 Open Access Article
Open Access ArticleImprovements in photoelectric performance of dye-sensitised solar cells using ionic liquid-modified TiO2 electrodes†
Tomohiko Inomata *a,
Ayaka Matsunagaa,
Guangzhu Jina,
Takuma Kitagawaa,
Mizuho Muramatsua,
Tomohiro Ozawaa and
Hideki Masuda
*a,
Ayaka Matsunagaa,
Guangzhu Jina,
Takuma Kitagawaa,
Mizuho Muramatsua,
Tomohiro Ozawaa and
Hideki Masuda *ab
*ab
aDepartment of Life Science and Applied Chemistry, Graduate School of Science, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya 466-8555, Japan
bDepartment of Applied Chemistry, Aichi Institute of Technology, 1247 Yachigusa, Yakusa-cho, Toyota 470-0392, Japan
First published on 6th July 2022
Abstract
One of the major problems in dye-sensitised solar cells (DSSCs) is the aggregation of dyes on TiO2 electrodes, which leads to undesirable electron transfer. Various anti-aggregation agents, such as deoxycholic acid, have been proposed and applied to prevent dye aggregation on the electrodes. In this study, we designed and synthesised a phosphonium-type ionic liquid that can be modified on the TiO2 electrode surface and used as a new anti-aggregation agent. Although the modification of the ionic liquid onto the electrode reduced the amount of dye adsorbed on the electrode, it showed a significant anti-aggregation effect, thereby improving the photovoltaic performance of DSSCs with N3 and J13 dyes. This finding suggests that ionic liquids are effective as anti-aggregation agents for DSSCs.
Introduction
Dye-sensitised solar cells (DSSCs) are gaining attention as next-generation low-cost and energy-saving solar cells. As the manufacturing process of DSSCs does not require high vacuum or temperature, it is expected to consume less energy than that of conventional solar cells.1,2 In 1993, Grätzel et al. reported the N3 dye, which is still widely used today because of its ability to absorb visible light up to 800 nm.3 The N719 dye, in which two protons on N3 are replaced by tetrabutylammonium ions (TBA+), was reported in 1999;2 its photoelectric conversion efficiency was as high as 11.2%.4,5 In addition, a conversion efficiency of 12.3% was achieved in a system using a DSSC that combined a zinc porphyrin complex (YD2-o-C8) with an organic dye (Y123), and a Co2+/Co3+ redox couple, [Co(bpy)3]2+/3+.6 The Z907 dye, in which the carboxyl group of one 4,4′-dicarboxy 2,2′-bipyridine (dcbpy) ligand on N3 is replaced with a C9H19– group, has a photoelectric conversion efficiency of over 7%. It has been widely studied and is now recognised as a thermally stable dye.7–9It is well known that one of the problems in DSSCs is the aggregation of dyes. The aggregation of dyes on the TiO2 surface prevents the efficient conversion of absorbed light into electrical energy, and/or causes excited electrons to transfer to nearby dye oxidants. This is attributed to the low photovoltaic performance of DSSCs. To improve the performance of DSSCs, various molecules have been explored and added to the dye solutions. For example, it was reported that the addition of cholic acid (CA) improved both the short-circuit current density (JSC) and open-circuit voltage (VOC) in a system containing porphyrin derivatives.10 Studies have also been conducted using hexadecylmalonic acid (HDMA),8 1-decylphosphonic acid (DPA),7 3-phenylpropionic acid (PPA),9 deoxycholic acid (DCA),11 and chenodeoxycholic acid (CDCA).12–14 Among these, CDCA is the most common anti-aggregation agent. When the surface of TiO2 is bare, reverse electron transfer can occur between the TiO2 electrode and the electrolyte. While CDCA has been found to suppress reverse electron transfer, studies on the introduction of various blocking layers on the TiO2 surfaces have also been conducted.15–17 In addition to the introduction of co-adsorbed molecules, it was reported that the stacking of metal oxides (such as SiO2, Al2O3, and ZrO2) on the TiO2 surface suppressed electron recombination.18 It was also found that the adsorption of imidazolium-based insulator on the electrode surface prevented the aggregation of the dye and suppressed reverse electron transfer.19 Studies involving the use of different-sized siloxane molecules to cover the TiO2 electrode surface have also been carried out.20
An ionic liquid (IL) is a molten salt that generally maintains a liquid state below 100 °C. ILs have recently attracted attention as an environmentally friendly solvent.21,22 ILs are non-volatile and flame resistant. They have wide potential windows and low viscosity despite their ionic nature. Because of the non-volatility of IL, it can be recovered with almost high purity after being used in material production and can be reused. Moreover, ILs have been extensively studied as a green solvent owing to their excellent flame retardancy and stability. In the 21st century, ionic conductivity and thermal stability have attracted considerable attention. Therefore, ILs have been actively studied as electrolytes and reaction solvents for fuel cells,23 DSSCs,24,25 and lithium-ion secondary batteries.26 However, ILs are difficult to purify due to their low volatility, and their preparation is expensive. Therefore, the large-scale industrial application of ILs in batteries and cells is difficult.
Recently, our group reported a few studies on electrodes modified with bulky phosphonium/ammonium-type ILs. These include the capture of exogenous compounds,27 four-electron reduction of molecular oxygen,28 NO sensing in water,29 and electrocatalytic CO2 reduction.30 The electrodes modified with bulky ILs provide a specific reaction field on the electrode surface that improves the stability and catalytic activity of the encapsulated compounds.
From the above-mentioned studies on IL-modified electrodes27–30 and the adsorption of the insulator which has the IL-like structure on the TiO2 electrode,19 we decided to study the application of ILs as an anti-aggregation agent for DSSCs. Fig. 1 shows the schematic structures of the ILs, dyes, and the conventional anti-aggregation agent, CDCA. The IL units modified on the electrode are expected to prevent dye aggregation. Their cationic character may be advantageous for the smooth transport of the anionic redox couple (I3−/I−). In this study, we prepared IL-modified TiO2 electrodes encapsulating the sensitising dyes N3 and J13. N3 is a dye with high photovoltaic performance,3 and J13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 is one of the ruthenium complex-based dyes in the J series that was previously synthesised by our group.32–34 We fabricated DSSCs using these dyes with IL-modified TiO2 electrodes and evaluated their photovoltaic performances. DSSCs using IL-modified TiO2 electrodes showed higher anti-aggregation effect than those using the conventional anti-aggregation agent, CDCA.
31 is one of the ruthenium complex-based dyes in the J series that was previously synthesised by our group.32–34 We fabricated DSSCs using these dyes with IL-modified TiO2 electrodes and evaluated their photovoltaic performances. DSSCs using IL-modified TiO2 electrodes showed higher anti-aggregation effect than those using the conventional anti-aggregation agent, CDCA.
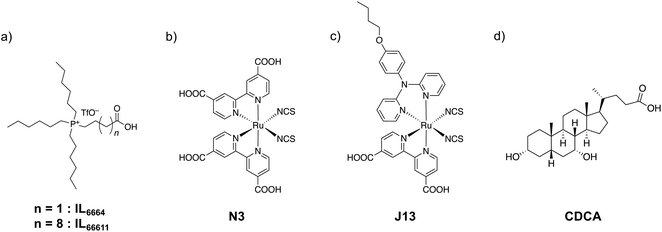 | ||
| Fig. 1 Schematic views of (a) ionic liquids used for the surface modification of electrodes (IL6664 and IL66611), (b) N3 dye, (c) J13 dye, and (d) chenodeoxycholic acid (CDCA). | ||
Experimental
General
1H NMR spectra were recorded on a Varian Gemini-2000 XL-300 MHz FT NMR spectrometer with TMS as the internal standard. Electronic absorption (UV/vis) spectra were recorded using a JASCO V-570 UV/vis spectrophotometer. Infrared (IR) spectra were recorded using a JASCO FT/IR-4200 spectrometer. Electrospray ionisation mass spectra (ESI-MS) were obtained with a Micromass LCT ESI-TOF MS. Wavelength-dispersive X-ray spectroscopy (WDS) measurements were recorded using JEOL electron probe microanalysers JXA-8230 and JXA-8530F. Elemental analysis was performed using an Elemental Vario El Cube CHNOS analyser.Chemicals
All reagents and organic solvents were purchased from Kanto Chemical, Kishida Chemical, Nacalai Tesque, Sigma-Aldrich, TCI, Wako Pure Chemical Industries, and Yoneyama Yakuhin Kogyo, and were used without further purification. Distilled water was obtained from an EYELA SA-2100E automatic water distillation apparatus. Thin-layer chromatography (TLC) was performed using a Merck TLC Silica 60 F254. Column chromatography was carried out with Kanto Chemical spherical silica gel 60 N (neutral, 63–210 μm).Solar cell fabrication
A Pilkington fluorine doped tin oxide (FTO) glass plate (Tec15, ohm per sq) was cut into a 2 × 2 cm piece and washed in MeCN for 30 min using an ultrasonic cleaning machine. The TiO2 paste (PST-18NR) was deposited on the FTO glass plate (as an adsorption layer for the dye chromophore) and heated at 100 °C for 10 min. The electrode was coated with TiO2 paste (PST-400C) as a light-scattering layer, and sintered at 530 °C for 1 h. After cooling, the TiO2/FTO electrode was immersed in a tert-butyl alcohol/MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution containing 0.3 mM of sensitised dye (N3 or J13) and various concentrations of ionic liquid (IL6664 or IL66611) for half a day. After washing with the tert-butyl alcohol/MeCN solution, the resultant dye/IL-modified TiO2/FTO electrode was used as the electrode of the DSSC. Reference cells were prepared by immersing the plate in a 0.3 mM tert-butyl alcohol/MeCN (1
1, v/v) solution containing 0.3 mM of sensitised dye (N3 or J13) and various concentrations of ionic liquid (IL6664 or IL66611) for half a day. After washing with the tert-butyl alcohol/MeCN solution, the resultant dye/IL-modified TiO2/FTO electrode was used as the electrode of the DSSC. Reference cells were prepared by immersing the plate in a 0.3 mM tert-butyl alcohol/MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution containing N3 or J13 for half a day. To prepare the platinum electrode, an FTO plate was dipped into a 30 mM solution of 2-propanol and H2PtCl6·6H2O, then burnt at 385 °C for 30 min. The Pt electrode and TiO2/FTO electrodes covered with dye or dye/IL were pasted using epoxy-based adhesive or UV-cured resin. An electrolyte comprising LiI (0.1 M), I2 (0.05 M), 1,2-dimethyl-3-propylimidazolium iodide (0.6 M), and 4-tert-butylpyridine (0.5 M) in MeCN (HPLC grade) was used.
1, v/v) solution containing N3 or J13 for half a day. To prepare the platinum electrode, an FTO plate was dipped into a 30 mM solution of 2-propanol and H2PtCl6·6H2O, then burnt at 385 °C for 30 min. The Pt electrode and TiO2/FTO electrodes covered with dye or dye/IL were pasted using epoxy-based adhesive or UV-cured resin. An electrolyte comprising LiI (0.1 M), I2 (0.05 M), 1,2-dimethyl-3-propylimidazolium iodide (0.6 M), and 4-tert-butylpyridine (0.5 M) in MeCN (HPLC grade) was used.
Estimation of surface coverage of adsorbed dyes and ILs on TiO2 electrodes
The amounts of N3 and J13 adsorbed were estimated using UV/vis spectroscopy after base treatment. A 0.1 M NaOH solution (for N3) and a 0.1 M NaOH solution in H2O/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (for J13) were used for the base treatments. WDS measurements were recorded to determine the surface ratios of the dyes and ILs. The surface coverages of the ILs were estimated from the ratio of P atoms (originating from ILs) to Ru atoms (originating from the dyes).
1) (for J13) were used for the base treatments. WDS measurements were recorded to determine the surface ratios of the dyes and ILs. The surface coverages of the ILs were estimated from the ratio of P atoms (originating from ILs) to Ru atoms (originating from the dyes).
Photovoltaic measurements
The photovoltaic performance of the DSSCs was measured with an Asahi Spectra IVP-0605 current–voltage (I–V) curve measurement recorder. An Asahi Spectra HAL-302 solar simulator was used to simulate the intensity of 1 sun (100 mW cm−2, AM 1.5) at the surface of the DSSC samples. To ensure that the irradiation area is identical across all samples, each DSSC was covered with a black tape containing a hole (size: 0.07 cm2) during the measurements to minimise the influence of stray light. Incident photo-to-current conversion efficiency (IPCE) spectra for DSSCs based on N3/TiO2 and N3 + IL66611/TiO2 were recorded with a Newport benchtop optical power meter model 1936-R using an Asahi Spectra PVL-4000 EX3 wavelength-tunable light source.EIS spectra measurements
The EIS spectra of N3/TiO2 and N3 + IL66611/TiO2 were carried out using an ALS/CH Instruments electrochemical analyzer model 660EKA. EIS measurements were performed at several potentials of the substrates with a frequency range from 10−2 to 106 Hz at an amplitude of 5 mV.Preparation of ILs and Ru complex dyes
The ILs (IL6664 and IL66611) were prepared according to Scheme S1† and the procedures are described below. The 1H NMR, FT-IR, and ESI-TOF MS spectra of the ILs are shown in Fig. S1–S6.† The Ru complex dyes, N3 and J13, were prepared according to previous methods.3,31 The ESI-TOF MS and 1H NMR spectra of the N3 and J13 are shown in Fig. S7–S9.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), giving a colourless viscous liquid. Yield: 2.50 g (15%).
1), giving a colourless viscous liquid. Yield: 2.50 g (15%).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield: 1.56 g (39%). 1H NMR (CD3OD, 300 MHz) δ ppm: 0.95 (t, 9H, –CH3), 1.31–1.63 (m, 24H, –CH2–), 1.88 (m, 2H, –CH2–), 2.23–2.35 (m, 8H, P+CH2–), 2.55 (t, 2H, –CH2COOH). FT-IR (DR, cm−1): 2934 (νC–H), 1732 (νC
1). Yield: 1.56 g (39%). 1H NMR (CD3OD, 300 MHz) δ ppm: 0.95 (t, 9H, –CH3), 1.31–1.63 (m, 24H, –CH2–), 1.88 (m, 2H, –CH2–), 2.23–2.35 (m, 8H, P+CH2–), 2.55 (t, 2H, –CH2COOH). FT-IR (DR, cm−1): 2934 (νC–H), 1732 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1256 (νSO3−), 1222 (νC–F), 1156(νC–F), 1031 (νSO3−). ESI TOF-MS (positive mode) m/z = 373.42 ([M]+).
O), 1256 (νSO3−), 1222 (νC–F), 1156(νC–F), 1031 (νSO3−). ESI TOF-MS (positive mode) m/z = 373.42 ([M]+).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1257 (νSO3−), 1223 (νC–F), 1157 (νC–F), 1030 (νSO3−). ESI TOF-MS (positive mode) m/z = 471.43 ([M]+).
O), 1257 (νSO3−), 1223 (νC–F), 1157 (νC–F), 1030 (νSO3−). ESI TOF-MS (positive mode) m/z = 471.43 ([M]+).Results and discussion
Preparation of ILs and sensitised Ru complex dyes
For the modification of ILs on the TiO2 electrode surface, tertiary phosphonium-type ILs with terminal carboxylic acids were synthesised. The terminal carboxylic acid is well known as the anchor group for the modification of the TiO2 electrode surface.20 The carboxylic acid and –OH groups on the TiO2 surface are condensed via dehydrogenation. As a result, molecules containing carboxyl groups are strongly bonded to the TiO2 surface. ILs with different alkyl chain lengths (Fig. 1a) were designed and synthesised to evaluate the effects of ILs on the photovoltaic performance of DSSCs. The numerical abbreviations of the ILs (IL6664 and IL66611) refer to the number of C atoms in each alkyl moiety (the linker moiety contains the C atom of the carboxyl group). The ILs were prepared based on our previous reports on ILs with disulfide groups for Au surface modification.27 All ILs were synthesised by reacting trihexylphosphine with carboxylic acids containing a terminal bromide group. IL6664 was obtained as a solid, likely because of its short alkyl chains. IL66611 was obtained as a viscous liquid owing to its long alkyl chains. The ILs were characterised by 1H NMR, FT-IR, and ESI-MS spectroscopy.N3 and J13 were chosen as the sensitising dyes in this study. The structures of the dyes are shown in Fig. 1b. N719 is often used as the standard dye in studies involving DSSCs. However, N719 contains two tetrabutylammonium ion (TBA+) units, which are introduced through a cation exchange reaction of N3. In this study, we used quaternary phosphonium-type ILs, which are very similar to quaternary ammonium cations, such as the TBA+ units in N719. Therefore, when N719 will be used in this study, an unfavourable cation exchange reaction will occur between TBA+ and the ILs. To exclude the cation exchange effect and evaluate the net effect of ILs, we decided to use the N3 dye in this study. The J13 dye, which we previously developed, has a relatively high conversion efficiency.31 In this study, N3 and J13 were used to study the effects for dye materials with different structures. Furthermore, the I−/I3− redox couple was used for all DSSCs fabricated in this study. Our previous studies using ILs-modified substrates indicate that the relatively large-sized molecules are blocked their transportation between the modified ILs layer on the electrode and the electrolyte solution interface.27–29 Thus, we chose the I−/I3− redox couple as the small and typical one for the DSSCs in this study. The dyes were synthesised according to a previously reported method and were characterised by 1H NMR, ESI-MS, FT-IR, UV/vis spectroscopy, and elemental analysis.
Preparation of IL- and N3-modified TiO2 electrodes
The modification of ILs and N3 onto the TiO2 surfaces was carried out by immersing the TiO2 electrodes in a solution of tert-butyl alcohol/acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing the ILs and N3. Table 1 lists the adsorption values of the ILs and N3. Solutions containing N3 and ILs of different alkyl chain lengths (IL6664 and IL66611) at various concentrations were prepared and the TiO2 electrodes were immersed in them. The concentration of N3 was fixed at 0.3 mM, and the concentration of ILs was varied in the range of 1–100 equivalents to N3. The amount of N3 adsorbed on the surface was estimated using the UV/vis spectra recorded after desorption from the TiO2 surface by treatment with a base. The ratio of N3 to ILs adsorbed was estimated using the WDS measurements of Ru atoms from N3 and P atoms from the ILs.
1) containing the ILs and N3. Table 1 lists the adsorption values of the ILs and N3. Solutions containing N3 and ILs of different alkyl chain lengths (IL6664 and IL66611) at various concentrations were prepared and the TiO2 electrodes were immersed in them. The concentration of N3 was fixed at 0.3 mM, and the concentration of ILs was varied in the range of 1–100 equivalents to N3. The amount of N3 adsorbed on the surface was estimated using the UV/vis spectra recorded after desorption from the TiO2 surface by treatment with a base. The ratio of N3 to ILs adsorbed was estimated using the WDS measurements of Ru atoms from N3 and P atoms from the ILs.
| TiO2 electrode | Immersion condition (N3 dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL) IL) |
Ratio of N3 dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL on TiO2 IL on TiO2 |
Amount of N3 dye adsorbed on TiO2 (mol cm−2) | N3 dye decrease rate (%) | Amount of IL adsorbed on TiO2 (mol cm−2) |
|---|---|---|---|---|---|
| N3/TiO2 | — | — | 8.73 × 10−8 | — | — |
| N3 + IL6664/TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.68 0.68 |
6.42 × 10−8 | 23 | 4.37 × 10−8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.85 0.85 |
5.95 × 10−8 | 32 | 5.06 × 10−8 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.87 0.87 |
5.64 × 10−8 | 35 | 4.91 × 10−8 | |
| N3 + IL66611/TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.18 0.18 |
7.95 × 10−8 | 8.9 | 1.43 × 10−8 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.47 0.47 |
8.12 × 10−8 | 7.0 | 3.83 × 10−8 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.59 0.59 |
8.18 × 10−8 | 6.3 | 4.83 × 10−8 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.78 0.78 |
8.03 × 10−8 | 8.0 | 6.26 × 10−8 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 1.00 |
8.32 × 10−8 | 4.7 | 8.32 × 10−8 |
As the ratio of IL6664 to N3 on the TiO2 electrode increased, the amount of N3 adsorbed decreased significantly, whereas the amount of IL6664 adsorbed did not change significantly (only N3 adsorption decreased by 35% at IL6664![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N3 = 1
N3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.87). There was no significant difference in the adsorption rate of N3 to IL6664 on the TiO2 surface. Therefore, we postulated that the adsorption of N3 was hindered to some extent when IL6664 was adsorbed on the surface. In our previous study, we reported that bulky ILs modified with thiol groups on Au electrodes can create adequate space between ILs for relatively large molecules to be introduced into them.27–29 As seen in Fig. 1a, the –CH2– linker chain in IL6664 is very short. When IL6664 is modified, there is not enough space between the modified ILs on TiO2 to adsorb the N3 dye; thus, the surface coverage of N3 decreases.
0.87). There was no significant difference in the adsorption rate of N3 to IL6664 on the TiO2 surface. Therefore, we postulated that the adsorption of N3 was hindered to some extent when IL6664 was adsorbed on the surface. In our previous study, we reported that bulky ILs modified with thiol groups on Au electrodes can create adequate space between ILs for relatively large molecules to be introduced into them.27–29 As seen in Fig. 1a, the –CH2– linker chain in IL6664 is very short. When IL6664 is modified, there is not enough space between the modified ILs on TiO2 to adsorb the N3 dye; thus, the surface coverage of N3 decreases.
In contrast, for IL66611, which has a long –CH2– linker chain, there was no significant decrease in the amount of N3 adsorbed, even when the ratio of IL in the soaking solution increased (the maximum decrease in the amount of N3 adsorbed was less than 10%). The amount of IL66611 adsorbed increased as the ratio of IL in the soaking solution increased. This indicates that N3 is first adsorbed on TiO2, followed by IL66611, which is adsorbed in the gaps between the adsorbed N3 particles. Due to the long –CH2– chain in IL66611 (Fig. 1a), the adsorption of IL66611 was not inhibited by the adsorption of N3 on the exposed TiO2 surface. Because all the ILs had the same headgroup (trihexyl-phosphonium moiety), it was suggested that the size of the space created on TiO2 depends on the length of the –CH2– linker chain, which had a significant effect on the adsorption surface area of N3.
Photovoltaic performance of DSSCs with IL-modified TiO2 electrodes
Based on the surface coverages of N3 and ILs on TiO2, IL66611 was found to be a suitable candidate as an anti-aggregation agent. Thus, we evaluated the photovoltaic performance of DSSCs using IL66611. Fig. 2 shows the I–V characteristics of the DSSCs with N3/TiO2 and N3 + IL66611/TiO2 electrodes. The photovoltaic parameters are listed in Table 2. The open-circuit voltage (VOC), short-circuit current density (JSC), fill factor (FF), and conversion efficiency (η) of the N3-based DSSC are 0.590 V, 13.9 mA cm−2, 0.673, and 5.54%, respectively. The introduction of IL66611 improved all the photovoltaic parameters of the DSSCs with N3 + IL66611/TiO2 electrodes. In particular, the greatest improvement in efficiency was observed for the electrode modified with N3 and IL66611 in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The VOC, JSC, FF, and η of the DSSC are 0.641 V, 15.9 mA cm−2, 0.705, and 7.20%, respectively. This shows a 30% increase in efficiency compared to that of N3 only. Although the surface coverage of N3 adsorbed on TiO2 was slightly decreased by IL modification, all the photovoltaic parameters of the DSSCs improved. This suggests that the modification with ILs achieved an anti-aggregation effect and suppressed reverse electron transfer. The reduction of dark current (Fig. 2b) suggests that ILs suppress the direct electron transfer between the TiO2 electrode and the I−/I3− redox couple. Fig. S10† shows the IPCE spectra of the DSSCs based on N3/TiO2 and N3 + IL66611/TiO2 (1
1. The VOC, JSC, FF, and η of the DSSC are 0.641 V, 15.9 mA cm−2, 0.705, and 7.20%, respectively. This shows a 30% increase in efficiency compared to that of N3 only. Although the surface coverage of N3 adsorbed on TiO2 was slightly decreased by IL modification, all the photovoltaic parameters of the DSSCs improved. This suggests that the modification with ILs achieved an anti-aggregation effect and suppressed reverse electron transfer. The reduction of dark current (Fig. 2b) suggests that ILs suppress the direct electron transfer between the TiO2 electrode and the I−/I3− redox couple. Fig. S10† shows the IPCE spectra of the DSSCs based on N3/TiO2 and N3 + IL66611/TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.78 and 1
0.78 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00). The IPCE values of two DSSCs based on N3 + IL66611/TiO2 were improved than that of N3/TiO2 in all measurement regions. This phenomenon also indicates that the IL66612 units modified on the TiO2 electrode surface work as a good anti-aggregation regent.
1.00). The IPCE values of two DSSCs based on N3 + IL66611/TiO2 were improved than that of N3/TiO2 in all measurement regions. This phenomenon also indicates that the IL66612 units modified on the TiO2 electrode surface work as a good anti-aggregation regent.
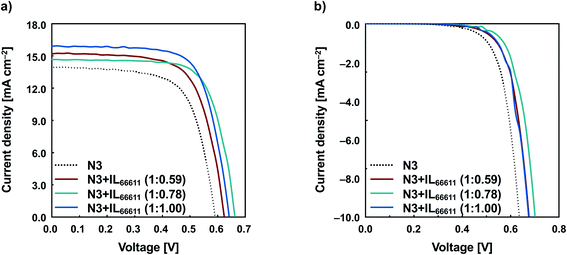 | ||
| Fig. 2 Current–voltage characteristics of DSSCs with N3 and N3 + IL66611 (a) under irradiation and (b) in the dark. | ||
| DSSC | Immersion condition (N3 dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL) IL) |
Ratio of N3 dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL on TiO2 IL on TiO2 |
VOC (V) | JSC (mA cm−2) | FF | η (%) | Increase rate of η (%) |
|---|---|---|---|---|---|---|---|
| N3 | — | — | 0.590 | 13.9 | 0.673 | 5.54 | — |
| N3 + IL66611/TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.59 0.59 |
0.593 | 15.2 | 0.682 | 6.47 | 17 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.78 0.78 |
0.663 | 14.7 | 0.715 | 6.95 | 25 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 1.00 |
0.641 | 15.9 | 0.705 | 7.20 | 30 |
The performances of the solar cells with the J13 dye are shown in Fig. 3 and Table 3. The VOC, JSC, FF, and η of the DSSC with only J13 are 0.574 V, 10.2 mA cm−2, 0.612, and 3.58%, respectively. Similarly, as in the case of N3, the introduction of ILs greatly improved the photovoltaic parameters. The most significant improvement was observed for the electrode modified by J13 and IL66611 at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.29 ratio, where VOC, JSC, FF, and η are 0.641 V, 11.6 mA cm−2, 0.648, and 4.80%, respectively. In this case, the conversion efficiency of the DSSC fabricated with J13 + IL66611/TiO2 was 34% higher than that of the DSSC fabricated with J13 only. The same effect was observed for different dyes, suggesting that the modification with ILs is very effective in inhibiting dye aggregation and reverse electron transfer. As in the case of N3, the dark current of J13 was also reduced upon modification with ILs (Fig. 2b). For both N3 and J13, the effect of IL66611 modification was significant. However, despite similar immersion conditions, the amount of IL66611 adsorbed on the TiO2 electrode with J13 was lower than that with N3 (Tables 2 and 3). This could be due to differences in the adsorption structures of the dyes on the TiO2 surface. The N3 dye had four carboxyl groups, whereas the J13 dye had only two carboxyl groups, as well as a terminal C4H9O– group. Thus, the orientation of J13 on the TiO2 surface is more suppressed than that of N3. These structural characteristics probably caused steric hindrance with the ILs. As a result, the unmodified area of the TiO2 surface of the DSSC with J13 + IL66611 appeared to be larger than that of the DSSC with N3 + IL66611.
0.29 ratio, where VOC, JSC, FF, and η are 0.641 V, 11.6 mA cm−2, 0.648, and 4.80%, respectively. In this case, the conversion efficiency of the DSSC fabricated with J13 + IL66611/TiO2 was 34% higher than that of the DSSC fabricated with J13 only. The same effect was observed for different dyes, suggesting that the modification with ILs is very effective in inhibiting dye aggregation and reverse electron transfer. As in the case of N3, the dark current of J13 was also reduced upon modification with ILs (Fig. 2b). For both N3 and J13, the effect of IL66611 modification was significant. However, despite similar immersion conditions, the amount of IL66611 adsorbed on the TiO2 electrode with J13 was lower than that with N3 (Tables 2 and 3). This could be due to differences in the adsorption structures of the dyes on the TiO2 surface. The N3 dye had four carboxyl groups, whereas the J13 dye had only two carboxyl groups, as well as a terminal C4H9O– group. Thus, the orientation of J13 on the TiO2 surface is more suppressed than that of N3. These structural characteristics probably caused steric hindrance with the ILs. As a result, the unmodified area of the TiO2 surface of the DSSC with J13 + IL66611 appeared to be larger than that of the DSSC with N3 + IL66611.
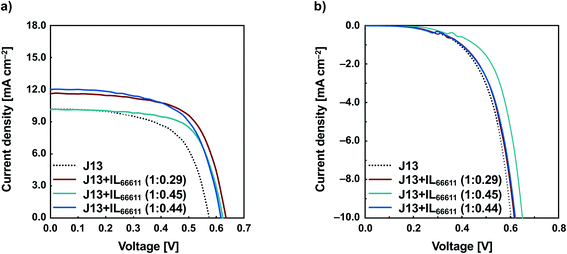 | ||
| Fig. 3 Current–voltage characteristics of DSSCs with J13 and J13 + IL66611 (a) under irradiation and (b) in the dark. | ||
| DSSC | Immersion condition (J13 dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL) IL) |
Ratio of J13 dye![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL on TiO2 IL on TiO2 |
VOC (V) | JSC (mA cm−2) | FF | η (%) | Increase rate of η (%) |
|---|---|---|---|---|---|---|---|
| J13 | — | — | 0.574 | 10.2 | 0.612 | 3.58 | — |
| J13 + IL66611/TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.29 0.29 |
0.641 | 11.6 | 0.648 | 4.80 | 34 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.45 0.45 |
0.614 | 10.2 | 0.671 | 4.22 | 18 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.44 1.44 |
0.617 | 12.0 | 0.622 | 4.62 | 29 |
Performance of ILs as anti-aggregation agents
The performance of IL66611 as an anti-aggregation agent was compared to that of solar cells fabricated using and chenodeoxycholic acid (CDCA), a conventional anti-aggregation agent. The results are summarised in Table 4. The performance of the solar cells with N3 decreased with the introduction of CDCA, which could be due to the decrease in the surface coverage of N3 (the surface coverage of N3 decreased to approximately 30% upon modification with CDCA). In contrast, the performance of the solar cells with IL66611 improved significantly. When IL66611 was used, the decrease in surface coverage of N3 was considerably lower (the N3 coverage only decreased to approximately 95%). This could be because IL66611 was able to enter the interstitial space of the N3 dye, preventing aggregation without reducing the amount of N3 adsorbed. When the same experiment was carried out with J13, the results were very similar to those of N3. This is because IL66611 can be adsorbed onto the surface without reducing the amount of dye adsorbed, as explained above. In other words, IL66611 can act as an anti-aggregation agent for various sensitising dyes. In addition, unlike CDCA, IL66611 has a positive charge, which may improve the conversion efficiency. To clarify the role of the ILs modified on the TiO2 surface, we measured the EIS spectra of N3/TiO2 and IL66611/TiO2 in the electrolyte solution. EIS spectra are often used to analyse the various interfaces on the electrodes.35,36 The results of the measurements of the EIS spectra at different potentials are shown in Fig. S11 and S12.† The several parameters related to the surface resistances and capacitances summarized in the figures were obtained by the curve-fitting of the Nyquist plots of N3/TiO2 and N3 + IL66611/TiO2 using the equivalent circuit shown in Fig. S13.† The surface-modified ILs did not affect the Rct1 values which are the resistances between the FTO and the TiO2 electrodes. On the other hand, the Rct2 values which are the resistances between the TiO2 surface and the electrolyte solution were lowered by the ILs on the TiO2 surfaces. These phenomena indicate that the positively-charged ILs on the TiO2 surface contribute to the decline of the interface resistance between the TiO2 surface and the electrolyte solution.| DSSC | VOC (V) | JSC (mA cm−2) | FF | η (%) | Increase rate of η (%) |
|---|---|---|---|---|---|
| N3 | 0.590 | 13.9 | 0.673 | 5.54 | — |
| N3 + CDCA/TiO2 | 0.633 | 10.0 | 0.679 | 4.30 | −22 |
| N3 + IL66611/TiO2 | 0.641 | 15.9 | 0.705 | 7.20 | +30 |
| J13 | 0.574 | 10.2 | 0.612 | 3.58 | — |
| J13 + CDCA/TiO2 | 0.578 | 8.78 | 0.627 | 3.18 | −11 |
| J13 + IL66611/TiO2 | 0.641 | 11.6 | 0.648 | 4.62 | +34 |
Furthermore, it is known that the introduction of a cation (such as TBA+) into some of the carboxylic acid moieties of N3 improves solar cell performance. From the DFT calculations, it was found that this was due to the increase in the energy level of N719 when the carboxylic acid moiety was replaced with TBA+.5 In this study, one of the possible reasons for the improved solar cell performance is also that the positive charge of the ILs has the same effect as TBA+ and interacted with the carboxylic acid moieties that were not adsorbed.
From our previous studies, the IL-modified substrates have enhanced the stability and durability of the entrapped compounds.28,29 The IL-modified TiO2 electrodes are expected to have a similar effect on the surface-modified dyes. However, due to factors such as the durability of the fabricated DSSC (e.g., electrolyte leakage) for long-time measurements, it is currently not achieved to evaluate the effect of the IL units modified on the TiO2 electrode. Now, we are attempting to rigorously evaluate the contribution of the IL-modified electrodes to the durability of DSSCs.
Conclusions
Ionic liquid-modified TiO2 electrodes were used to prevent the aggregation of dyes on the electrodes and improve the photovoltaic performance of DSSCs. The structures of the dyes and ILs had a significant effect on the surface coverage of the dyes adsorbed on the TiO2 surface. Using IL66611 with a long –CH2– linker chain, we succeeded in reducing the decrease in the amount of dye adsorbed. ILs were also found to outperform the conventional anti-aggregation agent, CDCA. It was shown that the use of positively-charged ILs as anti-aggregation agents enhanced the performance of solar cells without reducing the amount of dye adsorbed. The advantage of the ILs used in this study is that various structures can be synthetically designed, and we believe that they can be used as new anti-aggregation agents in the future.Author contributions
Conceptualization, T. I. and H. M.; methodology, T. I., A. M., G. J., and T. K.; validation, T. I. and T. O.; formal analysis, A. M., G. J., T. K., and M. M.; investigation, A. M., G. J., and A. M.; data curation, T. I., A. M., G. J. and M. M.; writing—original draft preparation, T. I.; writing—review and editing, T. I. and H. M.; visualization, T. I.; supervision, T. I. and T. O.; project administration, T. I. and H. M.; funding acquisition, T. I. and H. M. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Culture, Sports, Science, and Technology, Japan (MEXT/JSPS KAKENHI Grant Number (B) 20H02752 and (C) 21K04664 for H. M. and T. I., respectively). This study was also supported by the Murata Science Foundation. We would like to express our gratitude to them.Notes and references
- K. Kalyanasundaram, Dye-sensitized solar cells, EPFL Press, Lausanne, 2010 Search PubMed.
- Dye-Sensitized Solar Cells: Mathematical Modelling, and Materials Design and Optimization, ed. M. Soroush and K. K. S. Lau, 2019 Search PubMed.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Mueller, P. Liska, N. Vlachopoulos and M. Grätzel, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS.
- M. K. Nazeeruddin, S. M. Zakeeruddin, R. Humphry-Baker, M. Jirousek, P. Liska, N. Vlachopoulos, V. Shklover, C.-H. Fischer and M. Grätzel, Inorg. Chem., 1999, 38, 6298–6305 CrossRef CAS PubMed.
- M. K. Nazeeruddin, F. De Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, B. Takeru and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 16835–16847 CrossRef CAS PubMed.
- A. Yella, H. W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W. Diau, C. Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629–634 CrossRef CAS PubMed.
- P. Wang, S. M. Zakeeruddin, R. Humphry-Baker, J. E. Moser and M. Grätzel, Adv. Mater., 2003, 15, 2101–2104 CrossRef CAS.
- P. Wang, S. M. Zakeeruddin, P. Comte, R. Charvet, R. Humphry-Baker and M. Grätzel, J. Phys. Chem. B, 2003, 107, 14336–14341 CrossRef CAS.
- P. Wang, S. M. Zakeeruddin, R. Humphry-Baker and M. Grätzel, Chem. Mater., 2004, 16, 2694–2696 CrossRef CAS.
- A. Kay and M. Graetzel, J. Phys. Chem., 1993, 97, 6272–6277 CrossRef CAS.
- Z.-S. Wang, Y. Cui, Y. Dan-oh, C. Kasada, A. Shinpo and K. Hara, J. Phys. Chem. C, 2007, 111, 7224–7230 CrossRef CAS.
- N. R. Neale, N. Kopidakis, J. van de Lagemaat, M. Gratzel and A. J. Frank, J. Phys. Chem. B, 2005, 109, 23183–23189 CrossRef CAS PubMed.
- S. Qu, W. Wu, J. Hua, C. Kong, Y. Long and H. Tian, J. Phys. Chem. C, 2009, 114, 1343–1349 CrossRef.
- H. Matsuzaki, T. N. Murakami, N. Masaki, A. Furube, M. Kimura and S. Mori, J. Phys. Chem. C, 2014, 118, 17205–17212 CrossRef CAS.
- P. J. Cameron and L. M. Peter, J. Phys. Chem. B, 2003, 107, 14394–14400 CrossRef CAS.
- M. S. Góes, E. Joanni, E. C. Muniz, R. Savu, T. R. Habeck, P. R. Bueno and F. Fabregat-Santiago, J. Phys. Chem. C, 2012, 116, 12415–12421 CrossRef.
- P. Lellig, M. A. Niedermeier, M. Rawolle, M. Meister, F. Laquai, P. Muller-Buschbaum and J. S. Gutmann, Phys. Chem. Chem. Phys., 2012, 14, 1607–1613 RSC.
- E. Palomares, J. N. Clifford, S. A. Haque, T. Lutz and J. R. Durrant, J. Am. Chem. Soc., 2003, 125, 475–482 CrossRef CAS PubMed.
- M. Cai, X. Pan, W. Liu, J. Bell and S. Dai, RSC Adv., 2015, 5, 33855–33862 RSC.
- D. Song, H. An, J. H. Lee, J. Lee, H. Choi, I. S. Park, J. M. Kim and Y. S. Kang, ACS Appl. Mater. Interfaces, 2014, 6, 12422–12428 CrossRef CAS PubMed.
- Ionic liquids IV: not just solvents anymore, ed. J. F. Brennecke, R. D. Rogers and K. R. Seddon, American Chemical Society, Washington, 2008 Search PubMed.
- Ionic liquids for better separation processes, ed. H. Rodríguez, Springer, 2016 Search PubMed.
- S. Y. Lee, A. Ogawa, M. Kanno, H. Nakamoto, T. Yasuda and M. Watanabe, J. Am. Chem. Soc., 2010, 132, 9764–9773 CrossRef CAS PubMed.
- G. P. Lau, H. N. Tsao, S. M. Zakeeruddin, M. Gratzel and P. J. Dyson, ACS Appl. Mater. Interfaces, 2014, 6, 13571–13577 CrossRef CAS PubMed.
- T. C. Chu, R. Y. Lin, C. P. Lee, C. Y. Hsu, P. C. Shih, R. Lin, S. R. Li, S. S. Sun, J. T. Lin, R. Vittal and K. C. Ho, ChemSusChem, 2014, 7, 146–153 CrossRef CAS PubMed.
- H. Sakaebe and H. Matsumoto, Electrochem. Commun., 2003, 5, 594–598 CrossRef CAS.
- T. Kitagawa, T. Inomata, Y. Funahashi, T. Ozawa and H. Masuda, Chem. Commun., 2013, 49, 10184–10186 RSC.
- T. Kitagawa, J. Nishino, T. Inomata, T. Ozawa, Y. Funahashi and H. Masuda, Chem. Commun., 2016, 52, 4780–4783 RSC.
- T. Kitagawa, T. Yano, T. Inomata, T. Ozawa and H. Masuda, Chem. Lett., 2016, 45, 436–438 CrossRef CAS.
- G. Iijima, T. Kitagawa, A. Katayama, T. Inomata, H. Yamaguchi, K. Suzuki, K. Hirata, Y. Hijikata, M. Ito and H. Masuda, ACS Catal., 2018, 8, 1990–2000 CrossRef CAS.
- Z. Jin, H. Masuda, N. Yamanaka, M. Minami, T. Nakamura and Y. Nishikitani, J. Phys. Chem. C, 2009, 113, 2618–2623 CrossRef CAS.
- Z. Jin, H. Masuda, N. Yamanaka, M. Minami, T. Nakamura and Y. Nishikitani, ECS Trans., 2008, 16, 61–70 CrossRef.
- Z. Jin, H. Masuda, N. Yamanaka, M. Minami, T. Nakamura and Y. Nishikitani, ChemSusChem, 2008, 1, 901–904 CrossRef CAS PubMed.
- Z. Jin, H. Masuda, N. Yamanaka, M. Minami, T. Nakamura and Y. Nishikitani, Chem. Lett., 2009, 38, 44–45 CrossRef CAS.
- F. Fabregat-Santiago, G. Garcia-Belmonte, I. Mora-Sero and J. Bisquert, Phys. Chem. Chem. Phys., 2011, 13, 9083–9118 RSC.
- A. R. C. Bredar, A. L. Chown, A. R. Burton and B. H. Farnum, ACS Appl. Energy Mater., 2020, 3, 66–98 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03230a |
| This journal is © The Royal Society of Chemistry 2022 |
