. Thorat, H. M. Yadav, V. T. Magalad, M. A. Shinde, S. S. Bandgar, J. H. Kim and G. L. Agawane, Ceram. Int., 2016, 42, 5304–5311 CrossRef CAS.
- C. Cox, J. A. Thornby, G. J. Gibbons, M. A. Williams and K. K. Mallick, Mater. Sci. Eng., C, 2015, 47, 237–247 CrossRef.
- G. A. Fielding, A. Bandyopadhyay and S. Bose, Dent. Mater., 2012, 28, 113–122 CrossRef CAS PubMed.
- M. Jaishankar, T. Tseten, N. Anbalagan, B. B. Mathew and K. N. Beeregowda, Interdiscip. Toxicol., 2014, 7, 60–72 CrossRef PubMed.
- I. O. Igbokwe, E. Igwenagu and N. A. Igbokwe, Interdiscip. Toxicol., 2019, 12, 45–70 CrossRef CAS PubMed.
- X. Sun, Z. Cao, Q. Zhang, M. Li, L. Han and Y. Li, Chem.-Biol. Interact., 2016, 244, 9–15 CrossRef CAS PubMed.
- G. L. Klein, Osteoporos. Sarcopenia, 2019, 5, 2–5 CrossRef PubMed.
- C. S. Martinez, A. G. Escobar, J. A. Uranga-Ocio, F. M. Peçanha, D. V. Vassallo, C. Exley, M. Miguel and G. A. Wiggers, Reprod. Toxicol., 2017, 73, 128–141 CrossRef CAS PubMed.
- O. A. Taiwo, J. Occup. Environ. Med., 2014, 56, S71–S72 CrossRef CAS.
- Y. She, N. Wang, C. Chen, Y. Zhu, S. Xia, C. Hu and Y. Li, Biol. Trace Elem. Res., 2012, 147, 246–250 CrossRef CAS PubMed.
- H. Yu, J. Zhang, Q. Ji, K. Yu, P. Wang, M. Song, Z. Cao, X. Zhang and Y. Li, Ecotoxicol. Environ. Saf., 2019, 173, 131–141 CrossRef CAS PubMed.
- C. Exley, Expert Rev. Neurother., 2014, 14, 589–591 CrossRef CAS.
- C. Exley, G. Mamutse, O. Korchazhkina, E. Pye, S. Strekopytov, A. Polwart and C. Hawkins, Mult. Scler. J., 2006, 12, 533–540 CrossRef CAS.
- L. Tomljenovic and C. A. Shaw, J. Inorg. Biochem., 2011, 105, 1489–1499 CrossRef CAS PubMed.
- H. Zhou and J. Lee, Acta Biomater., 2011, 7, 2769–2781 CrossRef CAS PubMed.
- G. E. Poinern, R. K. Brundavanam, N. Mondinos and Z.-T. Jiang, Ultrason. Sonochem., 2009, 16, 469–474 CrossRef CAS PubMed.
- S. Samavedi, A. R. Whittington and A. S. Goldstein, Acta Biomater., 2013, 9, 8037–8045 CrossRef CAS PubMed.
- X. Pei, L. Ma, B. Zhang, J. Sun, Y. Sun, Y. Fan, Z. Gou, C. Zhou and X. Zhang, Biofabrication, 2017, 9, 045008 CrossRef PubMed.
- B. Zhang, H. Sun, L. Wu, L. Ma, F. Xing, Q. Kong, Y. Fan, C. Zhou and X. Zhang, Bio-Des. Manuf., 2019, 2, 161–171 CrossRef CAS.
- B. Ghiasi, Y. Sefidbakht, S. Mozaffari-Jovin, B. Gharehcheloo, M. Mehrarya, A. Khodadadi, M. Rezaei, S. O. Ranaei Siadat and V. Uskoković, Drug Dev. Ind. Pharm., 2020, 46, 1035–1062 CrossRef CAS PubMed.
- K. Fox, P. A. Tran and N. Tran, ChemPhysChem, 2012, 13, 2495–2506 CrossRef CAS PubMed.
- H. Siddiqui, K. Pickering and M. Mucalo, Materials, 2018, 11, 1813 CrossRef.
- S. V. Dorozhkin, Biomaterials, 2010, 31, 1465–1485 CrossRef CAS PubMed.
- J. Khotib, S. C. Cantika, S. Samirah and A. S. Budiatin, Folia Med., 2019, 55, 176–187 Search PubMed.
- W. S. W. Harun, R. I. M. Asri, A. B. Sulong, S. A. C. Ghani and Z. Ghazalli, Hydroxyapatite - Advances in Composite Nanomaterials, Biomedical Applications and Its Technological Facets, ed. J. Thirumalai, InTech, 2018, pp. 69–88 Search PubMed.
- Z. Zhang, M. F. Dunn, T. D. Xiao, A. P. Tomsia and E. Saiz, MRS Proc., 2001, 703, V7.5 CrossRef.
- C. Shuai, W. Yang, P. Feng, S. Peng and H. Pan, Bioact. Mater., 2021, 6, 490–502 CrossRef CAS PubMed.
- C. Shuai, B. Peng, P. Feng, L. Yu, R. Lai and A. Min, J. Adv. Res., 2022, 35, 13–24 CrossRef CAS PubMed.
- I. R. Gibson, Hydroxyapatite (Hap) for Biomedical Applications, Elsevier, 2015, pp. 269–287 Search PubMed.
- S. Liu, K. Li and R. Hughes, Ceram. Int., 2003, 29, 875–881 CrossRef CAS.
- N. Choksi and H. Desai, Int. J. Appl. Chem., 2017, 13, 377–384 Search PubMed.
- M. S. Lopes, A. L. Jardini and R. M. Filho, Chem. Eng. Trans., 2014, 38, 331–336 Search PubMed.
- A. Gregor, E. Filová, M. Novák, J. Kronek, H. Chlup, M. Buzgo, V. Blahnová, V. Lukášová, M. Bartoš, A. Nečas and J. Hošek, J. Biol. Eng., 2017, 11, 31–33 CrossRef.
- G. Wang and A. Li, Chin. J. Chem. Eng., 2008, 16, 929–933 CrossRef CAS.
- S. Yang, Z.-H. Wu, W. Yang and M.-B. Yang, Polym. Test., 2008, 27, 957–963 CrossRef CAS.
- T. T. Li, L. Ling, M.-C. Lin, Q. Jiang, Q. Lin, J.-H. Lin and C.-W. Lou, J. Nanomater., 2019, 9, 679 CrossRef CAS PubMed.
- M. C. Lin, C. W. Lou, J. Y. Lin, T. A. Lin, Y.-S. Chen and J. H. Lin, Mater. Sci. Eng., C, 2018, 91, 404–413 CrossRef CAS PubMed.
- S. Uma Maheshwari, V. K. Samuel and N. Nagiah, Ceram. Int., 2014, 40, 8469–8477 CrossRef CAS.
- R. P. Pawar, S. U. Tekale, S. U. Shisodia, J. T. Totre and A. J. Domb, Recent Pat. Regener. Med., 2014, 4, 40–51 CAS.
- D. da Silva, M. Kaduri, M. Poley, O. Adir, N. Krinsky, J. Shainsky-Roitman and A. Schroeder, Chem. Eng. J., 2018, 340, 9–14 CrossRef CAS PubMed.
- L. Wang, D. Barbieri, H. Zhou, J. D. de Bruijn, C. Bao and H. Yuan, J. Biomed. Mater. Res., Part A, 2015, 103, 1919–1929 CrossRef CAS PubMed.
- Z. Shi, X. Huang, Y. Cai, R. Tang and D. Yang, Acta Biomater., 2009, 5, 338–345 CrossRef CAS PubMed.
- Q. Tian, J. Lin, L. Rivera-Castaneda, A. Tsanhani, Z. S. Dunn, A. Rodriguez, A. Aslani and H. Liu, Sci. Rep., 2019, 9, 1–27 CrossRef CAS.
- Q. Hu, Z. Tan, Y. Liu, J. Tao, Y. Cai, M. Zhang, H. Pan, X. Xu and R. Tang, J. Mater. Chem., 2007, 17, 4690–4698 RSC.
- C. Rey, C. Combes, C. Drouet and H. Sfihi, Adv. Sci. Technol. Res. J., 2006, 49, 27–36 CAS.
- G. Ciobanu, G. Carja, O. Ciobanu, I. Sandu and A. Sandu, Micron, 2009, 40, 143–146 CrossRef CAS PubMed.
- L. Morejón, J. A. Delgado, A. A. Ribeiro, M. Varella de Oliveira, E. Mendizábal, I. García, A. Alfonso, P. Poh, M. van Griensven and E. R. Balmayor, Int. J. Mol. Sci., 2019, 20, 1790 CrossRef.
- B. Leukers, H. Gülkan, S. H. Irsen, S. Milz, C. Tille, M. Schieker and H. Seitz, J. Mater. Sci.: Mater. Med., 2005, 16, 1121–1124 CrossRef CAS PubMed.
- Y. W. Won, A. N. Patel and D. A. Bull, Biomaterials, 2014, 35, 5627–5635 CrossRef CAS PubMed.
- B. T. Smith, M. Santoro, E. C. Grosfeld, S. R. Shah, J. J. J. P. van den Beucken, J. A. Jansen and A. G. Mikos, Acta Biomater., 2017, 50, 68–77 CrossRef CAS.
- H. W. Kim, S.-Y. Lee, C.-J. Bae, Y.-J. Noh, H.-E. Kim, H.-M. Kim and J. S. Ko, Biomaterials, 2003, 24, 3277–3284 CrossRef CAS.
- G. L. Converse, T. L. Conrad, C. H. Merrill and R. K. Roeder, Acta Biomater., 2010, 6, 856–863 CrossRef CAS PubMed.
- G. Schmidmaier, B. Wildemann, P. Schwabe, R. Stange, J. Hoffmann, N. P. Südkamp, N. P. Haas and M. Raschke, J. Biomed. Mater. Res., 2002, 63, 168–172 CrossRef CAS PubMed.
- P. Feng, P. Wu, C. Gao, Y. Yang, W. Guo, W. Yang and C. Shuai, Adv. Sci., 2018, 5, 1700817 CrossRef PubMed.
- C. Feng, K. Zhang, R. He, G. Ding, M. Xia, X. Jin and C. Xie, J. Adv. Ceram., 2020, 9, 360–373 CrossRef CAS.
- C. Costa de Almeida, L. Á. Sena, M. Pinto, C. A. Muller, J. H. Cavalcanti Lima and G. de A. Soares, Braz. Dent. J., 2005, 16, 75–81 CrossRef PubMed.
- P. N. Chavan, M. M. Bahir, R. U. Mene, M. P. Mahabole and R. S. Khairnar, Mater. Sci. Eng., B, 2010, 168, 224–230 CrossRef CAS.
- P. Feng, S. Peng, C. Shuai, C. Gao, W. Yang, S. Bin and A. Min, ACS Appl. Mater. Interfaces, 2020, 12, 46743–46755 CrossRef CAS PubMed.
- Y. W. Gu, K. A. Khor and P. Cheang, Biomaterials, 2004, 25, 4127–4134 CrossRef CAS PubMed.
- V. Sarath Chandra, K. Elayaraja, R. V. Suganthi, M. I. Ahymah Joshy, I. Sulania, P. K. Kulriya, K. Asokan, D. Kanjilal and S. Narayana Kalkura, Adv. Mater. Lett., 2013, 4, 438–443 CrossRef.
- A. Ito, K. Maekawa, S. Tsutsumi, F. Ikazaki and T. Tateishi, J. Biomed. Mater. Res., 1997, 36, 522–528 CrossRef CAS.
- Z. Yang, L. Xia and J. Han, Adv. Biomed. Eng. Tech., 2015, 2, 13–19 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |